Abstract
Apolipoprotein E (apoE)-induced activation of low-density lipoprotein receptor (LDL) family members reduces inflammatory responses by suppressing c-Jun N-terminal kinase (JNK) activation. We aimed to identify which specific receptor family member mediates the effect of apoE on inflammation in primary cultures of microglia. Low-density lipoprotein receptor-related protein 1 (LRP1)-deficient (LRP1 −/−) microglia were derived from mice using tissue-specific loxP/Cre recombination. Using a peptide formed from the receptor-binding region of apoE (EP), we found that LRP1 mediates the effects of apoE on microglial inflammation. Microglial LRP1 was also essential for EP to suppress JNK activation induced by lipopolysaccharide.
Keywords: apolipoprotein E, LPS, Alzheimer's disease, signal transduction, nitric oxide, JNK
1. INTRODUCTION
Inflammation in the brain is characterized by activated microglial cells and increased levels of cytokines and chemokines. Activated microglia undergo morphological changes and become phagocytic, removing foreign particles and neurotoxic agents (Ladeby et al., 2005). Chronic activation of microglia can lead to overproduction of various neurotoxic molecules such as nitric oxide (NO), reactive oxygen species, excitotoxins, and pro-inflammatory cytokines that lead to neuronal death (Boje and Arora, 1992; Combs et al., 2001; Meda et al., 1995). Regulating microglial activation is a target for overcoming inflammation and neurodegeneration in the brain.
Apolipoprotein E (apoE), a 34 kDa protein that transports lipids, modulates the inflammatory response of microglia. ApoE is synthesized in the central nervous system (CNS) primarily by astrocytes and microglia. Single nucleotide polymorphisms in the APOE gene result in three common protein isoforms, termed apoE2, apoE3, and apoE4. These isoforms differ by the amino acids at residues 112 and 158 (Weisgraber, 1994). The APOE ε4 allele increases an individual's risk for Alzheimer's disease, which includes a dramatic increase in brain inflammation (Bales et al., 2000; McGeer and McGeer, 2001a; McGeer and McGeer, 2001b). In an isoform dependent manner, apoE reduces CNS inflammation, with apoE4 displaying the least anti-inflammatory activity. Several small apoE mimetic peptides have been developed which reduce glial inflammation (Laskowitz et al., 1997; Laskowitz et al., 2000; Laskowitz et al., 1998; Laskowitz et al., 2001; Lynch et al., 2001; Lynch et al., 2003; Mace et al., 2007; Pocivavsek et al., 2009).
ApoE modulates microglial inflammation through activation of the low-density lipoprotein (LDL) receptor family (Moon et al., 2007; Pocivavsek et al., 2009). The LDL receptor family includes the low-density lipoprotein receptor (LDLR), very-low density lipoprotein receptor (VLDLR), apolipoprotein E receptor 2 (ApoEr2), and the LDL receptor-related protein-1 (LRP1). Neuronal receptors (ApoEr2, VLDLR, and LRP1) (Christie et al., 1996; Kim et al., 1996; Rebeck et al., 1993) have been implicated in neurite outgrowth, calcium homeostasis, kinase activation and cell migration (Beffert et al., 2004). A different subset of receptors are expressed on astrocytes and microglia: LDLR, LRP1, and VLDLR (Christie et al., 1996; Rebeck et al., 1993). ApoE signaling through these receptors involves mitogen-activated protein kinase (MAPK) pathways in neurons and glia (Hoe et al., 2005; Hoe et al., 2006; Pocivavsek et al., 2009). However, it has not been determined whether all or some of LDL receptor family member expressed in microglia modulate the inflammatory response.
MAPK signaling pathways have been demonstrated in microglia activated with endotoxin lipopolysaccharide (LPS) (Bhat et al., 1998; Han et al., 2002; Pyo et al., 1998; Xie et al., 2004). In our previous study, we demonstrated that LDL receptor activation modulates glial inflammation by modulating MAPK (Pocivavsek et al., 2009). We used an apoE mimetic peptide to activate LDL receptor family members and showed that their anti-inflammatory effects specifically required reduction of MAPK family member c-Jun N-terminal kinase (JNK) activation (Pocivavsek et al., 2009).
The aims of this study were to determine which of the LDL receptor family members expressed in microglia affected the JNK signaling pathway. One appealing hypothesis was LRP1 because its cytoplasmic domain interacts with JNK-interacting proteins (JIPs) (Gotthardt et al., 2000), because JIPs modulate JNK activation. To investigate whether LRP1 mediates the microglial inflammatory response, we used a mouse model where LRP1 was deleted in cells of myeloid lineages, which include microglia. We monitored microglial activation by LPS-induced accumulation of nitric oxide and an increase in JNK activation. We used an apoE mimetic peptide (EP), consisting of a tandem repeat of the nine amino acid receptor-binding domain to induce activation of LRP1. Expression of LRP1 proved to be essential for EP to inhibit LPS-induced microglial inflammatory responses.
2. MATERIALS AND METHODS
Mice
Mice deficient in myeloid cell type-specific LRP1 were made using loxP/Cre-mediated recombination (Hu et al., 2006b); the mice were on an LDLR-deficient background as described previously (Lillis et al., 2008a). Briefly, F1 generation mice were generated by breeding LRP1-floxed mice on an LDLR-deficient background with LysMCre knock-in mice expressing Cre under the control of endogenous lysozyme M promoter to generate LRP1 flox+/− Cre+/− LDLR+/− mice. These mice were crossed with LRP1-floxed mice on an LDLR-deficient background to generate two F2 generation genotypes: LRP1 flox+/+ Cre+/− LDLR −/− and LRP1 flox+/+ Cre−/− LDLR −/− mice. This generation of mice was crossed with one another yielding half of the siblings that carry no copies of Cre and thus express LRP1 normally (termed ‘wild-type’). The other half of the siblings carried one copy of Cre recombinase under the lysosome M promoter and thus generated deletion of LRP1 in myeloid cell types (termed ‘LRP1 −/−’).
Primary Microglial Cell Culture
Microglial cells were prepared as previously described (Pocivavsek et al., 2009) from postnatal day 1 wild-type and LRP1 −/− mice. Briefly, cerebral cortices were carefully removed from pups, stripped of meninges and homogenized in minimum essential media (MEM, Invitrogen, Carlsbad, CA) supplemented with 5% horse serum (Invitrogen), 5% fetal bovine serum (FBS) (Invitrogen), 1% L-glutamine (Invitrogen), 1% sodium pyruvate (Invitrogen), 1% Pen/Strep (Invitrogen), and 1% Fungizone (Invitrogen). The homogenized cells were centrifuged at 2000 rpm for 5 min, resuspended in fresh media and plated into T75 flasks. Mixed glial cultures were grown to confluency over 3 weeks and microglia were harvested by shaking the flasks at 100 rpm for 1 h at 37°C. The microglia-enriched media was collected and cells were pelleted by centrifugation (2000 rpm, 5 min). For immunocytochemistry microglia were resuspended in MEM supplemented with 10% FBS, 1% L-glutamine, 1% sodium pyruvate, 1% Pen/Strep, and 1% Fungizone and plated at a density of 1 × 105 cells/ml on poly-D-lysine-coated glass coverslips. Microglial cell purity was confirmed to be >95% using immunofluorescence for OX42 or Iba1 (microglial markers, Serotec, Raleigh, NC) and DAPI (Vector Laboratories, Burlingame, CA) nuclear counterstain. For biochemical experiments, cells were plated in 24-well plates at 12.5 × 104 cells / well, grown overnight in supplemented media, and then the medium was replaced with serum-free Opti-MEM (Invitrogen) containing either control PBS or experimental agents.
Antibodies
Phosphorylation site-specific antibody against phospho-JNK (Thr183/Tyr185) (Cell Signaling Technologies, Beverly, MA) was used. Phospho-JNK antibody detected levels of p46 (JNK1) and p54 (JNK2 and JNK3) kinases when phosphorylated. Total JNK antibody (Cell Signaling Technologies) detected total levels of JNK, p46 and p54. Rabbit polyclonal antibody was used to detect iNOS (BD Biosciences Pharmigen, San Diego, CA). From the same blots, β-actin (Abcam, Cambridge, MA) was detected by monoclonal antibody to ensure equal protein levels in each lane. To detect LRP1, we used mouse monoclonal 5A6 that recognized the 85-kDa fragment of LRP1 (Misra et al., 1999). We also used a polyclonal antibody to detect the heavy chain of LRP1 (Newton et al., 2005). LRP1 was detected with goat anti-LRP (10ug/ml) by immunostaining (Newton et al., 2005). For receptor blocking experiments, an anti-VLDLR cocktail (antibodies 5F2, 1H5, 1H10) was used (Ruiz et al., 2005).
Immunocytochemistry
Primary microglia on glass coverslips were fixed with 4% formaldehyde and 5% sucrose in PBS for 20 min at room temperature (RT) and then washed 3 times with PBS. Fixed cells on coverslips were permeabilized with 0.4% Triton for 5 min at RT. Coverslips were blocked with 3% donkey serum for 1 hr and stained with mouse monoclonal Iba1 antibody (Alexa488 secondary) and goat anti-LRP antibody (Alexa568 secondary). Coverslips were mounted on glass slides with FluoroSave (Calbiochem, San Diego, CA) and analyzed with Zeiss confocal system Radiance2100 and LaserSharp2000 software.
Chemicals
EP, the apoE-derived dipeptide(141−149), consisting of a duplicated sequence of apoE amino acids 141 through 149, was synthesized by Johns Hopkins University of Medicine (Biosynthesis and Sequencing Facility, Baltimore, MD) (Hoe et al., 2005; Pocivavsek et al., 2009). Specific JNK inhibitor SP600125 was purchased from Invitrogen. Phosphatase inhibitor cocktails (Sigma) and protease inhibitor (Sigma) were used in cell lysis buffer. LPS was purchased from Calbiochem.
Western Blot Analyses
For analysis of cell-associated proteins, cells were harvested in ice-cold lysis buffer containing phosphatase inhibitor cocktails and protease inhibitors. Proteins were separated under denatured and reduced conditions using Tris-glycine SDS-polyacrylamide gel electrophoresis (Biorad, Hercules, CA). However, when detecting LRP, proteins were denatured but not reduced. Separated proteins were detected on PVDF membranes incubated with primary antibodies. Immunoreactivity was detected using horseradish peroxidase-conjugated secondary antibodies (anti-mouse and anti-rabbit from Jackson ImmunoResearch, West Grove, PA; anti-goat from Santa Cruz Biotechnology, Santa Cruz, CA) and visualized by enhanced chemiluminescence detection film. Band density was determined using Quantity One 1-D analysis software (Biorad).
Nitrite Quantification
The production of NO was assessed as the accumulation of nitrite from the spontaneous oxidation of NO in serum-free cell conditioned media after 24 h. Accumulation of nitrite was quantified using a colorimetric reaction with Griess reagent (Invitrogen). Absorbance was measured at 570nm by spectrophotometry.
Statistical Analysis
All experiments were repeated a minimum of three times. The data were analyzed using Graphpad Prism 4 software, with either Student's T-Test or ANOVA, using Newman-Keuls Multiple Comparison Test for posthoc analysis. Significance was determined at a P value of < 0.05.
3. RESULTS
LRP1 deletion in primary microglia
LRP1 floxed mice were crossed with LysMCre mice in recent studies to demonstrate deletion of LRP1 in macrophages and microglia (Lillis et al., 2008a; Zhang et al., 2009). Our work with these mice showed successful LRP1 deletion in primary microglial cultures by Western blot analysis (Fig. 1A) and immunocytochemistry (Fig. 1B). LRP1 expression was analyzed with two antibodies against LRP1, a mouse monoclonal antibody that recognized the 85-kDa fragment of LRP1 and a goat polyclonal antibody that recognized full length LRP1. Both analyses revealed strong LRP1 expression in cell lysates from wild-type primary microglia and faint LRP1 expression in LRP1 −/− microglia (Fig. 1A). Analysis of Western blots revealed that LRP1 levels in LRP1 −/− microglia remained at about 20 % of levels in wild-type microglia.
Figure 1. LRP1 knockout in primary microglia.
A) Cell lysates from wild-type (wt) and LRP1 −/− (ko) microglia were analyzed by Western blotting for LRP1 expression (denoted by the asterisks). The left panel shows a representative blot probed with 5A6, recognizing the 85 kDa band for LRP1. The right panel shows a blot probed with goat α-LRP1, recognizing full length LRP1 (n = 4). B) Primary microglia from wild-type (WT) and LRP1 −/− cultures were immunostained with monoclonal Iba1 (detected with Alexa488, green) and goat anti-LRP antibody (detected with Alexa568, red). C) Cells positive for Iba1 and LRP1 expression were counted. Quantification shows that 95 % of wild-type microglia and 25 % of LRP1 −/− microglia were LRP1 positive (mean ± SEM; *** P < 0.001 compared to wild-type; n = 5 for wild-type and n = 7 for LRP1 −/−).
Primary microglial cells were immunostained with a monoclonal antibody Iba1 to detect microglia and with a polyclonal antibody against LRP1. Robust Iba1 expression was detected in both wild-type and LRP1 −/− cultures; LRP1 expression was detected in 95 % of wild-type microglia and 25 % of LRP1 −/− microglia (Fig. 1B & C). Immunofluorescence revealed that in microglia derived from LRP1 −/− mice that express LRP1, the levels of LRP1 expression appeared to be reduced when compared to wild-type microglia (Fig 1B). Thus, LRP1 was reduced but not eliminated in microglia from the LRP1 floxed × LysMCre mice.
Activation of Microglia Increases NO Production in a JNK-Dependent Manner
Treatment of microglia with LPS induces intracellular signaling cascades, synthesis of inducible nitric oxide synthase (iNOS), and release of NO (Corradin et al., 1993). For the present experiments, wild-type and LRP1 −/− microglia were treated with 100 ng/ml LPS in serum-free media for 24 h. LPS caused significant and similar increases in NO from both wild-type and LRP1 −/− microglia (Fig 2A). Wild-type cells treated with LPS accumulated 6.4 ± 0.5 μM nitrite compared with 1.4 ± 0.3 μM nitrite from untreated cells. LRP1 −/− cells treated with LPS accumulated 7.2 ± 0.6 μM nitrite, compared with 1.6 ± 0.6 μM nitrite from untreated cells. The iNOS protein was undetected in untreated microglia, but easily observed in extracts from wild-type or LRP1 −/− cells treated with LPS for 24 h (Fig. 2B). Total protein levels of β-actin remained unchanged (Fig. 2B).
Figure 2. Stimulation of microglia with LPS increased NO through JNK activation.
A) In wild-type and LRP1 −/− microglia, LPS promoted nitrite accumulation in the conditioned media at 24 h (mean ± SEM; ***P < 0.001; n = 6 for each of 3 independent experiments). B) Cell lysates from wild-type and LRP1 −/− microglia were analyzed by Western blotting with antibodies to iNOS and β-actin (as a control). A representative blot shows that LPS increased iNOS levels in both cell types (n=6 for each of 3 independent experiments). C) Wild-type and LRP1 −/− microglia were treated with LPS alone (open bars) or LPS and SP600125 (colored bars). In a dose-dependent manner, SP600125 treatment reduced nitrite production in both wild-type and LRP1 −/− microglia to levels of unstimulated microglia (defined as the line at 100 %) (mean ± SEM; **P < 0.01 compared to control cultures; # P < 0.05, ## P < 0.01 compared to corresponding cultures; n=3 for each of 2 independent experiments).
Previously, we showed that activation of JNK was necessary for the LPS-induced NO accumulation in wild-type microglia (Pocivavsek et al., 2009). Here, we tested whether both wild-type and LRP1 −/− microglia respond to LPS treatment by activating JNK. As above, treatment of wild-type and LRP1 −/− microglia with LPS significantly increased nitrite accumulation over control cells (Fig. 2C). The JNK inhibitor SP600125 significantly reduced nitrite accumulation in both groups of microglia in a dose-dependent manner. With 10 μM SP600125 treatment, levels of nitrite production were reduced to control levels in wild-type cells and in LRP1 −/− cells (Fig. 2C).
EP Attenuates LPS Induced NO Production in LRP Expressing Microglia
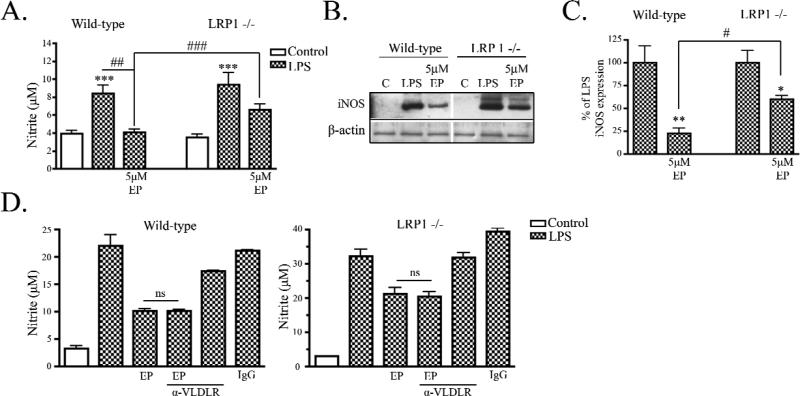
Previously we used EP to demonstrate that LDL receptor family members can attenuate LPS-induced nitrite accumulation in microglia (Pocivavsek et al., 2009). However, the specific receptor through which EP mediated its anti-inflammatory properties was unknown. Here, we tested whether LRP1 mediates these effects. For these studies, EP treatment was performed at the same time as LPS stimulation. Wild-type and LRP1 −/− microglia were treated with 1 to 5 μM EP. Wild-type microglia stimulated with LPS accumulated 8 ± 1 μM nitrite. When treated with 1 μM EP and LPS, wild-type cultures accumulated 6.5 ± 1 μM nitrite, a 22% decrease compared to wild-type cells treated with LPS alone (data not shown). Cells treated with 5 μM EP and LPS accumulated 4 ± 0.3 μM nitrite, a significant 52% decrease (p < 0.05) compared with cells treated with LPS alone (Fig. 3A). The magnitude of this effect of EP was consistent with our previous studies (Pocivavsek et al., 2009).
Figure 3. ApoE peptide attenuated LPS-induced NO production in LRP1 expressing microglia.
A) Wild-type and LRP1 −/− microglia were treated with 100 ng/mL LPS and nitrite was measured in the conditioned media at 24 h. LPS induced nitrite accumulation in both cell types and EP showed attenuation of LPS-induced nitrite production only in wild-type cultures (mean ± SEM; *** P < 0.001 compared to corresponding control cultures; ## P < 0.01, ### P < 0.001 compared with indicated cultures; n = 6 for each of 4 independent experiments). B) Cell lysates from wild-type and LRP1 −/− cultures were analyzed by Western blotting for iNOS and b-actin. Representative blots show that EP and LPS treatment reduced iNOS expression compared to LPS stimulation alone in wild-type stimulated cultures but not LRP1 −/− cultures. C) Western blot data were quantified as percent of control (mean ± SEM; * P < 0.05, ** P < 0.01 compared with LPS stimulated cultures; # < 0.05 compared with indicated cultures; n = 6 for each of 4 independent experiments). D) Wild-type (left panel) and LRP1 −/− (right panel) cultures were treated LPS and nitrite was measured in the conditioned media at 24 h. LPS induced nitrite accumulation in wild-type and LRP1 −/− cultures. EP and LPS treatment attenuated LPS-induced nitrite accumulation significantly in wild-type cultures and treatment with 500 nM VLDLR blocking antibody, EP and LPS did not prevent the effects of EP. Similarly in LRP1 −/− microglia, nitrite accumulation in cultures treated with VLDLR antibody cocktail did not significantly differ from cultures treated with EP and LPS (mean ± SEM; ns means not significant; n = 4).
LRP1 −/− primary microglia stimulated with LPS accumulated 9 ± 1 μM nitrite at 24 h. Cultures of LRP1 −/− microglia treated with 1 μM EP and LPS accumulated 8.1 ± 1 μM nitrite, a non-significant 14% decrease compared to LRP1 −/− microglia treated with LPS alone (data not shown). Cells treated with 5 μM EP and LPS accumulated 6.6 ± 1 μM nitrite, a non-significant 30% decrease compared to cells treated with LPS alone (Fig. 3A). At 5 μM EP, wild-type microglia cultures had significantly less nitrite accumulation (4 ± 0.3 μM) compared to LRP1 −/− cultures (6.6 ± 1 μM nitrite) (Fig. 3A).
Expression of iNOS was readily detected by Western blot analysis of primary microglia stimulated with LPS in both cell types. In wild-type microglia, EP treatment decreased expression of iNOS compared to cultures treated with LPS alone (Fig. 3B). Quantification of Western blot analysis revealed that expression of iNOS was reduced by 78 ± 6 % by EP (Fig. 3C). In LRP1 −/− microglia, EP also reduced iNOS (39 ± 4 % reduction) (Fig. 3B & C), but wild-type microglia treated with EP and LPS had significantly less iNOS than LRP1 −/− microglia treated with EP and LPS (Fig. 3C). This reduced response of LRP1 −/− microglia to EP treatment suggests that the anti-inflammatory effects of EP are at least partially mediated through LRP1.
Because we observed a reduction in NO production and iNOS expression in the LRP1 −/− microglia treated with EP, we hypothesized that, in addition to LRP1, there may be a second apoE receptor that mediates anti-inflammatory effects. We tested whether the apoE receptor, VLDLR, was also partially involved in mediating the inhibitory effects of EP on microglia activation. To block EP from binding VLDLR, we used an anti-VLDLR cocktail composed of monoclonal antibodies 5F3, 1H5, and 1H10, which are known to block the binding of apoE to VLDLR as described (Ruiz et al., 2005). In these experiments, wild-type microglia treated with LPS accumulated a significant increase in nitrite (22 ± 2 μM) when compared to control cultures (3 ± 0.4 μM). Similar to our previous experiments in Fig. 3A, wild-type cultures treated with EP and LPS showed a significant 54 % reduction in nitrite (10 ± 0.4 μM) when compared to cultures stimulated with LPS alone (Fig. 3D; p < 0.001). Cultures simultaneously treated with anti-VLDLR, EP and LPS accumulated 10 ± 0.2 μM nitrite, not significantly different from cultures treated with EP and LPS alone. Treatment with anti-VLDLR and LPS resulted in 17 ± 0.1 μM nitrite and treatment with cold IgG resulted in 21 ± 0.1 μM nitrite, both of which were not significantly different from wild-type cultures treated with LPS alone (Fig. 3D).
We observed similar effects in LRP1 −/− microglia. LRP1 −/− microglia treated with LPS accumulated an increase in nitrite (32 ± 2 μM) over control cultures (3 ± 0.1 μM), as we previously described in Fig 2. Further, treatment of LRP1 −/− microglia with EP and LPS reduced nitrite accumulation by 34% (21 ± 2 μM), similar to what we previously described in Fig. 3A; in this experiment the reduction was statistically significant (p < 0.05). We used anti-VLDLR in combination with EP and LPS in LRP1 −/− microglia, and observed that nitrite accumulation was not altered when VLDLR was blocked. LRP1 −/− cultures treated with anti-VLDLR, EP and LPS accumulated 20 ± 1 μM nitrite, similar to cultures treated with EP and LPS (Fig. 3D). Cultures of LRP1 −/− microglia treated with anti-VLDLR accumulated 32 ± 1 μM nitrite and cultures treated with cold IgG accumulated 39 ± 1 μM nitrite, both similar to LRP1 −/− microglia stimulated with LPS alone (32 ± 2 μM nitrite) (Fig. 3D). Taken together, our data support the idea that LRP1 mediates the inhibitory effects of EP on NO production and that VLDLR does not contribute to this effect.
EP reduces JNK activation only in LRP expressing microglia
We previously found that EP treatment decreased JNK activation through the LDL receptor family and counteracted LPS-induced inflammatory responses (Pocivavsek et al., 2009). The data in Fig. 3 show that EP significantly lowered the LPS-induced inflammatory response in wild-type microglia, but the effects of EP were less in LRP1 −/− microglia; we concluded that LRP1 mediated at least some of the anti-inflammatory effect of EP. We next asked whether EP treatment modulated JNK activation via LRP1. JNK phosphorylation in microglial cell lysates was examined 1 h after treatments. EP alone had minimal effects on phospho-JNK and total JNK levels in inactivated microglial cultures (Fig. 4A). Treatment of wild-type microglia with LPS for 1 h significantly increased phospho-JNK expression, while total levels of JNK remained unchanged (Fig. 4A) (591 ± 82% of control levels of phospho-JNK). Simultaneous treatment of wild-type microglia with EP and LPS induced a significant 48 % decrease in phospho-JNK levels (307 ± 50% of control) when compared with cultures treated with LPS alone (Fig. 4B). As with wild-type cells, treatment of LRP1 −/− cultures with EP did not reduce basal phospho-JNK expression when compared to control cultures (Fig. 4A). LRP1 −/− microglia stimulated with LPS had 555 ± 98 % of control levels of phospho-JNK, a significant increase that was similar to the increase observed in wild-type microglia stimulated with LPS (Fig. 4B). In contrast to wild-type microglia, LRP1 −/− microglia simultaneously treated with EP and LPS showed no reduction in phospho-JNK (567 ± 105 % of control) levels when compared with cultures treated with LPS alone (Fig. 4B). These data indicated that EP attenuates LPS-induced JNK activation via LRP1.
Figure 4. ApoE peptide signaling effects are LRP1 mediated.
Wild-type and LRP1 −/− primary microglia were treated with PBS (c), 1 μM EP, 100 ng/mL LPS, and 1 μM EP with LPS for 1 h. Cell lysates were analyzed by Western blotting with antibodies to phospho-JNK and total JNK. A) Wild-type cells treated with EP showed decreased phospho-JNK compared with cells treated with PBS (c). Treatment of wild-type cells with LPS increased phospho-JNK. Cells treated with EP and LPS showed reduced phospho-JNK compared to cells treated with LPS alone. LRP1 −/− cells treated with LPS showed increased phospho-JNK compared with cells treated with PBS (c). In LRP1 −/− cultures, EP and LPS treatment did not reduce phospho-JNK compared to cells treated with LPS alone. B) Western blot data were quantified as percent of control phospho-JNK (mean ± SEM; *** P < 0.001 compared with control cultures; ## P < 0.01 compared with indicated cultures; n = 3 for each of 2 independent experiments).
4. DISCUSSION
This study shows that activation of LRP1 can modulate microglial inflammation by reducing phosphorylation of JNK. We confirmed that LRP1 is expressed in microglia (Marzolo et al., 2000; Zhang et al., 2009) and sought to activate LRP1 by applying an apoE mimetic peptide, EP, which reduced microglial inflammation by LPS. In wild-type microglia, LPS caused activation of JNK, which led to increased NO production; EP treatment decreased activation of JNK and counteracted LPS-induced inflammation. In microglia lacking LRP1, EP did not decrease JNK and its inhibition of NO was significantly attenuated. EP still had some effect on activation in LRP1 −/− cells (Figure 3), possibly due to the 25% of cells that are still expressing LRP1 in the induced knockout cultures (as shown in Fig. 1), although it is also possible that a second, as yet unidentified, receptor is involved. Taken together, these data suggest that LRP1 is one mechanism for apoE's regulation of the microglial inflammatory response by suppressing activation of JNK.
While members of the LDL receptor family have previously been implicated in modulating the glial inflammatory response (LaDu et al., 2000; LaDu et al., 2001; Laskowitz et al., 2001; Pocivavsek et al., 2009), this is the first study to define an LRP1-dependent immunomodulatory cascade in microglia. LRP1 contains a single transmembrane domain and a cytoplasmic domain consisting of 100 amino acids, including two NPxY motifs that are tyrosine phosphorylated (Lillis et al., 2008b). This cytoplasmic tail interacts with various adaptor protein molecules implicated in cell signaling events including JNK-interacting proteins, JIP-1 and JIP-2 (Gotthardt et al., 2000). JIPs have been implicated as inhibitors and activators of the JNK signaling pathway (Dickens et al., 1997; Mooney and Whitmarsh, 2004; Whitmarsh et al., 2001; Willoughby et al., 2003). We postulate that upon LRP1 activation, the C-terminal fragment (CTF) of LRP1 is released from the plasma membrane, carrying with it JIP proteins. This LRP1-CTF-JIP complex could then travel to other subcellular domains and modulate the activation of JNK at those sites. We have observed ligand-induced proteolytic cleavage of LDL receptor family members (Hoe and Rebeck, 2005) and found that inhibition of that cleavage in neurons modulates JNK activation (Hoe et al., 2005). LRP1 undergoes proteolytic processing by several proteolytic activities, including γ-secretase, an intramembrane protease that processes many substrates (Parks and Curtis, 2007). Upon γ-secretase cleavage, the intracellular domain of LRP1 (LRP1-ICD) is released into the cytoplasm (May et al., 2002). A recent study showed that proteolytic processing of LRP1 by γ-secretase and the subsequent translocation of LRP1-ICD into the nucleus regulates inflammation in peritoneal macrophages (Zurhove et al., 2008). These findings support the hypothesis that proteolytic processing of LRP1 mediates its JNK signaling and anti-inflammatory effects.
In the CNS, LRP1 is expressed in neurons, astrocytes, and microglia. In neurons, LRP1 modulates Alzheimer's disease (AD) pathology by altering the trafficking of amyloid precursor protein (APP) and production of the toxic Aβ peptide (Pietrzik et al., 2002; Ulery et al., 2000; Ulery and Strickland, 2000). Studies have also implicated LRP1 in clearing Aβ by receptor-mediated endocytosis (Bu et al., 2006; Deane et al., 2004; Lillis et al., 2008b). Apart from its effects on AD pathological processes, neuronal LRP1 has been implicated in mediating long-term potentiation and calcium influx through the NMDA receptor (Bacskai et al., 2000; Herz, 2001). LRP1 contributes to blood-brain barrier (BBB) permeability through effects on perivascular astrocytes (Yepes et al., 2003). Recently, it was found that tissue-type plasminogen activator (tPA) regulates the permeability of the BBB via an interaction with LRP1 (Polavarapu et al., 2007; Yepes et al., 2003; Zhang et al., 2007). tPA is a serine proteinase (Bugge et al., 1996) that is used clinically to treat acute ischemic stroke. Increasing evidence shows that tPA has deleterious effects over time, and the interaction between tPA and LRP1 has been characterized as pro-inflammatory (Hu et al., 2006a; Wang et al., 2003; Yepes et al., 2003; Zhang et al., 2009; Zhang et al., 2007). While it was demonstrated that tPA mediates deleterious effects on the ischemic brain via LRP1-dependent activation of microglia (Zhang et al., 2009), the mechanism by which tPA induces LRP1 mediated microglial activation remains to be elucidated. However, our work suggests that LRP1 activation by an apoE mimetic reduces JNK activation and thus downregulates the microglial inflammatory response induced by LPS stimulation. The contrasting roles of different LRP1 ligands, apoE and tPA, suggests unique and specific ligand-induced physiological functions of LRP1 in microglia.
The immunomodulatory properties of LRP1 may have implications for AD. Astrocytes and microglia surround amyloid deposits in AD (Ard et al., 1996; Paresce et al., 1996; Schenk et al., 1999) and both cell types express LRP1 (Marzolo et al., 2000; Pocivavsek et al., 2009; Rebeck et al., 1993). Further, LRP1 associates with amyloid plaques in AD and its expression is upregulated in glial cells (Arelin et al., 2002). The glial activation seen in AD brains may demonstrate a failure of LRP1 immunomodulatory functions in the presence of chronic insults by Aβ oligomers (LaDu et al., 2000; LaDu et al., 2001). We hypothesize that LRP1 expressed on astrocytes may have similar immunomodulatory properties that are impaired when astrocytes surround amyloid plaques. Development of astrocyte-specific LRP1 knock-out mice would help elucidate the role of LRP1 on astrocytes specifically.
ApoE has been implicated in modulating the inflammatory response in the CNS in an isoform-dependent manner (apoE2 > apoE3 > apoE4) (Vitek et al., 2007). While apoE4 carriers have an increased risk of developing AD, the mechanisms for that increased risk are not known, but it could be related to the fact that apoE4 has worse anti-inflammatory properties compared to apoE2 or apoE3. The presence of arginines at amino acid positions 112 and 158 structural distinguish apoE4 from apoE2 or apoE3. Arg112 on apoE 4 affects the conformation of the Arg61 side chain, resulting in a domain interaction between the carboxyl-terminal and amino-terminal domains (Dong and Weisgraber, 1996). This altered structure reduces the stability of apoE (Hatters et al., 2006), resulting in preferential degradation of apoE4 by glial cells (Riddell et al., 2008). ApoE levels are also regulated by its state of lipidation through the ABC-A1 lipid transporter (Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004). Recent studies in the individual APOE targeted replacement mice show that levels of the apoE isoforms in the brain vary, with lowest expression of apoE in the APOE ε4 targeted replacement mice (Riddell et al., 2008; Vitek et al., 2007). In the current study, to mimic the effects of apoE, we used a synthetic peptide composed of only the receptor-binding domain, which is the same across apoE isoforms. Our work with EP suggests that the receptor-binding domain of apoE is responsible for signaling a reduction in JNK activation through LRP1, suggesting that the inferior anti-inflammatory properties of apoE4 are due to the diminished stability of the isoform. Mostly recently, a study suggested that in fact increasing apoE protein, regardless of isoform, may be an effective approach to treating AD (Bales et al., 2009). This hypothesis is further supported by our work on the mechanism of apoE signaling in microglia, implicating LRP1 in signal transduction and the JNK cascade as a downstream target for modulating inflammation in microglia. Regulating inflammation in AD may effectively reduce disease progression.
Our work provides insight into LRP1 signaling and shows that LRP1 activation modulates the inflammatory response of microglia. It demonstrates the important role of JNK activation in microglial NO production; JNK inhibitors may prove useful at reducing inflammation in vivo. We also found that EP suppressed JNK activation only in LRP1 expressing microglia, suggesting that LRP1 activation represents a target for anti-inflammatory therapies in AD and other CNS diseases.
ACKNOWLEDGEMENTS
This work was supported by NIH AG14473 (GWR), HL050784 (DKS) and HL054710 (DKS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD. Scavenging of Alzheimer's amyloid beta-protein by microglia in culture. J Neurosci Res. 1996;43:190–202. doi: 10.1002/(SICI)1097-4547(19960115)43:2<190::AID-JNR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Arelin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT. LRP and senile plaques in Alzheimer's disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002;104:38–46. doi: 10.1016/s0169-328x(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2000;97:11551–11556. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Du Y, Holtzman D, Cordell B, Paul SM. Neuroinflammation and Alzheimer's disease: critical roles for cytokine/Abeta-induced glial activation, NF-kappaB, and apolipoprotein E. Neurobiol Aging. 2000;21:427–432. doi: 10.1016/s0197-4580(00)00143-3. discussion 451−423. [DOI] [PubMed] [Google Scholar]
- Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Hansen JC, Sullivan PM, Paul SM. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29:6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Stolt PC, Herz J. Functions of lipoprotein receptors in neurons. J Lipid Res. 2004;45:403–409. doi: 10.1194/jlr.R300017-JLR200. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- Bu G, Cam J, Zerbinatti C. LRP in amyloid-beta production and metabolism. Ann N Y Acad Sci. 2006;1086:35–53. doi: 10.1196/annals.1377.005. [DOI] [PubMed] [Google Scholar]
- Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmback K, Danton MJ, Colbert MC, Witte DP, Fujikawa K, Davie EW, Degen JL. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci U S A. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie RH, Chung H, Rebeck GW, Strickland D, Hyman BT. Expression of the very low-density lipoprotein receptor (VLDL-r), an apolipoprotein-E receptor, in the central nervous system and in Alzheimer's disease. J Neuropathol Exp Neurol. 1996;55:491–498. doi: 10.1097/00005072-199604000-00012. [DOI] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin SB, Mauel J, Donini SD, Quattrocchi E, Ricciardi-Castagnoli P. Inducible nitric oxide synthase activity of cloned murine microglial cells. Glia. 1993;7:255–262. doi: 10.1002/glia.440070309. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Davis RJ. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- Dong LM, Weisgraber KH. Human apolipoprotein E4 domain interaction. Arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J Biol Chem. 1996;271:19053–19057. doi: 10.1074/jbc.271.32.19053. [DOI] [PubMed] [Google Scholar]
- Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- Han IO, Kim KW, Ryu JH, Kim WK. p38 mitogen-activated protein kinase mediates lipopolysaccharide, not interferon-gamma, -induced inducible nitric oxide synthase expression in mouse BV2 microglial cells. Neurosci Lett. 2002;325:9–12. doi: 10.1016/s0304-3940(02)00218-5. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Herz J. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001;29:571–581. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Harris DC, Rebeck GW. Multiple pathways of apolipoprotein E signaling in primary neurons. J Neurochem. 2005;93:145–155. doi: 10.1111/j.1471-4159.2004.03007.x. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Pocivavsek A, Dai H, Chakraborty G, Harris DC, Rebeck GW. Effects of apoE on neuronal signaling and APP processing in rodent brain. Brain Res. 2006;1112:70–79. doi: 10.1016/j.brainres.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Rebeck GW. Regulation of ApoE receptor proteolysis by ligand binding. Brain Res Mol Brain Res. 2005;137:31–39. doi: 10.1016/j.molbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006a;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- Hu L, Boesten LS, May P, Herz J, Bovenschen N, Huisman MV, Berbee JF, Havekes LM, van Vlijmen BJ, Tamsma JT. Macrophage low-density lipoprotein receptor-related protein deficiency enhances atherosclerosis in ApoE/LDLR double knockout mice. Arterioscler Thromb Vasc Biol. 2006b;26:2710–2715. doi: 10.1161/01.ATV.0000249641.96896.e6. [DOI] [PubMed] [Google Scholar]
- Kim DH, Iijima H, Goto K, Sakai J, Ishii H, Kim HJ, Suzuki H, Kondo H, Saeki S, Yamamoto T. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem. 1996;271:8373–8380. doi: 10.1074/jbc.271.14.8373. [DOI] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res Brain Res Rev. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, van Eldik LJ. Apolipoprotein E receptors mediate the effects of beta-amyloid on astrocyte cultures. J Biol Chem. 2000;275:33974–33980. doi: 10.1074/jbc.M000602200. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, Van Eldik LJ. Apolipoprotein E and apolipoprotein E receptors modulate A beta-induced glial neuroinflammatory responses. Neurochem Int. 2001;39:427–434. doi: 10.1016/s0197-0186(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J Neuroimmunol. 1997;76:70–74. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Lee DM, Schmechel D, Staats HF. Altered immune responses in apolipoprotein E-deficient mice. J Lipid Res. 2000;41:613–620. [PubMed] [Google Scholar]
- Laskowitz DT, Matthew WD, Bennett ER, Schmechel D, Herbstreith MH, Goel S, McMillian MK. Endogenous apolipoprotein E suppresses LPS-stimulated microglial nitric oxide production. Neuroreport. 1998;9:615–618. doi: 10.1097/00001756-199803090-00010. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Thekdi AD, Thekdi SD, Han SK, Myers JK, Pizzo SV, Bennett ER. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp Neurol. 2001;167:74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- Lillis AP, Greenlee MC, Mikhailenko I, Pizzo SV, Tenner AJ, Strickland DK, Bohlson SS. Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J Immunol. 2008a;181:364–373. doi: 10.4049/jimmunol.181.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008b;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–113. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Mace BE, Wang H, Lynch JR, Moss J, Sullivan P, Colton H, Morgan K, Renauld JC, Laskowitz DT. Apolipoprotein E modifies the CNS response to injury via a histamine-mediated pathway. Neurol Res. 2007;29:243–250. doi: 10.1179/016164107X158974. [DOI] [PubMed] [Google Scholar]
- Marzolo MP, von Bernhardi R, Bu G, Inestrosa NC. Expression of alpha(2)-macroglobulin receptor/low density lipoprotein receptor-related protein (LRP) in rat microglial cells. J Neurosci Res. 2000;60:401–411. doi: 10.1002/(SICI)1097-4547(20000501)60:3<401::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–18743. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001a;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Polymorphisms in inflammatory genes and the risk of Alzheimer disease. Arch Neurol. 2001b;58:1790–1792. doi: 10.1001/archneur.58.11.1790. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr., Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Misra UK, Gawdi G, Pizzo SV. Ligation of low-density lipoprotein receptor-related protein with antibodies elevates intracellular calcium and inositol 1,4, 5-trisphosphate in macrophages. Arch Biochem Biophys. 1999;372:238–247. doi: 10.1006/abbi.1999.1521. [DOI] [PubMed] [Google Scholar]
- Moon DO, Park SY, Lee KJ, Heo MS, Kim KC, Kim MO, Lee JD, Choi YH, Kim GY. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol. 2007;7:1092–1101. doi: 10.1016/j.intimp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Mooney LM, Whitmarsh AJ. Docking interactions in the c-Jun N-terminal kinase pathway. J Biol Chem. 2004;279:11843–11852. doi: 10.1074/jbc.M311841200. [DOI] [PubMed] [Google Scholar]
- Newton CS, Loukinova E, Mikhailenko I, Ranganathan S, Gao Y, Haudenschild C, Strickland DK. Platelet-derived growth factor receptor-beta (PDGFR-beta) activation promotes its association with the low density lipoprotein receptor-related protein (LRP). Evidence for co-receptor function. J Biol Chem. 2005;280:27872–27878. doi: 10.1074/jbc.M505410200. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Parks AL, Curtis D. Presenilin diversifies its portfolio. Trends Genet. 2007;23:140–150. doi: 10.1016/j.tig.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Pietrzik CU, Busse T, Merriam DE, Weggen S, Koo EH. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 2002;21:5691–5700. doi: 10.1093/emboj/cdf568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Burns MP, Rebeck GW. Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia. 2009;57:444–453. doi: 10.1002/glia.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo H, Jou I, Jung S, Hong S, Joe EH. Mitogen-activated protein kinases activated by lipopolysaccharide and beta-amyloid in cultured rat microglia. Neuroreport. 1998;9:871–874. doi: 10.1097/00001756-199803300-00020. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH, Strickland DK. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res. 2005;46:1721–1731. doi: 10.1194/jlr.M500114-JLR200. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Ulery PG, Beers J, Mikhailenko I, Tanzi RE, Rebeck GW, Hyman BT, Strickland DK. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J Biol Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- Ulery PG, Strickland DK. LRP in Alzheimer's disease: friend or foe? J Clin Invest. 2000;106:1077–1079. doi: 10.1172/JCI11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Kuan CY, Kennedy NJ, Kelkar N, Haydar TF, Mordes JP, Appel M, Rossini AA, Jones SN, Flavell RA, Rakic P, Davis RJ. Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 2001;15:2421–2432. doi: 10.1101/gad.922801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby EA, Perkins GR, Collins MK, Whitmarsh AJ. The JNK-interacting protein-1 scaffold protein targets MAPK phosphatase-7 to dephosphorylate JNK. J Biol Chem. 2003;278:10731–10736. doi: 10.1074/jbc.M207324200. [DOI] [PubMed] [Google Scholar]
- Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004;45:170–179. doi: 10.1002/glia.10314. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, An J, Strickland DK, Yepes M. The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am J Pathol. 2009;174:586–594. doi: 10.2353/ajpath.2009.080661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Polavarapu R, She H, Mao Z, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation. Am J Pathol. 2007;171:1281–1290. doi: 10.2353/ajpath.2007.070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci Signal. 2008;1:ra15. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]