Abstract
Signaling of bone morphogenetic protein (BMP) via type I and type II receptors is involved in multiple processes contributing to cardiogenesis. To investigate the role of the BMP type II receptor (BMPRII) in heart development, the BMPRII gene was deleted throughout the embryo during gastrulation using a Mox2-Cre transgene. BMPRIIflox/−;Mox2-Cre mice exhibited cardiac defects including double-outlet right ventricle, ventricular septal defect (VSD), atrioventricular (AV) cushion defects, and thickened valve leaflets. To characterize the tissue-specific functions of BMPRII in cardiogenesis, a series of Cre transgenes (αMHC-, Tie2-, Wnt1-, and SM22α-Cre) was employed. Interestingly, myocardial development was normal when the BMPRII gene was deleted in myocardial cells using Mox2-Cre, αMHC-Cre, or SM22α-Cre transgenes, suggesting that signaling by other BMP type II receptors may compensate for the absence of BMPRII in the myocardial cells. AV cushion defects including atrial septal defect, membranous VSD, and thickened valve leaflets were found in BMPRIIflox/−;Tie2-Cre mice. Abnormal positioning of the aorta was observed in BMPRIIflox/−;Wnt1-Cre and BMPRIIflox/−;SM22α-Cre mice. Taken together, these results demonstrate that endocardial BMPRII expression is required for septal formation and valvulogenesis. Moreover, mesenchymal BMPRII expression in the outflow tract cushion is required for proper positioning of the aorta.
Keywords: cardiogenesis, bone morphogenetic protein, Cre-loxP, endocardial cushion, valvulogenesis, cardiac outflow tract
Introduction
Congenital heart defects (CHDs) occur in approximately 1% of live births and are the most common birth defects (Hoffman and Kaplan, 2002). Most CHDs are caused by abnormalities in septation and valvulogenesis. Endocardial-mesenchymal transformation (EMT) is a critical process for normal septation of the outflow tract (OFT) and atrioventricular (AV) canal. In EMT, endocardial cells invade into the cardiac jelly between the endocardium and myocardium and become mesenchymal cells, subsequently contributing to the formation of septa and valves (de Lange et al., 2004). Neural crest-derived cells migrate into the cardiac OFT and differentiate into smooth muscle cells (SMCs) of the great vessels and mesenchymal cells, which contribute to the septation of OFT and formation of semilunar valves. In addition, cells derived from the secondary heart field play a critical role in the orientation and patterning of the cardiac outflow tract (Zhou et al., 2007). The tubular OFT rotates counterclockwise during the formation of the great arteries (Bajolle et al., 2006). Failure of rotation of the OFT can lead to abnormal positioning of the aorta and pulmonary artery (PA).
Transforming growth factor (TGF)-β and bone morphogenetic proteins (BMPs) are known to play important roles in cardiogenesis (Bartram et al., 2001; Chen et al., 2004). BMPs bind to two different serine/threonine kinase receptors (type I and type II), both of which are required for signaling (Miyazono et al., 2005). BMPs signal via three type I receptors (i.e., activin receptor-like kinase (ALK) 2, 3, and 6) and three type II receptors (i.e., BMP type II receptor (BMPRII), activin type IIA receptor (ActRIIA), and activin type IIB receptor (ActRIIB)). Activated type I receptors phosphorylate Smads 1, 5, and 8 to transduce BMP signals into the nucleus (Miyazono et al., 2005). Since conventional gene targeting for most of the BMP receptors causes early embryonic lethality (Beppu et al., 2000; Gu et al., 1999; Mishina et al., 1999; Mishina et al., 1995), conditional gene targeting techniques have been employed to identify the roles of BMP signaling in cardiogenesis during mid-to-late gestation. For example, BMP4 is expressed in the myocardium of the OFT during cardiogenesis. Myocardium-specific deletion of the BMP4 gene using cardiac troponin T-Cre results in double-outlet right ventricle (DORV) and AV cushion defects (Jiao et al., 2003). Cardiac myocyte-specific deletion of the ALK3 gene using α-myosin heavy chain (αMHC)-Cre causes defects in the development of trabeculae, compact myocardium, and endocardial cushion (Gaussin et al., 2002). The endocardial cushion defect was associated with a decreased expression of TGF-β2 in cardiac myocytes. Endocardial deletion of ALK2 or ALK3 has shown that BMP signals are required for EMT and the subsequent development of the AV cushion (Ma et al., 2005; Song et al., 2007; Wang et al., 2005). BMP signaling is also required for the development of cardiac OFT and great arteries: tissue-specific deletion of ALK2 or ALK3 using Wnt1-Cre causes persistent truncus arteriosus (PTA) due to the defect of OFT septation (Kaartinen et al., 2004; Stottmann et al., 2004).
Mice homozygous for a BMPRII null mutation die during gastrulation without forming mesoderm (Beppu et al., 2000). Mice homozygous for a BMPRII hypomorphic allele, which has residual BMP signaling activity, die at mid-gestation and exhibit skeletal and cardiovascular abnormalities including PTA, interrupted aortic arch, and absent semilunar valve formation (Delot et al., 2003).
To evaluate the impact of BMPRII deficiency on cardiogenesis, BMPRII conditional knockout mice were generated using the Cre-loxP system (Beppu et al., 2005). A series of Cre transgenes were used to achieve epiblast-specific gene deletion during gastrulation or tissue-specific gene deletion. We report that BMPRII deficiency, when induced throughout the embryo using Mox2-Cre (Tallquist and Soriano, 2000), causes severe cardiac defects including DORV, ventricular septal defect (VSD), and abnormal AV cushion remodeling. AV cushion defects (membranous VSD, atrial septal defect (ASD), and thickened valves) were also detected in mice with endocardium-specific BMPRII deficiency. Abnormal positioning of the aorta was observed following deletion of the BMPRII gene with either Wnt1-Cre or SM22α-Cre, indicating that mesenchymal BMPRII in the OFT cushion is required for the appropriate rotation and/or positioning of the OFT. Taken together, these findings suggest that BMPRII has critical roles in both OFT and AV cushion development.
Materials and Methods
Mice
Mice carrying a BMPRII allele in which exons 4 and 5 are flanked by loxP sequences were generated previously (Beppu et al., 2005). Mox2-, Tie2-, and Wnt1-Cre transgenic mice and ROSA26 reporter (R26R) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). αMHC-Cre mice were obtained from Dr. Abel (Abel et al., 1999). SM22α-Cre mice were generated as described previously (Lepore et al., 2005). All control and mutant mice used in this study carried one copy of the R26R transgene. Experiments using animals were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Measuring BMPRII gene expression
Total RNA was extracted from embryonic hearts at E14.5 using TRIzol reagent (Invitrogen), and cDNA was synthesized by the reverse transcriptase reaction. Primer sequences for BMPRII exons 4 and 5 were described previously (Yu et al., 2005).
Morphology
Cardiac or embryonic tissues were fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at 4°C overnight, embedded in paraffin, and sectioned at 6-µm thickness. Sections were stained with hematoxylin and eosin. Staining for β-galactosidase activity was performed as described previously (Beppu et al., 2008).
Measuring DNA synthesis
BrdU (bromodeoxyuridine) was injected intraperitoneally into pregnant females (100 µg/gram of body weight) at E16.5. The females were sacrificed 4 hours after injection, and the embryos were fixed in 4% PFA, embedded in paraffin, and sectioned at 6-µm thickness. The sections were incubated with anti-BrdU antibodies conjugated with horseradish peroxidase (Roche Applied Science, IN). Binding of the antibodies was visualized with Tyramide Signal Amplification kit (Perkin Elmer Life and Analytical Sciences, MA) and 3,3’-diaminobenzidine (DAB Substrate Kit, Vector Laboratories, CA). The sections were counterstained with hematoxylin. DNA synthesis was reflected in the percentage of cell nuclei which were stained with anti-BrdU antibody. The DNA-binding dye Hoechst H33258 was used to count the number of dividing cells in semilunar valves at E17.5–18.5, as a quantitative index of the cell proliferation.
Immunohistochemistry
Tissue sections from mouse embryos were reacted with primary antibodies, including phospho-Smad1/5/8 antibodies (Cell Signaling Technology, MA) and NFATc1 antibodies (BD Biosciences, CA). Bound primary antibodies were detected using biotinylated secondary antibodies with Fluorescein-Avidin D (Vector Laboratories) for phospho-Smad1/5/8 or Vectastain Elite ABC kit with DAB (Vector Laboratories) for NFATc1.
Results
BMPRII deletion in the epiblast causes perinatal lethality
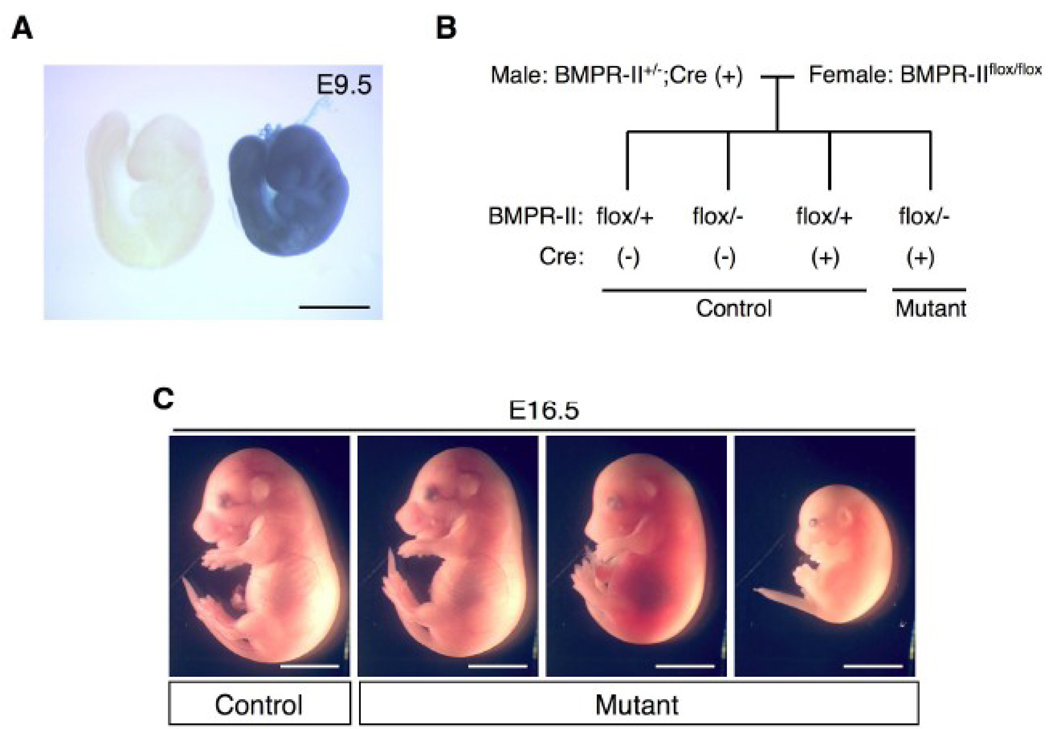
The BMPRII conditional mutant allele was previously generated using Cre-loxP system, in which a genomic fragment containing exons 4 and 5 of the BMPRII gene was flanked by two loxP sites (Beppu et al., 2005). Deletion of the BMPRII gene in the epiblast during gastrulation was performed at as early as embryonic day (E) 5 using a Mox2-Cre transgene (Tallquist and Soriano, 2000). Using this approach, the BMPRII gene was disrupted in all of the embryonic tissues during or after the gastrulation stage, but not in some extra-embryonic tissues including the primitive endoderm. Cell lineages in which the transgene was expressed were identified by staining for β-galactosidase activity, which is expressed when the ROSA26 reporter (R26R) allele undergoes Cre-mediated recombination. Gene recombination activity of Mox2-Cre in the entire embryo was confirmed at E 9.5 (Fig. 1A). BMPRII heterozygous mutant (BMPRII+/−) male mice carrying the Mox2-Cre transgene were crossed with female mice homozygous for a BMPRII conditional mutant (“floxed”) allele (BMPRIIflox/flox) and the R26R allele (Fig. 1B). BMPRIIflox/−;Mox2-Cre mice and control mice were examined at various embryonic stages and after birth. The numbers of live embryos for each genotype are shown in Table 1. The BMPRIIflox/−;Mox2-Cre embryos were observed in the anticipated Mendelian ratios through E12.5. However, there were only two BMPRIIflox/−;Mox2-Cre mice among 48 live littermates recovered after birth at postnatal day (P) 2. Some BMPRIIflox/−;Mox2-Cre embryos were found to be dead at E16.5 with varying degrees of resorption, whereas other BMPRIIflox/−;Mox2-Cre mutant embryos remained normal in gross appearance (Fig. 1C).
Figure 1. BMPRII deletion by the Mox2-Cre transgene causes perinatal lethality.
(A) Mox2-Cre-mediated recombination was detected by staining for β-galactosidase activity using a ROSA26 reporter allele. At E9.5, gene recombination was detected throughout the embryo (blue staining in the embryo on the right). An embryo without the Mox2-Cre transgene was used as a negative control (embryo on the left). (B) The breeding strategy used to obtain the mutant mice is shown. The three genotypes other than BMPRIIflox/−;Mox2-Cre were used as control mice. (C) Photographs of control and mutant embryos at E16.5 are shown. One of the three mutant mice was alive (left), but other two were found to be dead in utero (middle and right). Scale bars: 1 mm (A) and 5 mm (C).
Table 1.
Number of embryos obtained from crosses between BMPRII+/−; Mox2-Cre mice and BMPRIIflox/flox mice
| BMPRIIflox/+;Cre− | BMPRIIflox/−;Cre− | BMPRIIflox/+;Cre+ | BMPRIIflox/-;Cre+ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E9.5–12.5 | 77 | 82 | 75 | 78 | ||||||
| E13.5–16.5 | 43 | 50 | 49 | 27* | ||||||
| E17.5–18.5 | 17 | 13 | 10 | 3* | ||||||
| P1–2 | 13 | 19 | 14 | 2* | ||||||
The number of live embryos recovered from at various developmental stages is shown.
P < 0.01 compared with the expected Mendelian ratio.
Global deletion of BMPRII with Mox2-Cre causes cardiac defects in the outflow tract and AV canal
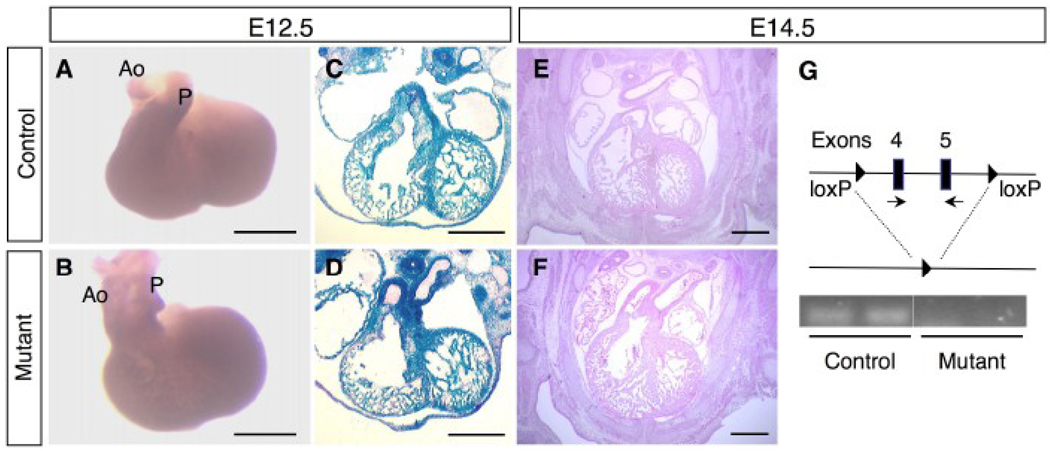
While only a fraction of the BMPRIIflox/−;Mox2-Cre embryos survived to late gestation without obvious growth retardation, all of the embryos showed cardiac defects including DORV with a VSD. DORV was observed as early as E12.5 (Fig. 2B, D), whereas the OFT developed normally in control mice (Fig. 2A, C). At E12.5, Cre recombinase activity was observed throughout the cardiac tissues in both control (BMPRIIflox/+;Mox2-Cre) and mutant (BMPRIIflox/−;Mox2-Cre) embryos (Fig. 2C, D). At E14.5, sections of developing hearts at the level of the PA showed persistent DORV in the BMPRIIflox/−;Mox2-Cre embryos (Fig. 2F) in contrast to the control hearts (Fig. 2E). Septation between the ascending aorta and PA occurred normally in the mutant heart (Fig. 2F). At E14.5, mRNA containing exons 4 and 5 of BMPRII (Fig. 2G) was detected in control, but not in the BMPRIIflox/−;Mox2-Cre mutant hearts using the reverse transcription-polymerase chain reaction (RT-PCR).
Figure 2. Outflow tract defects in BMPRIIflox/−;Mox2-Cre mice at E12.5–14.5.
(A, B) Photographs of embryonic hearts from control (A) and mutant (B) mice at E12.5. Ao: aorta, P: pulmonary artery. (C, D) Staining for β-galactosidase activity was performed to detect gene recombination on the frozen sections of the hearts from control (C) and mutant (D) mice at E12.5. (E, F) Hematoxylin and eosin staining of the embryonic hearts from control (E) and mutant (F) mice at E14.5. DORV was observed in the mutant hearts at E12.5 (D) and 14.5 (F). (G) Schematic diagram of the “floxed” allele and the deleted allele with loxP sites (filled triangles). BMPRII gene expression was detected by RT-PCR using a primer set for exons 4 and 5 (arrows). Ethidium bromide staining of PCR products for the BMPRII gene expression was shown for control and mutant (n=2 for each). Scale bars: 0.5 mm (A–F).
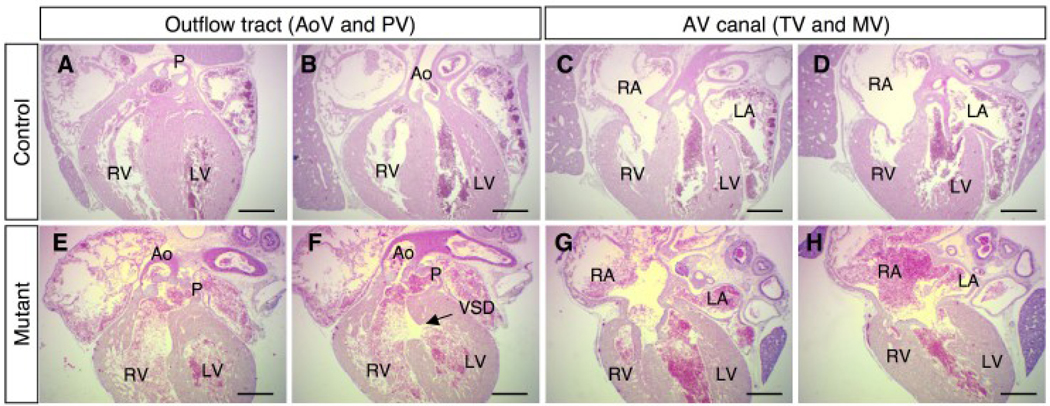
Normal development of the OFT and AV canal is complete by E16.5; however, persistent DORV and AV canal defects were observed in BMPRIIflox/−;Mox2-Cre mutant hearts at this stage (Fig. 3). The OFT of a control heart is shown at the level of the pulmonary valve (Fig. 3A) and aortic valve at E16.5 (Fig. 3B). In the BMPRIIflox/−;Mox2-Cre mutant heart, DORV (Fig. 3E, F) with a VSD (Fig. 3F, arrow) was observed. The tricuspid valve (TV) and mitral valve (MV) of a control heart are shown in Fig. 3C, D. In contrast, in BMPRIIflox/−;Mox2-Cre mutants the right atrium was shifted leftward (Fig. 3G, H), and atrial septal formation was disrupted (Fig. 3H). Aside from a VSD, there were no obvious structural defects in the myocardium in BMPRIIflox/−;Mox2-Cre mutant embryos (Fig. 3). The great arteries and the aortic arch in BMPRIIflox/−;Mox2-Cre embryos appeared normal when examined by Indian ink injection at E14.5 (data not shown). Quantitative RT-PCR revealed that expression of genes encoding cardiac transcription factors including Nkx2.5, MEF2C, GATA4, Tbx2, Tbx3, Tbx5, and dHand did not differ in control and BMPRIIflox/−;Mox2-Cre mutant hearts at E10.5 (data not shown).
Figure 3. Defects of the outflow tract and AV canal in BMPRIIflox/−;Mox2-Cre mice.
Hematoxylin and eosin staining is shown for control (A, B, C, D) and mutant (E, F, G, H) mice at E16.5. DORV (E, F), VSD (F, arrow), and AV canal defect with abnormal atrial septation (G, H) were observed in BMPRIIflox/−;Mox2-Cre mice. Ao: aorta, P: pulmonary artery, LA: left atrium, RA: right atrium, LV: left ventricle, RV: right ventricle. Scale bars: 0.5 mm (A–H).
Normal initiation of AV cushion formation and cell proliferation in BMPRII mutant embryos
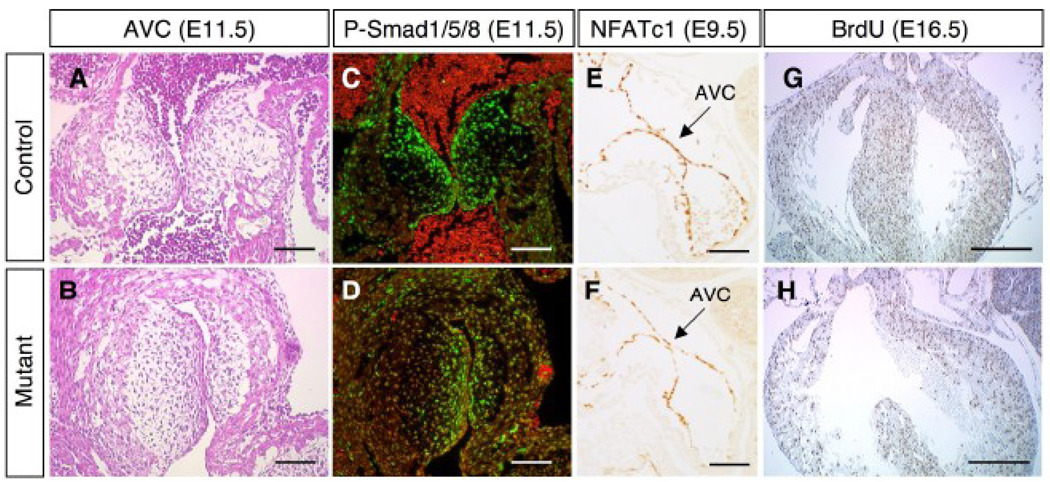
Histological analysis revealed that EMT occurred and progressed normally in the AV cushion of the BMPRIIflox/−;Mox2-Cre embryos at E11.5 (Fig. 4A, B). Mesenchymal cells transformed from endocardial cells were stained for phospho-Smad1/5/8 to detect active BMP signaling in the AV cushion (Fig. 4C, D). Smad1/5/8 phosphorylation was detected in the absence of BMPRII (Fig. 4D), suggesting that BMP signaling is transduced via alternate BMP type II receptors (i.e., Activin type IIA or IIB receptors). At E9.5, NFATc1 is known to regulate the initiation of EMT. However, no difference in NFATc1 expression between control and BMPRIIflox/−;Mox2-Cre mutant embryos was observed (Fig. 4E, F). Labeling of proliferating cells with BrdU revealed a similar number of cardiac cells undergoing DNA synthesis in both control and BMPRIIflox/−;Mox2-Cre mutant embryos (Fig. 4G, H). The fraction of cells that are actively synthesizing DNA (BrdU-positive) did not differ in control and mutant hearts (35±3% (n=2) versus 34±1% (n=3), respectively).
Figure 4. AV cushion development and cardiac cell proliferation in BMPRIIflox/−;Mox2-Cre mice.
Cardiac tissues from control (A, C, E, G) and mutant (B, D, F, H) are shown. (A, B) Sections of the AV cushion at E11.5 were stained with hematoxylin and eosin. (C, D) Sections shown in A and B were reacted with antibody directed against phosphorylated Smads1, 5, and 8 (green) and counterstained with propidium idodide (red). (E, F) Immunohistostaining for NFATc1 (brown) at E9.5 is shown. AVC: AV cushion. (G, H) BrdU incorporation for 4 hours was performed to detect proliferating cardiac cells (brown nuclei) at E16.5. Scale bars: 0.1 mm (A–F) and 0.5 mm (G, H).
Endocardial BMPRII is required for remodeling of the AV cushion
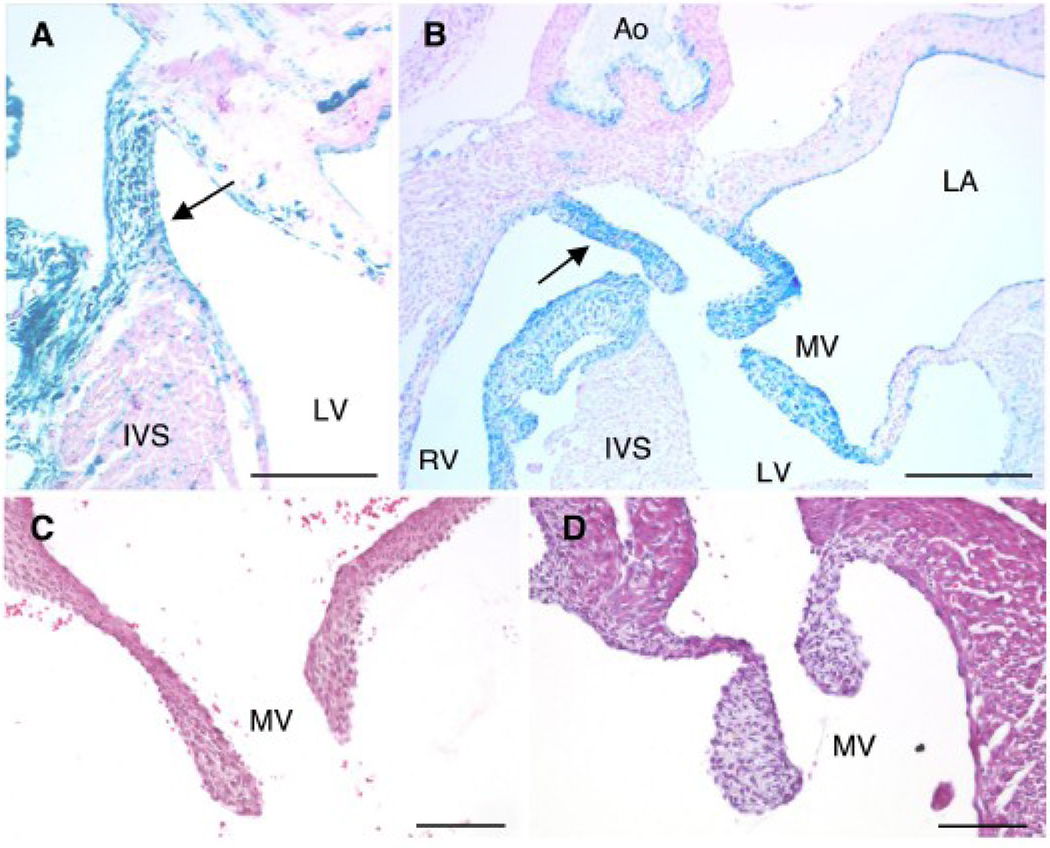
To determine the role of BMPRII in endocardial cell regulation of AV cushion and valve formation, the BMPRII gene was deleted specifically in the endocardial cell lineage using a Tie2-Cre transgene. The majority of BMPRIIflox/−;Tie2-Cre mice died within one week after birth. In mice carrying the Tie2-Cre transgene, Cre-mediated recombination occurs in endocardial cells at E9.5 (de Lange et al., 2004), many of which subsequently transform into mesenchymal cells and contribute to the formation of AV cushion and valves (semilunar and AV). The membranous portion of the interventricular septum (IVS) was stained for β-galactosidase activity from the R26R allele in BMPRIIflox/+;Tie2-Cre control mice at P2 (Fig. 5A, arrow). In BMPRIIflox/−;Tie2-Cre mutant mice, however, the membranous portion of the IVS failed to connect to the muscular portion of the IVS (Fig. 5B, arrow). Expression of β-galactosidase from the R26R allele was detected in both tricuspid (data not shown) and mitral valves (Fig. 5B). The mitral valve leaflets were significantly thickened in the BMPRIIflox/−;Tie2-Cre mutant mice (Fig. 5D) compared to those in control mice (Fig. 5C). Similar findings were found in the tricuspid valves of BMPRIIflox/−;Tie2-Cre mutant mice (data not shown). These results indicate that BMPRII deletion in the endocardial cell lineage does not block the initiation of EMT, but does lead to abnormal remodeling of the AV cushion forming the ventricular septum and aberrant formation of the tricuspid and mitral valves.
Figure 5. BMPRII deletion in endocardial lineage cells using a Tie2-Cre transgene.
(A, B) Staining for β-galactosidase activity (blue) with nuclear fast red counterstaining (pink) was performed at P2. Tie2-Cre activity was detected in the membranous portion of the interventricular septum in control mice (A, arrow). In BMPRIIflox/−;Tie2-Cre mutant mice at P2, the membranous portion of the septum failed to connect to the muscular portion of the septum (B, arrow). Mitral valve leaflets stained positively for β-galactosidase activity (B). Hematoxylin and eosin staining of a mitral valve from a control mouse (C) and a BMPRIIflox/−;Tie2-Cre mutant mouse (D) at P2 is shown. RV: right ventricle, LV: left ventricle, IVS: interventricular septum, Ao: aorta, LA, left atrium, MV: mitral valve. Scale bars: 0.2 mm (A, B) and 0.1 mm (C, D).
BMPRII deletion causes thickened semilunar valve formation
During late embryonic and postnatal development, valve leaflets elongate and remodel to form mature valve leaflets (Hinton et al., 2006). Normal aortic (E17.5) and pulmonary (P3) valves are shown in Fig. 6A and 6C, respectively. BMPRIIflox/−;Mox2-Cre mutant mice (E17.5; Fig. 6B) and BMPRIIflox/−;Tie2-Cre mutant mice (P3; Fig. 6D) have abnormally-thickened semilunar valves as compared to control littermates. The thickening of semilunar valves was obvious after E16.5 in BMPRIIflox/−;Mox2-Cre mutant mice. At E17.5–18.5, the number of dividing cells in the semilunar valves of BMPRIIflox/−;Mox2-Cre mutant mice was greater than that of the control mice (1.4±0.2 versus 3.7±0.3 cells per section in control and mutant, respectively; 4 sections from each of 4 mice in both genotypes, p<0.05). There was no difference in cell density or evidence of apoptosis (as measured using TUNEL staining) in normal and thickened valve leaflets at E16.5 (Mox2-Cre) and P2 (Tie2-Cre) (data not shown).
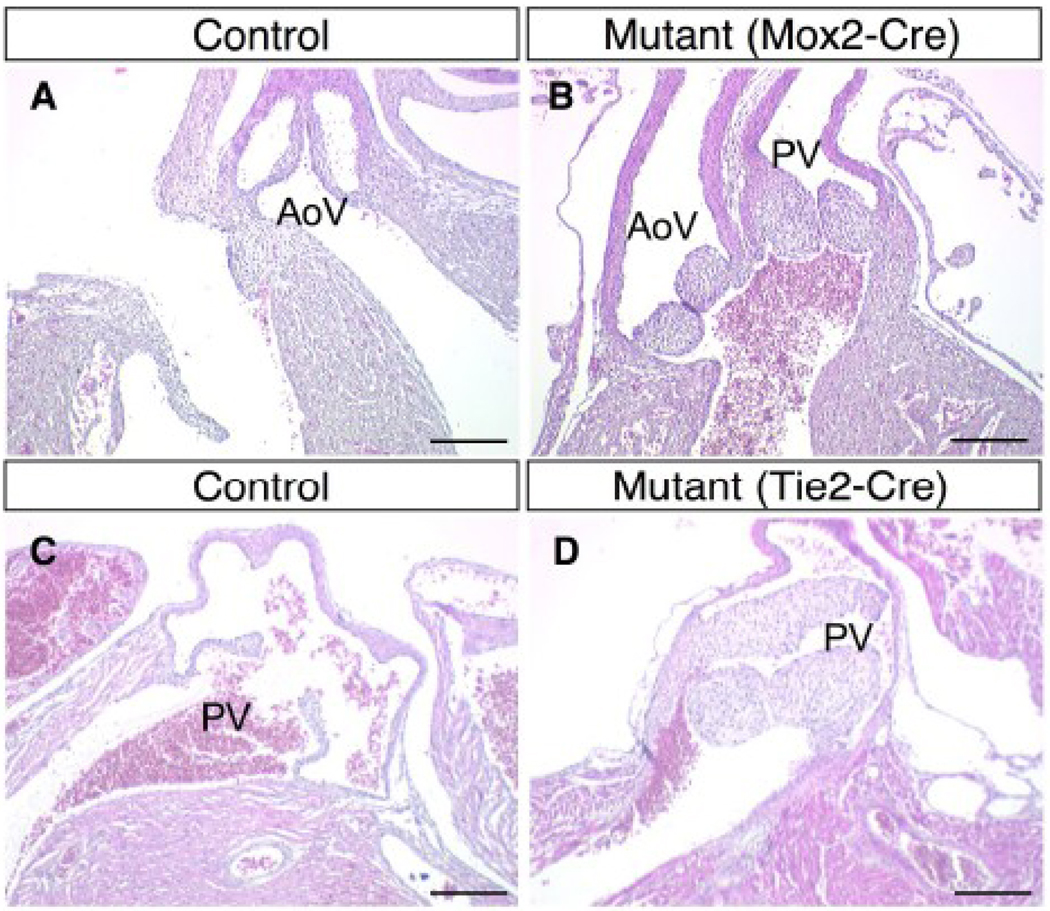
Figure 6. Thickened semilunar valves in BMPRIIflox/−;Mox2-Cre and BMPRIIflox/−;Tie2-Cre mice.
(A–D) Hematoxylin and eosin staining is shown for the aortic valve in a control mouse at E17.5 (A), for aortic and pulmonary valves with DORV in a BMPRIIflox/−;Mox2-Cre mouse at E17.5 (B), for a pulmonary valve in a control mouse at P3 (C), and for a pulmonary valve in a BMPRIIflox/−;Tie2-Cre mouse (D). AoV: aortic valve, PV: pulmonary valve. Scale bars: 0.2 mm (A–D).
Mesenchymal BMPRII in the OFT cushion regulates positioning of outflow tract
The SM22α-Cre transgene is expressed in vascular and visceral smooth muscle cells. During the development of murine embryos, the SM22α-Cre transgene is also expressed in all cardiac tissues except for endocardial cells (Lepore et al., 2005). The majority of BMPRIIflox/−;SM22α-Cre mice died shortly after birth. Abnormal positioning of the OFT (Fig. 7B, arrow) and VSDs (data not shown) were observed in the BMPRIIflox/−;SM22α-Cre mice at E14.5. In the control heart at E14.5 (Fig. 7A), the OFT from the left ventricle (LV) to the ascending aorta is positioned nearly vertically above the LV outflow tract and the IVS. In the mutant heart, the OFT deviates rightward, resulting in abnormal positioning of the aortic valve and ascending aorta (Fig 7B). These results indicate that deletion of BMPRII in the OFT cushion causes the rotation of the OFT to be stopped prematurely after formation of the aorto-pulmonary septum (Fig. 8).
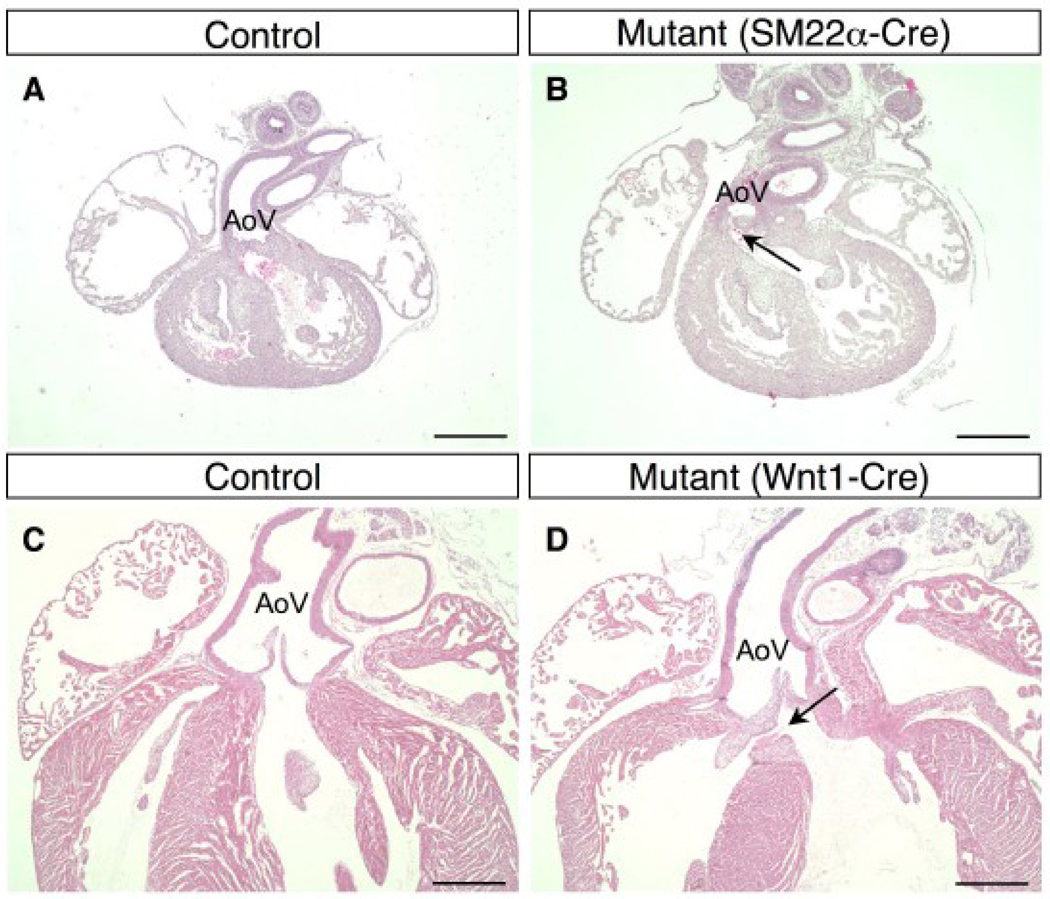
Figure 7. Overriding aorta in BMPRIIflox/−;SM22α-Cre and BMPRIIflox/−;Wnt1-Cre mice.
(A–D) Hematoxylin and eosin staining is shown for a control mouse (A) and a BMPRIIflox/−;SM22α-Cre mouse (B) at E14.5, and a control mouse (C) and a BMPRIIflox/−;Wnt1-Cre mouse (D) at P3. The OFT from the left ventricle was severely deviated to the right side of the heart in the BMPRIIflox/−;SM22α-Cre mouse. The aortic valve is located over the right ventricle (B, arrow) in this long axis cross section, whereas the aortic valve in a control mouse is located centrally (A). An overriding aorta with a small VSD (D, arrow) is observed in BMPRIIflox/−;Wnt1-Cre mice, whereas the normal position of the aorta is shown in control mice at P3 (C). AoV: aortic valve. Scale bars: 0.5 mm (A–D).
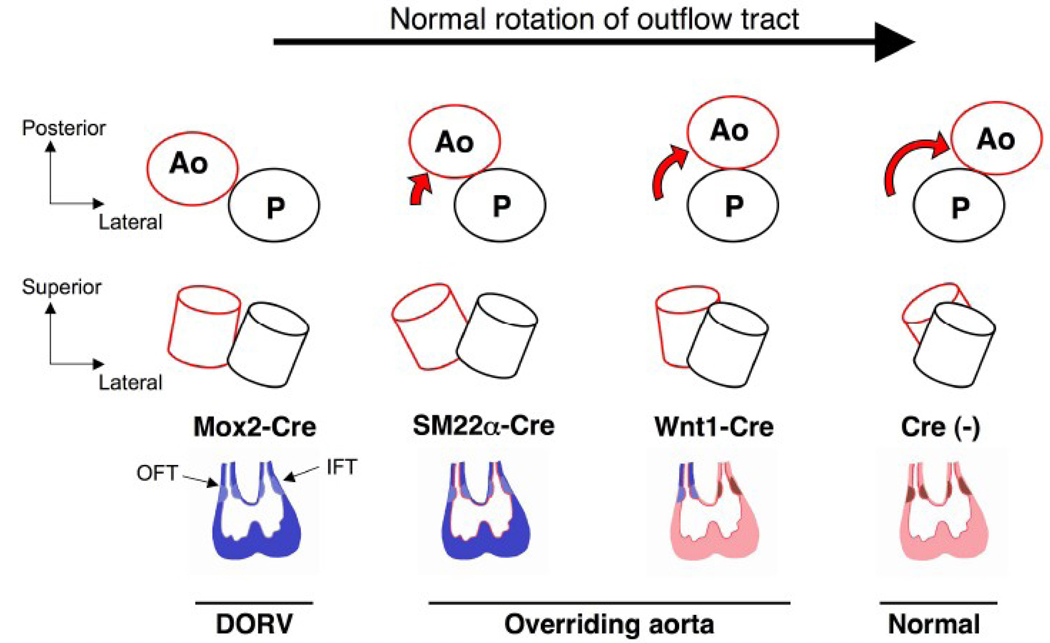
Figure 8. BMPRII deficiency interrupts normal rotation of the OFT in a tissue-specific manner.
The normal rotation of the aorta (red) and the pulmonary artery (black) during cardiac development is shown from a cranial view (upper panel) and a lateral view (middle panel). In this illustration of embryonic hearts (looping stage), BMPRII-deficient cardiac tissues are colored in blue for each Cre transgene (lower panel). Normal tissues (Cre-negative) are shown in pink (myocardium), red (endocardium), and brown (OFT and AV cushions). The rotational process is interrupted at different stages for each Cre transgenic strain as shown in the diagrams. Note that neural crest-derived cells expressing Wnt1-Cre partially contribute to the formation of the OFT cushion. Varying degrees of malrotation are observed with BMPRIIflox/− mice with the Mox2-Cre, SM22α-Cre, and Wnt1-Cre transgenes. The severity of aortic malpositioning likely correlates with the number of BMPRII-deficient mesenchymal cells in the OFT cushion and/or the timing of BMPRII deletion. Ao: aorta, P: pulmonary artery, IFT: inflow tract, OFT: outflow tract.
Neural crest-derived cells migrate to great arteries and to the cardiac OFT. The cells populate the truncus arteriosus at E9.5 and invade into OFT cushions at E10.5 (Jiang et al., 2000). To determine whether the observed OFT patterning defect results from abnormalities in neural crest-derived SMCs and mesenchymal cells populating the OFT, the BMPRII gene was deleted in neural crest-derived cells using a Wnt1-Cre transgene. An overriding aorta and small VSD (Fig. 7D, arrow) were observed in BMPRIIflox/−;Wnt1-Cre mice, and the rightward deviation of the OFT was less severe than that of the BMPRIIflox/−;SM22α-Cre mice (Fig. 7B, C, D). Of note, the positioning of the OFT appeared to be normal in the BMPRIIflox/−;Tie2-Cre and BMPRIIflox/−;αMHC-Cre mutant mice (data not shown). These results suggest that expression of BMPRII in neural crest-derived cells contributes to normal positioning of the aorta.
BMPRII is not essential for normal myocardial development
Absence of obvious myocardial defects in BMPRIIflox/−;Mox2-Cre mutant hearts suggested the possibility that BMPRII is not necessary for the development of myocardial cells. To further test this hypothesis, we examined the myocardium of BMPRII floxed mice carrying the SM22α-Cre or αMHC-Cre transgene, both of which express the Cre recombinase in myocardial cells during development. There were no myocardial defects in the BMPRII floxed mice carrying the SM22α-Cre (Fig. 7B) or αMHC-Cre transgene (data not shown). BMPRIIflox/−;αMHC-Cre mutant mice had a normal life span.
Discussion
BMP signaling plays important roles in many aspects of cardiogenesis including endocardial cushion and valve maturation (Delot, 2003; Schneider et al., 2003). Since BMPRII homozygous mutant embryos die during gastrulation (Beppu et al., 2000), our knowledge of the role of the BMPRII gene in the development of murine embryos during mid-to-late gestation has been largely incomplete. In this study, we examined the roles of BMPRII in cardiogenesis using a BMPRII conditional mutant allele (Beppu et al., 2005). We demonstrate the essential role of BMPRII in key aspects of cardiogenesis including the regulation of OFT position, AV cushion remodeling, and valve formation.
BMPRII is primarily required for cardiac development after gastrulation
To examine the role of the BMPRII gene in murine development after the gastrulation stage, the Mox2-Cre transgene was employed to delete the gene in epiblast cells during gastrulation (E5.5–7.5), but in not extra-embryonic tissues such as primitive endoderm and extra-embryonic ectoderm derivatives (Tallquist and Soriano, 2000). Previous reports have suggested that Mox2-Cre-induced DNA recombination is incomplete resulting in genetic mosaicism (Davis et al., 2004). However, we found that expression of the BMPRII gene was reduced by more than 90% at E12.5 (data not shown) and E14.5 (Fig. 2G) in the cardiac tissues of the mutant embryos. After deletion of the BMPRII gene in the epiblast cells at around E6.5, BMPRIIflox/−;Mox2-Cre embryos continued to develop through E16.5. The most obvious phenotypes observed in BMPRIIflox/−;Mox2-Cre embryos were cardiac defects, including DORV, VSD, abnormal AV cushion remodeling, and thickened valve leaflets. These results suggest that BMPRII is required for development of extra-embryonic tissues during gastrulation and for cardiac development after the gastrulation stage. The phenotypes observed in BMPRIIflox/−;Mox2-Cre embryos appear to be less severe than those observed in embryos in which the ALK3 gene was deleted using the same Mox2-Cre transgene (Davis et al., 2004). The ALK3 mutant embryos exhibited defects in endodermal morphogenesis and ectodermal patterning and died around E10.5 (Davis et al., 2004). Although BMP signaling is required for many developmental processes, most of the embryonic organs other than the heart appear to develop normally in the BMPRIIflox/−;Mox2-Cre embryos. Since both type I and type II receptors are required for BMP signaling (Kawabata et al., 1998), other BMP type II receptors (i.e., ActRIIA or ActRIIB) may partially compensate for loss of BMPRII. We previously reported the ability of ActRIIA to substitute, in part, for BMPRII for the activation of the Smad pathway in murine pulmonary artery smooth muscle cells (Yu et al., 2005). The gastrulation defects in the BMPRII−/− embryos and/or cardiac defects in BMPRIIflox/−;Mox2-Cre embryos may occur in tissues where the other type II receptors for BMPs are not available to compensate for the function of BMPRII. Alternatively, BMP ligand-specific gain of function in BMPRII-deficient cardiac cells, similar to that which we observed in pulmonary artery smooth muscle cells (Yu et al., 2005), could contribute to the defects observed in embryos deficient in BMPRII. However, we cannot exclude the possibility that the cardiac defects observed in the BMPRIIflox/−;Mox2-Cre embryos are a result of disrupted non-Smad pathways, including molecules which may directly interact with BMPRII (Chan et al., 2007; Foletta et al., 2003; Hassel et al., 2004; Machado et al., 2003).
BMPRII is not necessary for myocardial development
BMPs play important roles in myocardial development. Myocardium-specific deletion of ALK3 using the αMHC-Cre transgene showed that ALK3 is required for normal development of trabeculae, compact myocardium, interventricular septum, and endocardial cushion (Gaussin et al., 2002). However, the development of myocardium seems to be unaffected in the BMPRIIflox/−;Mox2-Cre embryos, in which the BMPRII gene was deleted in epiblast cells before the initiation of cardiac development. Levels of mRNA encoding Nkx2.5, GATA4, Tbx2, Tbx3, Tbx5, Mef2c, and dHand in BMPRIIflox/−;Mox2-Cre hearts did not differ from those in control hearts at E10.5 (data not shown). Furthermore, there were no obvious myocardial defects in BMPRIIflox/−;αMHC-Cre nor BMPRIIflox/−;SM22α-Cre mice, in which Cre recombinase is expressed in the myocardium. Together, these results suggest that BMPRII is dispensable for the development of myocardium and that BMP signaling in myocardial cells may be transduced via ActRIIA or ActRIIB in the absence of BMPRII.
BMPRII is required for remodeling of AV cushion
Formation of endocardial cushions is one of the key events in cardiogenesis. ALK2 and ALK3 regulate the initiation of EMT, and endocardial-specific deletion of ALK2 or ALK3 blocks the initiation of EMT with few, if any, mesenchymal cells observed in the AV cushions (Song et al., 2007; Wang et al., 2005). However, in the AV cushion of BMPRIIflox/−;Mox2-Cre mutant embryos, initiation of EMT appears to be normal, and comparable levels of Smad1/5/8 phosphorylation were detected in the AV cushions of mutant and control embryos (Fig. 4). Similarly, the progression of EMT seemed to be normal in BMPRIIflox/−;Tie2-Cre embryos, but deletion of the BMPRII gene specifically in endocardial cells results in a membranous VSD (Fig. 5B, arrow). These results suggest that BMPRII is required for AV cushion remodeling to form an intact interventricular septum.
Tissue-specific deficiency of BMPRII causes muscular or membranous VSDs
Presence of a muscular VSD was associated with abnormal rotation of the OFT, such as DORV (BMPRIIflox/−;Mox2-Cre) and severely overriding aorta (BMPRIIflox/−;SM22α-Cre), raising the possibility that muscular VSD formation was “secondary” to abnormal OFT rotation. Absence of a VSD in BMPRIIflox/−;αMHC-Cre mutant mice also suggests that BMPRII deficiency in cardiac myocytes is insufficient to induce VSD formation.
The location of the membranous VSD in BMPRIIflox/−;Tie2-Cre and BMPRIIflox/−;Wnt1-Cre mutant mice correlates with the expression of the Cre recombinase transgene in the IFT and OFT cushions. These results suggest a role for BMPRII in the maturation of endocardial cushions, and specifically, the membranous portion of the IVS.
BMPRII deficiency causes thickening of valve leaflets
BMP signaling is known to regulate cardiac valvulogenesis (Armstrong and Bischoff, 2004). In late gestation, valve remodeling begins and continues into postnatal life to form mature valve leaflets (Hinton et al., 2006). Valvular cell proliferation is dramatically decreased during the remodeling stage (Hinton et al., 2006). We found that BMPRII deficiency causes thickening of valve leaflets (Fig. 5D and Fig. 6), which was most evident after E16.5. We observed that the number of dividing cells in the thickened semilunar valves of BMPRIIflox/−;Mox2-Cre mutant mice was greater than that of control mice at E17.5–18.5. In contrast, there were no detectable apoptotic cells in the control and mutant valves. Moreover, there was no difference in the valve cell density between the two genotypes (data not shown). These results suggest that BMPRII deficiency leads to increased and/or persistent proliferation of mesenchymal cells, resulting in the development of thickened semilunar valve leaflets.
Delot and colleagues reported mice homozygous for a truncated_BMPRII mutation, which die at mid-gestation with various defects, including skeletal abnormalities, PTA, and an interrupted aortic arch (Delot et al., 2003). The truncated BMPRII (expressed from the mutant allele) has reduced BMP signaling capacity compared with wild-type BMPRII. In contrast to our findings, semilunar valves did not form in mice homozygous for the truncated BMPRII. On the other hand, mice with augmented BMP signaling due to deficiency of Smad6 or Noggin, which act as endogenous inhibitors of BMP signaling, also had thickened heart valves (Choi et al., 2007; Galvin et al., 2000). These results suggest that thickened valve leaflets observed in the BMPRIIflox/−;Mox2-Cre embryos and BMPRIIflox/−;Tie2-Cre mice may be due to augmented BMP signaling in the BMPRII-deficient cells (Yu et al., 2005). It is possible that presence of a truncated BMPRII could interfere with components of BMP signaling pathways (i.e., through sequestration of type I BMP receptors) resulting in more severe phenotypes than the complete absence of BMPRII, for which ActRIIA or ActRIIB may compensate. However, we cannot exclude the possibility that the different gene-targeting strategies (which lead to the expression of truncated BMPRII from the beginning of life in all cells including extra-embryonic tissues versus induced BMPRII deficiency at certain developmental stages in tissue-specific manner) contribute to the differing valve leaflet phenotypes.
BMPRII regulates OFT development and positioning of the aorta
Rotation of the OFT is critical for the proper positioning of ascending aorta and pulmonary artery (Bajolle et al., 2006). Although neural crest-derived cells are known to play a role in this process (Restivo et al., 2006), the mechanisms by which the rotation is initiated and regulated are largely unknown. Conditional deletion of ALK2 or ALK3 using a Wnt1-Cre transgene both cause severe defects in the OFT with failure to septate the aorto-pulmonary trunk leading to PTA (Kaartinen et al., 2004; Stottmann et al., 2004). Compared to these phenotypes, the hearts in BMPRIIflox/−;Mox2-Cre embryos showed normal aorto-pulmonary septation, but exhibited DORV. This result suggests that BMPRII is required for the normal rotation of the OFT and other type II BMP receptors are sufficient for aorto-pulmonary septation. Furthermore, tissue-specific deletion of the BMPRII gene using Wnt1-Cre or SM22α-Cre also results in abnormal positioning of the aorta (i.e., overriding aorta) with varying degrees of severity. However, endocardial-specific deletion of BMPRII with a Tie2-Cre transgene does not cause the abnormal positioning of the ascending aorta likely because the Tie2-Cre transgene is expressed in only a small fraction of the mesenchymal cells of the OFT cushion. Deletion of the BMPRII gene using Mox2-,SM22α-, and Wnt1-Cre interrupted the normal rotational process of the aorta and pulmonary artery and resulted in the malpositioning of the aorta ranging from DORV to overriding aorta (Fig. 8). BMPRIIflox/−;SM22α-Cre mutant mice have more severe rightward deviation of the overriding aorta than do BMPRIIflox/−;Wnt1-Cre mutant mice. The severity of the abnormal positioning of the aorta may correlate with the fraction of OFT mesenchymal cells in which the BMPRII gene is deleted and/or the timing of the BMPRII deletion in the OFT cushion.
In summary, BMPRII deletion using conditional gene targeting revealed various congenital defects including DORV, overriding aorta, VSD, and thickened valve leaflets. Our results show that mesenchymal BMPRII in the OFT cushion is responsible for the proper positioning of the OFT. These findings further contribute to our understanding of the tissue-specific role of BMPRII during cardiogenesis and may have future clinical implications for patients with CHD and/or valvular heart disease.
Acknowledgments
The authors thank Drs. Calum MacRae and Donald B. Bloch for valuable discussions and experimental advice, and Dr. E. Dale Abel for αMHC-Cre transgenic mice. This work was supported by American Heart Association Scientist Development Grant 0535079N (HB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajolle F, Zaffran S, Kelly RG, Hadchouel J, Bonnet D, Brown NA, Buckingham ME. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ Res. 2006;98:421–428. doi: 10.1161/01.RES.0000202800.85341.6e. [DOI] [PubMed] [Google Scholar]
- Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de Groot AC. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- Beppu H, Lei H, Bloch KD, Li E. Generation of a floxed allele of the mouse BMP type II receptor gene. Genesis. 2005;41:133–137. doi: 10.1002/gene.20099. [DOI] [PubMed] [Google Scholar]
- Beppu H, Mwizerwa ON, Beppu Y, Dattwyler MP, Lauwers GY, Bloch KD, Goldstein AM. Stromal inactivation of BMPRII leads to colorectal epithelial overgrowth and polyp formation. Oncogene. 2008;27:1063–1070. doi: 10.1038/sj.onc.1210720. [DOI] [PubMed] [Google Scholar]
- Chan MC, Nguyen PH, Davis BN, Ohoka N, Hayashi H, Du K, Lagna G, Hata A. A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol Cell Biol. 2007;27:5776–5789. doi: 10.1128/MCB.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, Chen Z, Yang Z, Schneider MD, Chien KR, Conway SJ, Yoder MC, Haneline LS, Franco D, Shou W. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Stottmann RW, Yang YP, Meyers EN, Klingensmith J. The bone morphogenetic protein antagonist noggin regulates mammalian cardiac morphogenesis. Circ Res. 2007;100:220–228. doi: 10.1161/01.RES.0000257780.60484.6a. [DOI] [PubMed] [Google Scholar]
- Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol. 2004;270:47–63. doi: 10.1016/j.ydbio.2004.01.048. [DOI] [PubMed] [Google Scholar]
- de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95:645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- Delot EC. Control of endocardial cushion and cardiac valve maturation by BMP signaling pathways. Mol Genet Metab. 2003;80:27–35. doi: 10.1016/j.ymgme.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development. 2003;130:209–220. doi: 10.1242/dev.00181. [DOI] [PubMed] [Google Scholar]
- Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massague J, Bernard O, Soosairaiah J. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol. 2003;162:1089–1098. doi: 10.1083/jcb.200212060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Jr, Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- Hassel S, Eichner A, Yakymovych M, Hellman U, Knaus P, Souchelnytskyi S. Proteins associated with type II bone morphogenetic protein receptor (BMPR-II) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004;4:1346–1358. doi: 10.1002/pmic.200300770. [DOI] [PubMed] [Google Scholar]
- Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Lepore JJ, Cheng L, Min Lu M, Mericko PA, Morrisey EE, Parmacek MS. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-Cre transgenic mice. Genesis. 2005;41:179–184. doi: 10.1002/gene.20112. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Machado RD, Rudarakanchana N, Atkinson C, Flanagan JA, Harrison R, Morrell NW, Trembath RC. Functional interaction between BMPR-II and Tctex-1, a light chain of Dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum Mol Genet. 2003;12:3277–3286. doi: 10.1093/hmg/ddg365. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol. 1999;213:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Restivo A, Piacentini G, Placidi S, Saffirio C, Marino B. Cardiac outflow tract: a review of some embryogenetic aspects of the conotruncal region of the heart. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:936–943. doi: 10.1002/ar.a.20367. [DOI] [PubMed] [Google Scholar]
- Schneider MD, Gaussin V, Lyons KM. Tempting fate: BMP signals for cardiac morphogenesis. Cytokine Growth Factor Rev. 2003;14:1–4. doi: 10.1016/s1359-6101(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Song L, Fassler R, Mishina Y, Jiao K, Baldwin HS. Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol. 2007;301:276–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 2004;131:2205–2218. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]