Abstract
Understanding the role of peripheral defocus on central refractive development is critical because refractive errors can vary significantly with eccentricity and peripheral refractions have been implicated in the genesis of central refractive errors in humans. Two rearing strategies were used to determine whether peripheral hyperopia alters central refractive development in rhesus monkeys. In intact eyes, lens-induced relative peripheral hyperopia produced central axial myopia. Moreover, eliminating the fovea by laser photoablation did not prevent compensating myopic changes in response to optically imposed hyperopia. These results show that peripheral refractive errors can have a substantial impact on central refractive development in primates.
Keywords: myopia, hyperopia, peripheral refraction, refractive error, emmetropization
1. Introduction
Visual feedback associated with the eye’s effective refractive state regulates emmetropization (Norton, 1999; Smith III, 1998; Wallman & Winawer, 2004). In many species, including primates, the effects of vision on refractive development appear to be mediated primarily by local retinal mechanisms that integrate visual signals in a spatially restricted manner and that exert their influence selectively on the subjacent sclera (Diether & Schaeffel, 1997; Hodos & Kuenzel, 1984; Siegwart & Norton, 1993; Smith III, Huang, Hung, Ramamirtham, Blasdel, Humbird & Bockhorst, 2009; Wallman, Gottlieb, Rajaram & Fugate-Wentzek, 1987). Although it has generally been assumed that visual signals from the fovea or central retina dominate refractive development in primates (Stone & Flitcroft, 2004), several lines of evidence indicate that peripheral visual signals can have a substantial effect on axial growth and central refractive development.
Clinical observations provide support for the idea that visual signals from the peripheral retina can have a significant impact on emmetropization at the fovea and possibly the genesis of common refractive errors. For example, patients who have natural or treatment-induced peripheral retinal abnormalities frequently exhibit larger than normal ranges of central refractive errors and, on average, larger central refractive errors (Connolly, Ng, McNamara, Regillo, Vander & Tasman, 2002; Knight-Nanan & O’Keefe, 1996; Nathan, Kiely, Crewther & Crewther, 1985; Nissenkorn, Yassur, Mashkowski, Sherf & Ben-Sira, 1983; Sieving & Fishman, 1978). It is possible that these central refractive errors come about because the treatment and/or disease processes interfere with the mechanisms responsible for emmetropization. In this respect, children who have conditions or diseases that primarily affect the peripheral retina usually exhibit larger central refractive errors than children with eye diseases that primarily affect central vision (Nathan et al., 1985).
The most direct evidence that peripheral vision can influence central refractive development come from animal experiments in which the visual signals from the fovea were eliminated or from experiments in which peripheral vision was selectively manipulated. For example, laser photoablation of the fovea in infant monkeys does not 1) interfere with emmetropization in animals reared with unrestricted vision, 2) prevent form-deprivation from producing central axial myopia, or 3) alter the recovery from experimentally induced refractive errors (Smith III, Kee, Ramamirtham, Qiao-Grider & Hung, 2005; Smith III, Ramamirtham, Qiao-Grider, Hung, Huang, Kee, Coats & Paysee, 2007). These results demonstrate that visual signals from the fovea are not essential for many vision-dependent changes in refractive development and that visual signals from the periphery, in isolation, can be used to determine the direction of axial growth required to eliminate central refractive errors and to determine when ocular growth has eliminated that refractive error, i.e., when emmetropia has been established. Moreover, when experimental manipulations impose conflicting visual signals between the central and peripheral retina, peripheral vision can dominate overall ocular growth. For example, chicks and monkeys that were reared with diffuser lenses that selectively deprived the peripheral retina of form vision, but allowed unrestricted central vision, typically developed central axial myopia (Smith III et al., 2005; Stone, Pendrak, Sugimoto, Lin, Gill, Capehart & Liu, 2006). Overall, these results indicate that peripheral vision can have a substantial influence on foveal refractive development in macaques.
However, Schippert and Schaeffel (Schippert & Schaeffel, 2006) recently reported that optically imposed peripheral hyperopic defocus does not affect central refractive development in chicks. They found that although lenses that defocused the entire retina consistently produced central myopic compensation in chicks, lenses with central apertures that allowed unrestricted central vision did not. This apparent discrepancy between the effects of peripheral form deprivation and peripheral defocus could reflect differences in the mechanisms that mediate the effects of defocus and form deprivation. In this respect, several observations suggest that the effects of defocus and form deprivation are mediated by different, but overlapping, vision-dependent processes (Bartmann, Schaeffel, Hagel & Zrenner, 1994; Kee, Marzani & Wallman, 2001; Schaeffel, Hagel, Bartmann, Kohler & Zrenner, 1994).
Understanding the potential effects of peripheral defocus on central refractive development is critical because refractive error can vary significantly with eccentricity (i.e., the visual signals that regulate eye growth can vary across the retina) (Ferree, Rand & Hardy, 1931; Ferree, Rand & Hardy, 1932; Millodot, 1981; Millodot & Lamont, 1974) and the pattern of peripheral refractive errors has been implicated in the genesis of refractive errors at the fovea in humans. In particular, relative peripheral hyperopia appears to be a risk factor for the onset and/or progression of myopia in children and adults (Hoogerheide, Rempt & Hoogenboom, 1971; Mutti, Hayes, Mitchell, Jones, Moeschberger, Cotter, Kleinstein, Manny, Twelker & Zadnik, 2007; Schmid, 2004). Consequently, the purpose of this investigation was to determine whether optically imposed, peripheral hyperopic defocus alters refractive development in infant monkeys.
2. Methods
2.1 Subjects
Data are presented for 59 infant rhesus monkeys (Macaca mulatta). The animals were obtained at 1 to 3 weeks of age and housed in our primate nursery that was maintained on a 12-hour light/12-hour dark lighting cycle. The details of the nursery care for our infant monkeys have been described previously (Smith III & Hung, 1999). After the initial biometry measurements at about 3 weeks of age, the monkeys were randomly assigned to either the control or treatment groups. All of the rearing and experimental procedures were reviewed and approved by the University of Houston’s Institutional Animal Care and Use Committee and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
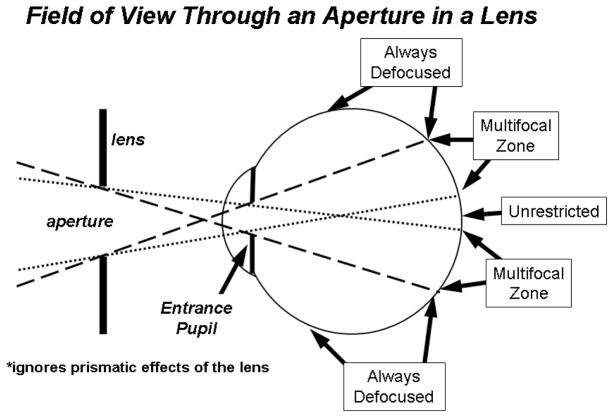
We employed two strategies to examine the impact of peripheral hyperopic defocus on eye growth and refractive development. First, relative peripheral hyperopia was optically imposed on both eyes of 8 infant monkeys by securing −3.0 D spectacle lenses that had 6 mm circular apertures that were centered on each eye’s entrance pupil (-3D-aperture group)(See Smith and Hung (1999) for details of our helmet rearing procedures.). As discussed by Carkeet (1998) (Carkeet, 1998), to determine the extent of the visual field affected by a lens aperture it is necessary to take into account interactions between the eye’s pupil and the lens aperture (see Figure 1). Our helmets held the lenses at a 14 mm vertex distance so ignoring the prismatic effects of the lens and assuming an entrance pupil diameter of 3.0 mm and an anterior chamber depth of 2.58 mm, the resulting “unrestricted” field of view through the 6 mm apertures was 10.3 deg (i.e., all the rays that formed the retinal images within the central 10.3 deg passed through the lens aperture and were unaffected by the power of the treatment lens)(dotted lines in Figure 1). For objects at field eccentricities of 31 deg or beyond (dashed lines in Figure 1), the retinal images were composed exclusively of rays that passed through the negative-powered portion of the treatment lens and, thus, relative to the central retina were always hyperopically defocused by -3 D. For objects located at eccentricities between about 10 and 31 deg, the resulting image was produced by rays that passed through the lens aperture and by rays refracted by the powered portion of the lens. Consequently, objects within this “multifocal” zone resulted in two images; one at a focal plane determined by the optics of the eye alone and a second at a more hyperopically located plane. Between 10 and 31 deg, the relative proportion of rays that were affected by the negative-powered portion of the lens increased in a systematic fashion with eccentricity.
Figure 1.
Schematic diagram of the extent of the effects of the treatment lens aperture on retinal imagery. The dotted lines represent the projection of the eye’s entrance pupil through the lens aperture and demark the object eccentricities that are imaged exclusively through the lens aperture (i.e., the “unrestricted” portion of the field). The dashed lines delineate the object eccentricities that are imaged exclusively through the powered portion of the lens. Within the “multifocal” zone between the dotted and dashed lines, objects will be imaged at two focal planes, one determined by the eye’s optics alone and a second located at a more hyperopic plane determined by the powered portion of the treatment lens. The diagram does not include any possible prismatic effects associated with the powered portion of the lens.
The apertures allowed binocular convergence of the pupillary axes for viewing distances as short as 15.7cm (assuming an interpupillary distance of 22 mm and that the center of rotation for each eye was 8.4 mm from the cornea). Although foveal vision was potentially degraded when the infant’s eyes were not aligned with the diffuser apertures (e.g., It is likely that the monkeys fixated objects closer than 15.7 cm with one eye; as a consequence, the foveal image of the non-fixating eye would have been degraded.), we believe that the refractive changes described below came about as a result of peripheral optical effects. First, the lenses were worn over both eyes and, because all of the animals were moderately hyperopic at the start of the treatment period, it was always to the animal’s advantage to fixate through the apertures (i.e., the animals were motivated to view through the apertures). Second, the infants rapidly adapted to the treatment lenses and observations throughout the rearing period demonstrated that the animals consistently fixated through the lens apertures. Third, even if foveal vision was occasionally degraded, the non-linear temporal integration properties of the emmetropization process would make it unlikely that brief episodes of defocus at the fovea would produce myopia (Kee, Hung, Qiao-Grider, Ramamirtham, Winawer, Wallman & Smith III, 2007; Napper, Brennan, Barrington, Squires, Vessey & Vingrys, 1997; Schmid & Wildsoet, 1996; Smith III, Harwerth, Wensveen, Ramamirtham, Kee & Hung, 2002).
The second strategy (-3D-laser group) that we employed to examine the impact of peripheral hyperopic defocus on refractive development was a lens compensation experiment in which we isolated the contribution of the periphery by eliminating visual signals from the fovea via laser photoablation (i.e., can the periphery, in isolation, mediate compensating ocular growth in response to optically imposed defocus?). Specifically, a blue-green argon laser with a nominal spot size of 500 microns was employed to ablate the fovea of one eye in each of 6 experimental monkeys. The laser procedures were performed immediately after the initial biometric measurements with the intent of eliminating all of the fovea and part of the perifovea. To make the foveal ablations, the monkeys were anesthetized (intramuscular injection: ketamine hydrochloride, 15–20 mg/kg, and acepromazine maleate, 0.15–0.2 mg/kg; topical: 1–2 drops of 0.5% tetracaine hydrochloride) and the laser was delivered to the eye via a slit lamp. The laser was presented in 50 msec pulses and the power of the laser was varied between 100 and 250 mW to produce soft white retinal burns. The foveal burns were overlapped to ensure complete ablation of the fovea. Subsequently, ophthalmoscopy, optical coherence tomography, and fundus photography confirmed the size and positions of the lesions. We have previously demonstrated that this laser ablation protocol is effective in destroying the neural retina within the treatment zones (Smith III et al., 2007). The diameters of the ablation zone were approximately 2 times the horizontal diameter of the optic disc and corresponded to about the central 10–12 degrees of the retina.
Immediately following the laser procedures, the monkeys were fitted with helmets that secured −3.0 D lenses over both eyes. Unlike the lenses employed in the -3D-aperture group, these treatment lenses imposed relative hyperopic defocus across the entire visual field. Like the animals in the first treatment group, these lens-reared monkeys wore the goggles continuously from about 3 weeks of age until about 5 months of age. The average age at the end of the treatment period was 138 ± 27 and 149 ± 7 days for the -3D-aperture group and -3D-laser group, respectively.
The control group consisted of 28 infant monkeys that were reared with normal unrestricted vision and 4 monkeys that were reared wearing helmets that held zero-powered spectacle lenses in front of both eyes. The lens wells provided monocular and binocular fields of view in the horizontal plane of 80 and 62 deg, respectively, and an 87-deg vertical field. The plano-lens-reared monkeys served as controls for our helmet rearing procedures and the resulting restrictions in visual field. Refractive data for 24 of the normal monkeys and 3 of the plano-lens-reared monkeys have been previously reported (Hung, Ramamirtham, Huang, Qiao-Grider & Smith III, 2008; Kee, Hung, Qiao-Grider, Roorda & Smith III, 2004; Kee, Hung, Qiao, Habib & Smith III, 2002; Kee et al., 2007; Smith III et al., 2009; Smith III & Hung, 1999).
Comparison data for the effects of optically imposed hyperopic defocus were obtained from 8 monkeys with intact retinas that were reared with binocular −3.0 D lenses that imposed relative hyperopic defocus across the entire visual field (Kee et al., 2007; Smith III & Hung, 1999). Control data for the effects of the laser photoablation procedures were obtained from 5 infants that had the fovea of one eye ablated at 3 weeks of age and allow unrestricted visual experience (Smith III et al., 2007).
2.2 Ocular Biometry
Each subject’s refractive status and their eye’s axial dimensions were measured at the start of the lens wear/treatment period and then every 2–4 weeks throughout the observation period using methods that have been described in detail previously (Smith & Hung, 1999). To make these measurements, the monkeys were anesthetized (intramuscular injection: ketamine hydrochloride, 15–20 mg/kg, and acepromazine maleate, 0.15–0.2 mg/kg; topical: 1–2 drops of 0.5% tetracaine hydrochloride) and cyclopleged (1% tropicamide). The refractive status of each eye, both the spherical and cylindrical components, were measured along the pupillary axis independently by two experienced investigators using a streak retinoscope and averaged (Harris, 1988). An eye’s refractive error was defined as the mean spherical-equivalent, spectacle-plane refractive correction. We have previously estimated that the 95% limits of agreement for our retinoscopy measures (spherical-equivalent refractive error) were ±0.6 D (Hung et al., 2008). The eye’s axial dimensions were measured by A-scan ultrasonography implemented with a 7 MHz transducer (Image 2000; Mentor, Norwell, MA). Ten separate measurements were averaged.
2.3 Statistical Analysis
Statistical analyses were performed using Minitab software (Release 12.21, Minitab Inc.). Paired t-tests were employed for interocular comparisons. Two-sample t-tests and nonparametric Mann-Whitney tests were used to test for significant differences between animal groups. Linear regression analysis was employed to examine the relationship between refractive error and vitreous chamber depth.
3. Results
Prior to the onset of the experimental rearing strategies, the eyes of the control and experimental monkeys were, on average, moderately hyperopic (right eyes; controls = +4.04 ± 1.91 D;-3D-aperture group = +3.54 ± 1.30 D;-3D-laser group = +4.45 ± 0.89 D). The two eyes of the treated and control monkeys were also well matched in terms of refractive error (paired t-test, P = 0.22 to 0.61) and vitreous chamber depth (paired t-test, P = 0.46 to 0.92) and there were no between group differences in refractive error (two-sample t-test, P = 0.15 to 0.42) or vitreous chamber depth (two-sample t-test, P = 0.28 to 0.99). Over the next 4 months, the two eyes of each of the control monkeys grew in a coordinated manner toward a low degree of hyperopia, i.e., emmetropization occurred. At ages corresponding to the end of the lens-rearing period for the experimental monkeys, 28 of the 32 control monkeys exhibited refractive errors between +1.25 and +3.25 D (average = +2.57 ± 1.07 D) and the average degree of anisometropia was 0.19 ± 0.13 D (range = 0 to 0.50 D).
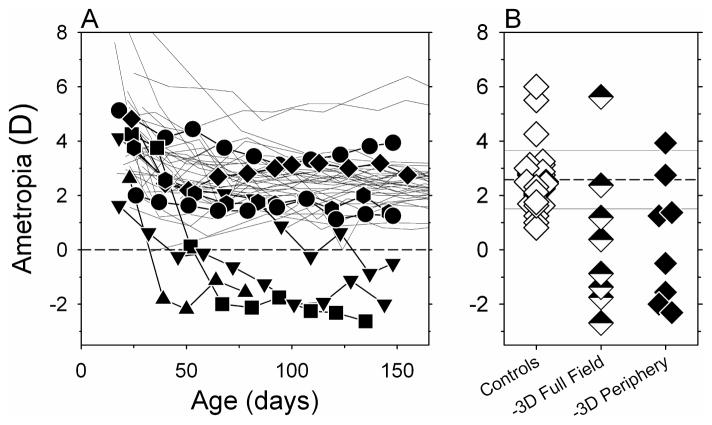
The treatment lenses produced obvious alterations in the course of emmetropization in many of the animals in the -3D-aperture group. Figure 2A shows the spherical-equivalent refractive corrections plotted as a function of age for the right eyes of the control (thin lines) and the -3D-aperture animals (filled symbols). Four of the monkeys in the -3D-aperture group developed myopic refractive errors that were outside the control range throughout most of the lens-rearing period and, by the end of the treatment period, 2 other experimental monkeys showed refractive errors that were less hyperopic/more myopic than 94% of the age-matched control animals.
Figure 2.
A. Spherical-equivalent refractive corrections obtained along the pupillary axis plotted as a function of age for the right eyes of individual control (thin lines) treated monkeys (filled symbols) reared with binocular −3.0 D spectacle lenses with 6 mm apertures centered on the pupils of each eye (-3D-aperture group). B. Right eye refractions obtained at ages corresponding to the end of the lens-rearing period for control animals (open diamonds) and the monkeys in the -3D-aperture group (filled diamonds). For comparison purposes, the half-filled diamonds represent monkeys that were reared with intact −3.0 D lenses that altered the focus of both eyes across the entire field. The horizontal dashed line represents the average refractive error for the control monkeys; the solid lines denote ±1 SD from the control mean.
The refractive corrections obtained at ages corresponding to the end of the lens-rearing period are shown for the right eyes of individual control (open diamonds) and treated monkeys (filled diamonds) in Figure 2B. For comparison purposes, the refractive errors obtained at the end of the lens-rearing period for monkeys that wore binocular, full-field, −3.0 D treatment lenses are also shown (half-filled diamonds). At the end of the treatment period, there were no systematic interocular differences in the refractive errors (paired t-test, P = 0.32) or vitreous chamber depths (paired t-test, P = 0.12) for the monkeys in the -3D-aperture group. However, the median refractive errors for the right and left eyes of the monkeys in the -3D-aperture group were significantly more myopic than those for the control animals (Mann-Whitney test; right eyes, +0.38 D vs +2.50 D, P = 0.01; left eyes, +1.28 D vs +2.56 D, P = 0.008). The range of refractive errors exhibited by the monkeys in the -3D-aperture group compared favorable to the range of end-of-treatment refractive errors found in monkeys reared with full-field -3D lenses (−2.69 to +5.63 D vs −2.61 to +3.93 D). Although the average (+0.36 ± 2.69 D vs +0.46 ± 2.49 D; two-sample t-test, P = 0.94) and median refractive errors (−0.25 D vs +0.38 D; Mann-Whitney test, P = 0.87) for the monkeys in the -3D-aperture group were slightly less myopic/more hyperopic than those for the monkeys reared with full-field, -3D lenses, these differences were not statistically significant.
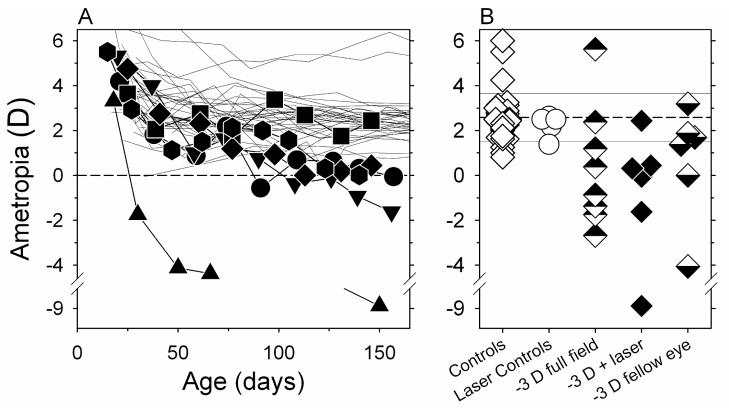
Laser photoablation of the fovea did not prevent the monkeys in the -3D-laser group from becoming myopic. As illustrated in Figure 3A, which shows the spherical-equivalent refractive corrections plotted as a function of age for the right eyes of the control animals and the laser-treated eyes of the monkeys in the -3D-laser group, 5 of the 6 experimental monkeys developed myopic errors that were outside the control range. The relative myopic changes were apparent in all five of these animals by about 100 days of age.
Figure 3.
A. Spherical-equivalent refractive corrections obtained along the pupillary axis plotted as a function of age for the right eyes of individual control monkeys (thin lines) and the laser-treated eyes of the monkeys (filled symbols) reared with binocular −3.0 D spectacle lenses (-3D-laser group). B. Refractions obtained at ages corresponding to the end of the lens-rearing period for the right eyes of control animals (open diamonds), the laser-treated eyes of monkeys reared with unrestricted vision (open circles), and the laser-treated (filled diamonds) and fellow eyes (bottom-filled diamonds) of the monkeys in the -3D-laser group. For comparison purposes, the top-filled diamonds represent monkeys that were reared with intact −3.0 D lenses that altered the focus of both eyes across the entire field. The horizontal dashed line represents the average refractive error for the control monkeys; the solid lines denote ±1 SD from the control mean.
Figure 3B shows the refractive errors obtained at ages corresponding to the end of the lens-rearing period for the laser-treated eyes of the animals in the -3D-laser group (filled diamonds) and the right eyes of the control monkeys (open diamonds). For reference, the open circles show the refractive corrections obtained at equivalent ages for the laser-treated eyes of monkeys that were reared with unrestricted vision; the top-filled diamonds show the refractive corrections obtained at the end of the lens-rearing period for the right eyes of monkeys reared with full-field -3 D lenses. At the end of the lens-rearing period, there was a tendency for the laser-treated eyes of the monkeys in the -3D-laser group to be more myopic than their fellow intact eyes that were also treated with -3D lenses (bottom-filled diamonds), however, these differences were not statistically significant (paired t-test, P = 0.09). The median refractive errors for the fellow and laser-treated eyes of the monkeys in the -3D-laser group were also comparable to those for the left and right eyes, respectively, of the monkeys with intact retinas and reared with full-field -3 D lenses (Mann-Whitney test, P = 0.85 and 1.00). On the other hand, the median refractive errors for the laser-treated eyes of the monkeys in the -3D-laser group were significantly more myopic than the right eyes of the control animals (+0.13 D vs +2.50 D, Mann-Whitney test, P = 0.001) and the laser-treated eyes of the laser-control monkeys that were reared with unrestricted vision (+0.13 D vs +2.50 D, Mann-Whitney Test, P = 0.02).
The range of refractive errors exhibited by the laser-treated eyes of the monkeys in the -3D-laser group was larger than that for the monkeys reared with full-field, -3D lenses primarily because one of the monkeys in the -3D-laser group developed relatively high levels of myopia in its treated (−8.87 D) and fellow eyes (−4.06 D).
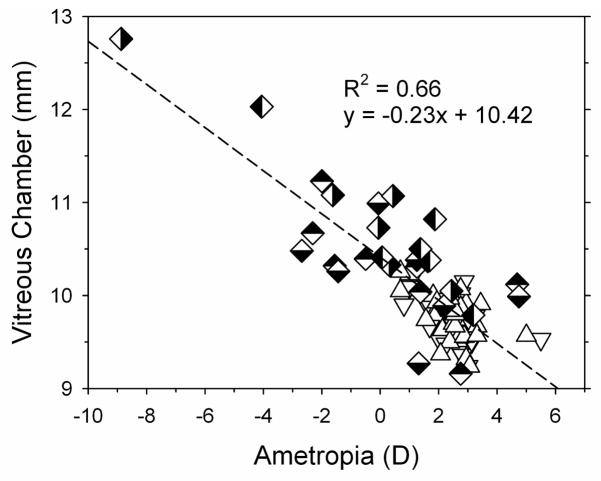
The relative myopic refractive errors observed in the experimental monkeys were axial in nature and due primarily to increases in vitreous chamber depth. In comparison to the control monkeys (right eye median = 9.82 mm), the median vitreous chamber depth in the right eyes of the monkeys in the -3D-aperture group (10.32 mm, Mann-Whitney test, P = 0.004) and in the laser-treated eyes of the animals in the -3D-laser group (10.90 mm, Mann-Whitney test, P = 0.0003) were significantly longer. In Figure 4, vitreous chamber depth is plotted as a function of refractive error for individual control monkeys (open triangle) and for monkeys in the -3D-aperture and -3D-laser treatment groups. All of the data were obtained at ages corresponding to the end of the lens-rearing period for the experimental monkeys; data for both the left and right eyes of each animal are shown. As represented by the dashed line vitreous chamber depth and refractive error were significantly correlated (regression analysis, P < 0.0001) with vitreous chamber depth accounting for 66% of the variance in refractive error. Each millimeter increase in vitreous chamber was associated with a 4.3 D myopic shift in refractive error.
Figure 4.
Vitreous chamber depths plotted as a function of spherical-equivalent refractive corrections for the right (open, down triangles) and left eyes of control monkeys (open, up triangles), the right (top-filled diamonds) and left eyes (bottom-filled diamonds) of monkeys in the -3D-aperture group and the laser-treated (right-filled diamond) and fellow eyes (left-filled diamond) of the monkeys in the -3D-laser group. The dashed line represents the best fitting regression line.
4. Discussion
Overall, the results from the two experimental groups demonstrate that peripheral vision can have a substantial impact on central refractive development. In particular, the results from the monkeys in the -3D-aperture group demonstrate that relative peripheral hyperopia can promote the development of central axial myopia in primates even in the presence of potentially clear images in the central retina. The fact that the magnitude of the relative central myopia produced by the optically imposed peripheral hyperopia was slightly smaller, but comparable, to that produced by treatment lenses that imposed the same degree of hyperopia across the entire field indicates that the overall contribution of the fovea to central refractive development is probably small. It is clear that unrestricted central vision is not sufficient to ensure normal emmetropization. Moreover, when there are conflicting visual signals between the fovea and peripheral retina, the direction of axial growth is dominated by the peripheral retina.
Selective peripheral form deprivation has been shown to cause central axial myopia in infant monkeys (Smith III et al., 2005) and chickens (Stone et al., 2006). Although several observations suggest that the mechanisms that mediate the effects of form deprivation and optical defocus are not identical (Wallman & Winawer, 2004), the results from our monkeys in the -3D-aperture group appear to be qualitatively similar to those produced by peripheral form deprivation. This similarity indicates that the spatial distribution across the retina and relative effectiveness of the mechanisms that mediate the effects of form deprivation and defocus are also similar.
In contrast to the results from our monkeys in the -3D-aperture group and the effects of peripheral form deprivation in chicks, Schippert and Schaeffel (2006) have reported that peripheral defocus does not necessarily affect central refractive development in chicks. Although it is possible that the effects of peripheral vision are more localized in chicks, and that any changes are restricted to the periphery of the chick eye, it is also likely that this apparent discrepancy reflects methodological differences in rearing strategies rather than an interspecies difference in sensitivity to peripheral defocus or a difference between the effects of defocus and form deprivation in chicks. In this study, as in our previous study of peripheral form deprivation (Smith III et al., 2005), our helmet system held the treatment lenses at a vertex distance of 14 mm. In contrast, the treatment lenses employed by Schippert and Schaeffel (2006) were held at a vertex distance of only 2 mm. Even though the nominal aperture sizes (4, 6, and 8 mm diameter apertures) used by Schippert and Schaeffel (2006) were similar to those that we have employed in monkeys, the greater vertex distance of our lenses would reduce the amount of retina viewing through the aperture, as Schippert and Schaeffel note. The effect of an aperture in a diffuser or a lens can be expressed either as a field stop, which limits the part of the visual field visible to the animal, or as the fraction of the retina illuminated, ascertained by calculating the effect of the eye’s peripheral optics on light rays entering the eye at the edge of the aperture. We used the former method because it is not affected significantly by eye growth and does not require modeling the peripheral optics, which would require estimating the peripheral corneal and lens curvatures and the refractive index distribution of the lens, parameters that are changing in growing eyes. Using this method, we estimate that in our monkeys all objects outside the central 31 deg were defocused relative to the central retina, whereas in Schippert and Schaeffel’s chicks wearing lenses with 4 mm apertures, a central field of about 95 deg included at least some rays that passed through the aperture, i.e., more than 3 times larger than with the aperture that we employed. It is likely that Schippert and Schaeffel (2006) failed to observe any effects of peripheral defocus on central refractive development because only the extreme retinal periphery was consistently defocused. In agreement with this argument, Stone et al. (2006) were able to produce central axial myopia by rearing chicks with diffuser lenses that had 5 mm diameter apertures that were held a vertex distance of about 8.5 mm. With this greater vertex distance, all images outside about the central 43 deg were consistently degraded by their diffusers. Thus, it appears that when comparable eccentricities are manipulated, peripheral optical effects have similar effects on central refraction in chicks and monkeys.
The results from the monkeys in our -3D-laser group provide additional insights into the contribution of foveal signals to vision-dependent eye growth. Four basic observations have provided the foundation for the idea that refractive development is regulated by visual feedback associated with the eye’s refractive status. Specifically, the observations that emmetropization requires vision, that chronic image degradation interferes with emmetropization typically resulting in axial myopia (i.e., form deprivation myopia), that the eyes of young animals can recover from experimentally induced refractive errors, and that optically imposed defocus can predictably alter the course of emmetropization (i.e., “lens compensation phenomenon) provide strong evidence for the vision-dependent nature of refractive development (Smith III, 1998; Wallman & Winawer, 2004). We have previously shown that laser ablation of the fovea in infant monkeys does not interfere with emmetropization in animals reared in unrestricted vision, does not prevent central axial myopia in response to form deprivation, and does not alter the recovery from experimentally induced refractive errors (Smith III et al., 2005; Smith III et al., 2007). The findings from the monkeys in the -3D-laser group demonstrate that visual signals from the fovea are also not required to produce compensating axial myopia in response to optically imposed hyperopia. Thus, visual signals from the fovea are not essential for any of the basic phenomena that support the idea that refractive development is a vision-dependent process. On the other hand, the results from all of these studies indicate that visual signals from the periphery are sufficient to mediate these basic phenomena. Given the dominance of the fovea in primate vision, these results may seem counterintuitive. However, it is important to keep in mind that the mechanisms that regulate eye growth appear to have evolved from fish, to have been largely conserved across species, and to operate very effectively in species without foveas. So it is unlikely that signals from the fovea contribute to vision-dependent refractive development in a qualitatively unique manner. It is very likely that visual signals from the fovea do contribute to the overall growth process and to refractive development. It is reasonable to suppose that the contribution of the fovea reflects its absolute area and/or the absolute number of neurons in the fovea (exactly which neurons are critical is not known) (Wallman & Winawer, 2004). Regardless, in both respects the fovea represents a relatively small portion of the retina. The periphery dominates primarily as a consequence of its relative size and issues related to spatial summation. However, because accommodation is controlled mostly by visual signals from the central retina, the fovea plays a key role in determining the overall quality of the retinal image.
The findings from both of our experimental animal groups support the idea that peripheral refractive errors, in particular relative peripheral hyperopia, can influence central refractive development. Several observations in humans suggest that relative peripheral hyperopia is a risk factor for myopia. Specifically, adults and children who exhibit relative peripheral hyperopia are more likely to develop central myopia than individuals who exhibit relative peripheral myopia (Hoogerheide et al., 1971; Mutti et al., 2007). However, from the available human data, it is not possible to determine whether the relationship between peripheral hyperopia and central myopia is causal in nature. Observations in form-deprived infant monkeys suggest that in some cases peripheral hyperopia may reflect changes in eye shape that are associated with the process of axial elongation (Huang, Hung, Ramamirtham, Blasdel, Humbird, Bockhorst & Smith III, 2009). However, regardless of whether peripheral hyperopia is a concomitant change that occurs during axial elongation or if peripheral hyperopia develops independently, because selective peripheral hyperopic defocus can produce axial myopia in infant monkeys, it is reasonable to hypothesize that the presence of peripheral hyperopia in children will promote myopic progression and increase the severity of myopia.
This idea is supported by recent observations that show that vision-dependent refractive development in primates is mediated by local retinal mechanisms that integrate visual information in a spatially restricted manner and that exert their influence selectively on the subjacent sclera (Smith III et al., 2009). This is significant because the refractive state at the fovea is dependent on ocular changes at the posterior pole and in the periphery (i.e., an expansion of the sclera in the periphery would displace the central retina in a posterior direction along the visual axis). As a consequence, peripheral visual signals can influence central refractive development in a manner that is independent of the nature of central vision.
Acknowledgments
This work was supported by NIH Grants EY-03611, EY-07551 and funds from the Vision CRC and the UH Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartmann M, Schaeffel F, Hagel G, Zrenner E. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Visual Neuroscience. 1994;11:199–208. doi: 10.1017/s0952523800001565. [DOI] [PubMed] [Google Scholar]
- Carkeet A. Field restriction and vignetting in contact lenses with opaque peripheries. Clinical and Experimental Optometry. 1998;81:151–158. doi: 10.1111/j.1444-0938.1998.tb06773.x. [DOI] [PubMed] [Google Scholar]
- Connolly BP, Ng EY, McNamara JA, Regillo CD, Vander JF, Tasman W. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: part 2 Refractive outcome. Ophthalmology. 2002;109:936–941. doi: 10.1016/s0161-6420(01)01015-6. [DOI] [PubMed] [Google Scholar]
- Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Research. 1997;37:659–668. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- Ferree CE, Rand G, Hardy C. Refraction for the peripheral field of vision. Archives of Ophthalmology. 1931;5:717–731. [Google Scholar]
- Ferree CE, Rand G, Hardy C. Refractive asymmetry in the temporal and nasal halves of the visual field. American Journal of Ophthalmology. 1932;15:513–522. [Google Scholar]
- Harris WF. Algebra of sphero-cylinders and refractive errors, and their means, variance, and standard deviation. American Journal of Optometry and Physiological Optics. 1988;65:794–902. doi: 10.1097/00006324-198810000-00003. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kuenzel WJ. Retinal-image degradation produces ocular enlargement in chicks. Investigative Ophthalmology and Visual Science. 1984;25:652–659. [PubMed] [Google Scholar]
- Hoogerheide J, Rempt F, Hoogenboom WP. Acquired myopia in young pilots. Ophthalmologica. 1971;163:209–215. doi: 10.1159/000306646. [DOI] [PubMed] [Google Scholar]
- Huang J, Hung L-F, Ramamirtham R, Blasdel T, Humbird T, Bockhorst K, Smith EL., III Form deprivation alters peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta) Investigative Ophthalmology & Visual Science. 2009 doi: 10.1167/iovs.08-3162. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LF, Ramamirtham R, Huang J, Qiao-Grider Y, Smith EL., III Peripheral refraction in normal infant rhesus monkeys. Investigative Ophthalmology & Visual Science. 2008;49:3747–3757. doi: 10.1167/iovs.07-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee C-s, Hung LF, Qiao-Grider Y, Roorda A, Smith EL., III Effects of optically imposed astigmatism on emmetropization in infant monkeys. Investigative Ophthalmology & Visual Science. 2004;45:1647–1659. doi: 10.1167/iovs.03-0841. [DOI] [PubMed] [Google Scholar]
- Kee C-s, Hung LF, Qiao Y, Habib A, Smith EL., III Prevalence of astigmatism in infant monkeys. Vision Research. 2002;42:1349–1359. doi: 10.1016/s0042-6989(02)00060-3. [DOI] [PubMed] [Google Scholar]
- Kee C-s, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J, Smith EL., III Temporal constraints on experimental emmetropization in infant monkeys. Investigative Ophthalmology & Visual Science. 2007;48:957–962. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee C-s, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Investigative Ophthalmology & Visual Science. 2001;42:757–583. [PubMed] [Google Scholar]
- Knight-Nanan DM, O’Keefe M. Refractive outcome in eyes with retinopathy of prematurity treated with cryotherapy or diode laser: 3 year follow up. British Journal of Ophthalmology. 1996;80:998–1001. doi: 10.1136/bjo.80.11.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Troilo D, Glasser A, Howland H. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Research. 1995;35:1203–1209. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- Millodot M. Effect of ametropia on peripheral refraction. American Journal of Optometry & Physiological Optics. 1981;58:691–695. doi: 10.1097/00006324-198109000-00001. [DOI] [PubMed] [Google Scholar]
- Millodot M, Lamont A. Refraction of the periphery of the eye. Journal of the Optical Society of America. 1974;64:110–111. doi: 10.1364/josa.64.000110. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Investigative Ophthalmology & Visual Science. 2007;48:2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys A. The effect of an interrupted daily period of normal visual stimulation on form deprivation myopia in chicks. Vision Research. 1997;37:1557–1564. doi: 10.1016/s0042-6989(96)00269-6. [DOI] [PubMed] [Google Scholar]
- Nathan J, Kiely PM, Crewther SG, Crewther DP. Disease-associated image degradation and spherical refractive errors in children. American Journal of Optometry and Physiological Optics. 1985;62:680–688. doi: 10.1097/00006324-198510000-00003. [DOI] [PubMed] [Google Scholar]
- Nissenkorn I, Yassur Y, Mashkowski O, Sherf I, Ben-Sira I. Myopia in premature babies with and without retinopathy of prematurity. British Journal of Ophthalmology. 1983;67:170–173. doi: 10.1136/bjo.67.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT. Animal Models of Myopia: Learning How Vision Controls the Size of the Eye. Journal of the Institute for Laboratory Animal Research. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee C-s, Ramamirtham R, Smith E., III Normal ocular development in young rhesus monkeys (Macaca mulatta) Vision Research. 2007;47:1424–1444. doi: 10.1016/j.visres.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-Hydroxydopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Research. 1994;34:143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Schippert R, Schaeffel F. Peripheral defocus does not necessarily affect central refractive development. Vision Research. 2006;46:3935–3940. doi: 10.1016/j.visres.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Schmid G. Retinal steepness vs. myopic shift in children. Optometry and Vision Science. 2004;12S:23. doi: 10.1097/OPX.0b013e3182152646. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Research. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Norton TT. Refractive and ocular changes in tree shrews raised with plus or minus lenses. Investigative Ophthalmology & Visual Science. 1993;34(Suppl):1208. [Google Scholar]
- Sieving PA, Fishman GA. Refractive errors of retinitis pigmentosa patients. British Journal of Ophthalmology. 1978;62:163–167. doi: 10.1136/bjo.62.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL., III . Environmentally induced refractive errors in animals. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Oxford: Butterworth-Heinemann; 1998. pp. 57–90. [Google Scholar]
- Smith EL, III, Harwerth RS, Wensveen JM, Ramamirtham R, Kee C-s, Hung LF. Effects of brief daily periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Investigative Ophthalmology & Vision Science. 2002;43:291–299. [PubMed] [Google Scholar]
- Smith EL, III, Huang J, Hung L-F, Ramamirtham R, Blasdel T, Humbird T, Bockhorst K. Hemi-retinal form deprivation: Evidence for Local Control of Eye Growth and Refractive Development in Infant Monkeys. Investigative Ophthalmology & Vision Science. 2008 doi: 10.1167/iovs.08-3232. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Research. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Kee C-s, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Investigative Ophthalmology & Visual Science. 2005;46:3965–3972. doi: 10.1167/iovs.05-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee C-s, Coats D, Paysee E. Effects of foveal ablation on emmetropization and form-deprivation myopia. Investigative Ophthalmology & Visual Science. 2007;48:3914–3922. doi: 10.1167/iovs.06-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Flitcroft DI. Ocular shape and myopia. Annals of the Academy of Medicine Singapore. 2004;33:7–15. [PubMed] [Google Scholar]
- Stone RA, Pendrak K, Sugimoto R, Lin T, Gill AS, Capehart C, Liu J. Local patterns of image degradation differentially affect refraction and eye shape in chick. Current Eye Research. 2006;31:90–105. doi: 10.1080/02713680500479517. [DOI] [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek L. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]