Abstract
Understanding the capabilities and limitations of nuclear import is crucial to efficient delivery of macromolecules and nanoparticles for diagnosis and targeted therapy of diseases. Here we report the Tat peptide-mediated import of different cargos into cell nucleus, including dye-labeled streptavidin protein, 43 and 90 nm fluorescent beads, as well as ∼20 nm quantum dots for kinetic measurements. Our results revealed significant differences between Tat- and NLS-mediated nuclear import: unlike delivery with the NLS, Tat peptide-based delivery is not inhibited by WGA blockage nor does it require ATP. Surprisingly, Tat peptide was able to import 90 nm beads into the nuclei of digitonin-permeabilized cells, suggesting that its interaction with the nuclear envelope follows a mechanism different from that of NLS. The import kinetics was quantified using Tat peptide-conjugated QDs, yielding a kinetic constant of 0.0085 s−1. Taken together, our results suggest that, compared with NLS, Tat peptide-mediated nuclear import is faster, follows a different pathway, and is capable of importing large nanoparticles. These results have significant implications for the development of new approaches for delivery of cargo into the nuclei of living cells.
Keywords: Nuclear delivery, Nanoparticles, Nuclear localizing sequence, Tat
Introduction
The ability of eukaryotic cells to transport large macromolecules across the nuclear membrane is essential for many cellular functions, and the mechanism of such a process has received extensive study.1,2,7,10,16,17,21,23 The nuclear membrane contains specialized protein complexes called nuclear pore complexes (NPC), which control the transport of macromolecules larger than 30–40 kDa from the cytoplasm to the nucleus.1 The transport of such macromolecules typically requires a nuclear localization signal (NLS) domain either in the primary sequence of a protein or attached to the macromolecule exogenously. The transport process involves two steps: (1) bringing a macromolecule to the NPC, and (2) translocating the macromolecule through the pore. The first step is energy-independent, whereas the second requires energy.5 It has been shown that conventional nuclear transport requires the presence of various cytosolic factors such as importins, karyopherin and ATP, and can be inhibited by blockage of the NPC with wheat germ agglutinin (WGA), cold incubation, or depletion of ATP and importins.2,5,10,23
The efficient delivery of molecular imaging probes and therapeutic agents into the nuclei of living cells is crucial for development of novel disease diagnosis and treatment strategies. In addition to the use of the NLS peptide for nuclear transport, it has been shown that the Tat peptide, an 11-amino-acid peptide of the HIV-1 Tat protein, can carry cargo across both the plasma membrane and the nuclear membrane.9,19 To reveal the mechanism responsible for Tat-based cargo delivery, extensive studies have been carried out using different constructs, including the Tat peptide alone, the Tat peptide complexed with a fluorescent protein or other reporters, the HIV-1 Tat protein, and the Tat peptide conjugated to nanoparticles or liposomes.3,6,9,11,13,18,20,25 Although most of these studies aimed to understand the interaction between the Tat peptide with the plasma membrane, and the mechanisms of its membrane translocation,6,8,11–13,20 very limited effort has been made to understand the function of Tat peptide for nuclear import.9,25 Since Tat peptide and its small molecular weight conjugates (e.g. with fluorescent dyes, short oligonucleotides) can diffuse through the NPC, studies using these constructs are not able to provide a better understanding of the import mechanism associated with the NPC.
To elucidate the process of Tat-mediated nuclear transport and understand the differences between the NLS- and Tat peptide-based nuclear import mechanisms,2,5,10 we delivered cargos with different sizes into the cell nucleus using Tat peptide (see Table 1) and studied the effect of WGA blockage on nuclear import. We also quantified the kinetics of Tat peptide using Tat-conjugated quantum dots (QDs) of 15–25 nm. Surprisingly, we found that the Tat peptide can deliver fluorescent beads of 43 and 90 nm into the nuclei of digitonin-permeabilized cells, which could not be blocked by WGA, suggesting that the nuclear import mechanism of Tat peptide is different from that of NLS peptide. These results provide insight into the import process of Tat-linked cargos to the nuclei of living cells, and demonstrate the difference between Tat- and NLS-mediated nuclear import, thus facilitating the development of Tat-based delivery of imaging agents and drug molecules into the cell nucleus.
Table 1.
Characteristics of cargos for nuclear import studies.
| Cargo | Size (nm) | Fluorescence reporter and wavelength |
|---|---|---|
| Streptavidin | ∼5 | Alexa-647 conjugated to streptavidin, peak excitation = 647 nm, peak emission = 660 nm |
| Polymer nanoparticles coated with streptavidin | 43 | Formulation proprietary, peak excitation = 488 nm, peak emission = 515 nm |
| Polymer nanoparticles coated with streptavidin | 90 | Alexa-647 conjugated to streptavidin, peak excitation = 647 nm, peak emission = 660 nm |
| Streptavidin-coated quantum dots | ∼20 | Excitation = 488 nm, peak emission = 580 nm |
Experimental Procedures
Peptide Synthesis, Conjugation and Labeling
The SV-40 NLS peptide (PKKKRKVKC) was synthesized by SynPep Inc. with purity >98%. The peptide was modified to introduce a cysteine group at its carboxyl terminus to allow for specific conjugation. The cysteine-modified NLS peptide was conjugated to the fluorescently labeled streptavidin protein using the heterobifunctional crosslinker SMCC (succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate) (Pierce Biotechnology). The peptide concentration was 2.5-fold higher than that of the fluorescently labeled streptavidin protein (10 μM) so that on average more than one peptide is conjugated to a streptavidin molecule.
Conjugates were purified using YM-30 centrifugal filter (Millipore, MA) units to remove excess peptide. The conjugates were recovered as retentate on the filter membrane. The retentate was washed three times in 1× PBS buffer, followed by centrifugal separation at each stage to ensure removal of conjugating agents and excess peptide.
The Tat peptide (N-terminus–TyrGlyArgLysLysArgArgGlnArgArgArg-C-terminus) was synthesized by Sigma-Aldrich, Corp. As shown in Fig. 1, the Tat peptide was modified with biotin at its carboxyl terminus to allow for direct conjugation with either a fluorescently labeled streptavidin protein (∼50 kDa, Molecular Probes) (Fig. 1a), streptavidin-coated fluorescent beads of 43 and 90 nm diameter (Fig. 1b), or streptavidin-coated QDs (Quantum Dot Corp.) (Fig. 1c). The biotin-modified Tat peptides were reacted to fluorescently labeled streptavidin with a 2-fold excess of concentration compared with that of streptavidin. It was assumed that all biotin-modified Tat peptides reacted with streptavidin, which has four biotin binding sites. Due to high affinity of biotin for the streptavidin protein, the conjugates were used without further purification.
Figure 1.
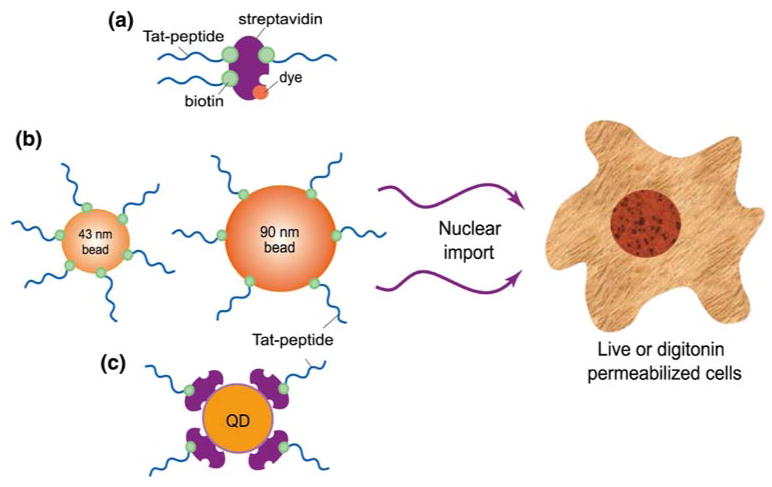
A schematic illustration of various cargos designed for Tat-mediated nuclear import.
For conjugating Tat peptides to nanoparticles, we used the reaction between Tat peptide-biotin and streptavidin protein on particle surface. The amount of streptavidin molecules on bead surface was provided by the manufacturer (Molecular Probes, OR). For the 43 nm nanoparticles, there are about 5–7 streptavidin molecules per bead, and for the 90 nm nanoparticles, there are about 30 streptavidin molecules per bead (Invitrogen). For both 43 and 90 nm nanoparticles, Tat peptide was conjugated using 1:1 stoichiometric ratio of Tat-biotin and streptavidin (but not the beads), with a conjugation of ∼1.31 μM. Due to the high affinity of the reaction and the limited availability of Tat-biotin molecules compared with biotin binding sites (4 biotin binding sites per streptavidin), it is expected that all the Tat peptides were conjugated to the nanoparticle surface. Therefore, on average there were at least 5 Tat peptides per bead. The 90 nm streptavidin-coated beads were first labeled with Alexa-647 (Molecular Probes) using a concentration 5 times that of the streptavidin (1.31 μM) on the surface of the beads. The excess dye was removed for the mixture using YM-100 centrifugal filters. The retentate was washed three times in 1× PBS buffer followed by centrifugal separation at each stage to remove any excess dye.
For conjugating biotin-modified Tat peptide to the streptavidin-coated QDs (each has about 4–6 streptavidin molecules according to the vendor), Tat peptide was reacted at twice the concentration of streptavidin (1 μM), resulting in approximately 8–12 Tat peptides per QD. The Tat-conjugated QDs and beads were not further purified and used directly for nuclear import assays.
Nuclear Import Assay and WGA Blockage
The nuclear import assay developed by Adam et al.1,2 was adapted in this study. Specifically, HeLa cells were permeabilized using 40 μg/mL digitonin in transport buffer for 5 min at 4 °C. The concentration of digitonin (40–80 μg/mL) and the incubation time (5–15 min) for permeabilization were chosen based on previous studies.2,10 For nuclear import in digitonin-permeabilized cells, the permeabilized cells were washed with transport buffer (formulated according to Adam et al.1,2) and then incubated with 1 μM of the Tat-conjugated, fluorescently labeled streptavidin for 30 min at 37 °C. In addition, experiments were conducted to validate the transport of NLS conjugated macromolecules in the presence of 50% rabbit reticulocyte lysate (RRL) in import buffer (Promoega Corp., WI) with 0.5 mM ATP, 0.2 mM GTP, 5 mM creatine phosphate, 1 unit creatine phosphokinase (Calbiochem, MA). The transport buffer with import factor was formulated according to the published protocol.1,2 The cells were imaged without further washing. The nuclear import assay for streptavidin-coated fluorescent beads was carried out with similar conditions except that a much lower bead concentration was used and that 1% bovine serum albumin (BSA) was added to the transport buffer to reduce non-specific interactions of the beads with either the cytoplasm or the membranes in digitonin-permeabilized cells. The 90 nm beads were sonicated before incubation with cells to eliminate aggregation. For measurement of the rate of QD import into the nuclei of digitonin-permeabilized cells, an estimated 0.5 nM QD concentration was used.
In using WGA as a blocker to inhibit the nuclear import process, permeabilized cells were first incubated in transport buffer with WGA (1 mg/mL) alone for 10 min, then with both WGA (1 mg/mL) and the Tat protein conjugate (1 μM) for 30 min. The import of Tat-conjugated, dye-labeled streptavidin was then imaged.
Fluorescence Imaging
Imaging was carried out using a Zeiss LSM 510 confocal microscope. Confocal microscopy, with its ability to control slice thickness and intracellular focal volume, helps distinguish between cargos inside the cell nucleus and those on the surface. For the Alexa 647-labeled streptavidin protein and 90 nm beads (Polysciences Inc.), fluorescence imaging was performed with excitation at 638 nm and emission detection at 660 nm using a long pass filter. For the 43 nm fluorescent beads (Molecular Probes), excitation was at 488 nm and emission detection was performed using a band pass filter of 510–530 nm. QDs were excited at 488 nm and emission detection was at 565 nm using a long pass filter. Table 1 summarizes the excitation and emission wavelengths for all the cargos used in this study.
Determination of Nuclear Import Kinetics
To determine the kinetic rate constant of nuclear import, the fluorescence signal intensity of Tat-conjugated QDs was quantified as a function of time over a defined region of interest (ROI) at room temperature. This curve was normalized and fitted to a first-order exponential function of the form y = 1 − exp(−kt) to obtain the characteristic parameter k describing the rate of import of the QD-linked Tat peptide, where t is time in seconds, and y is the normalized average intensity of the fluorescence signal in the nuclei of digitonin-permeabilized cells.
Results and Discussion
Nuclear Import of Tat-Conjugated, Fluorescently Labeled Streptavidin
As a first step to characterize Tat-based nuclear import, we studied the Tat-based delivery of fluorescently labeled streptavidin into the nuclei of digitonin-permeabilized cells. Digitonin permeabilization has been used in nuclear import assays to characterize the delivery of various cargos into permeabilized cells. Digitonin interacts with cholesterol-rich membranes and leaves the nuclear envelope intact,7 allowing for the direct study of nuclear import using a variety of approaches. Streptavidin was chosen as a cargo of interest as it has 4 binding sites for biotin, allowing for conjugation of multiple peptides. Streptavidin also has a molecular weight greater than 50 kDa, preventing it from simply diffusing across the nuclear membrane.1 Streptavidin was labeled with Alexa-647 to minimize autofluorescence from the cells during imaging. The digitonin-permeabilized cells were incubated with the Tat-conjugated, fluorescently labeled streptavidin as described in the “Experimental procedures” section.
To test if Tat-based nuclear import uses the same mechanism as NLS, Tat-streptavidin delivery assays were carried out in a transport buffer without the addition of cytoplasmic extracts or an ATP regenerating system. It has been shown1,2 that during permeabilization of cells with digitonin, nuclear import factors and other cytosolic factors are removed from the cells. Thus to reconstitute the nuclear import system for classical NLS-based import, it is essential to add back the cytoplasmic extract along with an ATP regenerating system.1 To directly compare the import of Tat–streptavidin with that of NLS-mediated import under the same experimental conditions, a construct of the NLS peptide conjugated to fluorescently labeled streptavidin was prepared according to the conjugation protocol described in the Experimental Procedures section and incubated under the same conditions as for the Tat-streptavidin complex.
As demonstrated by the confocal fluorescence image in Fig. 2a, the delivery of Tat-streptavidin to the nuclei of digitonin-permeabilized HeLa cells was successful without the addition of any cytoplasmic extracts (reconstituted cytosolic nuclear import machinery) or an ATP regenerating system. This is in sharp contrast to the result shown in Fig. 2b for NLS–streptavidin delivery to the nucleus under the same experimental conditions. It is clear that the nuclear import of NLS–streptavidin was inhibited in the absence of cytoplasmic extracts. Most of the signal observed in Fig. 2b was in the perinuclear region of the cytoplasm of HeLa cells, indicating that the NLS peptide could mediate the binding of the NLS–streptavidin complex to the nuclear envelope but not actively transport the cargo through the nuclear membrane pore.2 The result shown in Fig. 2b also indicates that macromolecules were not able to enter the nucleus without ATP and nuclear import factors, suggesting that the integrity of the nuclear membrane was not compromised as a result of digitonin permeabilization. Additional experiments were carried out to block the import of the Tat-streptavidin complex by using excess (50-fold) NLS peptide, but this had no effect on the import of Tat-streptavidin complex (data not shown). These results suggest that the mechanism responsible for nuclear import of Tat-streptavidin complex is different from that of the classic NLS-based import.
Figure 2.
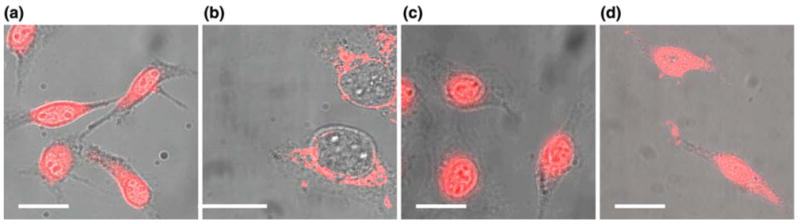
Confocal microscopy images of nuclear import of fluorescently labeled streptavidin into digitonin-permeabilized cells. (a) The positive result of Tat-peptide mediated nuclear import of dye-labeled streptavidin without the addition of cytoplasmic extracts or ATP regenerating system. (b) NLS-peptide conjugated, dye-labeled streptavidin was not able to enter the nuclei of digitonin-permeabilized cells without the addition of cytoplasmic extracts or ATP regenerating system. (c) WGA was not effective in blocking the import of the Tat-conjugated streptavidin complex. The digitonin-permeabilized cells were pre-treated with WGA of 1 mg/mL concentration for 10 min. In all the import assays, the permeabilized cells were incubated with 1 μM of the Tat-conjugated fluorescent streptavidin for 30 min at 37 °C. (d) NLS-peptide conjugated, dye labeled streptavidin was able to enter the nuclei of digitonin-permeabilized cells with the addition of ATP and other nuclear transport factors. Scale bar represents 20 μm.
WGA Does Not Block Tat-Mediated Nuclear Import
Assays were performed to study the effect of WGA blockage on Tat-mediated nuclear import. WGA is a generic blocker of the nuclear import process in digitonin-permeabilized cells.14,22,24 It binds specifically to the glycosylated proteins of the NPC and thus competes off the nuclear import machinery or blocks its interaction with the pore complex.2,7 In our study, the WGA treatment of digitonin-permeabilized cells was carried out with a WGA concentration 20-fold higher than the concentrations reported in previous studies7 to ensure effective blockage. As shown in Fig. 2c, even with this high concentration, WGA was not able to block the Tat peptide-mediated nuclear import of dye-labeled streptavidin. This clearly indicates that, compared with NLS-mediated import, the Tat peptide uses a different mechanism in interacting with the nuclear envelope and its pore complexes. Since WGA only binds to glycosylated proteins of the nuclear pore complex, it is possible that Tat interacts with different proteins within the pore complex for nuclear import. It is also possible that the interaction between Tat peptide and the NPC or the nuclear envelope has either a higher affinity than WGA blockage or significantly different interaction than the classical NLS pathway.
Transport of Macromolecules Through Nuclear Pore Was Not Compromised
To confirm the functional integrity of nuclear membrane and its ability to import NLS conjugated cargo after digitonin permeabilization, we carried out an experiment using NLS conjugated streptavidin protein. This conjugate was introduced into digitonin-permeabilized cells in the presence of ATP and other nuclear transport factors present in rabbit reticulocyte lysate.1,2,16 As shown in Fig. 2d, NLS conjugated streptavidin molecules accumulated in cell nucleus, which clearly indicates that the transport of macromolecules to the nucleus in digitonin-permeabilized cells was not compromised. Similar results have been reported in the literature.1
Tat-Mediated Import of 43 nm Fluorescent Beads into Digitonin-Permeabilized and Live Cells
To further characterize the differences between NLS-and Tat-mediated nuclear import, experiments were performed to evaluate the limitation on the size of cargo that can be imported into the cell nucleus by the Tat peptide. Previous work has indicated that Tat peptide can transport large cargo across the plasma membrane13 and the nuclear membrane25 of living cells. The size of the nuclear pore, frequently assumed to be the maximum size for molecules entering the nucleus, has been determined indirectly using electron microscopy based measurements of the import of NLS-linked gold nanoparticles.8,15 Without taking into account the cytoplasmic proteins such as importin alpha/beta that bind to the NLS sequence, the size of the nuclear pore was initially estimated to be ∼26 nm.8 However, more recent work has estimated the size of the nuclear pore in the Xenopus oocyte to be ∼39 nm,15 since intact hepatitis B cores of ∼36 nm can be imported through the NPC. In our study, we used Tat-conjugated, streptavidin-coated fluorescent beads of two sizes, 43 nm (±2 nm) and 90 nm (±10 nm), respectively (Fig. 1b). The 43 nm beads are just slightly larger than the maximum cargo size of NLS-mediated import in Xenopus oocytes (∼39 nm).15 As shown in Fig. 3a, Tat peptide was able to deliver the 43 nm beads to the nucleoplasm of digitonin-permeabilized cells without the presence of cytosolic proteins or ATP. Further, WGA did not block the import of 43 nm beads into the nuclei of digitonin-permeabilized cells, as shown in Fig. 3b.
Figure 3.
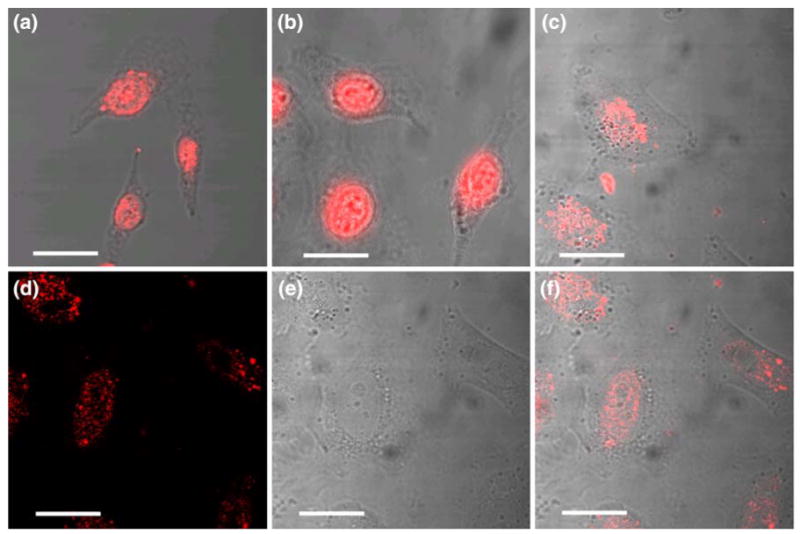
Confocal microscopy images of nuclear import of Tat-conjugated 43 nm streptavidin-coated fluorescent beads. (a) Tat-peptide was able to import 43 nm fluorescent beads into the nuclei of digitonin-permeabilized cells. (b) WGA was not effective in blocking the Tat-mediated nuclear import of 43 nm fluorescent beads. (c) Successful import of Tat-conjugated 43 nm fluorescent beads into the nuclei of living cells without plasma membrane permeabilization. (d) A confocal image shows the nucleoli in the image plane of cells. (e) Fluorescence signal in the same image plane. (f) The overlay of the images in (d) and (e). The results shown in (d–f) indicate that the nanoparticles were in the cell nucleus, and there was only a small amount of 43 nm particles entered the nucleolus. Scale bar represents 20 μm.
To demonstrate the import of large beads into the nucleus under normal physiological conditions and to compare the results with digitonin-permeabilized cells, delivery assays were performed in which we imaged the import of 43 nm streptavidin-coated, Tat-conjugated beads into the nuclei of live HeLa cells. For this assay, we used the same concentration of fluorescent beads as that used in the digitonin-permeabilized cells. The Tat-conjugated beads were incubated with live cells without any permeabilization. The images were collected 1.5 h after initial incubation. Due to the presence of more barriers to transport (such as the plasma membrane and cytoskeletal structures) in live cells, the live-cell images of bead delivery were taken after 1.5 h of incubation in contrast to the incubation time of 0.5 h for the studies with digitonin-permeabilized cells.
As demonstrated by Fig. 3c, accumulation of 43 nm beads is clearly visible in the nucleoplasm of live HeLa cells, in agreement with the results obtained previously12,25 with iron oxide nanoparticles in a similar size range. This confirms that large cargos (>40 nm) can be imported to the nuclei of living cells by Tat peptide. Note that the localization of the beads within the cell nucleus is different in live and permeabilized cells: in permeabilized cells, Tat-conjugated beads showed significant accumulation in the nucleoli (Figs. 3a and 3b), whereas in live cells, there was a widespread distribution in the nucleoplasm, with less accumulation in the nucleolus (Fig. 3c).
To drive this point home, in Figs. 3d and 3e, the fluorescent signal (Fig. 3d) and the white light image of cells (Fig. 3e) were shown separately, and the overlay of the two is shown in Fig. 3f. The confocal image in Fig. 3d shows clearly the nucleoli in the image plane, and the fluorescence signal shown in Fig. 3d in the same image plane indicates that there was only a small amount of 43 nm particles, if any, entered the nucleolus. Evidently, the fluorescence images show that the nanoparticles were indeed in the cell nucleus, for otherwise the images would not correlate so well with the shape of the cell nuclei and the locations of the nucleoli.
Tat-Mediated Import of 90 nm Fluorescent Beads into Digitonin-Permeabilized Cells
To determine if cargos larger than 40 nm in diameter could be imported into the cell nucleus using Tat peptide, we delivered streptavidin-coated, Alexa-647 dye labeled 90 nm beads into digitonin-permeabilized HeLa cells without the presence of cytosolic factors or ATP. As shown clearly in Fig. 4, Tat peptide could import 90 nm beads into digitonin-permeabilized cells, defying the conventional ‘wisdom’ that only cargos smaller than 40 nm can be delivered into the cell nucleus. While most of the 90 nm beads were delivered into the cell nucleus (some localized in the nucleoli), a small amount of the conjugate remained in the cytoplasm, suggesting that there was a reduced mobility or increased resistance for the nuclear import of large particles.
Figure 4.
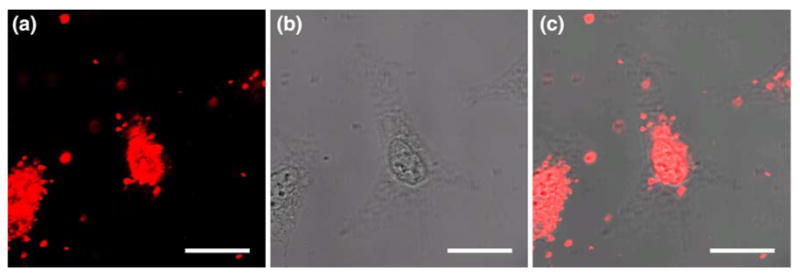
Import of Tat-conjugated 90 nm streptavidin-coated fluorescent beads into the nuclei of digitonin-permeablized cells. (a) Fluorescence signal indicating the internalization of Tat-peptide conjugated 90 nm beads into cell nuclei. (b) The white-light image of cells. (c) The overlap of white-light and fluorescence images in (a) and (b). Scale bar represents 20 μm.
An attempt was made to deliver 90 nm beads into the nuclei of live HeLa cells. However, we found that in cell culture media large clumps of beads were formed on the surface of cells, resulted in a significant amount of non-specific binding of the beads on cell surface as well as non-specific uptake of beads into cells via the endocytic pathway. Consequently, it was difficult to visualize nuclear localization of 90 nm beads in living cells since the large aggregates of beads either trapped in endosomes and other intracellular compartments, or stayed on cell surface. In contrast, with digitonin-permeabilized cells, the absence of cell culture media reduced significantly the formation of large aggregate, and digitonin helped overcome the plasma membrane barrier, thus eliminating the trapping of beads into endosomes.
The observed import of 90 nm beads into the nucleus in this study raises several important questions concerning Tat-peptide-based cargo delivery and its interaction with the NPC. For example, what is the biochemical basis of the interaction between Tat-peptide conjugates and the nuclear envelope, and does the Tat peptide use an alternative pathway to import cargo from the cytoplasm to the nucleus? This observation revealed that the particle-size limit on nuclear import might have been underestimated; a detailed biochemical study of the underlying mechanism(s) of Tat-based nuclear import is left to the future work.
Rate of Import of Tat-Conjugated Cargo
Quantitative measurement was carried out to characterize the uptake kinetics of Tat conjugated macromolecules in the nuclei of digitonin-permeabilized cells and determine whether the Tat-peptide mediated import process has characteristics similar to, or different from, that of NLS-based import. Initial experiments were performed using Tat-conjugated, fluorescently labeled streptavidin proteins at a low concentration (∼50 nM). This method is similar to that used in studying the import of NLS–peptide linked cargo.16 However, photobleaching of the dye had a significant effect on the fluorescence intensity in the cell nuclei and thus the overall kinetic measurement. To overcome this limitation, we used quantum dots (QDs) as a model cargo, which has high signal intensity and essentially no photobleaching.4 The use of QDs allowed us to determine the kinetic rates of import at a nanomolar concentration of the Tat-conjugated QDs (physiological levels). Further, QDs with ∼20 nm diameter represent a macromolecular cargo with an intermediate size between individual streptavidin proteins and 43 nm streptavidin-coated nanoparticles. We conjugated Tat peptide to streptavidin-coated QDs (∼20 nm in diameter) and delivered the resulting QD construct into digitonin-permeabilized cells to observe cargo import as a function of time. This approach has the potential to perform single molecule imaging of the import process without significantly perturbing the physiological condition of the cell.
In Fig. 5, the import of the Tat-QD complex at different time points is shown, indicating increased fluorescence intensity with time. Note that the use of QDs with a diameter >15 nm excludes the possibility of passive transport into the nucleus. Figure 5 also shows the background signal due to incubation of QDs with digitonin-permeabilized cells without any Tat peptide. There was no nuclear localization of QDs observed in this control case, demonstrating the integrity of the nuclear envelope and the low level of non-specific interactions. During the experiment we did observe an increase in non-specific binding of Tat-conjugated QDs to the glass slide. We also observed an increase in fluorescence intensity in the cytoplasm of cells. The increase in fluorescence intensity of QDs in the cytoplasm can be attributed to two reasons: (1) initial accumulation of QDs in the cytoplasmic region, particularly in the per-nuclear region, due to the affinity of Tat peptide for the nuclear membrane; (2) non-specific interaction of QDs with cytoplasmic contents.
Figure 5.
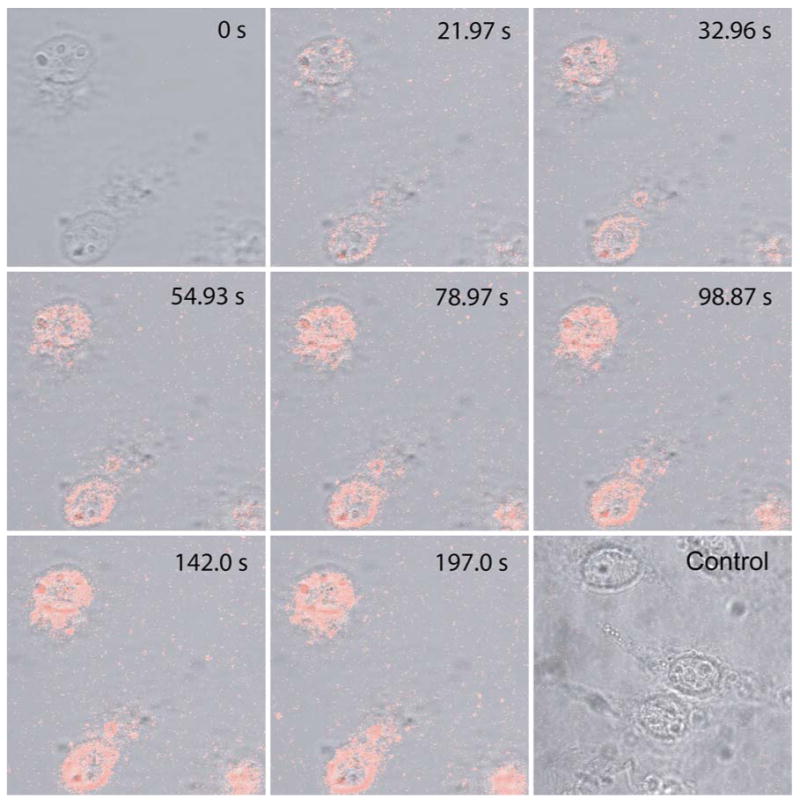
A series of confocal images showing an increase in the fluorescence intensity with time due to import of Tat-peptide conjugated quantum dots (QDs) into the nuclei of digitonin-permeablized cells, providing a basis for kinetic analysis. Included also is a fluorescence image of the control study, carried out after 150 s following the addition of unconjugated QDs, indicating the limited non-specific internalization of QDs into cells without Tat peptide.
The increase in fluorescence intensity of the cell free area (on the glass slide) was only observed right after addition of QDs; the background fluorescence reached a plateau in approximately 35 s after addition of QDs. This background signal was possibly due to non-specific interaction of Tat-conjugated QDs with the glass surface, since the Tat peptide is positively charged. In a control experiment using QDs without Tat peptide, we did not observe any increase in non-specific binding of QDs with the glass slide.
Shown in Fig. 6 is the quantitative measurement of the increase in fluorescence intensity with time averaged over three independent experiments. Specifically, the plot of normalized fluorescence intensity as a function of time indicates an initial increase in fluorescence intensity followed by a saturation level; the curve can be fitted to a first-order exponential function:
Figure 6.
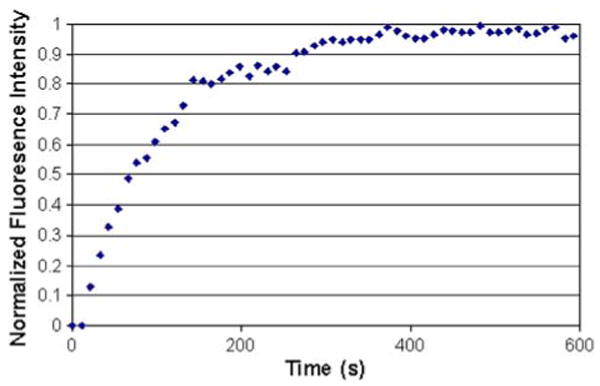
Normalized fluorescence intensity vs. time as a result of the nuclear import of Tat-conjugated quantum dots into digitonin-permeabilized cells.
where k is a characteristic parameter (rate constant) describing the rate of import of the Tat-conjugated QDs, t is time in seconds, and y is the normalized average intensity of the fluorescent signal in the nuclei of digitonin-permeabilized cells. Using this equation, the kinetic rate constant k was determined to be 0.008488 s−1 by fitting the curve in Fig. 6.
There are a few interesting features of the results shown in Fig. 6. The curve shown in Fig. 6 has the characteristics of a receptor-mediated process, although Tat peptide-based nuclear import does not require the presence of cytoplasmic components such as importin, and blockage with WGA does not inhibit nuclear import. Further, comparison of the curve in Fig. 6 for Tat-mediated import with that of NLS-mediated import16 reveals similar characteristics, although the concentration of the probe used in this study is 1000-fold lower than that used in studying the kinetics of NLS-mediated import. Our data suggest that the kinetic rate constant for Tat peptide-mediated import is much higher than that of NLS-mediated import, since we used a much lower probe concentration with comparable saturation times.
In summary, in this study we performed nuclear import assays with different cargos using the Tat peptide and compared the results with NLS-mediated cargo delivery into the cell nucleus. Specifically, the Tat-mediated import process was studied using a range of cargo molecules, including dye-labeled streptavidin protein, 43 and 90 nm beads and ∼15–20 nm QDs. The results of this study revealed significant differences between Tat- and NLS-mediated nuclear import. For example, Tat peptide mediated import is not inhibited by WGA blockage and does not require supplementation with ATP, whereas WGA blockage and ATP depletion have been shown to inhibit the nuclear import of NLS-linked cargo. The results also demonstrate the ability of Tat peptide to import 43 and 90 nm beads in digitonin-permeabilized cells, suggesting the significant difference in the import process of Tat-linked cargo and its interaction with nuclear envelope compared with NLS-mediated import. To quantify the rate of nuclear import of Tat-linked cargo, we used Tat-conjugated QDs for imaging the import process as a function of time. The measured kinetic curve (amount of QDs in the nucleus as a function of time) indicates that the characteristics of Tat-mediated import can be modeled as a first-order kinetic process, which is similar to that obtained with the NLS peptide. However, our results suggest that Tat-mediated import has much faster kinetics than the NLS mediated nuclear import. Taken together, our results demonstrate significant differences between the Tat- and NLS-mediated nuclear import pathways. These results have significant implications in developing new approaches for delivery of macromolecules into the nuclei of cells.
The molecular mechanism of how Tat peptide imports cargo into the cell nucleus remains elusive. Clearly, the import process of Tat peptide conjugates is not diffusion-driven.25 It has been suggested9 that Tat peptide conjugated with beta-galactosidase is imported into the cell nucleus by a novel mechanism which does not require the presence of cytosolic factors but requires ATP. However, our experimental results indicate that Tat peptide-mediated nuclear import does not require ATP or cytosolic factors. Clearly, Tat peptide carries cargo into the cell nucleus using a mechanism different from that of NLS. Even more striking is that Tat peptide can import beads of 90 nm into the cell nucleus, challenging the current understanding of import through the NPC. Although establishing the exact mechanism for Tat peptide-mediated nuclear import is beyond the scope of this report, we believe that the discoveries made in this study will help develop a fundamental understanding of Tat peptide-based nuclear import as well as new approaches to deliver nanoparticles into the nuclei of living cells.
The results of this study have demonstrated import of Tat conjugated nanoparticles to the nuclei of digitonin permeabilized cells. Additional studies are needed to confirm that large nanoparticles can be delivered into the nuclei of living cells by Tat peptide. For example, 3D multi-color confocal imaging studies with spectrally distinct fluorophores shall be performed to individually track the fate of Tat peptide and nanoparticles in permeabilized and living cells to provide a more detailed characterization of Tat mediated nuclear import process. It is also desirable to gain a mechanistic insight into the interactions of Tat peptide with nuclear membrane by which import of nanoparticles across nuclear membrane can occur. This may be achieved by combining fluorescence imaging with electron microscopy to characterize the nuclear import of Tat conjugated nanoparticles with higher spatial resolution.
Acknowledgments
This work was supported by NIH Roadmap Initiative in Nanomedicine through a Nanomedicine Development Center award, 1PN2EY018244 (GB), and by the Office of Science, Department of Energy grant DE-FG02-04ER63785 (GB).
References
- 1.Adam SA, Marr RS, Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam SA, Sterne-Marr R, Gerace L. In vitro nuclear protein import using permeabilized mammalian cells. Methods Cell Biol. 1991;35:469–482. doi: 10.1016/s0091-679x(08)60584-1. [DOI] [PubMed] [Google Scholar]
- 3.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24:247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- 4.Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 5.Cserpan I, Udvardy A. The mechanism of nuclear transport of natural or artificial transport substrates in digitonin-permeabilized cells. J Cell Sci. 1995;108(Pt 5):1849–1861. doi: 10.1242/jcs.108.5.1849. [DOI] [PubMed] [Google Scholar]
- 6.Curnow P, Mellor H, Stephens DJ, Lorch M, Booth PJ. Translocation of the cell-penetrating Tat peptide across artificial bilayers and into living cells. Biochem Soc Symp. 2005:199–209. doi: 10.1042/bss0720199. [DOI] [PubMed] [Google Scholar]
- 7.Duverger E, Pellerin-Mendes C, Mayer R, Roche AC, Monsigny M. Nuclear import of glycoconjugates is distinct from the classical NLS pathway. J Cell Sci. 1995;108(Pt 4):1325–1332. doi: 10.1242/jcs.108.4.1325. [DOI] [PubMed] [Google Scholar]
- 8.Dworetzky SI, Lanford RE, Feldherr CM. The effects of variations in the number and sequence of targeting signals on nuclear uptake. J Cell Biol. 1988;107:1279–1287. doi: 10.1083/jcb.107.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efthymiadis A, Briggs LJ, Jans DA. The HIV-1 Tat nuclear localization sequence confers novel nuclear import properties. J Biol Chem. 1998;273:1623–1628. doi: 10.1074/jbc.273.3.1623. [DOI] [PubMed] [Google Scholar]
- 10.Hagstrom JE, Ludtke JJ, Bassik MC, Sebestyen MG, Adam SA, Wolff JA. Nuclear import of DNA in digitonin-permeabilized cells. J Cell Sci. 1997;110(Pt 18):2323–2331. doi: 10.1242/jcs.110.18.2323. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Koch AM, Reynolds F, Kircher MF, Merkle HP, Weissleder R, Josephson L. Uptake and metabolism of a dual fluorochrome Tat-nanoparticle in HeLa cells. Bioconjug Chem. 2003;14:1115–1121. doi: 10.1021/bc034123v. [DOI] [PubMed] [Google Scholar]
- 13.Levchenko TS, Rammohan R, Volodina N, Torchilin VP. Tat peptide-mediated intracellular delivery of liposomes. Methods Enzymol. 2003;372:339–349. doi: 10.1016/s0076-6879(03)72019-9. [DOI] [PubMed] [Google Scholar]
- 14.Newmeyer DD, Forbes DJ. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988;52:641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- 15.Pante N, Kann M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol Biol Cell. 2002;13:425–434. doi: 10.1091/mbc.01-06-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribbeck K, Gorlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 2001;20:1320–1330. doi: 10.1093/emboj/20.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribbeck K, Gorlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 2002;21:2664–2671. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torchilin VP. TAT peptide-modified liposomes for intracellular delivery of drugs and DNA. Cell Mol Biol Lett. 2002;7:265–267. [PubMed] [Google Scholar]
- 19.Vaysse L, Gregory LG, Harbottle RP, Perouzel E, Tolmachov O, Coutelle C. Nuclear-targeted minicircle to enhance gene transfer with non-viral vectors in vitro and in vivo. J Gene Med. 2006;8:754–763. doi: 10.1002/jgm.883. [DOI] [PubMed] [Google Scholar]
- 20.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 21.Wilson GL, Dean BS, Wang G, Dean DA. Nuclear import of plasmid DNA in digitonin-permeabilized cells requires both cytoplasmic factors and specific DNA sequences. J Biol Chem. 1999;274:22025–22032. doi: 10.1074/jbc.274.31.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff B, Willingham WC, Hanover JA. Nuclear protein import: specificity for transport across the nuclear pore. Exp Cell Res. 1988;178:318–334. doi: 10.1016/0014-4827(88)90402-8. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto N, Deng XW. Protein nucleocytoplasmic transport and its light regulation in plants. Genes Cells. 1999;4:489–500. doi: 10.1046/j.1365-2443.1999.00282.x. [DOI] [PubMed] [Google Scholar]
- 24.Yoneda Y, Imamoto-Sonobe N, Yamaizumi M, Uchida T. Reversible inhibition of protein import into the nucleus by wheat germ agglutinin injected into cultured cells. Exp Cell Res. 1987;173:586–595. doi: 10.1016/0014-4827(87)90297-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhao M, Kircher MF, Josephson L, Weissleder R. Differential conjugation of tat peptide to superparamagnetic nanoparticles and its effect on cellular uptake. Bioconjug Chem. 2002;13:840–844. doi: 10.1021/bc0255236. [DOI] [PubMed] [Google Scholar]


