Abstract
Musculoskeletal procedures often show wide variation in rates across geographic areas, which begs the question, “Which rate is right?” Clearly, there is no simple answer to this question. We summarize a conceptual framework for thinking about how to approach this question for different types of interventions. One guiding principle is the “right rate” is usually the one that results from the choices of a fully informed and empowered patient population. For truly effective care without substantial tradeoffs, the right rate may approach 100%. The rate of operative treatment of hip fracture, for example, approaches the underlying incidence of disease; however, the rate of some forms of effective care, like osteoporosis evaluation and treatment after a fragility fracture, is often quite low and undoubtedly reflects underuse. The recommended approach to underuse is to improve the reliability and accountability of the delivery system. Many other musculoskeletal interventions fall into the category of “preference-sensitive care.” These interventions involve important tradeoffs between risks and benefits. Variations in these procedure rates may represent insufficient focus on patient values and preferences, relying instead on the enthusiasm of the physician for treatment alternatives. The recommended approach in this setting is the use of decision aids and other approaches to informed choice.
Level of Evidence: Level V, expert opinion. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Wide geographic variations in the rates of musculoskeletal procedures have been repeatedly documented [3, 9, 14, 25, 27]. In addition, variations have been found in the rates of certain musculoskeletal procedures among racial, ethnic, and socioeconomic groups [4, 8, 24]. These large variations have not been explainable by differences among the patient populations [7, 28], and the persistence of these large differences in the number of procedures performed in different regions begs the question, “Which rate is right?”
The inherent uncertainty involved in most medical decisions and the variability in patients’ preferences for different treatments and values for different outcomes makes any simple answer to this question impossible. This is not a topic amenable to a systematic review or meta-analysis. A key first step is having a conceptual framework within which to look at the variety of musculoskeletal care and procedures and to consider what criteria one might use for assessing the appropriateness of the delivery of that care. One key principle that can serve as a guide for questioning this process is the “right rate” for most procedures is the one resulting from a fully informed patient population fully engaged in the decision-making process [26].
Fisher and Wennberg and their colleagues have outlined a useful analytic framework that classifies three different types of care: effective care, supply-sensitive care, and preference-sensitive care [7]. Effective care consists of those interventions where there is reasonable scientific evidence of efficacy and minimal tradeoffs between risks and benefits; supply-sensitive care indicates those parts of care for which patients do not have strong preferences, clinical science has little to offer in terms of guidance as far as the most efficient methods of delivery, and the rate of the care delivered is dependent almost entirely on the extent of local resources; and preference-sensitive care offers a distinct choice between at least two treatments with different risks and benefits, and the preferred therapy will vary based on the patients’ values. Each of these three categories will have different criteria for the “right rate” and different methods for achieving it.
In this narrative review, we summarize this conceptual framework, analyze the three categories of care as applied to musculoskeletal procedures, and synthesize some of the relevant literature on the current state of musculoskeletal practice using this framework.
Effective Care
Effective care consists of those interventions where there is reasonable scientific evidence of efficacy and minimal tradeoffs between risks and benefits; this is the type of care that nearly all well-informed patients would want (eg, prompt administration of appropriate antibiotics for a serious infection) [7]. For truly effective care, the appropriate rate approaches 100% and the major problem in clinical practice is underuse.
For effective care to be reliably delivered, providers must be educated regarding best practices. This is often pursued through the used of evidence-based practice guidelines. However, this is often insufficient and health delivery systems must be designed to ensure the reliable delivery of effective care. As a result, rates of effective care often serve as standard “quality measures.”
One example of effective musculoskeletal care is the use of appropriately timed perioperative antibiotics and thromboembolic prophylaxis. With few clinical exceptions, the rate in appropriately defined patients would be 100% and the gap between this ideal and the rate in practice represents both a failure of reliable delivery and an opportunity to improve the system.
Surgical repair of a hip fracture is another example of effective care. Here the diagnosis is relatively certain, as is the efficacy of the intervention; very few patients would prefer nonoperative therapy given the difference in functional outcomes. As a result, the surgical treatment of hip fracture approaches the incidence rate of disease and there is very little variation in these rates across geographic areas [25]. Similarly, there is no substantial difference in the treatments received following hip fracture among racial groups [5].
An example of musculoskeletal procedures in the “effective but underused” category is the evaluation and treatment of osteoporosis in patients following hip fracture. Patients with a hip fracture have a 2.5-fold increase in the risk of a subsequent osteoporotic fracture and appropriate intervention improves outcomes; however, few patients receive adequate treatment for osteoporosis post hip fracture [2]. In one Finnish study, only 39% of patients used antiosteoporotic medications and only 53% took calcium and vitamin D in the 2- to 3-year period following an incident hip fracture [18]. In a Belgian study only 6% received osteoporosis treatment in the year following a hip fracture [21] and a study from Manitoba found that in 2001–2002 only 20.5% of women received any identifiable osteoporosis intervention (bone mineral density assessment or pharmacotherapy) following hip fracture [19].
With some types of effective care, the most reliable delivery mechanism may be to bypass the physician entirely, such as with flu vaccination. However, in most clinical situations, the physician remains critical to the proper delivery of care since even highly effective care will have caveats, exceptions, and/or contraindications that the physician may be in the best position to understand. Thus, while systems of care are important for reminding, supporting and reliably delivering effective care, such as perioperative antibiotics, they cannot and probably should not try to completely absolve the physician from the responsibility of making final decisions regarding the specifics of care received by their patients.
Supply-sensitive Care
Some musculoskeletal services fall into the category of so-called “supply-sensitive” care; this is a particularly thorny issue as there are no specific clinical theories about the optimal rate of this type of procedure [7]. Examples of supply-sensitive care include nonemergent physician visits, subspecialty referrals, hospital admissions for chronic conditions, and many diagnostic imaging studies. The rate of these services varies dramatically with the capacity of the local healthcare system [8]. While things like physician visits are often not “big ticket” items, overall Medicare spending in a region is strongly associated with these supply-sensitive services. For example, the regions in the highest and lowest deciles of per-capita Medicare spending varied dramatically with regard to the number of visits to medical specialists (2.7 fold difference), days in the hospital (1.8 fold difference), and days in the ICU (1.6 fold difference) for patients during the last 6 months of life [9]; however, regional per-capita Medicare spending is not associated with greater provision of effective care nor with health outcomes or satisfaction [9]. In fact, in areas with high intensity care (ie, having many more hospital beds and more specialists), physicians felt less able to obtain an elective hospital admission, a timely specialty referral, or maintain good ongoing patient relationships [23].
We are aware of very little direct research looking at supply-sensitive care in relation to specific musculoskeletal procedures. The rates of advanced spinal imaging, CT, and MRI across geographic areas within the Medicare population varies over sevenfold [17]. The relationship between these rates and Medicare spending is not known, however spine surgery rates were substantially higher in those areas with higher imaging rates [17].
Preference-sensitive Care
Finally, many if not most musculoskeletal procedures fall into the category of preference-sensitive care. These are interventions in which there is a real choice between at least two treatments with different risks and benefits, and the preferred therapy will vary based on the patients’ values for different outcomes and their attitude toward risk [7]. Hawker and colleagues demonstrated the profound degree to which preferences can affect the rates of musculoskeletal procedures; in a survey of patients with documented severe arthritis considered candidates for arthroplasty, less than 15% were willing to consider undergoing arthroplasty [9].
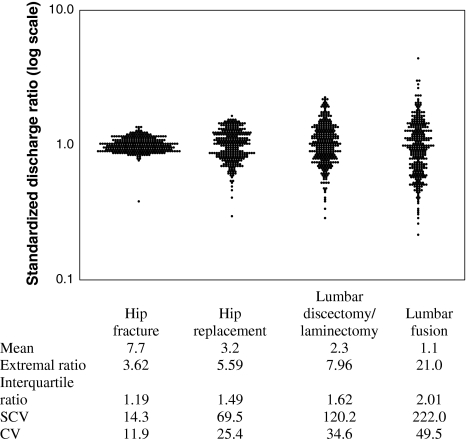
Preference-sensitive procedures such as spine surgery or total joint arthroplasty show much greater variation across geographic areas than surgery for hip fractures (Fig. 1). The variability in rates of preference-sensitive procedures results in a relatively haphazard geographic distribution. but a number of characteristics can be seen. Procedures with the most scientific uncertainty regarding their effectiveness tend to show more geographic variability in rates [25]. Hip replacement, which has a strong evidence base, shows about a fivefold variation in rates while spinal fusion surgery shows 20-fold variation [25] and vertebroplasty have 100-fold variation in rates across geographic areas (Lurie, unpublished data) [16].
Fig. 1.
The geographic variation in rates of orthopaedic procedures varies dramatically between different procedures with greater variability seen for procedures having greater scientific uncertainty about their effectiveness. All data are 2002–2003. (Reprinted with permission from Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31:2707–2714.)
Variability in the rates of different procedures across geographic areas is often idiosyncratic, such that one region may have a high rate of one procedure and a low rate of a different procedure while a nearby geographic area has the opposite relationship. This variability can result in a so-called “surgical signature” for a specific geographic area [25]. These patterns can remain quite stable over time. Weinstein et al. reported that for degenerative diseases of the hip, knee, and spine, the most powerful predictor of the rate of a specific procedure in a geographic area in 2000-2001 was the rate of that same procedure in 1992-1993, illustrating that these “surgical signatures” were stable over time [25].
While the ideal rate of these procedures would be the rate that occurred if patients were fully and impartially informed about the risks and benefits of each procedure and empowered to fully participate in the choice of treatment, the evidence suggests that this does not routinely happen in practice. The magnitude of the differences in rates suggests that the decision making is likely concentrated among a few decision makers (ie, the physicians) rather than diffused among a large number of decision makers (ie, the patients). Wright et al. directly studied this for rates of total knee arthroplasty in Ontario, Canada; after controlling for characteristics of the population and access to care in different regions, the orthopaedic surgeons’ enthusiasm for and optimistic perception of the outcomes of total knee arthroplasty was the dominant modifiable determinant of regional variability in rates [29]. Studies of independent decision aids, which seek to educate patients about a procedure and engage them in a process of informed choice, suggest they can substantially alter the rate at which procedures are performed [20, 26].
Disparities in Care
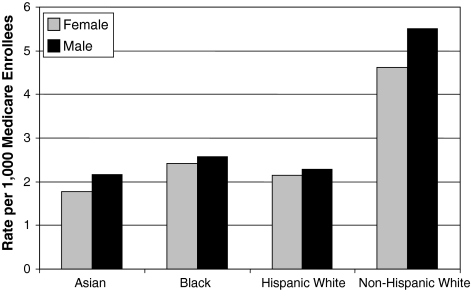
The Institute of Medicine report, “Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care” documented large and consistent disparities in care between racial and ethnic groups across a wide range of healthcare conditions and procedures [11]. As far back as 1991, Gittelsohn and colleagues reported major differences in the rate of discretionary surgery such as lumbar laminectomy according to race [8]. They found that the rate of laminectomy among blacks was less than half the rate among whites. Similarly, Skinner et al. reported the rate of knee arthroplasty among black men in particular was substantially lower than for whites [24]. A more recent evaluation of the rates of spine surgery among Medicare beneficiaries in 1999 and 2000 (Fig. 2) finds similar spine surgery rates for Asian, black, and Hispanic beneficiaries, which were all about half the rate for non-Hispanic whites (Lurie, unpublished data). However hip fracture repair, where there is strong consensus and low geographic variation, was not associated with racial disparities in treatment received [5].
Fig. 2.
The rates of spine surgery among Medicare beneficiaries 1999-2000, stratified by race and gender, show much higher rates among whites.
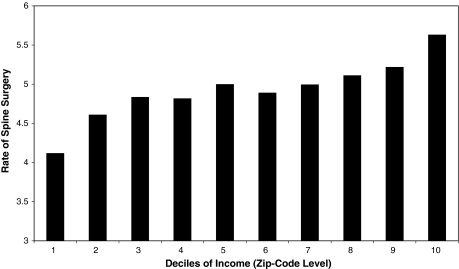
Disparities are also seen across income groups. Gittelsohn et al. found considerable variability in discretionary surgery across income groups; for example high-income areas had a 42% higher rate of laminectomy than did low-income areas [8]. Similarly, a more recent evaluation of spine surgery finds a strong monotonic increase in the rate of spine surgeries by deciles of zip code level income (Fig. 3); this relationship persists after controlling for age, gender, race and hospital referral region (Lurie, unpublished data).
Fig. 3.
The rates of spine surgery increase monotonically across deciles of zip-code level income with areas of higher income showing higher rates of surgery.
The reasons for these disparities remain unclear. Because these tend to be preference-sensitive procedures, differences in patient preferences may differ by race and socioeconomic status, thereby explaining the differences. Evidence to date, however, suggests that this is not likely to explain the observed differences. Among patients with disc herniation in the Spine Patient Outcomes Research Trial (SPORT), preference for surgery versus nonoperative treatment did not differ by race [15]. Among SPORT patients with spinal stenosis, the proportion of white patients preferring surgery was similar to those without a surgical preference (86% versus 83%); similarly, income did not differ between preference groups (Lurie, unpublished data). In another study, Hawker et al. found lower income was associated with greater likelihood of potential need for total joint arthroplasty but was not associated with willingness to consider arthroplasty, resulting in greater potential unmet need among those with lower income [10].
Disparity in care is, however, an extremely complex issue. While often conceptualized as simple differential or biased treatment at the level of the provider, Baicker et al. have demonstrated the complex interaction between racial disparities and geographic variation in care [1]. Evaluating the proportion of effective care received within hospital referral regions, they demonstrated substantial geographic clustering where blacks receive healthcare nationally. Both white patients and black patients received less effective care (in this case eye exams for diabetics) in geographic areas with a high proportion of blacks than in those with a very low proportion of blacks. When Baicker et al. [1] tried to deconstruct the variability in care, they found the majority of the racial disparities in eye exams among diabetics (56%) were due to geographic differences in care rather than within-region disparities between care received by blacks and whites.
Discussion
Wide geographic variations have been found in the rates of many different musculoskeletal procedures. We summarized a conceptual framework that groups different types of medical care into the categories of effective care, supply-sensitive care, and preference-sensitive care using illustrative examples, where possible, from the musculoskeletal literature. We then examined what potential policy approaches might be indicated for decreasing unwarranted variation for the different types of care.
We note several limitations in the existing literature. While we believe this conceptual framework provides a good starting point for thinking about ways to approach defining and achieving the “right rate” for different type of medical interventions, the categories of effective, supply-sensitive, and preference-sensitive care are not mutually exclusive and there are not always clear criteria for determining which category is most appropriate for a given procedure or intervention. Diagnostic imaging, for example, often behaves as an example of supply-sensitive care though certain circumstances may be an example of preference-sensitive care. The list of procedures in the effective care category can change with new information or with different interpretations of the existing information. While some interventions, such as joint replacement, have had substantial research identifying and studying patient preferences, for many other procedures there are little or no data. And while there is a vast literature on decision aids, there are limited data on how to identify and overcome the barriers to their widespread adoption by practicing clinicians. Finally, there are a lack of data that directly address supply-sensitive care in musculoskeletal conditions.
How can we move forward? Large and unwarranted variations in the rates of care across geographic regions occur for effective care, supply-sensitive care, and preference-sensitive care. The solutions vary markedly for different types of services.
For effective care procedures, there can be substantial problems of underuse. The solutions are a combination of awareness of the problem, redesign of healthcare delivery systems to improve the coordination and reliability of care, and monitoring/feedback systems that improve the accountability for the quality of care delivered.
For supply-sensitive care, the route forward is much less clear. However, given the relationship between supply-sensitive care and healthcare costs, healthcare reform that ignores the influence of local capacity on practice patterns is not likely to be meaningful or successful. Coordination of care appears to be a key ingredient for prudent use of local resources. One suggestion to address the problems of supply-sensitive care has been to focus on what have been described as “accountable care organizations” or “accountable care systems” [6, 22]. Currently, virtual networks of physicians, hospitals, and other institutions often function in a fragmented way and are thus unaware of the results they create or how local supply factors affect those results. By creating incentives and accountability at an aggregated level, policy reforms could help to stimulate the integration of care across current practice silos and allow these systems/organizations to consciously manage both local healthcare process and capacity decisions.
For preference-sensitive care there is a roadmap for change but considerable practical challenges [25]. Improvement in the scientific basis of clinical decision making is critical. Pragmatic clinical trials, those that help to demonstrate the comparative effectiveness of commonly used interventions, could provide the foundation for patient-informed choice. Then, systems must be built which allow the ongoing development and implementation of decision aids and other unbiased patient education strategies that can be applied uniformly in order to help patients identify and utilize their values and preferences in the process of medical decision making. Surveys of surgeons’ attitudes toward a shared decision-making model have revealed broad support for the idea of decision aids but little movement toward widespread use due to a lack of practical implementation strategies [26].
An important caveat to relying upon patient preferences to drive the rate of musculoskeletal procedures is the requirement that they be informed patient preferences. As Katz has pointed out, merely incorporating patient preferences into the decision making process may not improve disparities in care if those preferences rely on either misconceptions regarding the risks and benefits of care or even accurate perceptions of historically inferior care [12, 13]. Addressing disparities in care will require both improving the quality of underperforming hospitals and healthcare providers as well as accurately educating patients on the risks and outcomes of available procedures to allow fair and authentic choices to be made.
Acknowledgments
We thank Tamara Morgan for her help with preparing this manuscript.
Footnotes
Drs. Lurie and Weinstein have both served as consultants for the Foundation for Informed Medical Decision Making (FIMDM).
References
- 1.Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med. 2005;48(1 Suppl):S42–S53. [PubMed]
- 2.Bruyere O, Brandi ML, Burlet N, Harvey N, Lyritis G, Minne H, Boonen S, Reginster JY, Rizzoli R, Akesson K. Post-fracture management of patients with hip fracture: a perspective. Current Medical Research and Opinion. 2008;24:2841–2851. [DOI] [PubMed]
- 3.Dartmouth Medical School, Weinstein JN. Birkmeyer JD, ed. Dartmouth Atlas of Musculoskeletal Health Care. Chicago, IL: AHA Press; 2000.
- 4.Escalante A, Barrett J, del Rincon I, Cornell JE, Phillips CB, Katz JN. Disparity in total hip replacement affecting Hispanic Medicare beneficiaries. Med Care. 2002;40:451–460. [DOI] [PubMed]
- 5.Fanuele JC, Lurie JD, Zhou W, Koval KJ, Weinstein JN. Variations in hip fracture treatment: are black and white patients treated equally? Am J Orthop. 2009;38:E13–E17. [PubMed]
- 6.Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: the extended hospital medical staff. Health Aff (Millwood). 2007;26:w44–w57. [DOI] [PMC free article] [PubMed]
- 7.Fisher ES, Wennberg JE, Stukel TA, Sharp SM. Hospital readmission rates for cohorts of Medicare beneficiaries in Boston and New Haven. N Engl J Med. 1994;331:989–995. [DOI] [PubMed]
- 8.Gittelsohn AM, Halpern J, Sanchez RL. Income, race, and surgery in Maryland. Am J Public Health. 1991;81:1435–1441. [DOI] [PMC free article] [PubMed]
- 9.Hawker GA, Wright JG, Coyte PC, Williams JI, Harvey B, Glazier R, Wilkins A, Badley EM. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients’ preferences. Med Care. 2001;39:206–216. [DOI] [PubMed]
- 10.Hawker GA, Wright JG, Glazier RH, Coyte PC, Harvey B, Williams JI, Badley EM. The effect of education and income on need and willingness to undergo total joint arthroplasty. Arthritis Rheum. 2002;46:3331–3339. [DOI] [PubMed]
- 11.Insitute of Medicine, ed. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003. [PubMed]
- 12.Katz J. Preferences, disparities, and the authenticity of patient choices. J Rheumatology. 2003;30(S68):12–14. [PubMed]
- 13.Katz JN. Patient preferences and health disparities. JAMA. 2001;286:1506–1509. [DOI] [PubMed]
- 14.Koval KJ, Lurie J, Zhou W, Sparks MB, Cantu RV, Sporer SM, Weinstein J. Ankle fractures in the elderly: what you get depends on where you live and who you see. J Orthop Trauma. 2005;19:635–639. [DOI] [PubMed]
- 15.Lurie J, Berven S, Gibson J, Tosteson TD, Tosteson ANA, Hu SS, Weinstein J. Patient preferences and expectations for care: determinants in patients with lumbar intervertebral disc herniation. Spine. 2008;33:2663–2668. [DOI] [PMC free article] [PubMed]
- 16.Lurie J, Tosteson ANA, Zhou W, Weinstein J. Patterns of Vertebroplasty Use Among Medicare Beneficiaries. International Society for Study of the Lumbar Spine. Hong Kong, PRC; 2007.
- 17.Lurie JD, Birkmeyer NJ, Weinstein JN. Rates of advanced spinal imaging and spine surgery. Spine. 2003;28:616–620. [DOI] [PubMed]
- 18.Lüthje P, Nurmi-Lüthje I, Kaukonen JP, Kuurne S, Naboulsi H, Kataja M. Undertreatment of osteoporosis following hip fracture in the elderly. Arch Gerontol Geriatr. 2008 Aug 13. [Epub ahead of print]. [DOI] [PubMed]
- 19.Metje CJ, Leslie WD, Manness L-J, Yogandran M, Yuen CK, Kvern B. Postfracture care for older women: gaps between optimal care and actual care. Can Fam Physician. 2008;54:1270–1276. [PMC free article] [PubMed]
- 20.Phelan EA, Deyo RA, Cherkin DC, Weinstein JN, Ciol MA, Kreuter W, Howe JF. Helping patients decide about back surgery: a randomized trial of an interactive video program. Spine. 2001;26:206–211;discussion 212. [DOI] [PubMed]
- 21.Rabenda V, Vanoverloop J, Fabri V, Mertens R, Sumkay F, Vannecke C, Deswaef A, Verpooten GA, Reginster JY. Low incidence of anti-osteoporosis treatment after hip fracture. J Bone Joint Surg Am. 2008;90:2142–2148. [DOI] [PubMed]
- 22.Shortell SM, Casalino LP. Health care reform requires accountable care systems. JAMA. 2008;300:95–97. [DOI] [PubMed]
- 23.Sirovich BE, Gottlieb DJ, Welch HG, Fisher ES. Regional variations in health care intensity and physician perceptions of quality of care. Ann Intern Med. 2006;144:641–649. [DOI] [PubMed]
- 24.Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349:1350–1359. [DOI] [PubMed]
- 25.Weinstein JN, Bronner KK, Morgan TS, Wennberg JE. Trends and geographic variations in major surgery for degenerative diseases of the hip, knee, and spine. Health Aff (Millwood). 2004;Suppl Web Exclusives:VAR81-9. [DOI] [PubMed]
- 26.Weinstein JN, Clay K, Morgan TS. Informed patient choice: patient-centered valuing of surgical risks and benefits. Health Aff (Millwood). 2007;26:726–730. [DOI] [PMC free article] [PubMed]
- 27.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31:2707–2714. [DOI] [PMC free article] [PubMed]
- 28.Wennberg JE, Fisher ES, Skinner JS. Geography and the debate over Medicare reform. Health Aff (Millwood). 2002;Suppl Web Exclusives:W96-114. [DOI] [PubMed]
- 29.Wright JG, Hawker GA, Bombardier C, Croxford R, Dittus RS, Freund DA, Coyte PC. Physician enthusiasm as an explanation for area variation in the utilization of knee replacement surgery. Med Care. 1999;37:946–956. [DOI] [PubMed]