Abstract
Objective
To determine the relation between exposure to glycaemia over time and the risk of macrovascular or microvascular complications in patients with type 2 diabetes.
Design
Prospective observational study.
Setting
23 hospital based clinics in England, Scotland, and Northern Ireland.
Participants
4585 white, Asian Indian, and Afro-Caribbean UKPDS patients, whether randomised or not to treatment, were included in analyses of incidence; of these, 3642 were included in analyses of relative risk.
Outcome measures
Primary predefined aggregate clinical outcomes: any end point or deaths related to diabetes and all cause mortality. Secondary aggregate outcomes: myocardial infarction, stroke, amputation (including death from peripheral vascular disease), and microvascular disease (predominantly retinal photo-coagulation). Single end points: non-fatal heart failure and cataract extraction. Risk reduction associated with a 1% reduction in updated mean HbA1c adjusted for possible confounders at diagnosis of diabetes.
Results
The incidence of clinical complications was significantly associated with glycaemia. Each 1% reduction in updated mean HbA1c was associated with reductions in risk of 21% for any end point related to diabetes (95% confidence interval 17% to 24%, P<0.0001), 21% for deaths related to diabetes (15% to 27%, P<0.0001), 14% for myocardial infarction (8% to 21%, P<0.0001), and 37% for microvascular complications (33% to 41%, P<0.0001). No threshold of risk was observed for any end point.
Conclusions
In patients with type 2 diabetes the risk of diabetic complications was strongly associated with previous hyperglycaemia. Any reduction in HbA1c is likely to reduce the risk of complications, with the lowest risk being in those with HbA1c values in the normal range (<6.0%).
Introduction
The UK prospective diabetes study (UKPDS), a clinical trial of a policy of intensive control of blood glucose after diagnosis of type 2 diabetes, which achieved a median haemoglobin A1c (HbA1c) of 7.0% compared with 7.9% in those allocated to conventional treatment over a median 10.0 years of follow up, has shown a substantial reduction in the risk of microvascular complications, with a reduction in the risk of myocardial infarction of borderline significance.1 Complementary information for estimates of the risk of complications at different levels of glycaemia can be obtained from observational analyses of data during the study.
In patients with type 2 diabetes previous prospective studies have shown an association between the degree of hyperglycaemia and increased risk of microvascular complications,2,3 sensory neuropathy,3,4 myocardial infarction,2,5,6 stroke,7 macrovascular mortality,8–10 and all cause mortality.9,11–14 Generally, these studies measured glycaemia as being high or low or assessed glycaemia on a single occasion, whereas repeated measurements of glycaemia over several years would be more informative.
The existence of thresholds of glycaemia—that is, concentrations above which the risk of complications markedly increases—has not been studied often in patients with type 2 diabetes. The relative risk for myocardial infarction seems to increase with any increase in glycaemia above the normal range,15,16 whereas the risk for microvascular disease is thought to occur only with more extreme concentrations of glycaemia.17–19 The diabetes control and complications trial (DCCT) research group showed an association between glycaemia and the progression of microvascular complications in patients with type 1 diabetes for haemoglobin A1c over the range of 6-11% after a mean of six years of follow up.20 No specific thresholds of glycaemia were identified above which patients were at greater risk of progression of retinopathy, increased urinary albumin excretion, or nephropathy.19–21 Nor has any threshold of fasting plasma glucose concentration been identified for cardiovascular deaths.22,23
We evaluated the relation between exposure to glycaemia over time and the development of macrovascular and microvascular complications and compared this with the results of the UKPDS trial of a policy of intensive control of blood glucose control.1
Methods
Participants recruited to the UKPDS
Details are presented in the companion paper (UKPDS 36) published in this issue (see page 412).
Participants in observational analysis
Of 5102 patients, 4585 white, Asian Indian, and Afro-Caribbean patients who had haemoglobin A1c (HbA1c) measured three months after the diagnosis of diabetes were included in analyses of incidence rates. Of these, 3642 with complete data for potential confounders were included in analyses of relative risk. Complete data were required for all participants included in the multivariate observational analyses. For this reason there are fewer (3642) participants in these analyses than in the clinical trial, despite the inclusion of patients not randomised in the trial. Their characteristics are presented in table 1.
Table 1.
Characteristics of patients included in proportional hazards model measured after three month dietary run-in after diagnosis of diabetes and those included in UKPDS glucose control study.1 Figures are means (SD) unless stated otherwise
| Proportional hazards model of observational data (n=3642) | Clinical trial of intensive v conventional blood glucose control policy (n=3867) | |
|---|---|---|
| Age (years) | 53 (8) | 53 (9) |
| Proportion of men (%) | 60 | 61 |
| Ethnicity (% white/Asian Indian/Afro-Caribbean/other) | 82/10/8/0 | 81/10/8/1 |
| Body mass index (kg/m2) | 27.7 (5.3) | 27.5 (5.2) |
| Fasting plasma glucose (mmol/l)* | 7.9 (6.6-10) | 8.0 (7.1-9.7) |
| Haemoglobin A1c (%) | 7.1 (1.8) | 7.1 (1.5) |
| Systolic blood pressure (mm Hg) | 135 (19) | 135 (20) |
| Low density lipoprotein cholesterol (mmol/l) | 3.5 (1.0) | 3.5 (1.0) |
| High density lipoprotein cholesterol (mmol/l) | 1.06 (0.24) | 1.07 (0.24) |
| Triglyceride (mmol/l)† | 1.5 (0.9-2.5) | 1.5 (0.9-2.5) |
| Albuminuria (%)‡ | 13.3 | 11.4 |
Median (interquartile range).
Geometric mean (1 SD range).
>50 mg/l in single morning sample.
Participants in UKPDS blood glucose control study
After a three month dietary run-in period patients were stratified on the basis of fasting plasma glucose concentration and body weight. The 3867 patients who had fasting plasma glucose concentrations between 6.1 and 15.0 mmol/l and no symptoms of hyperglycaemia were randomised to a policy of conventional glucose control, primarily with diet, or to an intensive policy with sulphonylurea or insulin.1,24–26 The aim in the group allocated to conventional control (n=1138) was to obtain fasting plasma glucose concentration <15 mmol/l, but if concentrations rose to ⩾15 mmol/l or symptoms of hyperglycaemia developed patients were secondarily randomised to non-intensive use of these pharmacological treatments, with the aim of achieving fasting plasma glucose concentrations <15 mmol/l without symptoms. The aim in the group allocated to intensive control (n=2729) was to achieve fasting plasma glucose concentration <6 mmol/l, primarily with a single pharmacological treatment. Details of treatments and their effect on glucose control have been published elsewhere.1
Biochemical methods
Biochemical methods have been reported previously.27 Haemoglobin A1c was measured by high performance liquid chromatography (Biorad Diamat automated glycosylated haemoglobin analyser), the range for people without diabetes being 4.5% to 6.2%.27,28 Baseline variables are quoted for measurements after the initial dietary run-in period.
Glycaemic exposure
Exposure to glycaemia was measured firstly at baseline as haemoglobin A1c concentration and secondly over time as an updated mean of annual measurements of haemoglobin A1c concentration, calculated for each individual from baseline to each year of follow up. For example, at one year the updated mean is the average of the baseline and one year values and at three years is the average of baseline, one year, two year, and three year values.
Clinical complications
The clinical end points and their definitions are shown in the box in the companion paper (UKPDS 36) published in this issue (see page 412).
Statistical analysis
Incidence rates by category of glycaemia
The unadjusted incidence rates were calculated by dividing the number of people with a given complication by the person years of follow up for the given complication within each category of updated mean haemoglobin A1c concentration and reported as events per 1000 years of follow up.29 The categories were defined (median values in parentheses) as: <6% (5.6%), 6-<7% (6.5%), 7-<8% (7.5%), 8-<9% (8.4%), 9-<10% (9.4%), and ⩾10% (10.6%) over the range of updated mean haemoglobin A1c of 4.6-11.2% (1st-99th centile). Follow up time was calculated from the end of the initial period of dietary treatment to the first occurrence of that complication or loss to follow up, death from another cause, or to the end of the study on 30 September 1997 for those who did not have that complication. Hence, follow up time is equivalent to duration of diabetes. For myocardial infarction and stroke for participants who had a non-fatal followed by a fatal event, the time to the first event was used. The rates were therefore for single and not recurrent events. The median follow up time for all cause mortality was 10.4 years.
We calculated adjusted incidence rates for each category of updated mean haemoglobin A1c using a Poisson regression model adjusted for male sex, white ethnic group, age at diagnosis 50-54 years, and duration of diabetes 7.5-12.5 years and expressed in events per 1000 person years of follow up. These parameters were chosen to reflect the median age and duration of diabetes and the modal ethnic group and sex.
Hazard ratio and risk reduction
To assess potential associations between updated mean haemoglobin A1c and complications we used proportional hazards regression (Cox) models. Potential confounding risk factors included in all Cox models were sex, age, ethnic group, smoking (current/ever/never) at time of diagnosis of diabetes, and baseline high and low density lipoprotein cholesterol, triglyceride, presence of albuminuria (> 50 mg/l measured in a single morning urine sample) measured after three months' dietary treatment, and systolic blood pressure represented by the mean of measures at two and nine months after diagnosis. The hazard ratio was used to estimate the relative risk. At each event time, the updated mean haemoglobin A1c value for individuals with an event was compared with the updated value of those who had not had an event by that time. The updated mean value was included as a time dependent covariate to evaluate glucose exposure during follow up.20,29,30 It was included as a categorical variable in the categories of glycaemia listed above, with the lowest category (<6%) as the reference category assigned a hazard ratio of 1.0 and with the highest category ⩾9%. (This is reflected in the point estimates as shown in figures 3 and 4.) Separate models, with updated mean haemoglobin A1c as a continuous variable, were used to determine reduction in risk associated with a 1% reduction in haemoglobin A1c (see regression lines in figures 3 and 4). We evaluated the presence of thresholds by visual inspection. The 95% confidence intervals were calculated on the basis of the floating absolute risk.31 Log linear relations are reported by convention.1,32 The risk reduction associated with a reduction of 1% updated mean haemoglobin A1c was calculated as 100% minus the reciprocal of the hazard ratio expressed as a percentage. The risk reduction from the continuous variable model associated with a 1% reduction in observed haemoglobin A1c was compared with the risk reduction seen in the UKPDS intervention trial of an intensive versus a conventional policy of blood glucose control, for which no adjustment for potential confounders was required as they were balanced by randomisation.1
Figure 3.
Hazard ratios, with 95% confidence intervals as floating absolute risks, as estimate of association between category of updated mean haemoglobin A1c concentration and any end point or deaths related to diabetes and all cause mortality. Reference category (hazard ratio 1.0) is haemoglobin A1c <6% with log linear scales. P value reflects contribution of glycaemia to multivariate model. Data adjusted for age at diagnosis of diabetes, sex, ethnic group, smoking, presence of albuminuria, systolic blood pressure, high and low density lipoprotein cholesterol, and triglycerides
Figure 4.
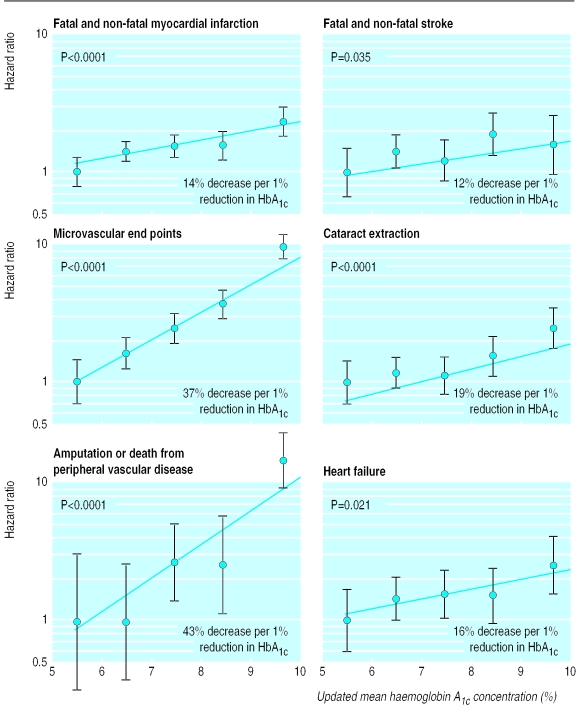
Hazard ratios, with 95% confidence intervals as floating absolute risks, as estimate of association between category of updated mean haemoglobin A1c concentration and myocardial infarction, stroke, microvascular end points, cataract extraction, lower extremity amputation or fatal peripheral vascular disease, and heart failure. Reference category (hazard ratio 1.0) is haemoglobin A1c <6% with log linear scales. P value reflects contribution of glycaemia to multivariate model. Data adjusted for age at diagnosis of diabetes, sex, ethnic group, smoking, presence of albuminuria, systolic blood pressure, high and low density lipoprotein cholesterol, and triglycerides
To assess whether the association between mean updated haemoglobin A1c and complications was independent of randomisation, separate models included mean updated haemoglobin A1c and randomisation to either intensive or conventional policy, as well as all potential confounders listed above. The model for all end points related to diabetes included 3005 individuals.
Statistical analyses were performed with SAS version 6.12.33
Results
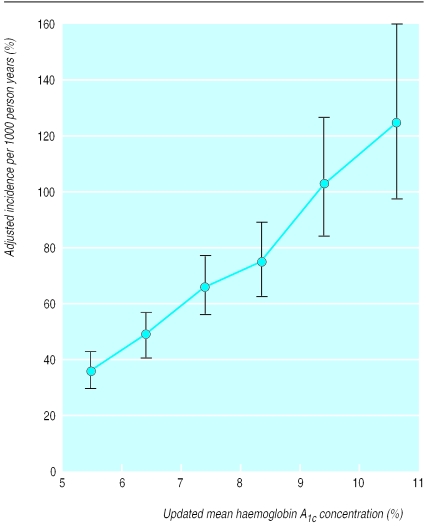
The risk of each of the microvascular and macrovascular complications of type 2 diabetes and cataract extraction was strongly associated with hyperglycaemia as measured by updated mean haemoglobin A1c. The incidence rates for any end point related to diabetes, adjusted for age, sex, ethnic group, and duration of diabetes, increased with each higher category of updated mean haemoglobin A1c, with no evidence of a threshold and with a threefold increase over the range of updated mean haemoglobin A1c of <6% (median 5.6%) to ⩾10% (median 10.6%) (figs 1 and 2). The unadjusted and adjusted incidence rates are shown in table 2. Figure 2 shows the adjusted incidence rates for myocardial infarction and microvascular end points. The increase in the incidence rate for microvascular end points was greater over the range of increasing glycaemia than was the increase in the incidence rate for myocardial infarction. Thus at near normal concentrations of updated mean haemoglobin A1c the risk of myocardial infarction was twice to three times that of a microvascular end point, whereas in the highest category of haemoglobin A1c concentration (⩾10%) the risks were of the same order.
Figure 1.
Incidence rate and 95% confidence intervals for any end point related to diabetes by category of updated mean haemoglobin A1c concentration, adjusted for age, sex, and ethnic group, expressed for white men aged 50-54 years at diagnosis and with mean duration of diabetes of 10 years
Figure 2.
Incidence rates and 95% confidence intervals for myocardial infarction and microvascular complications by category of updated mean haemoglobin A1c concentration, adjusted for age, sex, and ethnic group, expressed for white men aged 50-54 years at diagnosis and with mean duration of diabetes of 10 years
Table 2.
Incidence of complications in patients with type 2 diabetes by category of updated mean haemoglobin A1c concentration (%). Rates per 1000 person years' follow up adjusted in Poisson regression model to white men aged 50 to 54 years at diagnosis of diabetes and followed up for 7.5 to <12.5 years, termed “10 years” (n=4585)
| <6% | 6% to <7% | 7% to <8% | 8% to <9% | 9% to <10% | ⩾10% | |
|---|---|---|---|---|---|---|
| Aggregate end points | ||||||
| Complications related to diabetes: | ||||||
| Events/person years | 229/9195 | 391/11 432 | 369/8464 | 268/5605 | 159/2542 | 88/1334 |
| Unadjusted rate | 24.9 | 34.2 | 43.6 | 47.8 | 62.5 | 65.9 |
| Adjusted rate (95% CI) | 35.9 (29.9 to 43.1) | 48.7 (41.3 to 57.3) | 65.5 (55.5 to 77.2) | 74.5 (62.6 to 88.8) | 103.2 (84.2 to 126.5) | 124.9 (97.3 to 160.3) |
| Deaths related to diabetes: | ||||||
| Events/person years | 56/10 113 | 101/13 143 | 116/10 054 | 84/6595 | 47/3137 | 19/1537 |
| Unadjusted rate | 5.5 | 7.7 | 11.5 | 12.7 | 15.0 | 12.4 |
| Adjusted rate (95% CI) | 8.9 (6.3 to 12.7) | 12.0 (8.9 to 16.3) | 19.9 (14.8 to 26.7) | 23.5 (17.2 to 32.0) | 29.5 (20.4 to 42.6) | 33.0 (19.8 to 55.1) |
| All cause mortality: | ||||||
| Events/person years | 112/10 113 | 207/13 143 | 188/10 054 | 123/6595 | 64/3137 | 26/1537 |
| Unadjusted rate | 11.1 | 15.8 | 18.7 | 18.7 | 20.4 | 16.9 |
| Adjusted rate (95% CI) | 17.0 (13.1 to 22.0) | 23.3 (18.5 to 29.2) | 30.0 (23.8 to 37.7) | 31.8 (24.7 to 40.8) | 37.0 (27.3 to 50.2) | 40.7 (26.5 to 64.5) |
| Fatal or non-fatal myocardial infarction: | ||||||
| Events/person years | 100/9870 | 163/12 590 | 159/9579 | 101/6331 | 60/3016 | 23/1490 |
| Unadjusted rate | 10.1 | 13.0 | 16.6 | 16.0 | 19.9 | 15.4 |
| Adjusted rate (95% CI) | 16.0 (12.1 to 21.2) | 20.8 (16.2 to 26.7) | 29.2 (22.8 to 37.4) | 30.0 (22.9 to 39.4) | 39.6 (28.8 to 54.5) | 38.6 (24.4 to 61.0) |
| Fatal or non-fatal stroke: | ||||||
| Events/person years | 32/9916 | 67/12 869 | 59/9822 | 32/6424 | 13/3062 | 9/1509 |
| Unadjusted rate | 3.2 | 5.2 | 6.0 | 5.0 | 4.2 | 6.0 |
| Adjusted rate (95% CI) | 4.3 (2.6 to 7.0) | 6.6 (4.4 to 10.1) | 8.3 (5.4 to 12.7) | 7.4 (4.5 to 11.9) | 6.7 (3.5 to 12.7) | 12.0 (5.7 to 25.3) |
| Amputation or death from peripheral vascular disease: | ||||||
| Events/person years | 3/10 018 | 7/12 993 | 7/9897 | 9/6492 | 15/3061 | 7/1502 |
| Unadjusted rate | 0.3 | 0.5 | 0.7 | 1.4 | 4.9 | 4.7 |
| Adjusted rate (95% CI) | 1.2 (0.4 to 3.2) | 1.2 (0.5 to 3.1) | 2.6 (1.1 to 5.8) | 4.0 (1.8 to 9.0) | 10.9 (5.0 to 23.7) | 12.2 (4.6 to 32.4) |
| Fatal or non-fatal microvascular disease: | ||||||
| Events/person years | 38/9814 | 77/12 707 | 86/9438 | 91/6185 | 73/2855 | 47/1432 |
| Unadjusted rate | 3.9 | 6.1 | 9.1 | 14.7 | 25.6 | 32.8 |
| Adjusted rate (95% CI) | 6.1 (4.1 to 9.0) | 9.3 (6.7 to 12.9) | 14.2 (10.3 to 19.5) | 22.8 (16.7 to 31.3) | 40.4 (28.9 to 56.5) | 57.8 (39.3 to 85.1) |
| Single end points | ||||||
| Heart failure: | ||||||
| Events/person years | 17/9967 | 34/12 928 | 36/9782 | 20/6432 | 10/3062 | 10/1514 |
| Unadjusted rate | 1.7 | 2.6 | 3.7 | 3.1 | 3.3 | 6.6 |
| Adjusted rate (95% CI) | 2.3 (1.2 to 4.5) | 3.4 (1.9 to 5.8) | 5.0 (2.9 to 8.6) | 4.4 (2.4 to 8.2) | 5.0 (2.3 to 10.6) | 11.9 (5.5 to 25.8) |
| Cataract extraction: | ||||||
| Events/person years | 35/9841 | 59/12 763 | 49/9692 | 45/6355 | 19/3009 | 19/1495 |
| Unadjusted rate | 3.6 | 4.6 | 5.1 | 7.1 | 6.3 | 12.7 |
| Adjusted rate (95% CI) | 4.1 (2.5 to 6.5) | 4.5 (3.0 to 6.9) | 4.9 (3.1 to 7.6) | 6.9 (4.4 to 10.8) | 6.6 (3.8 to 11.6) | 14.4 (8.1 to 25.7) |
Person years, events, and unadjusted rates are for all patients.
The estimated hazard ratios associated with different categories of updated mean haemoglobin A1c concentration, relative to the lowest category, are shown as log linear plots in figures 3 and 4. Mortality related to diabetes and all cause mortality were both strongly associated with glycaemia (P<0.0001). The risk of each of the complications evaluated rose with increasing updated mean haemoglobin A1c concentration both before and after adjustment for baseline variables including age, sex, ethnic group, lipid concentrations, blood pressure, smoking, and albuminuria. The decrease in risk for each 1% reduction in updated mean haemoglobin A1c concentration is shown in table 3 and figures 3 and 4. The glycaemia associated reduction in risk for microvascular end points and for amputation or death from peripheral vascular disease was greater (by 37% and 43% per 1% reduction in haemoglobin A1c concentration, respectively, each P<0.0001) than it was for myocardial infarction, stroke, and heart failure (by 14% (P<0.0001), 12% (P=0.035), and 16% (P=0.021) per 1% haemoglobin A1c, respectively) (fig 4). In models that included a variable for conventional control of blood glucose or intensive control with either sulphonylurea or insulin, updated mean haemoglobin A1c remained associated with all complications, although for stroke and heart failure, where the numbers of events were lower than in the previous analyses, these were no longer significant. In these models, treatment of blood glucose per se had no association with any complication beyond that of mean updated haemoglobin A1c.
Table 3.
Observational analysis of relation between glycaemic exposure and complications of diabetes as estimated by decrease in risk for 1% reduction in haemoglobin A1c (HbA1c) concentration, measured at baseline and as updated mean, controlled for age at diagnosis of diabetes, sex, ethnic group, smoking, albuminuria, systolic blood pressure, high and low density lipoprotein cholesterol, and triglycerides (n=3642) compared with results of clinical trial of intensive v conventional glucose control policy (n=3867)1
| Observational analysis
|
Clinical trial of intensive v conventional policy1
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No of events | Baseline HbA1c
|
Updated mean HbA1c
|
No of events | Decrease in risk (%) seen for 0.9% difference in HbA1c (95% CI) | P value | |||||
| Decrease in risk (%)/1% reduction (95% CI) | P value | Decrease in risk (%)/1% reduction (95% CI) | P value | |||||||
| Aggregate end points | ||||||||||
| Any end point related to diabetes | 1255 | 11 (8 to 13) | <0.0001 | 21 (17 to 24) | <0.0001 | 1401 | 12 (1 to 21) | 0.029 | ||
| Deaths related to diabetes | 346 | 9 (3 to 14) | 0.0018 | 21 (15 to 27) | <0.0001 | 414 | 10 (−11 to 27) | 0.34 | ||
| All cause mortality | 597 | 6 (2 to 10) | 0.0081 | 14 (9 to 19) | <0.0001 | 702 | 6 (−10 to 20) | 0.44 | ||
| Myocardial infarction | 496 | 5 (0 to 9) | 0.067 | 14 (8 to 21) | <0.0001 | 573 | 16 (0 to 29) | 0.052 | ||
| Stroke | 162 | −4 (−14 to 6) | 0.44 | 12 (1 to 21) | 0.035 | 203 | −11 (−49 to 19) | 0.52 | ||
| Peripheral vascular disease* | 41 | 28 (18 to 37) | <0.0001 | 43 (31 to 53) | <0.0001 | 47 | 35 (−18 to 64) | 0.15 | ||
| Microvascular disease | 323 | 23 (20 to 27) | <0.0001 | 37 (33 to 41) | <0.0001 | 346 | 25 (7 to 40) | 0.0099 | ||
| Single end points | ||||||||||
| Heart failure | 104 | 0 (−12 to 11) | 0.99 | 16 (3 to 26) | 0.016 | 116 | 9 (−35 to 39) | 0.63 | ||
| Cataract extraction | 195 | 9 (2 to 16) | 0.013 | 19 (11 to 26) | <0.0001 | 229 | 24 (0 to 42) | 0.046 | ||
Lower extremity amputation or fatal peripheral vascular disease.
There was no indication of a threshold for any complication below which risk no longer decreased nor a level above which risk no longer increased. The updated mean haemoglobin A1c showed steeper relations than did baseline haemoglobin A1c (table 3), and when both glycaemic variables were included in a model for all complications of diabetes only updated mean haemoglobin A1c reached significance (P<0.0001).
Discussion
This observational analysis shows highly significant associations between the development of each of the complications of diabetes, including mortality, across the wide range of exposure to glycaemia that occurs in patients with type 2 diabetes. This association remained after adjustment for other known risk factors, including age at diagnosis, sex, ethnic group, systolic blood pressure, lipid concentrations, smoking, and albuminuria. Each 1% reduction in haemoglobin A1c was associated with a 37% decrease in risk for microvascular complications and a 21% decrease in the risk of any end point or death related to diabetes. The association with glycaemia was less steep for stroke and heart failure, for which blood pressure is a major contributing factor.32,34,35 In patients within the lowest category of updated mean haemoglobin A1c the incidence of myocardial infarction was higher than that of microvascular disease.5 These results suggest that, in these people, the effect of hyperglycaemia itself may account for at least part of the excess cardiovascular risk observed in diabetic compared with non-diabetic people beyond that explained by the conventional risk factors of dyslipidaemia, hypertension, and smoking.36 The rate of increase of relative risk for microvascular disease with hyperglycaemia was greater than that for myocardial infarction, which emphasises the crucial role of hyperglycaemia in the aetiology of small vessel disease and may explain the greater rate of microvascular complications seen in populations with less satisfactory control of glycaemia.
Relation to trial data
This observational analysis provides an estimate of the reduction in risk that might be achieved by the therapeutic lowering of haemoglobin A1c by 1.0%, but it is important to realise that epidemiological associations cannot necessarily be transferred to clinical practice. Tissue damage from previous hyperglycaemia may not promptly be overcome, but the results are not inconsistent with those achieved by the policy of intensive glucose control in the clinical trial.1 This suggests that the reduction in glycaemia obtained over a median 10 years of follow up of the trial, comparing median haemoglobin A1c 7.0% with 7.9%, provided much of the benefit that could be expected from that degree of improved glycaemic control. Our results suggest that intensive treatment with sulphonylurea or insulin does not have an effect beyond that of lowering blood glucose concentration with respect to altering risk. The 16% risk reduction (P=0.052) in myocardial infarction in the clinical trial in the group allocated to a policy of intensive blood glucose control (associated with a 0.9% difference in haemoglobin A1c) was similar to the 14% risk reduction seen in the epidemiological analysis, which was associated with a 1% reduction in concentration of updated mean haemoglobin A1c. The UKPDS clinical trial evaluated a policy of intensive glucose control based primarily on single pharmacological treatments to enable evaluation of the individual treatments. Now that the UKPDS has shown that improved glucose control reduces the risk of complications and that the treaments used are safe in clinical practice, a larger reduction in haemoglobin A1c might be achieved by the earlier use of combination treatments or by the use of newer treatments, which could further reduce the risk of myocardial infarction.
What is already known on this topic
The risk of developing complications of diabetes increases with increasing concentrations of hyperglycaemia
Reduction of hyperglycaemia in these individuals reduces the risk of complications
What this study adds
There is a direct relation between the risk of complications of diabetes and glycaemia over time
No threshold of glycaemia was observed for a substantive change in risk for any of the clinical outcomes examined
The lower the glycaemia the lower the risk of complications
The rate of increase of risk for microvascular disease with hyperglycaemia is greater than that for macrovascular disease
The observational analysis extends the range of hyperglycaemia studied in the UKPDS by including participants who, throughout the study, had near normal glucose concentrations on dietary treatment alone and participants who could never be treated by dietary treatment alone.37 The UKPDS population was likely to be at lower risk of complications than other diabetic populations. Hence, the incidence rates we report are perhaps lower than might be observed in other diabetic populations as the cohort was newly diagnosed with diabetes, excluded old or ill patients, and contained a small proportion (6%) of participants with impaired fasting glycaemia.38 None the less, the decrease in relative risk is unlikely to be different from other diabetic populations.
Lack of thresholds
We observed no thresholds of glycaemia for any type of complication of diabetes. This suggests that there is no specific target value of haemoglobin A1c for which one should aim but that the nearer to normal the haemoglobin A1c concentration the better. In reality, it is difficult to obtain and maintain near normal concentrations of haemoglobin A1c in patients with type 2 diabetes, particularly in those with a high concentration of haemoglobin A1c at diagnosis of diabetes.37 Intensification of treatment by adding insulin to improve the relatively modest reduction in glycaemia achieved with oral hypoglycaemic treatments can be constrained by reluctance from patients and providers because, in part, of side effects such as hypoglycaemia or weight gain. These observational analyses, together with the results of the clinical trial, however, indicate that any improvement in a raised haemoglobin A1c concentration is likely to reduce the risk of diabetic complications.
The magnitude of the risk reduction associated with a 1% reduction in haemoglobin A1c concentration for myocardial infarction and microvascular disease (mostly retinopathy) was consistent with that observed in a cohort of patients from Wisconsin.2 As in this analysis, a stronger association with haemoglobin A1c concentration was observed for amputation than for ischaemic heart disease, possibly because glycaemia increases the risk of microvascular disease, neuropathy, and peripheral arterial disease, each of which increases the risk of amputation.4,8,18,39–41 The estimated 14% decrease in all cause mortality per 1% reduction in haemoglobin A1c concentration was similar to that seen in other studies that have assessed glycaemia as haemoglobin A1c as a continuous variable (per 1% change) in multivariate proportional hazards models.9
Summary
Both the observational and clinical trial analyses of an intensive glucose control policy suggest that even a modest reduction in glycaemia has the potential to prevent deaths from complications related to diabetes as cardiovascular and cerebrovascular disease account for 50-60% of all mortality in this and other diabetic populations.8,42–47 Individuals with very high concentrations of glycaemia would be most likely to benefit from reduction of glycaemia as they are particularly at risk from the complications of type 2 diabetes, but the data suggest that any improvement in glycaemic control across the diabetic range is likely to reduce the risk of diabetic complications.
Supplementary Material
Acknowledgments
The cooperation of the patients and many NHS and non-NHS staff at the centres is much appreciated. We thank Mr Dick Jelfs for the measurement of haemoglobin A1c. Details of participating centres can be found on the BMJ 's website.
Editorial by Tuomilehto
Footnotes
Professor Turner died unexpectedly after completing work on this paper
Funding: The major grants for this study were from the UK Medical Research Council, the British Diabetic Association, the UK Department of Health, The National Eye Institute and The National Institute of Digestive, Diabetes and Kidney Disease in the National Institutes of Health, United States, The British Heart Foundation, Novo Nordisk, Bayer, Bristol-Myers Squibb, Hoechst, Lilly, Lipha, and Farmitalia Carlo Erba. Details of other funding companies and agencies, the supervising committees, and all participating staff can be found on the BMJ's website.
Competing interests: AIA has received fees for speaking from Bristol-Myers Squibb, SmithKline Beecham, and Pfizer. IMS has received support for attending conferences from Zeneca and Hoechst and fees for speaking from Hoechst. CAC has received support for attending conferences from Bristol-Myers Squibb, Novo Nordisk, and Pfizer and fees for speaking from Bristol-Myers Squibb and Novo Nordisk. DRM has received fees for speaking from Bristol-Myers Squibb, Novo Nordisk, SmithKline Beecham, and Lilly and research funding from Lilly. SEM has received support for attending conferences from Bayer and Novo Nordisk. RRH has received fees for consulting from Bayer, Boehringer Mannheim, Bristol-Myers Squibb, Hoechst, Lilly, Novo Nordisk, Pfizer, and SmithKline Beecham; support for attending conferences from Bayer, Bristol-Myers Squibb, Hoechst, Lilly, Lipha, Novo Nordisk, and SmithKline Beecham; and research funding from Bayer, Bristol-Myers Squibb, Lilly, Lipha, and Novo Nordisk.
Details of participating centres, staff, and committees and additional funding agencies are on the BMJ's website.
References
- 1.UKPDS Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2.Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18:258–268. doi: 10.2337/diacare.18.2.258. [DOI] [PubMed] [Google Scholar]
- 3.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973 (part 1) Diabetes Care. 1978;1:168–188. [Google Scholar]
- 4.Adler AI, Boyko EJ, Ahroni AJ, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle prospective diabetic foot study. Diabetes Care. 1997;20:1162–1167. doi: 10.2337/diacare.20.7.1162. [DOI] [PubMed] [Google Scholar]
- 5.UKPDS Group. Risk factors for coronary artery disease in non-insulin dependent diabetes (UKPDS 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuusisto J, Mykkänen L, Pyörälä K, Laakso M. NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes. 1994;43:960–967. doi: 10.2337/diab.43.8.960. [DOI] [PubMed] [Google Scholar]
- 7.Lehto S, Ronnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke. 1996;27:63–68. doi: 10.1161/01.str.27.1.63. [DOI] [PubMed] [Google Scholar]
- 8.Standl E, Balletshofer B, Dahl B, Weichenhain B, Stiegler H, Hormann A, et al. Predictors of 10-year macrovascular and overall mortality in patients with NIDDM: the Munich general practitioner project. Diabetologia. 1996;39:1540–1545. doi: 10.1007/s001250050612. [DOI] [PubMed] [Google Scholar]
- 9.Groeneveld Y, Petri H, Hermans J, Springer MP. Relationship between blood glucose level and mortality in type 2 diabetes mellitus: a systematic review. Diabet Med. 1999;116:2–13. doi: 10.1046/j.1464-5491.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 10.Uusitupa MI, Niskanen LK, Siitonen O, Voutilainen E, Pyörälä K. Ten-year cardiovascular mortality in relation to risk factors and abnormalities in lipoprotein composition in type 2 (non-insulin-dependent) diabetic and non-diabetic subjects. Diabetologia. 1993;36:1175–1184. doi: 10.1007/BF00401063. [DOI] [PubMed] [Google Scholar]
- 11.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycaemia on all-cause and cardiovascular mortality. Diabetes Care. 1998;21:1167–1172. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 12.Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, Schmechel H, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the diabetes intervention study, 11-year follow-up. Diabetologia. 1996;39:1577–1583. doi: 10.1007/s001250050617. [DOI] [PubMed] [Google Scholar]
- 13.Knuiman MW, Welborn TA, Whittall DE. An analysis of excess mortality rates for persons with non-insulin-dependent diabetes mellitus in Western Australia using the Cox proportional hazards regression model. Am J Epidemiol. 1992;135:638–648. doi: 10.1093/oxfordjournals.aje.a116343. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki A, Uehara M, Horiuchi N, Hasegawa K. A long-term follow-up study of diabetic patients in Osaka, Japan: mortality and causes of death. Tohoku J Exp Med. 1983;141(suppl):639–644. [PubMed] [Google Scholar]
- 15.Balkau B, Shipley M, Jarrett RJ, Pyorala K, Pyorala M, Forhan A, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. Diabetes Care. 1998;21:360–367. doi: 10.2337/diacare.21.3.360. [DOI] [PubMed] [Google Scholar]
- 16.Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: the Whitehall study. BMJ. 1983;287:867–870. doi: 10.1136/bmj.287.6396.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarrett RJ, Keen H. Hyperglycaemia and diabetes mellitus. Lancet. 1976;ii:1009–1012. doi: 10.1016/s0140-6736(76)90844-8. [DOI] [PubMed] [Google Scholar]
- 18.Pettitt DJ, Knowler WC, Lisse JR, Bennett PH. Development of retinopathy and proteinuria in relation to plasma glucose concentration in Pima Indians. Lancet. 1980;ii:1050–1052. doi: 10.1016/s0140-6736(80)92274-6. [DOI] [PubMed] [Google Scholar]
- 19.Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;332:1251–1255. doi: 10.1056/NEJM199505113321902. [DOI] [PubMed] [Google Scholar]
- 20.DCCT Research Group. The absence of a glycemic threshold for the development of long-term complications: the perspective of the diabetes control and complications trial. Diabetes. 1996;45:1289–1298. [PubMed] [Google Scholar]
- 21.Orchard T, Forrest K, Ellis D, Becker D. Cumulative glycemic exposure and microvascular complications in insulin-dependent diabetes mellitus. Arch Intern Med. 1997;157:1851–1856. [PubMed] [Google Scholar]
- 22.Balkau B, Bertrais S, Ducimitière P, Eschwège E. Is there a glycemic threshold for mortality risk? Diabetes Care. 1999;22:696–699. doi: 10.2337/diacare.22.5.696. [DOI] [PubMed] [Google Scholar]
- 23.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 24.UKPDS Group. UK prospective diabetes study VIII: study design, progress and performance. Diabetologia. 1991;34:877–890. [PubMed] [Google Scholar]
- 25.Manley SE, Cull CA, Holman RR. Relation of fasting plasma glucose on patients with type 2 diabetes in UKPDS randomised to and treated with diet or oral agents. Diabetes. 2000;49(suppl 1):A180. [Google Scholar]
- 26.UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 27.UKPDS Group. UK prospective diabetes study XI: biochemical risk factors in type 2 diabetic patients at diagnosis compared with age-matched normal subjects. Diabet Med. 1994;11:534–544. [PubMed] [Google Scholar]
- 28.Cull CA, Manley SE, Stratton IM, Neil HAW, Ross IS, Holman RR, et al. Approach to maintaining comparability of biochemical data during long-term clinical trials. Clin Chem. 1997;43:1913–1918. [PubMed] [Google Scholar]
- 29.Breslow NE, Day NE. The design and analysis of cohort studies. Statistical methods in cancer research II. Oxford: Oxford University Press; 1987. [PubMed] [Google Scholar]
- 30.Agresti A. Categorical data analysis. New York: Wiley; 1990. [Google Scholar]
- 31.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 32.UKPDS Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes (UKPDS 38) BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 33.SAS. Version 6. Cary, North Carolina: SAS Institute; 1990. [Google Scholar]
- 34.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1: prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 35.Adler AI, Stratton IM, Neil HAW, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamler J. Epidemiology, established major risk factors and the primary prevention of coronary heart disease. In: Parmley WW, Chatterjee K, editors. Cardiology. Philadelphia: JB Lippincott; 1987. pp. 1–41. [Google Scholar]
- 37.UKPDS Group. UK prospective diabetes study 24: relative efficacy of sulfonylurea, insulin and metformin therapy in newly diagnosed non-insulin dependent diabetes with primary diet failure followed for six years. Ann Intern Med. 1998;128:165–175. doi: 10.7326/0003-4819-128-3-199802010-00001. [DOI] [PubMed] [Google Scholar]
- 38.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1998;21(suppl 1):5–19. [Google Scholar]
- 39.Franklin GF, Shetterly SM, Cohen JA, Baxter J, Hamman RF. Risk factors for distal symmetric neuropathy in NIDDM. The San Luis Valley diabetes study. Diabetes Care 1994:1172-7. [DOI] [PubMed]
- 40.Harris M, Eastman R, Cowie C. Symptoms of sensory neuropathy in adults with NIDDM in the US population. Diabetes Care. 1993;16:1446–1452. doi: 10.2337/diacare.16.11.1446. [DOI] [PubMed] [Google Scholar]
- 41.Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower extremity amputation in diabetes mellitus: the independent effects of peripheral vascular disease, sensory neuropathy and foot ulcers. Diabetes Care. 1999;22:1029–1035. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- 42.Adler AI, Matthews D, Holman RR, Turner RC. Type 2 diabetes and death: causes, estimated life expectancy and mortality rates—the UK prospective diabetes study. Diabetes. 1998;47(suppl 1):A71. [Google Scholar]
- 43.Palumbo PJ, Elveback LR, Chu CP, Connolly DC, Kurland LT. Diabetes mellitus: incidence, prevalence, survivorship and causes of death in Rochester, Minnesota, 1945-1970. Diabetes. 1976;25:566–573. doi: 10.2337/diab.25.7.566. [DOI] [PubMed] [Google Scholar]
- 44.Panzram G. Mortality and survival in type 2 (non-insulin-dependent) diabetes mellitus. Diabetelogia. 1987;30:123–131. doi: 10.1007/BF00274216. [DOI] [PubMed] [Google Scholar]
- 45.Goodkin G. Mortality in diabetes. A 20 year mortality study. J Occup Med. 1975;17:716–721. [PubMed] [Google Scholar]
- 46.Wetterhall SF, Olson DR, DeStefano F, Stevenson JM, Ford ES, German RR, et al. Trends in diabetes and diabetic complications, 1980-1987. Diabetes Care. 1992;15:960–967. doi: 10.2337/diacare.15.8.960. [DOI] [PubMed] [Google Scholar]
- 47.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12 year cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.