Abstract
Purpose
To review institutional outcomes for patients treated for differentiated thyroid cancer with post-operative conformal external beam radiotherapy (EBRT).
Methods
This is a single institution retrospective review of one hundred thirty-one consecutive patients with differentiated thyroid cancer who underwent EBRT between 1/1996 and 12/2005. Histologic diagnoses included 104 papillary, 21 follicular, and 6 mixed papillary-follicular. AJCC stage distribution was 2 stage III, 128 stage IVa–c, and not accessible in 1. Thirty-four (26%) patients had high-risk histology and 76 (58%) had recurrent disease. Extraglandular disease spread was seen in 126 (96%) cases, microscopically positive surgical margins in 62 (47%) and gross residual disease in 15 (11%). Median EBRT dose was 60 Gy [range: 38–72 Gy]. Fifty-seven (44%) patients were treated with IMRT to a median dose of 60 Gy [range: 56–66 Gy]. Median follow-up was 38 months (range: 0–134).
Results
Kaplan-Meier estimates of locoregional relapse free survival (LRFS), disease specific survival (DSS), and overall survival (OS) at 4 years were 79%, 76%, and 73%, respectively. On multivariate analysis, high-risk histologic features and gross residual disease predicted for inferior LRFS, while high-risk histologic features, M1 disease, and gross residual disease predicted for inferior DSS and OS. IMRT did not impact survival outcomes, but was associated with less frequent severe late morbidity (12% vs.2%).
Conclusions
Post-operative conformal EBRT provides durable locoregional disease control for high-risk differentiated thyroid cancer if disease is reduced to microscopic burden. Patients with gross disease face significantly worse outcomes. IMRT may significantly reduce chronic radiation morbidity, but requires further study.
Keywords: Thyroid Cancer, Differentiated, Radiotherapy, IMRT, Conformal, Postoperative, Adjuvant
Introduction
Thyroid cancer constitutes 1% of newly diagnosed cancers in the United States, representing over 20,000 cases each year. More than 90% of patients present with differentiated disease, which encompasses papillary, follicular, or mixed papillary-follicular histologies. The cornerstone of management is surgery, complemented by ablative radioactive iodine treatment as indicated by adverse histopathologic or clinical risk factors.
Post-operative external beam radiotherapy (EBRT) potentially improves locoregional disease control in the setting of incompletely resected high-risk disease 1–14. However, available data originate from retrospective institutional series with varying case selection criteria. Conflicting data from a small number of older reports 15–17 also suggest that adjuvant radiotherapy may not necessarily improve treatment outcomes. This has led to continuing controversy regarding the utility of adjuvant EBRT, especially for patients following their first surgical procedure and/or with microscopic residual disease. Our general institutional practice has been to offer postoperative EBRT to patients with recurrent disease and/or gross/microscopic residual disease, especially diffusely infiltrative disease which would preclude reoperation for subsequent recurrences.
The challenging location of the thyroid bed plays a key role in determining the efficacy and therapeutic ratio of post-operative radiotherapy. The distribution of surgical dissection necessitated by locally advanced disease requires a large, irregularly shaped treatment volume which includes or closely approximates normal structures in the central neck compartment and superior mediastinum. Parotid glands and skull base may also be at risk for radiation related morbidity in cases of diffuse cervical nodal involvement. The geometry of the thyroid operative bed has historically led to undesirable dose reductions and/or treatment morbidity with older radiotherapy techniques. These challenges can now be better met with modern radiation delivery methods such as three-dimensionally guided radiation therapy (3DRT) and, more recently, intensity modulated radiation therapy (IMRT). In this report, we review our institutional treatment and morbidity outcomes for differentiated thyroid cancer patients treated with these conformal techniques over a ten-year period following 1995.
Methods
After approval from our Institutional Review Board, we retrospectively reviewed the medical records of 239 consecutive patients who presented to our department for definitive treatment following surgical management of thyroid malignancy between January 1996 and December 2005. We excluded 108 patients with non-differentiated histologies (including anaplastic, medullary, unclassified, or lymphoma) from analysis, yielding a study cohort of 131 patients. High-risk histology was defined as Hurthle cell, tall cell, clear cell, or poorly differentiated features.
All patients received 3DRT or IMRT following CT-based simulation. CT imaging included the entire thorax to permit incorporation of both lung volumes into treatment planning. Every case in this series was formally presented to members of the full head and neck clinical service for physical examination, discussion, and quality assurance.
Seventy-four patients received conventional EBRT via extended opposed anterior/posterior (AP/PA) fields supplemented by off-cord photon boost fields or en face electron fields after delivery of 40–45 Gy. Fifty-seven patients received intensity modulated radiotherapy (IMRT) after 7/2000. With either technique, the superior mediastinum was treated to a prophylactic dose of 45–50 Gy. Definitive post-operative doses and extended inferior coverage of the middle or inferior mediastinum was pursued if these regions were involved with disease and/or if the patient underwent mediastinal nodal dissection.
IMRT was delivered via a step-and-shoot, multileaf collimation through a static treatment gantry using a mono-isocentric technique. From 7/2000 through 8/2003, IMRT treatment planning was performed with a CORVUS treatment planning system (CORVUS v.4.0, Nomos Corporation, Pittsburgh, PA). A Pinnacle3 system (version 6.2b or later, Philips Medical Systems, Andover, MA) was used after 8/2003. Regions of grossly positive margins or gross residual disease were typically prescribed 63–66 Gy in 30 daily fractions to a high dose clinical target volume (CTV)1. An intermediate dose CTV2 encompassing regions of the operative bed directly involved with disease, as well as immediately adjacent soft tissues and draining nodal basins were treated to a dose of 60 Gy in 30 daily fractions. An adjuvant dose of 56 Gy in 30 daily fractions was prescribed to a CTV3 encompassing the entirety of the surgical resection bed with a 1–2 cm (minimum 0.5 cm) margin. Finally, prophylactic coverage of at-risk cervical and mediastinal nodal stations was provided by a CTV4 treated to 54 Gy in 30 daily fractions. A representative treatment plan is demonstrated in Figure 1. Constraints for normal tissue structures were set at 45 Gy for spinal cord, mean dose of 26 Gy for parotid glands, and 20 Gy to whole lungs, while larynx and esophagus were contoured as avoidance structures with low priority weighting to prevent compromising intended CTV1 or CTV2 coverage. The mean dose delivered to CTV1 ranged from 62.1 to 74.9 Gy [median: 68.6 Gy], while the mean dose delivered to CTV2 ranged from 59.8 to 72.6 Gy [median: 62.4 Gy]. The mean percentage of the prescribed dose delivered to CTV1 ranged from 101.0% to 107.9% [mean: 103.4%]. The volume of CTV1 receiving less than the prescribed dose ranged from 0.0% to 7.7% [mean: 0.9%].
Figure 1. Representative IMRT treatment plan.
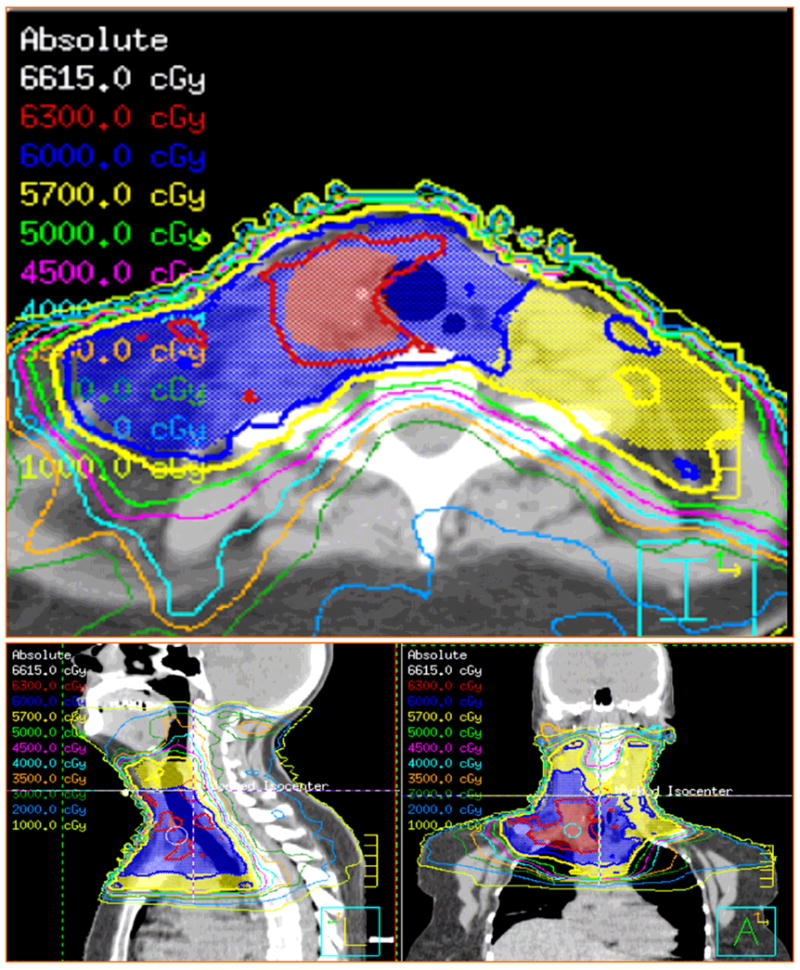
IMRT treatment plan for a 52 year old female patient with papillary thyroid carcinoma initially treated via thyroidectomy, bilateral paratracheal dissection, left-sided cervical neck dissection, and radioactive iodine ablation at an outside hospital. Patient presented to our institution with a recurrent 7.5 cm paratracheal mass inferior to the right clavicular head, which was grossly resected via a combined neck and thoracic surgical approach. Surgical margins were close, and disease was noted to infiltrate into soft tissues. An accompanying right-sided cervical neck dissection confirmed 5/22 sampled nodes from levels 3–4 to contain metastatic disease with extracapsular extension. Patient subsequently received 63 Gy IMRT to a CTV1 (demarcated red) encompassing the surgical bed directly involved with gross disease, 60 Gy (blue) to a CTV2 encompassing adjacent soft tissues and right-sided neck dissection bed, and 57 Gy to a CTV3 encompassing the high right-sided cervical neck, previously dissected left cervical neck, and superior mediastinum. All doses were delivered over 30 daily fractions.
The endpoints of this study were local relapse-free survival (LRFS), disease specific survival (DSS), and overall survival (OS). These were defined from the date of completion of radiotherapy and were estimated by the Kaplan-Meier method. Any evidence of residual disease following irradiation of gross disease was scored as an immediate local failure. Univariate correlates were determined using the log-rank test and Cox proportional hazards testing. Multivariate analysis was performed using Cox proportional hazards models. We included covariates in our multivariate models if they were significant on univariate analysis to a p-value corrected for multiple comparisons by the Bonferroni method (p-value ≤ 0.004, derived from p = 0.05/12 univariate tests). To minimize bias and inaccuracy with retrospective grading of post-treatment morbidity, late radiation-related toxicity occurring 3 months or later following completion of treatment was defined according to three-point scale as absent, minor-to-moderate, or severe. The latter designation was assigned if the patient was hospitalized for medical management, required surgical intervention, or died as a direct result of a late radiotherapy-related morbid event (consistent with a toxicity grade of 4 or greater). Analyses were performed using StatView v.5 (SAS Institute, Inc) and significance determined at a p-value < 0.05, with the exception of a Bonferroni corrected p-value < 0.004 which was used for populating our proportional hazards models, as described above.
Results
Patients
Patient characteristics are summarized in Table 1. Median age was 57 years (range: 18–83, with 106 (81%) patients ≥ 45 years old). There were 75 (57%) male and 56 (43%) female patients. Disease histology consisted of 104 (79%) papillary, 21 (16%) follicular, and 6 (5%) mixed papillary-follicular cancers. Thirty-four (26%) patients had high-risk histology (12 Hurthle cell, 11 poorly differentiated, 9 tall cell, and 2 clear cell). Seventeen (81%) of the 21 patients with follicular disease had high-risk histologic features. Seventy-six (58%) patients had recurrent disease at presentation to our institution; these patients underwent a median of 2 (range: 1–6) definitive surgical resections prior to radiotherapy. Forty-four (34%) patients presented for definitive surgery at initial diagnosis, and 9 (7%) patients presented for additional treatment following incomplete initial surgical management at an outside facility. There were 5 T2, 11 T3, 109 T4, and 6 Tx stage cases. Ninety-five (73%) patients had confirmed node positive disease with a median number of 3 positive nodes (range: 1–47), and 37 (28%) patients were M1. AJCC stage distribution was 2 stage III, 128 stage IVa–c, and not accessible in 1 case. Extraglandular disease spread from primary tumor was present in 126 (96%) cases, and positive surgical margins were present in 62 (47%). Forty-two (44%) of N1 patients had nodal extracapsular disease spread.
Table 1.
Characteristics of Study Cohort
| N (%) | |
|---|---|
| Gender | |
| Male | 75 (57) |
| Female | 56 (43) |
| Age | |
| Median | 57 years |
| Range | 18–83 years |
| ≥45 Years-Old | 106 (81) |
| Histology | |
| Papillary | 104 (79) |
| Follicular | 21 (16) |
| Mixed | 6 (5) |
| High-Risk Histology | |
| Hurthle Cell | 12 (9) |
| Poorly Differentiated | 11 (8) |
| Tall Cell | 9 (7) |
| Clear Cell | 2 (2) |
| T Stage | |
| T2 | 5 (4) |
| T3 | 11 (8) |
| T4 | 109 (83) |
| Tx | 6 (5) |
| N Stage | |
| N0 | 29 (22) |
| N1a | 18 (14) |
| N1b | 77 (59) |
| Nx | 7 (5) |
| M Stage | |
| M0 | 94 (72) |
| M1 | 37 (28) |
| AJCC stage | |
| III | 2 (2) |
| IVa | 87 (67) |
| IVb | 2 (2) |
| IVc | 37 (28) |
| N/A | 1 (1) |
| Recurrent Disease | |
| Yes | 76 (58) |
| No | 55 (42) |
| # Positive Nodes | |
| Median | 3 |
| Range | 1–47 |
| Nodal Extracapsular Spread | |
| Positive ECE | 42 (44) |
| Negative ECE | 53 (56) |
| Primary Tumor Size | |
| Median | 4 cm |
| Range | 0.5–11.3 cm |
| Extraglandular Spread | |
| Yes | 126 (96) |
| No | 5 (4) |
| Surgical Margin Status | |
| Gross + | 25 (19) |
| Microscopic + | 37 (28) |
| Negative | 68 (52) |
| Unknown | 1 (1) |
| Gross Residual Disease | |
| Yes | 15 (11) |
| No | 116 (89) |
| XRT Dose | |
| Median | 60 Gy |
| Range | 38–72 Gy |
| # XRT Fractions | |
| Median | 30 |
| Range | 19–40 |
| XRT Technique | |
| 3DRT | 74 (56) |
| IMRT | 57 (44) |
Abbreviations: AJCC = American Joint Committee on Cancer, N/A = Not Available, ECE = Extracapsular Extension, XRT = Radiotherapy, Gy = Grey, 3DRT = Three Dimensionally-Guided Radiotherapy, IMRT = Intensity Modulated Radiotherapy
Treatment
One-hundred nineteen (91%) patients underwent definitive resection at our institution consisting of primary or completion thyroidectomy with or without nodal dissection of the central compartment, cervical neck, and/or superior mediastinum. One-hundred-fifteen (88%) patients had a central compartment dissection, and 76 (58%) patients underwent formal dissection of great vessels. Fifty-six (43%) patients underwent unilateral cervical neck dissection, while 42 (32%) patients underwent bilateral neck procedures. Forty-eight (37%) patients underwent exploration of the superior mediastinum, while 50 (38%) patients had a tracheal shave or resection performed. Twelve (9%) patients underwent either incomplete resection, or had surgery deferred at our institution following subtotal resection at an outside facility when consensus surgical opinion deemed disease to be technically unresectable. A total of 15 (11%) patients received radiotherapy for gross unresectable or residual disease.
Median EBRT dose was 60 Gy [range: 38–72 Gy] in 30 fractions [range: 19–40]. Seventy-four (56%) patients received conventional EBRT to a median dose of 60 Gy [range: 38–72 Gy]. Fifty-seven (44%) patients received intensity modulated radiotherapy (IMRT) to a median dose of 60 Gy [range: 56–66 Gy] after 7/2000. Patients treated at time of initial diagnosis received a median dose of 60 Gy [range: 56–72 Gy], patients with recurrent disease received a median dose of 60 Gy [range: 38–70 Gy], and patients with distant metastases received a median dose of 60 Gy [range: 56–66 Gy]. Patients with negative or microscopically positive surgical margins received a median dose of 60 Gy [range: 38–72 Gy], while patients with gross disease received a median dose of 66 Gy [range: 60–70 Gy]. One patient received less than 56 Gy; this was a 69 year-old male patient with a completely excised small volume nodal recurrence of poorly differentiated papillary disease with extracapsular extension. The surgical bed was prescribed an intended dose of 60 Gy via 3DRT. Treatment was halted at 38 Gy following hospitalization for an unrelated GI bleeding episode. At the patient’s request, treatment was not restarted. The patient remains alive and disease free after 67 months follow-up.
All patients received thyroid hormone replacement titrated to suppress thyroid stimulating hormone (TSH). Radioactive iodine (RAI) was given to 107 (82%) patients prior to radiotherapy; the median dose/number of courses was 150 mCi (range: 30–700 mCi) over 1 course (range: 1–5). An additional 12 (9%) patients received RAI following radiotherapy for disease progression. Six patients received concurrent chemotherapy during radiotherapy. Four patients with gross disease received treatment as follows: two patients received 60 Gy 3DRT/cisplatin, one patient received 60 Gy IMRT/carboplatin/paclitaxel, and one patient received 66 Gy 3DRT/cisplatin. One patient with poorly differentiated follicular disease received 60 Gy 3DRT/cisplatin, while another patient with poorly differentiated papillary disease received 60 Gy 3DRT/carboplatin. An additional 9 patients received systemic cytotoxic chemotherapy following radiation treatment for disease progression.
Survival Endpoints
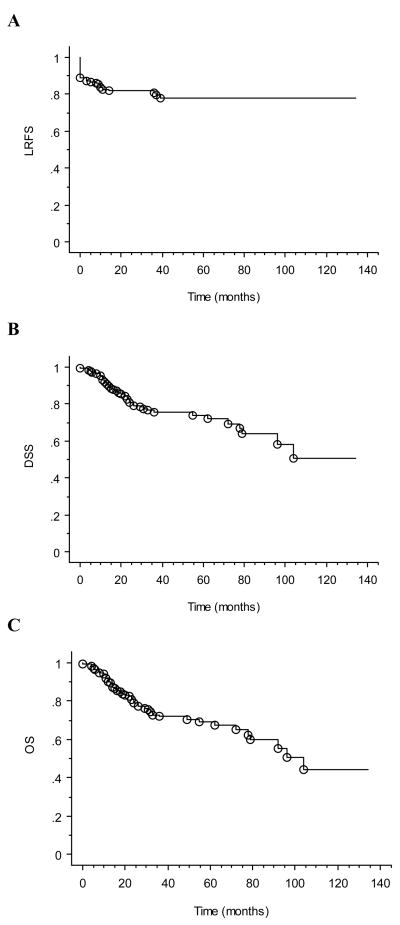
Median follow-up from completion of radiotherapy was 38 months (range: 0–134 months) for the study cohort, 47 months (range: 2 –134 months) for surviving patients, and 34 months (range: 5–85 months) for patients receiving IMRT. At last follow-up, 90 (69%) patients were alive, and 106 (81%) patients had no evidence of locoregional disease progression. Kaplan-Meier estimates of locoregional relapse free survival (LRFS), disease specific survival (DSS), and overall survival (OS) at 4 years were 79%, 76%, and 73%, respectively (Figure 2).
Figure 2. Survival outcomes of study cohort.
Kaplan-Meier curves demonstrating (A) LRFS, (B) DSS, and (C) OS. Open circles designate events.
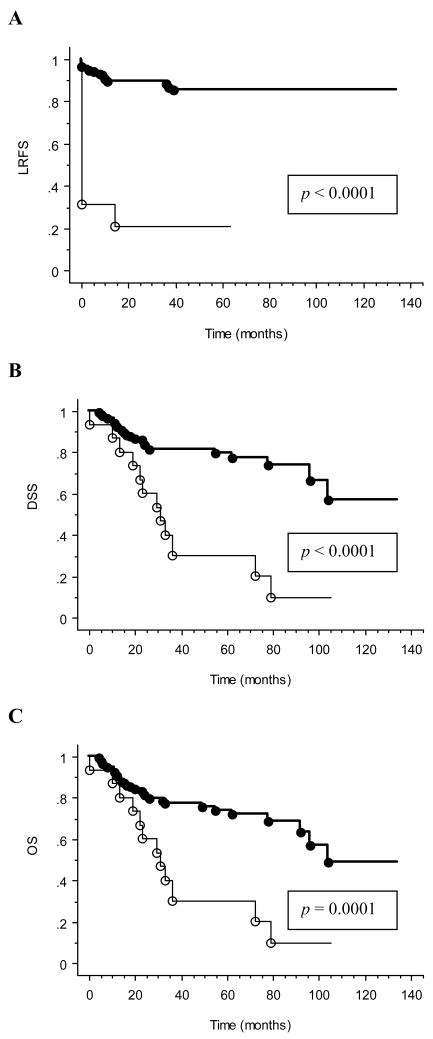
Seventy-one (76%) of 94 M0 patients remained free of distant failure following radiotherapy. Of the 15 patients treated definitively for gross disease, 4 demonstrated complete response, 3 had partial response, 6 had stable disease, and 2 progressed through treatment. All 4 patients with complete disease response remained free of locoregional relapse with follow-up durations of 13, 14, 29, and 63 months. Four of six patients with stable disease ultimately progressed locoregionally within 3–21 months. The strong interaction (p ≤ 0.0001) between presence of gross disease and treatment outcomes is demonstrated in Figure 3.
Figure 3. Univariate analysis of the interaction between presence of gross disease and survival outcomes.
Kaplan-Meier curves are shown with p-values determined by log-rank test for (A) LRFS, (B) DSS, and (C) OS. Absence of gross disease at the time of radiation treatment is indicated by heavy solid line (), while presence of gross disease is indicated by thin solid line (). Circles designate events.
Prognostic Factors
The following covariates were examined in our statistical models: gender, age ≥ 45 years, follicular histology, high-risk histologic features, recurrent disease, M stage, number of positive nodes, nodal extracapsular disease spread, mediastinal surgical exploration (as a surrogate for surgical complexity), surgical margin status, gross residual disease, and IMRT vs. 3DRT. On univariate analysis, high-risk histologic features and gross residual disease predicted for inferior LRFS at a Bonferroni corrected p < 0.004. On multivariate analysis, these factors remained prognostic for inferior LRFS at p < 0.05. On univariate analysis, high-risk histologic features, M1 disease, and gross residual disease predicted for both inferior disease specific survival and overall survival at p < 0.004. On multivariate analysis for both DSS and OS, these factors remained prognostic for inferior outcome at p < 0.05. The results of univariate and multivariate analysis are summarized in Tables 2 and 3. Patients with gross residual disease had 22% LRFS, 32% DSS, and 32% OS at 4 years versus 86%, 82%, and 79%, respectively, for patients without gross residual disease.
Table 2.
Univariate and Multivariate Predictors of Locoregional Control and Disease Specific Survival.
| Locoregional Control |
Disease Specific Survival |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate‡ (25 events) |
Univariate |
Multivariate‡ (35 events) |
|||||||||
| Variable | HR* | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Gross Residual Disease | 10.94 | 4.72–25.3 | <.0001 | 10.84 | 4.45–26.3 | <.0001 | 4.34 | 2.12–8.88 | <.0001 | 2.53 | 1.19–5.35 | 0.0153 |
| High Risk Histology | 3.89 | 1.76–8.56 | 0.0008 | 3.61 | 1.60–8.17 | 0.0021 | 3.32 | 1.70–6.48 | 0.0004 | 2.85 | 1.45–5.60 | 0.0023 |
| M Stage (M0, vs. M1) | 0.38 | 0.17–0.83 | 0.0158 | 0.30 | 0.15–0.58 | 0.0004 | 0.38 | 0.19–0.77 | 0.0071 | |||
| Histology (PC, vs. FC)† | 0.37 | 0.16–0.86 | 0.0202 | 0.44 | 0.21–0.91 | 0.0267 | ||||||
| Age < 45 | 0.72 | 0.25–2.10 | 0.5442 | 0.37 | 0.13–1.07 | 0.0665 | ||||||
| Surgical Margins (+, vs. −) | 2.54 | 1.10–5.91 | 0.0295 | 1.34 | 0.68–2.64 | 0.4033 | ||||||
| Gender (Male, vs. Female) | 1.71 | 0.74–3.96 | 0.2122 | 1.42 | 0.72–2.81 | 0.3180 | ||||||
| Recurrent (No, vs. Yes) | 0.64 | 0.28–1.48 | 0.2931 | 0.77 | 0.39–1.53 | 0.4580 | ||||||
| Number + Nodes§ | 1.02 | 0.98–1.06 | 0.2972 | 0.97 | 0.92–1.02 | 0.1988 | ||||||
| Extranodal Spread | 1.35 | 0.56–3.22 | 0.5060 | 1.14 | 0.54–2.38 | 0.7323 | ||||||
| Mediastinal Exploration | 0.99 | 0.44–2.25 | 0.9893 | 0.88 | 0.42–1.84 | 0.7296 | ||||||
| 3DRT vs. IMRT | 0.68 | 0.31–1.50 | 0.3386 | 1.67 | 0.76–3.67 | 0.1983 | ||||||
HR-Hazard ratio, determined by Cox Proportional Hazards Model.
PC- papillary carcinoma; FC-follicular carcinoma
HR per each additional positive nodes
Covariates included in multivariate model if significant on univariate analysis to a Bonferroni corrected p-value ≤ 0.004 (derived from p = 0.05/12 univariate tests)
Table 3.
Univariate and Multivariate Predictors of Overall Survival.
| Overall Survival |
||||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate‡ (41 events) |
|||||
| Variable | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Gross Residual Disease | 3.54 | 1.77–7.08 | 0.0003 | 2.22 | 1.08–4.59 | 0.0306 |
| High Risk Histology | 3.02 | 1.62–5.61 | 0.0005 | 2.61 | 1.40–4.88 | 0.0027 |
| M Stage (M0, vs. M1) | 0.36 | 0.20–0.68 | 0.0014 | 0.45 | 0.23–0.87 | 0.0176 |
| Histology (PC, vs. FC)† | 0.48 | 0.24–0.97 | 0.0403 | |||
| Age < 45 | 0.30 | 0.11–0.87 | 0.0261 | |||
| Surgical Margins (+, vs. −) | 1.15 | 0.62–2.15 | 0.6582 | |||
| Gender (Male, vs. Female) | 1.60 | 0.84–3.04 | 0.1504 | |||
| Recurrent (No, vs. Yes) | 0.88 | 0.48–1.66 | 0.7101 | |||
| Number + Nodes§ | 0.97 | 0.92–1.01 | 0.1238 | |||
| Extranodal Spread | 1.34 | 0.66–2.76 | 0.4207 | |||
| Mediastinal Exploration | 0.93 | 0.47–1.82 | 0.8227 | |||
| 3DRT vs. IMRT | 1.72 | 0.82–3.60 | 0.1497 | |||
HR-Hazard ratio, determined by Cox Proportional Hazards Model.
PC- papillary carcinoma; FC-follicular carcinoma
HR per each additional positive nodes
Covariates included in multivariate model if significant on univariate analysis to a Bonferroni corrected p-value ≤ 0.004 (derived from p = 0.05/12 univariate tests)
Treatment Morbidity
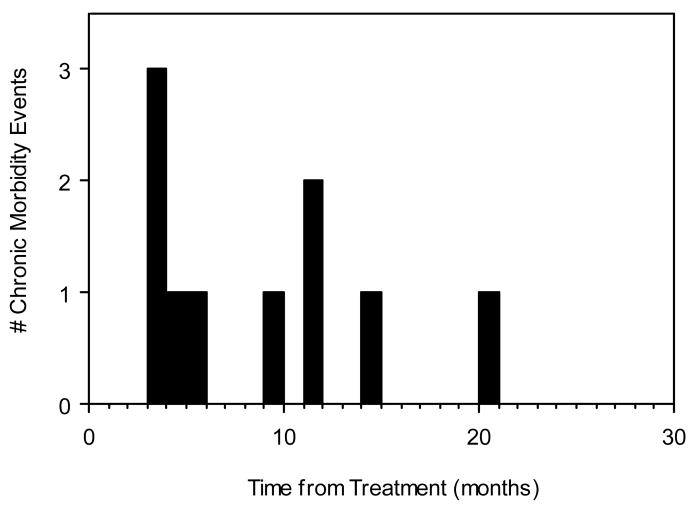
Late radiotherapy-related morbidity requiring supportive intervention is summarized in Table 4. IMRT was associated with less frequent severe late radiation morbidity than 3DRT. Nine (12%) patients treated with 58–60 Gy 3DRT without chemotherapy had late morbid events 3–60 months following completion of treatment. Six patients had esophageal stricture requiring dilatation, 1 patient had subglottic laryngeal stenosis requiring tracheostomy and dilatations, 1 patient had chronic laryngeal edema requiring tracheostomy 9 months post-treatment, and 1 patient remained feeding tube dependent secondary to chronic dysphagia. One (2%) patient treated with IMRT suffered morbidity of equivalent severity. This patient was treated with 60 Gy alone for a completely resected recurrent Hurthle cell disease and suffered an esophageal stricture 3 months post-treatment requiring dilatation. The patient recurred centrally 10 months post-treatment. The timing of all late morbidity events is plotted in Figure 4.
Table 4.
Severe Radiation-Related Morbidity.
| Patient | Stage/Histology | XRT Modality | XRT Dose (Gy) | Radiation Morbidity |
|---|---|---|---|---|
| 1 | T3N1bM0 Papillary | 3DRT | 60 | Esophageal stricture treated with dilatation. |
| 2 | T4aN0M0 Papillary | 3DRT | 60 | Subglottic laryngeal stenosis requiring tracheostomy and dilatation. |
| 3 | T2N1bM0 Follicular | 3DRT | 60 | Esophageal stricture treated with dilatation. |
| 4 | T4aN1bM1 Papillary | 3DRT | 60 | Esophageal stricture treated with dilatation. |
| 5 | T4aN1bM1 Papillary | 3DRT | 58 | Esophageal stricture treated with dilatation. |
| 6 | T4aN1bM0 Papillary | 3DRT | 60 | Esophageal stricture treated with dilatation. |
| 7 | T4aN1a M1 Papillary | 3DRT | 60 | Severe laryngeal edema requiring tracheostomy. |
| 8 | T4aN1bM1 Papillary | 3DRT | 60 | Esophageal stricture treated with dilatation. |
| 9 | T4aN1bM0 Papillary | 3DRT | 60 | Feeding tube dependent due to chronic dysphagia. |
| 10 | T4aN1bM0 Hurthle Cell | IMRT | 60 | Esophageal stricture treated with dilatation. |
Figure 4. Timing of severe late radiation-related morbidity.
Solid bars indicate number and timing of morbid events.
Discussion
Differentiated thyroid cancer is a surgical diagnosis, with most cases being effectively approached by thyroidectomy and RAI ablation. Management of the approximately 10% of patients presenting with locally advanced disease remains controversial. Indications for adjuvant radiotherapy generally include high risk presentations involving local invasion or encasement of surrounding structures (e.g. trachea, esophagus, larynx, mediastinum, and/or great vessels) with evidence of residual microscopic or gross disease, especially if this disease is iodine non-avid.
Support for adjuvant radiotherapy for microscopic residual disease is lent from retrospective reports which, taken together, suggest that curative doses of radiotherapy can reduce the 25–50% locoregional recurrence rates reported for surgery alone 1–14. Patient selection and use of RAI varied widely across these studies, with many study subjects not receiving the full complement of treatment used in modern therapy. Two of these reports evaluated high-risk patients with an aggressive multimodality approach akin to what our institution currently uses, including total thyroidectomy, RAI, and TSH suppression. Farhati, et. al. 3 reviewed 169 cases of pathologic T4 cases without evidence for distant metastasis. Ninety-nine of these patients received 50–60 Gy; these patients had improved locoregional and distant disease control. However, multivariate analysis demonstrated that locoregional disease control benefit was isolated to patients over 40 years-old with node positive papillary disease. Phlips, et. al. 5 reviewed 94 patients with microscopic residual differentiated disease or extranodal extension, of whom 38 received EBRT. Local regional failure was 3% in the irradiated cohort, compared with 21% in unirradiated patients. Our current series is in keeping with previously published results, with an estimated LRFS at 4 years of 85% for patients presenting with proven or suspected microscopic residual disease. These, in fact, represent encouraging results in a very high-risk patient population; 96% of our study subjects had extraglandular disease spread and almost 60% had recurrent disease.
Gross, unresectable residual disease is a relatively straightforward indication for adjuvant radiotherapy18, 19, but predicts for poor locoregional control and overall disease outcome regardless of adjuvant radiation 20, 21. This was confirmed in our current series. Actuarial locoregional disease control at one year was 33% for these patients, with only 4/15 cases demonstrating a complete response to treatment. Gross residual disease was predictive for inferior LRFS, DSS, and OS on univariate and multivariate analysis. This emphasizes the primacy of surgical management for high-risk disease. The ability to resect disease, no matter how involved the required surgery may be, outweighed all other clinical risk factors in our study other than adverse histologic features (Hurthle cell, tall cell, poorly differentiated disease, etc.) and distant metastasis. No surrogate measure of surgical complexity (e.g. cervical neck dissection, mediastinal exploration, great vessel dissection, tracheal resection, etc.) impacted outcome.
Locoregionally recurrent disease is typically managed similarly to locally advanced disease at initial presentation. One can expect local disease control, especially for isolated cervical nodal recurrences22, 23. In our current series, patients with recurrent disease did not appear to fare worse relative to patients treated at initial presentation, as long as all gross disease could be resected. This suggests that deferring EBRT in favor of RAI and TSH suppression alone at the time of initial disease presentation may not sacrifice ultimate local disease control or survival. However, subsequent recurrence necessitates reoperation, with significant attendant morbidity and costs. And not all recurrences can be successfully salvaged. Our institution has previously demonstrated that recurrences located in the thyroid bed and/or with soft tissue infiltration are less likely to be iodine avid and are controlled less frequently 23. Non-avid, centrally-located recurrences should be carefully considered for adjuvant EBRT. As for earlier treatment, EBRT provides a potential local control benefit at the time of initial presentation of high-risk disease. Nonetheless, an improved toxicity profile will be necessary for EBRT to gain wider acceptance among clinicians for this and other clinical indications.
Improvement of therapeutic index serves as a strong rationale for using advanced conformal radiotherapy techniques for thyroid cancer. The concave geometry and close proximity of the thyroid bed to critical normal structures such as esophagus, trachea, larynx, lungs, spinal cord, and (in cases with high cervical disease) parotid glands make delivery of high doses challenging and contribute to the considerable toxicity seen with conventional radiotherapy. Radiation side effects can compound healing and functional problems in reconstructed regions of esophageal and trachea, and can exacerbate the salivary and pulmonary sequelae of RAI administration. Morbidity outcomes for our current series suggest an improved therapeutic index with IMRT relative to 3DRT. IMRT was associated with less frequent severe late radiation morbidity than 3DRT (2% vs. 12%, respectively). Our methodology was purposely stringent to minimize the biases of retrospective chart review, which can be particularly significant for this diagnosis given the confounding contribution of post-operative and post-RAI morbidity. We focused on severe late toxicity temporally related to radiation treatment which necessitated medical or surgical intervention. This strategy afforded clearer assessment of late toxicity, but precluded formal assessment of acute toxicity and minor chronic treatment sequelae.
Published results for thyroid IMRT remain very limited. A pilot study from Memorial Sloan-Kettering 24 examined outcomes in 20 patients treated with IMRT for differentiated or medullary thyroid cancer. Median follow-up was limited to 13 months. There were 2 local failures, yielding a 2-year local progression free survival rate of 85%. Six patients died, yielding a 2-year overall survival rate of 60%. Reported acute toxicity was mostly self-limited, with the most severe presentations consisting of Grade 3 mucositis, pharyngitis, laryngitis, and skin toxicity. This is in keeping with our experience. No severe chronic toxicity was reported, although cases of mild-moderate radiation pneumonitis, Lhermitte syndrome, dysphagia, and xerostomia were seen. More recently, a small prospective thyroid IMRT experience was published by Urbano, et. al. 25, demonstrating encouraging results with mild, self-limited acute toxicity. Median follow-up was too short (37 weeks) for analysis of chronic toxicity.
Currently, we use IMRT for all curative treatment delivered at the UT M.D. Anderson Cancer Center for differentiated thyroid cancer. Treatment planning CT imaging extends caudally past the diaphragm to facilitate formal dose avoidance to the whole lungs. We manage locally advanced disease, especially recurrent disease, in an individualized fashion with multi-disciplinary input. In light of encouraging recent data 26, we are using FDG-PET/CT imaging more frequently to select patients for salvage surgical resection and to restage patients post-operatively to rule out occult residual locoregional gross disease or metastases. Our current practice is to recommend adjuvant irradiation for patients with recurrent and/or gross/microscopic residual disease, especially disease infiltrating into surrounding structures which would potentially preclude safe reoperation. Clinical features such as age > 45 years or high risk histologies (e.g. high grade or tall cell papillary variants, Hurtle cell, etc.) are relative indications for adjuvant irradiation. All operative beds are treated to adjuvant doses of 57–60 Gy. Immediately-adjacent nodal basins and uninvolved bystander structures can be treated to a reduced prophylactic dose of 54 Gy. Superior coverage typically extends no higher than level II to limit doses to oral cavity and parotid glands, unless the high neck is directly involved. Portions of the operative bed affected by positive surgical margins can be boosted to doses of approximately 63 Gy, while residual gross disease is typically treated to definitive doses of 66–70 Gy as dictated by neighboring normal tissue dose-limiting constraints.
Conclusions
Locally advanced differentiated thyroid cancer is an uncommon diagnosis for which the role of BRT still remains undefined. This institutional series demonstrates that multimodality approaches employing modern EBRT provide encouraging locoregional disease control and overall survival, even for very high-risk presentations. However, this holds true only if disease has been surgically reduced to microscopic levels; patients with gross residual disease face markedly inferior disease control outcomes. Our results suggest that use of IMRT may reduce chronic morbidity relative to 3DRT, but additional study will be required to validate this finding. Further work will also be necessary to define optimal IMRT target volume design, normal tissue dose constraints, and curative dose prescriptions for this technically challenging disease site.
Acknowledgments
This work was partly supported by P01 CA06294 awarded by the National Cancer Institute. Presented in part at the 7th International Conference on Head and Neck Cancer, San Francisco, CA, July, 2008 and the 50th Scientific Meeting of the American Society of Therapeutic Radiology and Oncology, Boston, MA, September, 2008. The authors report no conflicts-of-interest.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benker G, Olbricht T, Reinwein D, et al. Survival rates in patients with differentiated thyroid carcinoma. Influence of postoperative external radiotherapy. Cancer. 1990;65:1517–1520. doi: 10.1002/1097-0142(19900401)65:7<1517::aid-cncr2820650711>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Chung CT, Sagerman RH, Ryoo MC, et al. External irradiation for malignant thyroid tumors. Radiology. 1980;136:753–756. doi: 10.1148/radiology.136.3.6773105. [DOI] [PubMed] [Google Scholar]
- 3.Farahati J, Reiners C, Stuschke M, et al. Differentiated thyroid cancer. Impact of adjuvant external radiotherapy in patients with perithyroidal tumor infiltration (stage pT4) Cancer. 1996;77:172–180. doi: 10.1002/(SICI)1097-0142(19960101)77:1<172::AID-CNCR28>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Harmer CL. External beam radiotherapy for thyroid cancer. Ann Radiol (Paris) 1977;20:791–800. [PubMed] [Google Scholar]
- 5.Phlips P, Hanzen C, Andry G, et al. Postoperative irradiation for thyroid cancer. Eur J Surg Oncol. 1993;19:399–404. [PubMed] [Google Scholar]
- 6.Sheline GE, Galante M, Lindsay S. Radiation therapy in the control of persistent thyroid cancer. Am J Roentgenol Radium Ther Nucl Med. 1966;97:923–930. doi: 10.2214/ajr.97.4.923. [DOI] [PubMed] [Google Scholar]
- 7.Simpson WJ, McKinney SE, Carruthers JS, et al. Papillary and follicular thyroid cancer. Prognostic factors in 1,578 patients. Am J Med. 1987;83:479–488. doi: 10.1016/0002-9343(87)90758-3. [DOI] [PubMed] [Google Scholar]
- 8.Tsang RW, Brierley JD, Simpson WJ, et al. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer. 1998;82:375–388. [PubMed] [Google Scholar]
- 9.Wu XL, Hu YH, Li QH, et al. Value of postoperative radiotherapy for thyroid cancer. Head Neck Surg. 1987;10:107–112. doi: 10.1002/hed.2890100209. [DOI] [PubMed] [Google Scholar]
- 10.O’Connell ME, A’Hern RP, Harmer CL. Results of external beam radiotherapy in differentiated thyroid carcinoma: a retrospective study from the Royal Marsden Hospital. Eur J Cancer. 1994;30A:733–739. doi: 10.1016/0959-8049(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 11.Esik O, Nemeth G, Eller J. Prophylactic external irradiation in differentiated thyroid cancer: a retrospective study over a 30-year observation period. Oncology. 1994;51:372–379. doi: 10.1159/000227368. [DOI] [PubMed] [Google Scholar]
- 12.Ford D, Giridharan S, McConkey C, et al. External beam radiotherapy in the management of differentiated thyroid cancer. Clin Oncol (R Coll Radiol) 2003;15:337–341. doi: 10.1016/s0936-6555(03)00162-6. [DOI] [PubMed] [Google Scholar]
- 13.Meadows KM, Amdur RJ, Morris CG, et al. External beam radiotherapy for differentiated thyroid cancer. Am J Otolaryngol. 2006;27:24–28. doi: 10.1016/j.amjoto.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Yang DS, Jung KY, et al. Value of external irradiation for locally advanced papillary thyroid cancer. Int J Radiat Oncol Biol Phys. 2003;55:1006–1012. doi: 10.1016/s0360-3016(02)04203-7. [DOI] [PubMed] [Google Scholar]
- 15.Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70:511–518. doi: 10.1016/0002-9343(81)90573-8. [DOI] [PubMed] [Google Scholar]
- 16.Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–720. doi: 10.1210/jcem.75.3.1517360. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MH, Davis RK, Derrick L. Thyroid cancer: a computer-assisted review of 5287 cases. Otolaryngol Head Neck Surg. 1990;102:51–65. doi: 10.1177/019459989010200109. [DOI] [PubMed] [Google Scholar]
- 18.Chow SM, Yau S, Kwan CK, et al. Local and regional control in patients with papillary thyroid carcinoma: specific indications of external radiotherapy and radioactive iodine according to T and N categories in AJCC 6th edition. Endocr Relat Cancer. 2006;13:1159–1172. doi: 10.1677/erc.1.01320. [DOI] [PubMed] [Google Scholar]
- 19.Lee N, Tuttle M. The role of external beam radiotherapy in the treatment of papillary thyroid cancer. Endocr Relat Cancer. 2006;13:971–977. doi: 10.1677/ERC-06-0039. [DOI] [PubMed] [Google Scholar]
- 20.Simpson WJ, Panzarella T, Carruthers JS, et al. Papillary and follicular thyroid cancer: impact of treatment in 1578 patients. Int J Radiat Oncol Biol Phys. 1988;14:1063–1075. doi: 10.1016/0360-3016(88)90381-1. [DOI] [PubMed] [Google Scholar]
- 21.Tubiana M, Haddad E, Schlumberger M, et al. External radiotherapy in thyroid cancers. Cancer. 1985;55:2062–2071. doi: 10.1002/1097-0142(19850501)55:9+<2062::aid-cncr2820551406>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Coburn MC, Wanebo HJ. Prognostic factors and management considerations in patients with cervical metastases of thyroid cancer. Am J Surg. 1992;164:671–676. doi: 10.1016/s0002-9610(05)80732-9. [DOI] [PubMed] [Google Scholar]
- 23.Vassilopoulou-Sellin R, Schultz PN, Haynie TP. Clinical outcome of patients with papillary thyroid carcinoma who have recurrence after initial radioactive iodine therapy. Cancer. 1996;78:493–501. doi: 10.1002/(SICI)1097-0142(19960801)78:3<493::AID-CNCR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbluth BD, Serrano V, Happersett L, et al. Intensity-modulated radiation therapy for the treatment of nonanaplastic thyroid cancer. Int J Radiat Oncol Biol Phys. 2005;63:1419–1426. doi: 10.1016/j.ijrobp.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 25.Urbano TG, Clark CH, Hansen VN, et al. Intensity Modulated Radiotherapy (IMRT) in locally advanced thyroid cancer: acute toxicity results of a phase I study. Radiother Oncol. 2007;85:58–63. doi: 10.1016/j.radonc.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein SE, Grigsby PW, Siegel BA, et al. Combined [18F]Fluorodeoxyglucose positron emission tomography and computed tomography (FDG-PET/CT) for detection of recurrent, 131I-negative thyroid cancer. Ann Surg Oncol. 2008;15:286–292. doi: 10.1245/s10434-007-9611-5. [DOI] [PubMed] [Google Scholar]