Abstract
Anticonvulsant drugs have demonstrated efficacy in the management of irritability and aggression in a variety of psychiatric populations. We examined the acute effects of topiramate on aggression using a laboratory model of human aggression (PSAP) in individuals at high risk for aggressive and violent behavior. Twelve subjects, on parole/probation and with an Axis-II personality disorder and/or a substance use disorder, received 100, 200, 300, and 400 mg in an ascending sequence, with intervening placebo doses. Subjects participated 2–3 days per week over 4–6 weeks. Due to cognitive side effects at 300 mg, two subjects only completed through the 200 mg dose. Topiramate produced an inverted U-shaped dose response curve, with increases in aggression peaking at 200 mg and a modest decrease at 400 mg. Statistical analysis revealed a polynomial trend for dose (P = .001). The observed inverted U-shaped function in aggressive responding is consistent with non-human aggression studies of GABA-A modulators. Acute topiramate doses > 400 mg may have anti-aggressive effects, but dose levels in the 200–300 mg range may produce increases in aggression and side effects.
Keywords: human aggression, GABA, Topiramate, laboratory
INTRODUCTION
Direct stimulation of the GABA receptor system commonly produces suppression of aggressive behavior, though drugs that modulate the GABA-A receptor (alcohol, benzodiazepines) can also produce an inverted U-shaped dose-response function, with aggression-heightening properties at low to moderate doses and sedation and reduced aggression at sufficiently high doses (Miczek et al, 2003). Several GABA-modulating drugs, in particular those used as antiepileptics, have shown promise as anti-aggressive agents in both non-humans (Navaro et al., 2007; Rogers and Depaulis, 1982) and humans who are at higher than normal risk for aggressive behavior (Guay 2007; Stanford et al., 2005; Nickel and Loew, 2008). It has been suggested that the mechanism for these anti-aggressive effects may be an interaction of (a) the individual organism’s history of aggressive behavior and (b) available levels of CNS GABA, which putatively serve an inhibitory function in modulating other receptor systems (DA and 5-HT) and prefrontal-limbic circuits active during bouts of aggressive behavior (Bjork, 2001; Miczek et al., 2003; Siever, 2008). It should be emphasized that GABAergic effects on aggressive behavior are more pronounced for those whose aggression is extreme in intensity or frequency.
Epidemiological community-based studies indicate that people with DSM Axis-II personality disorders (e.g., antisocial personality disorder, borderline personality disorder), and substance use disorders (SUD) are at highest risk for physically violent and/or aggressive behavior. Individuals with antisocial personality disorder or SUD are 2–3 times more likely to commit a violent or aggressive act than individuals with schizophrenia or a mood disorder, and up to 10–15 times more likely than matched community controls without any psychiatric diagnosis (Arseneault et al., 2000; Steadman et al., 1998; Swanson et al., 1990). Notably, it is the combination of antisocial personality disorder and substance use disorders that confers greatest risk (Robins et al., 1993; Rasmussen & Levander, 1996; Steadman et al., 1998). These individuals are clearly an important target for pharmacological intervention for aggressive behavior.
The present study examined the acute effects of the GABA-modulating drug topiramate (Topomax ©, Ortho-McNeil, Raritan, New Jersey) using a laboratory model of aggressive behavior. Topiramate is primarily indicated as an anticonvulsant and for the treatment of migraine headache. Along with several other anticonvulsants, it has been utilized for mood stabilization. Topiramate’s actions involve several mechanisms, including blocking voltage-sensitive sodium channels, inhibiting excitatory transmission by antagonizing glutamate receptors, and enhancing the activity of the inhibitory neurotransmitter GABA, via modulation of GABA-A receptors, by potentiating GABA-evoked currents through interaction with the GABA-A ionophore (Perucca, 1997; Rosenfeld, 1997). However, in a rodent model topiramate was also shown to act on GABA-B receptors (Kim et al., 2005). In healthy humans, acute topiramate administration is known to increase H1 MRS measured cerebral GABA levels by as much as 72% (Kuznieky et al., 1998).
The GABA-enhancing actions of topiramate may be related to its reported effects on disorders related to deficient response inhibition/impulse control, including aggression, binge eating disorder (Carter et al., 2003; McElroy et al., 2003), and substance abuse of alcohol (Johnson et al., 2007), cocaine (Kampman et al., 2004), and nicotine (Johnson et al., 2005). Prior studies suggest that chronic administration of topiramate may help reduce irritability and aggression in populations with psychotic, mood, and personality disorders (Gobbi et al., 2006; Janowsky et al., 2003; Nickel et al., 2004; Nickel, 2007; Nickel & Loew, 2008).
Here we focused on individuals who, as suggested by community-based epidemiological data, are at high risk for violent and aggressive behavior (Arseneault et al., 2000; Robins et al., 1993; Rasmussen & Levander, 1996; Swanson et al., 1990). Individuals with histories of criminal prosecution and incarceration (on parole/probation), Axis-II personality disorders (ASPD, BPD), and substance use disorders were enrolled. Our primary aim was to characterize a dose-response curve following acute administration of topiramate in the range of 50 mg – 400 mg in individuals with these high-risk characteristics.
METHODS
Subject Selection
This study was approved by the local IRB (UTHSC-Houston Committee for the Protection of Human Subjects) and in accordance with the declaration of Helsinki. Male and female participants were recruited via local newspaper advertisements. Inital telephone screening was used to identify individuals with an incidence of past drug use and antisocial behavior. Based on information obtained during initial telephone interviews, potential participants were brought to the laboratory for more extensive interviews covering physical and mental health status, drug and alcohol use history, and criminal behavior. Exclusionary criteria included: (a) current or past medical problems (e.g., seizures, diabetes, high blood pressure, renal or cardiovascular disease); (b) current use of any medications; (c) current ongoing illicit drug use (measured by daily urinalysis); and (d) current or past history of an Axis I disorder other than substance abuse or dependence, as defined by the Structured Clinical Interview for the DSM-IV (SCID-I, version 2.0, First et al., 1996). Tweleve adults (7 M, 5 F) completed the project.
Subject Intake
At intake, subjects read and then signed a detailed informed consent form. After consent and mental and physical exams, urine drug screen analysis was carried out using enzyme multiple immunoassay (EMIT d.a.u ® - SYVA Corp), which tested for the following drug types: cocaine, stimulants, opiates, marijuana, and benzodiazepines. Temperature monitoring and creatinine level determinations were performed daily to detect attempts to alter urine samples. At intake, subjects were provided with information about potential earnings, urine drug testing, breath alcohol testing, psychiatric screening, and experimental procedures (including that they would receive a range of doses of topiramate). Subjects were told that they could expect to earn $30–40 per day based on earnings in the testing sessions, and that additional bonuses would be provided for drug-free breath and urine samples, attendance, and for completing the study.
Testing Schedule
Subjects participated 2–3 days a week, depending on their schedules, completing six 25-min PSAP sessions per day (described below) at 9:00 am, 10:00 am, 11:00 am, 12:00 pm, 1:30 pm and 2:30 pm. Each day of testing, urine (EMIT d.a.u ® - SYVA Corp) and breath alcohol samples were obtained from subjects when they arrived in the laboratory at approximately 8:00 am. Breath alcohol testing was conducted using an AlcoSensor III (Intoximeters, Inc), and acquired from a sustained 10-sec expired air sample. Participation was discontinued if a subject provided two consecutive drug-positive urine samples or positive breath-alcohol samples. One subject was removed for drug-positive urine tests; four other subjects dropped out of the study without notification. Any sample testing positive resulted in the subject being sent home and rescheduled for another day (or released if UA was positive on more than one occassion). Lunch was provided at 11:30 am. Between sessions, subjects waited in a common area containing a television and magazines. At the end of each day of participation, subjects were paid in cash the total amount earned during all sessions.
Drug Administration
Placebo or topiramate was administered orally in three #00 opaque capsules at 8:30 am, approximately 30 min prior to the first session. The peak plasma levels of topiramate are usually reached approximately two to three hours after administration (Perucca, 1997). Initial pilot data showed that no effects were observed under 25 mg and 50 mg; these doses were subsequently dropped. Using a within-subject design, four doses of topiramate were administered: 100, 200, 300, and 400 mg, separated by intervening placebo doses. This dose range is near the middle of the standard recommended clinical dose range for treatment of epilepsy (200–800 mg: Carter et al., 2003; Petroff et al., 2001; Rosenfeld, 1997), and near the upper end of the dose range for treatment in substance users (Johnson et al., 2005; Johnson et al., 2007; Kampman et al., 2004). An ascending dose sequence was used for the protection of human subjects so that possible side effects could be assessed in a dose escalating manner, as mandated by the University of Texas Health Science Center Institutional Review Board. Dose administration was completed by a research assistant unaware of both dose content and dose order to ensure that both subject and administrator were blind to dosing conditions.
Dosing Schedule
Responding under baseline (non-drug) was repeated for two to three days until behavior was stable and subjects had passed a complete medical screening. Next, placebo capsules were administered until the rate of responding on the A (monetary) and B (aggressive) options met stability criteria, defined by a coefficient of variation (sd/mean) of ≤ 0.25 and no monotonically increasing or decreasing trends in the data. The four topiramate doses were then administered, separated by a variable number of placebo doses. Stable response rates on all options were reestablished between each dose, with the number of intervening placebo doses determined by (a) meeting stability criteria, or in the event that stability was achieved within one day, (b) a predetermined number of placebo doses assigned randomly and ranging between one and three. This strategy was used to prevent subjects from determining or forming expectations about dosing order. Therefore, each topiramate dose was separated by at least one experimental day in which the placebo dose was administered. Placebo doses were administered to subjects at least seven times over the course of the experiment (range 7–15).
Aggression Testing Instructions
On the first day of participation, subjects were shown a diagram of the computer monitor and response panel and were read a set of scripted instructions (Cherek and Lane, 1999). Portions of the instructions were repeated if the subjects asked questions.
Aggression Testing Procedure
The Point Subtraction Aggression Paradigm (PSAP, Cherek, 1991) software program was used to measure aggressive, escape, and monetary-reinforced responding. During each session, 15 cents was subtracted from the subject’s counter at random intervals between 6 and 120 s. These monetary subtractions were attributed to a fictitious other person paired with the subject. No subtractions occurred when the subject’s counter was at zero. During experimental sessions, subjects were presented with choices between three response options: (1) a monetary-reinforced response option associated with the letter A; (2) an aggressive response option associated with the letter B, which ostensibly subtracted 15 cents from the fictitious other person (this functions as the presentation of an aversive stimulus to another person, in accord with standard operational definitions of human aggression, e.g., Baron & Richardson, 1994); and (3) an escape response option associated with the letter C, which ostensibly protected the subject’s counter for some period of time from subtractions. At the start of each session the letters A, B and C and a counter appeared on the computer screen. A single response on the button labeled A, B, or C disabled the other options and the associated letters disappeared from the screen. When option A (monetary-reinforced option) was selected, 100 consecutive presses on button A (fixed-ratio FR 100) added 15 cents to the counter. If option B (aggressive response option) or option C (escape response option) was selected, 10 (FR 10) consecutive responses on button B or C, respectively, produced an unsignaled time period that was free of provocations (i.e. a provocation-free interval). The provocation-free interval was 125 s on average (intervals ranged from 100 to 150 s). At the end of the provocation-free interval, monetary subtractions were again presented. At the start of a session and at the end of each provocation-free interval, at least one provocation had to occur before a provocation-free interval could be initiated. Responding on option B (aggressive option) or C (escape option) could therefore reduce the number of subtractions per session, but could not prevent all subtractions. In the absence of B (aggressive) or C (escape) responses, approximately 24 subtractions would occur per session.
Following the completion of the response requirement (FR 100 on A, FR 10 on B and C) the selected letter disappeared from the screen and, after 2 s, all three letters reappeared to signal that the three response options were again available. The PSAP contingencies engender mostly high-rate responding on the monetary-reinforced option (FR 100 on option A), as this is how subjects generate their earnings. Accordingly, the response rate on option A serves as an index of acute drug effects (stimulation or sedation), while responding on option C serves as a control for the effects of provocation on escape from subsequent provocations.
At the end of each day subjects were given a questionnaire designed to assess the instructional deception, asking questions regarding how many individuals the subject had been paired with and who had subtracted more money. Any indication in the end-of-day questionnaire that the subject did not believe the deception, e.g., “I think I was playing against a computer,” resulted in the removal of that subject from the study. In the present study, all 12 subjects reported being paired with other people during the experimental sessions.
Psychometric tests
Subjects completed a battery of questionnaires related to aggression and impulsivity at the end of the study: (1) the Buss-Perry Aggression Inventory (Buss and Perry, 1992); (2) the Lifetime History of Aggression Questionnaire (LHA; Coccaro et al., 1995); (3) the State-Trait Anger Expression Inventory (STAXI, Speilberger, 1988); (4) the Retrospective Overt Aggression Scale (Sorgi et al., 1991); the Barratt Impulsivity Scale (BIS-11, Patton et al., 1995); and (6) the Eysenck Impulsvity and Venturesomeness Questionnaire (EIVQ, Eysenck et al., 1985). Data from these scales are provided in Table 1. Generally, average scores were elevated across nearly all scales relative to published norms for healthy control populations.
Table 1.
Demographic and psychometric characteristics for the twelve subjects (7 male, 5 female) enrolled in the study.
| Variable | Mean ± SEM |
|---|---|
| Age (yrs) | 25.07 ± 1.77 |
| Education (yrs complete) | 11.44 ± 2.20 |
| Substance Use Disorder (#) | 9 |
| Conduct Disorder (#) | 6 |
| Antisocial Personality Disorder (#) | 5 |
| Borderline Personality Disorder (#) | 2 |
| Past criminal offense (#) | 12 |
| Cognitive Aptitude | |
| Shipley t-score | 41.50 ± 2.12 |
| WAIS estimate | 96.25 ± 2.19 |
| STAXI | |
| State Anger | 14.42 ± 2.32 |
| Trait Anger | 20.83 ± 2.33 |
| AX/EX1 | 32.75 ± 2.59 |
| ROAS | |
| Verbal Aggression | 2.17 ± 0.39 |
| Physical Aggression2 | 3.00 ± 0.71 |
| LHA | |
| Total Score | 14.83 ± 2.78 |
| BPAI | |
| Total Score | 76.33 ± 6.83 |
| BIS-11 | |
| Total Score | 72.50 ± 2.53 |
| EIVQ | |
| Impulsivity | 10.00 ± 0.93 |
| Venturesomeness | 7.42 ± 0.68 |
STAXI = State Trait Anger Expression Inventory
ROAS = Retrospective Overt Aggression Scale
LHA = Lifetime History of Aggression questionnaire
BPAI = Buss-Perry Aggression Inventory
BIS-11 = Barratt Impulsivity Scale
EIVQ = Eysenck Impulsivity and Venturesomeness Questionnaire
AX/EX represents a composite score that examines overall frequency of anger expression based on holding in, expressing, and controlling anger (Speilberger, 1985).
Composite score of physical agression towards both objects and other people.
Data analyses
Rates of responding on the monetary-reinforced option (responses per second) and aggressive option (responses per minute) were used as the primary dependent measures. Data were averaged across all six PSAP sessions completed in an experimental day. The escape response option (the C button) was not used in a sufficient number of subjects or sessions to be analyzable. The ascending dose sequence introduced the possibility that placebo response patterns changed over the course of the experiment (generally 4–5 weeks), possibly confounding the evaluation of drug response. To evaluate this possibility, repeated measures analysis of variance (ANOVAs) were conducted on monetary responses/sec and aggressive responses/min to assess possible time/order effects. Data were analyzed using the four placebo sessions that met stability criteria on days immediately preceding topiramate administration (e.g., those also included in the primary analyses used to examine dose effects).
For the primary data analyses, data for each individual subject were analyzed as a percent of placebo responding from the placebo dose immediately prior to the active dose day. For example, if the 200 mg dose was adminstered on a Friday, the six sessions from the (stable) placebo administration day on the preceding Wednesday was used calculate the percent of placebo for the 200 mg dose. A one-way repeated measures ANOVA was used with both linear and polynomial (quadratic) tests for trend as a function of dose (SAS v.9.1.3, Proc Mixed; Cary, NC, USA). The quadratic test was included based on the graphical analyses (see Figure 1). Also based on Figure 1, post-hoc contrasts were performed for the placebo, 200 mg, and 400 mg dose pairs.
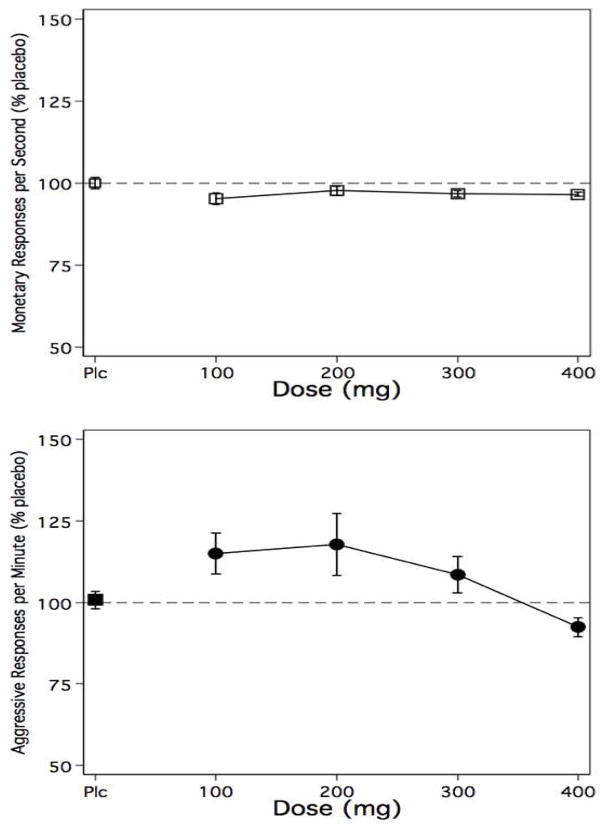
Figure 1.
The top panel shows data for monetary (A button) responses per second for the mean of six test sessions on a given experimental day. Active doses are expressed as percent of placebo responding from the preceding placebo administration day. The bottom panel shows data for aggressive (B button) resonses per minute, represented in the same manner. Error bars represent ± SEM. Data for 300 mg and 400 mg data represent only 10 subjects, as side effects prevented collection of data for two subjects See text for further details.
Side Effects
During testing, two subjects (one male, one female) reported adverse effects at the 300 mg dose. These side effects occurred within 60 minutes of adminsitration and included complaints of dizziness, memory loss, problems concentrating, nausea, and headache. In the female subject, these side effects precipitated the onset of anxiety symptoms (fear, mild hand tremor, and rapid pulse). No change in blood pressure was observed in either subject. Side effects and anxiety diminished within 180 minutes in both subjects. Following observation by a registered nurse and physician, both subjects were released from the study. Thus, data for these subjects were only obtained for 100 mg and 200 mg doses.
Results
A summary of subject demographic and psychomteric information is provided in Table 1. In summary, subjects averaged 25 years of age and 11 years of education. To assess cognitive functioning, all subjects were administered the Shipley Institute of Living Scale (Shipley-Boyle, 1967), a test of general intellectual aptitude that includes a 40-item vocabulary test and a 20-item abstraction test. Shipley score estimates of WAIS IQ correlate highly (0.76–0.87) with actual WAIS IQ scores (Zachary et al., 1985). The average Shipley age-adjusted t-score was 41.50 (± 2.12), corresponding to a WAIS-adjusted IQ of 96.25 (± 2.18). Nine subjects met DSM-IV criteria for a past substance use disorder, including marijuana (7), alcohol (3) and alprazolam (1). Six subjects met DSM-IV criteria for past childhood conduct disorder (CD), five met criteria for antisocial personality disorder (ASPD), and two females met criteria for borderline personality disorder. All subjects had at least one criminal offense. Rates of nicotine and caffeine consumption were low. The majority of subjects drank less than one caffeinated beverage per day. Four subjects were smokers, averaging 4.25 cigarettes per day (range 1–7). During the study, no reports of nicotine withdrawal were made. No subjects had positive breath alcohol samples on test days.
Figure 1 shows the outcome for monetary responses/sec (top panel) and aggressive responses/min (bottom panel) expressed as a percent of the corresponding placebo dose. The figure clearly shows that topiramate produced few changes in monetary reinforced responding, and shows an inverted U shaped dose-response function on aggressive responding. Relative to corresponding placebo doses, the 100 mg and 200 mg doses produced increases in aggression, while the 400 mg dose produced a modest decrease in aggressive responding. These response patterns suggest that the drug-related changes were specific to aggressive behavior rather than non-specific changes due to sedation or stimulation.
A repeated measures ANOVA on placebo doses only failed to reveal reliable change over time in monetary responding F (3, 43) = 0.68, ns or aggressive responding F (3, 43) = 0.21, ns. This outcome provides assurance that the ascending dose sequence did not produce changes in baseline (placebo) response patterns that would confound the interpretation of the dose effect curve.
For the primary data analyses, a one-way repeated measures ANOVA on monetary reinforced responding failed to show a linear [F (1, 42) = 2.02, ns] or quadratic trend [F (1, 42) = 1.86, ns]. The one-way repeated measures ANOVA on aggressive responding failed to reveal a linear trend [F (1, 42) = 0.93, ns], but identified a quadratic trend [F (1, 42) = 11.91, P = .001]. Results of post-hoc contrasts between placebo, 200 mg (largest increase) and 400 mg (decrease) were as follows: placebo vs. 200 mg, F (1, 40) = 5.26, P = .027; placebo vs. 400 mg, F (1, 40) = 0.76, ns; and 200 mg vs. 400 mg, F (1, 40) = 9.37, P = .004. Only the 200 mg vs. 400 mg contrast remained significant after Bonferroni correction.
Mean response rates for monetary and aggressive responding at each dose are provided in Table 2. In contrast to Figure 1, which provides the relative percent of the active dose to the placebo that preceeded it (usually by 24 to 48 hours), Table 2 shows the data represented as absolute values collapsed across all four placebos and all active doses. Analyzed in this manner via repeated measures ANOVA, there were no significant relationships (all p values > .15). These outcomes reflect two factors: (a) between subjects, absolute rates of aggressive responding (responses/min) were quite variable (note Table 2 standard errors); and (b) within subjects, some placebo and active doses were separated by as many as five weeks, and response rates can drift over this duration of time; thus the focus on the relationship to the immediately preceding placebo, as expressed in Figure 1.
Table 2.
Overall response rate data for monetary-reinforced responding (responses/sec) and aggressive responding (responses/min) for the 12 subjects, expressed as the mean ± SEM and averaged across six daily experimental test sessions. Placebo doses are collapsed across all four administrations, rather than presented as relative values compared to the corresponding active dose (as presented in Figure 1). Data for 300 mg and 400 mg data represent only 10 subjects, as side effects prevented collection of data for two subjects (see text for details).
| Dose | Monetary responding | Aggressive responding |
|---|---|---|
| Placebo | 5.31 ± 0.16 | 7.13 ± 1.14 |
| 100 mg | 5.14 ± 0.15 | 7.30 ± 1.10 |
| 200 mg | 5.14 ± 0.16 | 8.50 ± 1.62 |
| 300 mg | 5.23 ± 0.21 | 8.20 ± 1.42 |
| 400 mg | 5.24 ± 0.18 | 7.25 ± 1.51 |
Discussion
In a subject population of parolees with histories of substance use disorders and/or personality disorders (antisocial and borderline), topiramate produced an inverted U-shaped dose-response curve in aggressive responding; such curves are not uncommon in pharmacology (Calabrese & Baldwin, 2003). While increases occurred at the 100 mg and 200 mg doses, only the 400 mg dose produced decreases in aggression, and this decrease was modest. There were no changes in monetary response rates, indicating that the changes were specific to aggressive responding and were not due to non-specific stimulant or sedative effects.
The shape of the dose effect curve suggests that higher doses in the 600 mg to 800 mg range may have produced further declines in aggression. The inverted U pattern resembles dose-response functions observed in rodent models with other GABA-A modulating agents, in which low to moderate doses produced increases in aggression and higher doses engendered anti-aggressive and sedative effects (de Almeida et al., 2005; Miczek et al., 2003). The adverse effects observed in two subjects at 300 mg introduce the possibility that higher doses may not be advisable, at least in acute pharmacotherapy for aggressive behavior. It should be noted that both subjects who experienced side effects at the 300 mg met DSM-IV criteria for past alcohol abuse, and one also met criteria for past alprazolam abuse. Though other subjects (who did not report side effects) also met criteria for past alcohol abuse and/or dependence, it remains possible that for these two subjects, a dysregulated GABA system may have contributed to the oberved side effects. Additionally, at sufficiently high doses, topiramate has been well documented to produce disruptions in cognition (Martin et al., 1999; Meador et al., 2003; Reijs et al., 2004).
In contrast with the present results, other studies with topiramate using chronic dosing designs with escalating doses up to 250 mg have reported significant decreases in aggression in borderline personality disorder patients (Nickel, 2007; Nickel & Loew, 2008; Nickel et al., 2004; Nickel et al, 2005), aggressive and self-injurious individuals with developmental disabilities (Janowsky et al., 2003), and individuals with psychosis (Gobbi et al., 2006). Similar GABA-enhancing antiepileptic drugs were shown to be effective in reducing aggression in prisoners with personality disorders (Stanford et al., 2005). Though less common, prior studies have reported increases in aggression and/or hostility following chronic topiramate dosing for treatment of epilepsy (Mula et al., 2003) and acute dosing in healthy controls (Martin et al., 1999).
Importantly, the above-noted studies uniformly employed self-report based rating scales of aggression, primarily the STAXI and OAS, and data were based on behavior occurring in natural (e.g., non-laboratory) environments. In contrast, the PSAP measures ongoing dynamic patterns of aggressive responding acquired under laboratory conditions designed to serve as an operationally defined proxy for human aggression under naturalistic conditions (advantages and disadvantages of these approaches are beyond the scope of this report, but can be found in Cherek et al., 2002a).
Differences in measurement approach, measurement setting, and dosing protocol (acute vs. chronic) may therefore have contributed to the differences in outcomes. For example, in the 200 mg to 400 mg range acute topiramate effects may be transient and due to rapid changes in CNS GABA levels. Chronic dosing may otherwise produce more stable and regulated GABAergic activity due to adaptation and downregulation of GABA receptors. Thus, one limitation of the present study may be the dosing protocol. While we used an ascending sequence, the acute administration protocol may have deterred our ability to measure decreases in aggressive responding within this dose range. By contrast, previous studies using topiramate for substance abuse treatment (Johnson et al., 2007) and management of aggression in individuals with personality disorders (Nickel, 2007; Nickel & Loew, 2008) produced more efficacious results – within a similar dose range – by using an extended eight-week chronic dosing protocol, with escalating doses at regular intervals. Future studies examining populations at high-risk for aggressive behavior may consider utilizing similar chronic dose-escalation protocols.
The results are not entirely consistent with previous acute GABAergic drug administration studies in our own laboratory. Previous experiments with baclofen (Cherek et al., 2002b) and tiagabine (Lieving et al., 2007) have not shown an inverted U-shaped dose-response pattern, and these drugs were generally more efficacious in producing dose-related decreases in aggression. However, increases in aggression were also observed under lower doses of gabapentin (200 and 400 mg), with significant decreases occurring at 800 mg (Cherek et al., 2004). Additionally, due to variability in absolute response rates across subjects and the 4–5 week study duration, another limitation is that aggressive responding was only statistically significantly different between doses when analyzed as a percent of the immediately preceding placebo.
The GABA-A receptor is an important target for aggressive behavior (de Almeida et al., 2005; Guay, 2007; Miczek et al, 2003), but GABA-A modulating drugs may have both aggression-enhancing and anti-aggressive effects, depending on the individual organism, the social context, and as shown here, drug dose. With regard to individual differences, our subject population was selected to be at high risk for aggressive behavior (see Table 1), with past histories of aggression and other illegal activity, personality disorders, and substance use disorders. However, the sample was not entirely homogenous. While all had been convicted of criminal offenses and had elevated psychometric scores on measures of aggression and impulse control, not all met criteria for both a personality disorder and an SUD. These individual differences might account for some of the variablity in the range of outcomes – although shape of curve was reasonably similar across all 10 completers. This variability might also be partially responsible for the discrepancy between the current results and previous studies by Nickel et al with Borderline Personality Disorder patients. The current sample size precluded an analysis of differences among subjects with personality disorders, substance use disorders, or both disorders. Future work utilizing parametric designs will be needed to delineate the relative contributions of individual differences in clincial diagnosis, history of aggression, and biological and behavioral variation in response to topiramate and other GABAergic agents.
Acknowledgments
This research was supported by National Institutes of Health/NIDA grants R01 DA 003166 and P50 DA 009262. FGM is supported by and National Institutes of Health/NIDA merit award K02 DA 00403. FGM has received past contract, consultation, and speaker’s bureau support from Ortho-McNeil. No other authors have conflicts of interest to report. The authors wish to Darla Wentzel for assistance in dose preparation, and Veronica Aldana and Irshad Prasala for valuable technical assistance in conducting the experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arseneault L, Moffitt TE, Caspi A, Taylor PJ, Silva PA. Mental disorders and violence in a total birth cohort: results from the Dunedin Study. Arch Gen Psychiatry. 2000;57:979–986. doi: 10.1001/archpsyc.57.10.979. [DOI] [PubMed] [Google Scholar]
- Baron RA, Richardson DR. Human aggression. New York: Plenum Press; 1994. [Google Scholar]
- Bjork JM, Moeller FG, Kramer GL, Kram M, Suris A, Rush AJ, Petty F. Plasma GABA levels correlate with aggressiveness in relatives of patients with unipolar depressive disorder. Psychiatry Res. 2001;101:131–136. doi: 10.1016/s0165-1781(01)00220-7. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The Aggression Questionnaire. Journal of Personality and Social Psychology. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Carter WP, Hudson JI, Lalonde JK, Pindyck L, McElroy SL, Pope HG., Jr Pharmacologic treatment of binge eating disorder. Int J Eat Disord. 2003;34 (Suppl):S74–88. doi: 10.1002/eat.10207. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SD. Effects of d,l-fenfluramine on aggressive and impulsive responding in adult males with a history of conduct disorder. Psychopharmacology. 1999;146:473–481. doi: 10.1007/pl00005493. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SD, Pietras CJ. Laboratory measures: Point Subtraction Aggression Paradigm. In: Coccaro EF, editor. Aggression: Assessment and treatment into the 21st century. New York: Marcel Dekker; 2002a. pp. 215–228. [Google Scholar]
- Cherek DR, Lane SD, Pietras CJ, Sharon J, Steinberg JL. Acute effects of baclofen, a gamma-aminobutyric acid-B agonist, on laboratory measures of aggressive and escape responses of adult male parolees with and without a history of conduct disorder. Psychopharmacology (Berl) 2002b;164:160–167. doi: 10.1007/s00213-002-1167-2. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Spiga R, Roache JD, Cowan KA. Effects of triazolam on human aggressive, escape and point- maintained responding. Pharmacology, Biochemistry, and Behavior. 1991;40:835–839. doi: 10.1016/0091-3057(91)90094-i. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Tcheremissine OV, Lane SD, Pietras CJ. Acute effects of gabapentin on laboratory measures of aggressive and escape responses of adult parolees with and without a history of conduct disorder. Psychopharmacology (Berl) 2004;171:405–412. doi: 10.1007/s00213-003-1590-z. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL. Physiological responses to d-fenfluramine and ipsapirone challenge correlate with indices of aggression in males with personality disorder. International Clinical Psychopharmacology. 1995;10:177–179. doi: 10.1097/00004850-199510030-00007. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol. 2005;526:51–64. doi: 10.1016/j.ejphar.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Personality and Individual Differences. 1985;6:613–619. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Non patient edition (SCID-NP) New York: NY State Psychiatric Institute; 1996. [Google Scholar]
- Gobbi G, Gaudreau PO, Leblanc N. Efficacy of topiramate, valproate, and their combination on aggression/agitation behavior in patients with psychosis. J Clin Psychopharmacol. 2006;26:467–473. doi: 10.1097/01.jcp.0000237945.35022.45. [DOI] [PubMed] [Google Scholar]
- Guay DR. Newer antiepileptic drugs in the management of agitation/aggression in patients with dementia or developmental disability. Consult Pharm. 2007;22:1004–1034. doi: 10.4140/tcp.n.2007.1004. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Kraus JE, Barnhill J, Elamir B, Davis JM. Effects of topiramate on aggressive, self-injurious, and disruptive/destructive behaviors in the intellectually disabled: an open-label retrospective study. J Clin Psychopharmacol. 2003;23:500–504. doi: 10.1097/01.jcp.0000088906.24613.76. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA. Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch Intern Med. 2005;165:1600–1605. doi: 10.1001/archinte.165.14.1600. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kwak SE, Kim JE, Won MH, Choi HC, Song HK, Kim YI, Choi SY, Kang TC. The effect of topiramate on GABA(B) receptor, vesicular GABA transporter and paired-pulse inhibition in the gerbil hippocampus. Neurosci Res. 2005;53:413–420. doi: 10.1016/j.neures.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Hetherington H, Ho S, Pan J, Martin R, Gilliam F, Hugg J, Faught E. Topiramate increases cerebral GABA in healthy humans. Neurology. 1998;51:627–629. doi: 10.1212/wnl.51.2.627. [DOI] [PubMed] [Google Scholar]
- Lieving LM, Cherek DR, Lane SD, Tcheremissine OV, Nouvion SO. Effects of acute tiagabine administration on aggressive responses of adult male parolees. J Psychopharmacol. 2008;22:144–152. doi: 10.1177/0269881107078489. [DOI] [PubMed] [Google Scholar]
- Martin R, Kuzniecky R, Ho S, Hetherington H, Pan J, Sinclair K, Gilliam F, Faught E. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52:321–327. doi: 10.1212/wnl.52.2.321. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Arnold LM, Shapira NA, Keck PE, Jr, Rosenthal NR, Karim MR, Kamin M, Hudson JI. Topiramate in the treatment of binge eating disorder associated with obesity: a randomized, placebo-controlled trial. Am J Psychiatry. 2003;160:255–261. doi: 10.1176/appi.ajp.160.2.255. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Hulihan JF, Kamin M, Karim R. Differential cognitive and behavioral effects of topiramate and valproate. Neurology. 2003;60:1483–1488. doi: 10.1212/01.wnl.0000063308.22506.19. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–257. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Mula M, Trimble MR, Lhatoo SD, Sander JW. Topiramate and psychiatric adverse events in patients with epilepsy. Epilepsia. 2003;44:659–663. doi: 10.1046/j.1528-1157.2003.05402.x. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Buron E, Martin-Lopez M. Antiaggressive effects of topiramate in agonistic encounters between male mice. Methods Find Exp Clin Pharmacol. 2007;29:195–198. doi: 10.1358/mf.2007.29.3.1075351. [DOI] [PubMed] [Google Scholar]
- Nickel MK. Topiramate treatment of aggression in male borderline patients. Aust N Z J Psychiatry. 2007;41:461–462. doi: 10.1080/00048670701261251. [DOI] [PubMed] [Google Scholar]
- Nickel MK, Loew TH. Treatment of aggression with topiramate in male borderline patients, part II: 18-month follow-up. Eur Psychiatry. 2008;23:115–117. doi: 10.1016/j.eurpsy.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Nickel MK, Nickel C, Kaplan P, Lahmann C, Muhlbacher M, Tritt K, Krawczyk J, Leiberich PK, Rother WK, Loew TH. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biol Psychiatry. 2005;57:495–499. doi: 10.1016/j.biopsych.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Nickel MK, Nickel C, Mitterlehner FO, Tritt K, Lahmann C, Leiberich PK, Rother WK, Loew TH. Topiramate treatment of aggression in female borderline personality disorder patients: a double-blind, placebo-controlled study. J Clin Psychiatry. 2004;65:1515–1519. doi: 10.4088/jcp.v65n1112. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perucca E. A pharmacological and clinical review on topiramate, a new antiepileptic drug. Pharmacol Res. 1997;35:241–256. doi: 10.1006/phrs.1997.0124. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Hyder F, Rothman DL, Mattson RH. Topiramate rapidly raises brain GABA in epilepsy patients. Epilepsia. 2001;42:543–548. doi: 10.1046/j.1528-1157.2001.18800.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Levander S. Symptoms and personality characteristics of patients in a maximum security psychiatric unit. International Journal of Law Psychiatry. 1996;19:27–37. doi: 10.1016/0160-2527(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Reijs R, Aldenkamp AP, De Krom M. Mood effects of antiepileptic drugs. Epilepsy Behav. 2004;5 (Suppl 1):S66–76. doi: 10.1016/j.yebeh.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Robins LN. Childhood conduct problems, adult psychopathology, and crime. In: Hodgins S, editor. Mental disorder and crime. Newbury Park, CA: Sage; 1993. pp. 173–193. [Google Scholar]
- Rodgers RJ, Depaulis A. GABAergic influences on defensive fighting in rats. Pharmacol Biochem Behav. 1982;17:451–456. doi: 10.1016/0091-3057(82)90303-3. [DOI] [PubMed] [Google Scholar]
- Rosenfeld WE. Topiramate: a review of preclinical, pharmacokinetic, and clinical data. Clin Ther. 1997;19:1294–1308. doi: 10.1016/s0149-2918(97)80006-9. [DOI] [PubMed] [Google Scholar]
- Shipley-Boyle B. The Shipley Institute of Living Scale. Los Angeles CA: Western Psychological Services; 1967. [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165:429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgi P, Ratey JJ, Knoedler DW, Markert RJ, Reichman M. Rating aggression in the clinical setting: A retrospective adaptation of the overt aggression scale. Journal of Neuropsychiatry and Clinical Neuroscience. 1991;3:S52–S56. [PubMed] [Google Scholar]
- Spielberger CD, Jacobs G, Russell S, Crane RS, Butcher JN, Spielberger CD. Advances in personality assessment. Hillsdale NJ: Erlbaum; 1983. Assessment of anger: The state-trait anger scale; pp. 159–187. [Google Scholar]
- Stanford MS, Helfritz LE, Conklin SM, Villemarette-Pittman NR, Greve KW, Adams D, Houston RJ. A comparison of anticonvulsants in the treatment of impulsive aggression. Exp Clin Psychopharmacol. 2005;13:72–77. doi: 10.1037/1064-1297.13.1.72. [DOI] [PubMed] [Google Scholar]
- Steadman HJ, Mulvey EP, Monahan J, Robbins PC, Appelbaum PS, Grisso T, Roth LH, Silver E. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55:393–401. doi: 10.1001/archpsyc.55.5.393. [DOI] [PubMed] [Google Scholar]
- Swanson JW, Holzer CE, 3rd, Ganju VK, Jono RT. Violence and psychiatric disorder in the community: evidence from the Epidemiologic Catchment Area surveys. Hosp Community Psychiatry. 1990;41:761–770. doi: 10.1176/ps.41.7.761. [DOI] [PubMed] [Google Scholar]
- Zachary RA, Crumpton E, Spiegel DE. Estimating WAIS-R IQ from the Shipley Institute of Living Scale. Journal of Clinical Psychology. 1985;41:532–540. doi: 10.1002/1097-4679(198511)41:6<820::aid-jclp2270410616>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]