Abstract
Translational Relevance
We report that the expression pattern of inflammatory-related genes in tumors and paired noncancerous tissues was an independent prognostic marker for colon adenocarcinoma patients. This gene signature was associated with prognosis in early stage patients. Therefore, this gene signature may be useful to identify high risk, early stage patients to assist in decisions regarding appropriate therapeutic intervention. We also show that combining independent biomarkers can improve predictions over single biomarkers. The combination of the inflammatory gene signature with available microRNA-21 expression data improved predictions with prognosis over either alone. These findings demonstrate the potential of IRS and/or microRNA-21 to be used as prognostic biomarkers for early stage colon cancer.
Purpose
Inflammatory genes and microRNAs have roles in colon carcinogenesis; therefore, they may provide useful biomarkers for colon cancer. This study examines the potential clinical utility of an inflammatory gene expression signature as a prognostic biomarker for colon cancer in addition to previously examined microRNA-21 expression.
Experimental Design
Quantitative RTPCR measured the expression 23 inflammatory genes in colon adenocarcinomas and adjacent noncancerous tissues from 196 patients. These data were used to develop models for cancer-specific mortality on a training cohort (n=57) and this model was tested in both a test (n=56) and validation (n=83) cohort. Expression data for microRNA-21 was available for these patients and was compared to and combined with inflammatory gene expression.
Results
PRG1, IL-10, CD68, IL-23a, and IL-12a expression in noncancerous tissue and PRG1, ANXA1, IL-23a, IL-17a, FOXP3 and HLA-DRA expression in tumor tissues were associated with poor prognosis based on Cox regression (|Z-score| > 1.5) and were used to generate the inflammatory risk score (IRS). IRS was associated with cancer-specific mortality in the training, test (P=0.01) and validation (P=0.02) cohorts. This association was strong for stage II cases (P=0.002). microRNA-21 expression was associated with IL-6, IL-8, IL-10, IL-12a and NOS2a, providing evidence that the function of this microRNA and these inflammatory genes are linked. Both IRS and microRNA-21 expression were independently associated with cancer-specific mortality, including stage II patients alone.
Conclusion
IRS and microRNA-21 expression are independent predictors of colon cancer prognosis and may provide a clinically useful tool to identify high risk patients.
Colon adenocarcinoma is a leading cause of cancer mortality worldwide (1) and accounts for ~50,000 deaths annually in the United States (2). While current adjuvant treatment modalities improve survival for TNM stage III colon cancer patients, it remains controversial if stage II patients should be given these therapies (3, 4). Some stage II patients will benefit from therapy; but therapy for others will harm quality of life with little therapeutic benefit. Therefore, it is important to develop biomarkers to identify high risk, early stage patients that may be suitable for therapeutic intervention.
Inflammation plays a key role in tumor initiation, progression and metastasis (5, 6). Chronic inflammation is associated with increased rates of colon cancer for both ulcerative colitis and Crohn’s disease (7–9). Nonsteroidal anti-inflammatorydrugs can reduce colon cancer risk (10). Inflammation-modulating cytokines affect tumor development through roles in cell proliferation, angiogenesis and apoptosis (11). Cytokines can signal changes directly within the tumor or the tumor microenvironment to influence cancer progression (12). Since inflammation contributes to colon carcinogenesis, expression of inflammatory genes may serve as biomarkers for colon cancer. Infiltration of inflammatory cells in colorectal cancer have been associated with prognosis (13–15). Polymorphisms in inflammatory genes have been associated with colon cancer incidence and prognosis (16–18). Expression of inflammatory genes has also been associated with TNM staging and prognosis in colon cancer (19, 20). Previous studies identified unique expression signatures composed of a panel of inflammatory/immune-response genes that predict metastatic progression and survival of hepatocellular carcinoma (21) and lung adenocarcinoma (22) patients. Building on these findings in hepatocellular carcinoma and lung adenocarcinoma, we determined if expression of these inflammatory genes in tumors and the surrounding noncancerous tissue can be used as a prognostic biomarker for colon adenocarcinoma.
Combining multiple, independent prognostic biomarkers may improve the ability to identify cancer patients at high risk of disease progression and mortality. Therefore, adding an additional factor to an inflammatory gene biomarker may provide a more clinically useful biomarker than either alone. MicroRNA expression may serve this purpose. MicroRNAs are small, noncoding RNA molecules that have demonstrated potential as biomarkers in cancer (23–26). Expression levels of microRNAs have are altered in all cancers that have been studied. Alteration of specific microRNAs can alter tumor progression in mouse models (27), demonstrating their potential to be causal factors in carcinogenesis. We recently reported that patients with tumors expressing high levels of an oncogenic microRNA, miR-21, have worse survival prognosis for stage II or stage III colon adenocarcinoma, demonstrating its potential as a prognostic biomarker for colon cancer (28). The expression of miR-21 has previously been linked to inflammatory responses. Mir-21 expression is increased following lipopolysaccharide-induced inflammation (29) and increased miR-21 expression occurs during T-cell differentiation (30). Interleukin 6 (IL-6), a proinflammaotory cytokine, can drive miR-21 expression through a STAT3 dependent mechanism (31). Because miR-21 expression is linked to inflammation and both miR-21 and inflammatory gene expression are linked to colon cancer, combining inflammatory gene biomarkers with miR-21 may improve their clinical utility.
In this study, we set out to measure the expression of inflammatory genes in tumors and paired noncancerous tissue from 196 colon adenocarcinoma patients and use these data to develop an inflammatory risk model that could be used as a prognostic biomarker for colon cancer. In addition, we used previously acquired data on miR-21 to address two specific questions. First does miR-21 expression correlate with specific inflammatory genes as is predicted from mechanistic studies in cell culture? Secondly, does the combination of the inflammatory risk model with miR-21 expression have improved associations with cancer-specific mortality over either alone?
Materials and Methods
Tissue collection and RNA isolation
Pairs of primary colon tumor and adjacent noncancerous tissues came from 83 patients recruited from the University of Maryland Medical Center or Baltimore Veterans Administration Medical Center between 1993 and 2002, and from 113 patients recruited from Queen Mary Hospital in Hong Kong between 1991 and 2000. These patients have been described in a previous study (28). Cases with familial adenomatous polyposis were excluded. Tissues were grossly dissected and flash frozen after surgery, prior to any adjuvant therapy. Detailed backgrounds for each tissue donor, including age, gender, clinical staging, tumor location and survival time from diagnosis were collected. Final date of follow-up was 12-31-05 or 8-16-04 for the NCI-Maryland or Hong Kong Cohort, respectively. Tumor histopathology was classified according to the World Health Organization Classification of Tumor system (1). Informed consent was given by all participants. This study was approved by the Institutional Review Board of the National Institutes of Health, the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster and the Institutional Review Board for Human Subject Research at the University of Maryland.
RNA isolation and quantitative RT-PCR of inflammatory genes
RNA from frozen tissue samples was extracted using standard TRIZOL (Invitrogen, Carlsbad, CA) methods. RNA was reverse transcribed using the cDNA Archive Kit (Applied Biosystems, Foster City, CA) with a 50 ng/μl final concentration. Expression levels of inflammatory genes were measured with custom-designed, Taqman low-density-array real-time polymerase-chain reaction plates (Applied Biosystems, Foster City, CA) containing probes to 23 inflammatory genes: Annexin A1 (ANXA1) (assay ID Hs00167549_m1), colon stimulating factor 1 (CSF1) (assay ID Hs00174164_m1), major histocompatibility complex (MHC) class II antigen DRα (HLA-DRA) (ID Hs00219575_m1), MHC class II antigen DPα1 (HLA-DPA1) (ID Hs00410276_m1), interferon γ (IFN-γ) (ID Hs00174143_m1), interleukin 1α (IL-1A) (ID Hs00174092_m1), interleukin 1β (IL-1B) (ID Hs00174097_m1), interleukin 2 (IL-2) (ID Hs00174114_m1), interleukin 4 (IL-4) (ID Hs00174122_m1), interleukin 5 (IL-5) (ID Hs00174200_m1), interleukin 6 (IL-6) (ID Hs00174131_m1), interleukin 8 (IL-8) (ID Hs00174103_m1), interleukin 10 (IL-10) (ID Hs00174086_m1), interleukin 12p35 (IL-12A) (ID Hs00168405_m1), interleukin 12p45 (IL-12B) (ID Hs00233688_m1), interleukin 15 (IL-15) (ID Hs00542571_m1), interleukin 17A (IL-17A) (ID Hs00174383_m1), interleukin 23 (IL-23A) (ID Hs00372324_m1), proteoglycan 1 (PRG1) (ID Hs00160444_m1), nitric oxide synthase 2A (NOS2A) (ID Hs00167257_m1), forkhead box p3 (FOXP3) (ID Hs00203958_m1), cluster of differentiation 68 (CD68) (ID Hs00154355_m1), and tumor necrosis factor α (TNF-a) (ID Hs00174128_m1)with 18s rRNA (ID Hs99999901_s1) as a normalization control. Expression of inflammatory genes was measured while blinded to all clinical outcomes. For quality control, any tissue that had 18s threshold cycle values >15 were considered poor quality and were removed. A patient was removed from this study if either noncancerous or paired tumor tissues failed quality control.
Measurement of miR-21
In a previous study, microRNA expression levels were measured in all of these patient samples and is described in detail there (28). Briefly, microRNA expression levels in the NCI-Maryland cohort were measured using microRNA microarrays (Ohio State microRNA microarray version 2.0). For the Hong Kong Cohort, expression of miR-21 was measured using quantitative RT-PCR using Taqman MicroRNA assays (Applied Biosystems, Foster City, CA) according to manufacturer’s instructions. High expression cases for miR-21 were defined based on highest tertile separately for the microarrays and qRT-PCR results.
Statistical analyses
Expression data were imported into Biometric Research Branch Array Tools v3.6.0 and median normalized for the Hong Kong cohort and Maryland cohort separately. Paired t tests identified differentially expressed genes between tumor and noncancerous tissue for the Hong Kong and the Maryland cohort separately. To account for multiple comparisons, only differences that were found and validated in each cohort separately (P < 0.05) were considered significant. Graphpad Prism v5.0 (Graphpad Software Inc, La Jolla, CA) was used for correlation analysis.
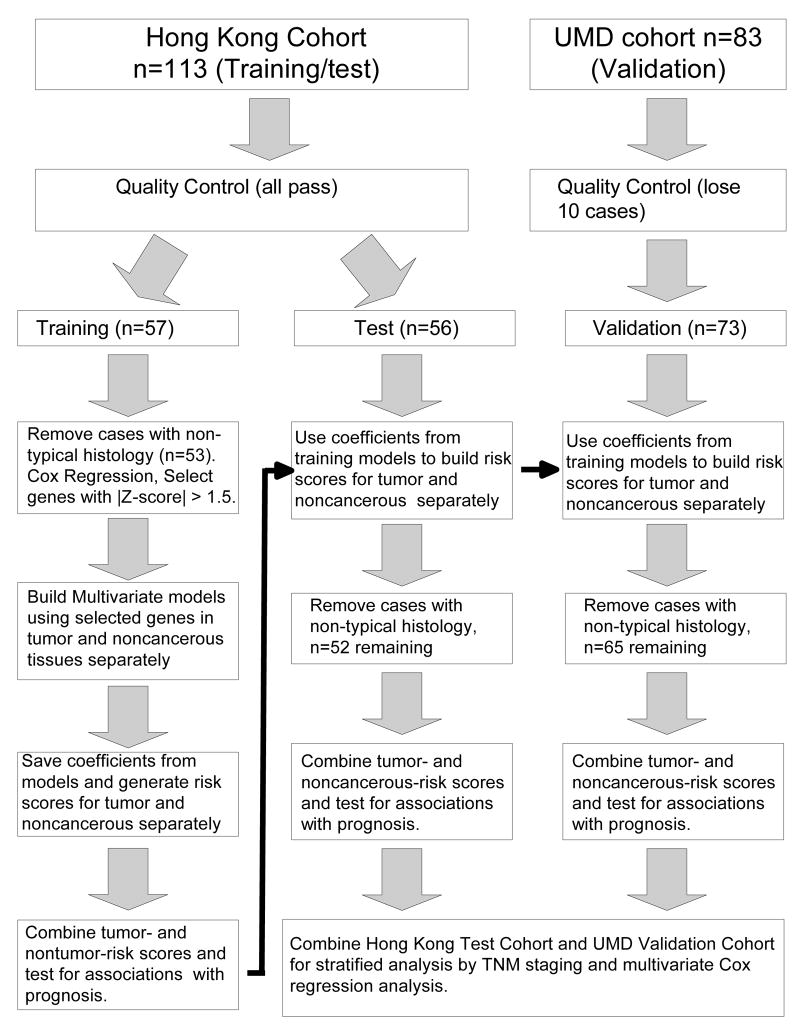
The Hong Kong cohort was divided randomly into a training and test cohort to identify a gene expression model associated with cancer-specific mortality. The Maryland cohort was used as the validation cohort for this model. Prior to beginning the analysis, the Hong Kong cohort was selected to divide into the training and test cohort since it was the larger of the two cohorts and likely result in a model with improved accuracy compared to the smaller, Maryland cohort. Univariate Cox regression analysis on the training cohort was used to select genes associated with cancer-specific mortality (|Z-score| > 1.5; p<0.13) to include in multivariate risk models using previously reported methods (32). All genes were included for these purposes and expression values for all analyses are continuous variables. For multivariate Cox regression models, missing values for genes were replaced with the average values. In the training cohort, selected genes were used to build multivariate models for tumor and noncancerous tissue separately. Coefficients from these models were multiplied with gene expression values and summed to build risk scores. Individuals were defined as high risk if they had higher than median risk scores for both tumor and noncancerous models. Kaplan Meier analysis was performed with WINSTAT 2007 (R Fitch Software, Bad Krozingen, Germany). Cox regression was performed in Stata 9.2 (StataCorp LP, College Station, TX).
Linear regression models identified associations between miR-21 expression and inflammatory gene expression in noncancerous tissue, tumor tissue and then a combined analysis of both tumor and noncancerous tissue adjusting for tumor status. Only the qRT-PCR data from the Hong Kong cohort was analyzed for these purposes sine th microarray data from the Maryland cohort was considered less reliable. IL-4, IL-5, and IL-12b were excluded since they were missing data for >25% of the samples. The Bonferroni-Holm method (33) was used to adjust for multiple comparisons in the combined tumor and noncancerous regression models.
Results
Expression of inflammatory genes are systematically altered in colon adenocarcinoma
This study used two independent cohorts, one consisting of 113 cases recruited from Hong Kong and a second cohort of 83 cases recruited from Maryland (table 1). The median follow-up time was 84.6 and 80.0 months for patients in the Hong Kong or NCI-Maryland cohort, respectively. The cohorts were similar in TNM staging (p = 0.65, Fisher’s exact) and cancer-specific mortality (p = 0.46; Kaplan Meier log rank) with 5 year survival rates of 49.5% (Hong Kong Cohort) and 59.7% (NCI-Maryland Cohort). Along with the racial, cultural and geographic differences of the two cohorts, the Maryland cohort was considerably older with a higher percentage of men.
Table 1.
Characteristics of Study Populations and Tumors
| Hong Kong Cohort | Maryland Cohort | ||
|---|---|---|---|
| Training (n=57) | Test (n=56) | Validation (n=83) | |
| Age (years) at enrollment | |||
| Mean (SD) | 57.8 (15.5) | 53.8 (14.1) | 64.4 (10.6) |
| Range | 32–84 | 30–82 | 32–87 |
| Gender, No (%) | |||
| Male | 30 (53) | 26 (46) | 65 (78) |
| Female | 27 (47) | 30 (54) | 18 (22) |
| Tumor locationa, No. (%) | |||
| Distal | 41 (72) | 49 (87.5) | 48 (59) |
| Proximal | 16 (28) | 7 (12.5) | 33 (41) |
| Adenocarcinoma Histology, No. (%) | |||
| Adenocarcinoma | 53 (93) | 52 (93) | 74 (89) |
| Mucinous Adenocarcinoma | 4 (7) | 3 (5) | 8 (10) |
| Adenosquamous | 0 (0) | 0 (0) | 1 (1) |
| Signet Ring Cell and Mucinous | 0 (0) | 1 (2) | 0 (0) |
| Adjuvant Chemotherapyb, No. (%) | |||
| Received | 22 (39) | 18 (32) | 22 (38) |
| Did not receive | 35 (61) | 38 (57) | 36 (62) |
| TNM stagingc, No. (%) | |||
| I | 2 (4) | 7 (13) | 8 (10) |
| II | 19 (33) | 18 (32) | 29 (35) |
| III | 27 (47) | 21 (38) | 36 (43) |
| IV | 9 (16) | 10 (18) | 9(11) |
| Removed during quality controld | 0 (0) | 0 (0) | 10 (12) |
Distal includes tumors located in or distal to the descending colon. Proximal tumors include tumors in or proximal to the splenic flexure. Tumor location was available for all cases of the Hong Kong Cohort and 81 cases in the Maryland Cohort.
Detailed information pertaining to receipt of chemotherapy was available for all patients in the Hong Kong cohorts and 58 in the Maryland cohort. Chemotherapy was primarily fluorouracil-based (in forms of either intravenous fluorouracil or oral drugs including tegafur with uracil) with or without levamisole or leucovorin.
For 1 patient in the Maryland Cohort, it was unclear if that patient had stage III or stage IV colon cancer, therefore this patient is removed from analyses stratifying by TNM stage.
Cases with poor quality data from quantitative RT-PCR for either the tumor or nontumorous tissue were removed.
We measured the expression of 23 inflammatory genes in primary colon tumor and paired noncancerous tissues using low-density-array real-time polymerase-chain reaction. Eighteen of these genes were selected because they were included in previous studies of hepatocellular carcinoma (21) and lung adenocarcinoma (22). The additional five genes (IL-17A, IL-23A, CD68, NOS2A, and FOXP3) were selected based on literature supporting their roles in colonic inflammation or cancer (34–36). IL-4 and IL-5 were not detectable in the majority of tissues and were removed from all further analyses.
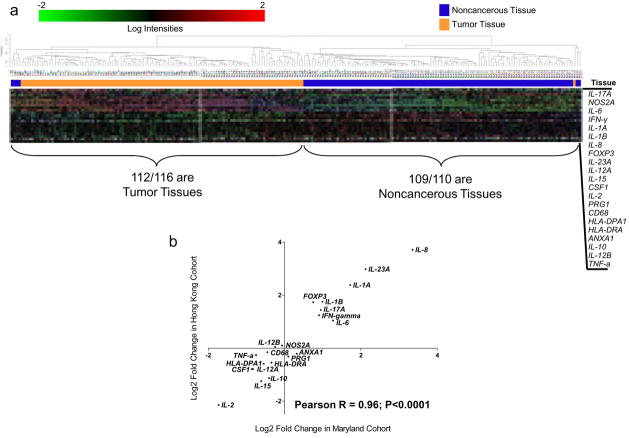
Inflammatory gene expression was systematically altered in tumors. Expression of the 21 inflammatory genes could distinguish tumor from noncancerous tissue pairs with 99 or 100% accuracy based on nearest centroid or 3-nearest neighbors class prediction algorithms, respectively (10-fold cross validation repeated 100 times) using the Hong Kong cohort. Unsupervised hierarchical clustering of the 21 genes separated tissues into two distinct groups; one composed of 97% tumor tissue and one composed of 99% noncancerous tissue (figure 1a). Of the 21 inflammatory genes examined, expression of 18 were altered in tumors in the Hong Kong Cohort (p < 0.05; paired t-test) (supplemental table 1). Of these, IL-8 demonstrated the largest fold-increase in tumors at ~13-fold higher levels in tumors while IL-2 demonstrated the largest reduction in tumor with ~80% less in tumors. These results indicate a systematic change in the expression of inflammatory genes during tumorigenesis.
Figure 1.
Inflammatory genes are consistently altered in colon tumors from both cohorts. a) Unsupervised hierarchical clustering (correlation, average linkage) using 21 inflammatory genes on 113 pairs of cancerous and noncancerous tissues in the Hong Kong Cohort. Clustering separates tissues into two distinct groups; one composed of 97% tumor tissues and one composed of 99% noncancerous tissue. b) Correlation of the tumor/noncancerous tissue expression ratio comparing Hong Kong Cohort with NCI-Maryland cohort indicates consistent changes in inflammatory gene expression in both cohorts.
We next analyzed the NCI-Maryland cohort. Fold changes in tumors for these inflammatory genes were consistent with the Hong Kong cohort (Pearson R = 0.96; Figure 1b) indicating that these changes in gene expression are likely representative of the majority of colon adenocarcinomas. Expression of IL-8, IL-23a, IL-1a, IL-1b, FOXP3 IL-17a, IFN-γ and IL-6 were significantly increased in tumors from both cohorts, IL-2, IL-15, IL-10, IL-12a, CSF1, HLA-DPA1, HLA-DRA, TNF-a, and CD68 were significantly decreased in tumors from both cohorts.
Colon adenomas represent an early, precancerous lesion of the colon. Changes in inflammatory gene expression in adenoma tissues may indicate early changes in the inflammatory state that can lead to cancer. We evaluated the expression levels of the 23 inflammatory-related genes in 18 pairs of colon adenomas and non-adenoma tissues. While there was limited power to detect differences in expression of these genes due to using a limited number of tissues, we found similar changes in genes expression in adenoma as compared to the colon cancer tissues (supplemental table 1). When using the Hong Kong cohort as a reference, expression changes in colon adenomas were consistent with colon cancer tissues for these inflammatory genes (Pearson R = 0.91; p<0.0001). Of the 10 genes significantly decreased in tumors, all showed decreased expression in adenomas and seven of these (IL-2, IL-10, IL-12a, CSF1, HLA-DPA1, HLA-DRA, and PRG1) were significantly reduced. Of the 8 genes significantly increased in tumors, all 8 were increased in adenomas and 6 of these (IL-8, IL-23a, IL-1a, IL-1b, FOXP3, and IL-17a) were significantly increased. Similar to colon cancer tissues, IL-8 showed the greatest increase and IL-2 showed the greatest decrease in adenoma tissues.
Inflammatory risk score (IRS) is associated with cancer-specific mortality
We evaluated the expression of these inflammatory genes for associations with cancer-specific mortality. Constructing a multi-gene signature using several genes with moderate associations can provide more accurate predictions than a model using a single gene. Therefore, we used univariate Cox regression to identify genes with moderate associations with prognosis following previously established methodologies (32). We randomly split the Hong Kong cohort into a training cohort (n = 57) and a test cohort (n = 56) (figure 2). These cohorts were similar in clinical characteristics including age at enrollment, gender and TNM staging. Based on univariate Cox regression on the training cohort, expression of PRG1, IL-10, CD68, IL-23a and IL-12a in noncancerous tissue and PRG1, ANXA1, IL-23a, IL-17a, FOXP3 and HLA-DRA in tumors were moderately associated with cancer-specific mortality (|Z-score| > 1.5; using criteria from (32)) (supplemental figure 1). These genes were selected to construct a multi-gene risk signature. Using the training cohort, multivariate Cox regression was performed on selected genes to develop risk models. The noncancerous risk model was [(0.855 * PRG1) + (0.720 * IL-10) + (0.458 * CD68) + (−0.494 * IL-23a) + (−0.635 * IL-12a)] = risk score. The tumor risk model was [(1.321 * PRG1) + (0.840 * ANXA1) + (0.123 * IL- 23a) + (0.484 * IL-17a) + (0.367 * FOXP3) + (−0.373 * HLA-DRA)] = risk score. Individuals having higher than median values for both models were classified as having high inflammatory risk score (IRS). All others were considered low. When evaluated separately, patients classified as high IRS had significantly worse cancer-specific mortality for the Hong Kong training cohort, the Hong Kong test cohort (p = 0.01, Kaplan Meier log rank) and NCI-Maryland validation cohort (p = 0.02, Kaplan Meier log rank) (Figure 3a).
Figure 2.
Strategy for building inflammatory risk scores. Genes were selected to be included in the risk score based on univariate Cox regression on the Hong Kong Training Cohort. Multivariate Cox regression on the training cohort was used to build the risk models. This model was then tested on the Hong Kong test and Maryland validation cohorts.
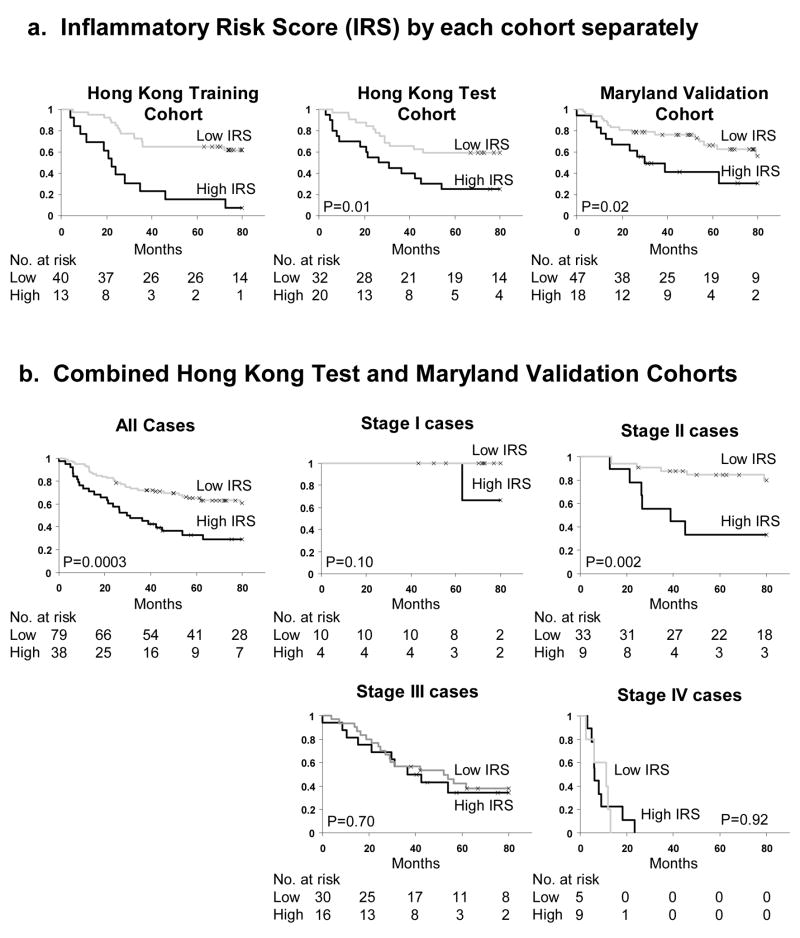
Figure 3.
Inflammatory risk score (IRS) is associated with cancer specific mortality in TNM stage II patients. (a) IRS in Hong Kong training, Hong Kong test and NCI-Maryland validation cohorts separately. (b) Combined analysis of Hong Kong test and NCI-Maryland validation cohorts, stratified by TNM stage. For 1 patient in the Maryland Cohort, it was unclear if that patient had stage III or stage IV colon cancer and therefore is removed from analyses stratifying by TNM stage.
To evaluate the potential use for IRS as a biomarker, we performed a stratified analysis by TNM staging. For these analyses, the Hong Kong training cohort is excluded to prevent over fitting. The Hong Kong test and NCI-Maryland validation cohorts were combined. IRS was associated with TNM stage (p = 0.03, Fisher’s exact test). Patients with more advanced TNM stage were more likely to be classified as high IRS. Four of 14 (29%) stage I, 9 of 42 (21%) stage II, 16 of 46 (35%) stage III, and 9 of 14 (64%) stage IV cases were classified as high IRS. High IRS was associated with poor cancer-specific mortality for all patients (p = 0.0003, Kaplan Meier log rank) (Figure 3b). When stratified by TNM stage, IRS was associated with cancer-specific mortality in stage II cases (p = 0.002, Kaplan Meier log rank) (figure 3b). IRS was not associated with prognosis in stage I, stage III, or stage IV patients.
We were unable to analyze associations with therapeutic outcome. Of the 34 stage II patients for which we had information about receipt of adjuvant therapy, only 7 received it. Only one of these seven patients was classified as high IRS. Therefore we did not have sufficient power to analyze associations between IRS and therapeutic outcome.
miR-21 expression is associated with the expression of IL-6, IL-8, IL-10, IL-12a and NOS2a
Expression of miR-21 was available for these samples from a previous study (28). miR-21 has previously been shown to be associated with inflammation. Therefore, we used linear regression to examine the associations of miR-21 expression with inflammatory genes in these tissues. This was evaluated in noncancerous tissues, tumor tissues, and then a combination of all tissues adjusting for tumor status (Supplemental table 2). In the combined model, expression of IL-6 (p<0.0005), IL-8 (p<0.0005) and IL-10 (p=0.002) were positively associated and IL-12a (p<0.0005) and NOS2a (p=0.0025) were negatively associated with miR-21 expression. Only IL-6 and IL-12a expression was statistically significant (p<0.05) in both the tumor and noncancerous tissues, separately.
IRS and miR-21 expression are independently associated with cancer-specific mortality, including stage II patients
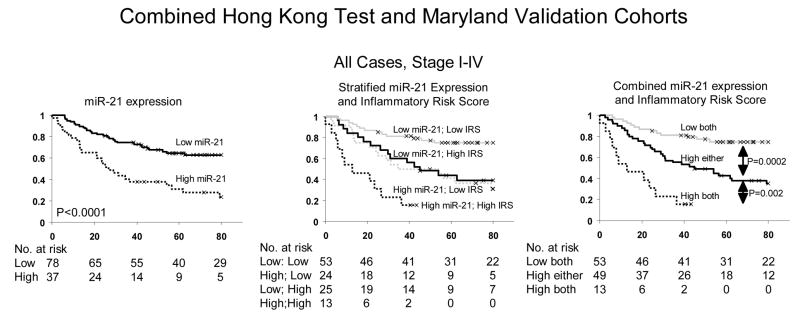
We previously reported that high miR-21 expression in tumors was associated with poor prognosis in colon adenocarcinoma (28). That study utilized the same patients as the current study and provides an opportunity to combine miR-21 and IRS to determine if together they have improved prognostic utility. High miR-21 expression was defined in our previous publication where the highest tertile (> 3.3-fold higher than average noncancerous tissue) is defined as high (28). Survival information for the NCI-Maryland cohort was updated from that study to include an additional 12 months of available survival information. Consistent with our previous report, high miR-21 expression is associated with cancer-specific mortality using all cases (p < 0.0001, Kaplan Meier log rank) or stage II cases (p = 0.006, Kaplan Meier log rank) (Figure 4). Due to the association between IL-6 and miR-21 expression, we investigated if combining IL-6 and miR-21 expression data into survival models would alter the association between miR-21and cancer-specific mortality and found that it did not (data not shown).
Figure 4.
Combined Inflammatory Risk Score (IRS) and miR-21 expression predict colon cancer specific mortality better than either alone. All patients from the Hong Kong test and NCI-Maryland validation cohorts are analyzed. Left is stratified by miR-21 expression; middle is stratified by IRS and miR-21 expression; right is combined IRS and miR-21 expression. miR-21 expression data was available for 115 of 117 patients and only those patients were used for this analysis. The Hong Kong training cohort is also excluded from this analysis.
While IRS and miR-21 expression were each associated with prognosis, they were not associated with one another (p = 0.83, Fisher’s exact). Therefore, combination of these biomarkers may identify high risk patients that would be misclassified by a single endpoint. We performed a stratified analysis of miR-21 and IRS (Figure 3). Patients with low miR-21 expression and low IRS had the best prognosis. Patients with high miR-21/low IRS or low miR-21/high IRS had an intermediate prognosis. Patients with high miR-21/high IRS had the worst prognosis. This was true when observing all cases or stage II cases alone. Upon combining intermediate groups, patients classified as high for either miR-21 or IRS score had significantly worse cancer-specific mortality than those classified as low miR-21/low IRS for all cases (p = 0.0002, Kaplan Meier log rank) or stage II cases (p = 0.002, Kaplan Meier log rank). Patients classified as high for both miR-21 and IRS had worse survival than patients classified as high for either using all cases (p = 0.002, Kaplan Meier log rank) or stage II alone (p = 0.02, Kaplan Meier log rank). Only two stage II patients were classified as high IRS and high miR-21. Therefore, one should be cautious interpreting the poor outcome of these stage II patients and future studies will explore this association.
Univariate Cox regression analysis for all cases found high IRS (Hazard Ratio [HR] = 2.4, 95% Confidence Interval [CI] = 1.4 to 4.2), high miR-21 (HR = 3.0, 95% CI = 1.7 to 5.1) and TNM staging (HR = 4.7, 95% CI = 2.5 to 8.8) were each associated with poor prognosis (table 2). Multivariate analyses demonstrated that both high IRS (HR = 2.2, 95% CI = 1.3 to 3.8) and high miR-21 (HR = 3.0, 95% CI = 1.7 to 5.2) were independent of one another and TNM staging. Additionally, the multivariate model including IRS, TNM staging and miR-21 performed significantly better than the model without miR-21 (p < 0.001; likelihood ratio test). When restricting the analysis to stage II cases, univariate analyses demonstrated high IRS (HR = 5.4, 95% CI = 1.7 to 17.2) and high miR-21 (HR = 4.8, 95% CI = 1.4 to 16.1) were each associated with poor prognosis. Multivariate analysis demonstrated that high IRS (HR = 7.5, 95% CI = 2.2 to 25.6) and high miR-21 (HR = 6.5, 95% CI = 1.9 to 21.9) were each associated with prognosis independent of one another. A multivariate model including both IRS and miR-21 in stage II patients performed significantly better than a model including only IRS (p = 0.004; likelihood ratio test). Therefore, IRS and miR-21 expression may be used together as a prognostic biomarker for stage II colon adenocarcinoma.
Table 2.
Cox regression of Inflammatory Risk Score (IRS) and miR-21 expression with cancer-specific mortality on combined Hong Kong test cohort and Maryland Validation cohort.
| All cases, regardless of TNM stage | ||||
|---|---|---|---|---|
| Univariate analysisa | Multivariate analysisb | |||
| Variable (comparison/referent) | HR (95% CI) | P-value | HR (95% CI) | P-value |
| IRS (high/low) | 2.4 (1.4 – 4.2) | 0.001 | 2.2 (1.3 – 3.8) | 0.005 |
| miR-21 expression (High/low) | 3.0 (1.7 – 5.1) | <0.0005 | 3.0 (1.7 – 5.2) | <0.0005 |
| Tumor Stage (III-IV/I-II) | 4.7 (2.5 – 8.8) | <0.0005 | 4.0 (2.1 – 7.5) | <0.0005 |
| Age (≥ 50/< 50) | 1.1 (0.6 – 2.1) | 0.82 | ||
| Gender (male/female) | 1.9 (1.0 – 3.5) | 0.06 | ||
| Tumor Location (Proximal/Distal) | 0.8 - (0.4 – 1.7) | 0.58 | ||
|
| ||||
| Stage II cases, adjusted for cohort membership | ||||
| Univariate analysisa | Multivariate analysisb | |||
| Variable (comparison/referent) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|
| ||||
| IRS (high/low) | 5.4 (1.7 – 17.2) | 0.005 | 7.5 (2.2 – 25.6) | 0.001 |
| miR-21 expression (High/low) | 4.8 (1.4 – 16.1) | 0.01 | 6.5 (1.9 – 21.9) | 0.002 |
| Age (≥ 50/< 50) | 2.9 (0.4 – 24.2) | 0.31 | ||
| Gender (male/female) | 1.4 (0.4 – 4.7) | 0.57 | ||
| Tumor Location (Proximal/Distal) | 0.4 (0.1 – 1.7) | 0.20 | ||
Univariate analysis is adjusted for cohort membership only.
Multivariate analysis is adjusted for cohort membership, IRS, miR-21 expression and (where appropriate) TNM stage. Multivariate analysis used stepwise addition and removal of clinical covariates found to be associated with survival in univariate models (P < 0.10) and final models include only those covariates that were significantly associated with survival (Wald statistic, P < 0.05). HR = Hazard Ratio. miR-21 measurements were available for 115 of 117 patients, including all 42 stage II patients, and only those patients are included in multivariate analyses.
Discussion
We find systematic changes in inflammatory gene expression in colon tumors. Of the 8 inflammatory genes consistently increased in tumors, seven (IL-8, IL-23a, IL-1a, IL-1b, IL-17a, INFγ and IL-6) are proinflammatory cytokines and the other is FOXP3, a marker for regulatory T cells. These results are consistent with other reports evaluating their expression in colon cancer (37). Therefore, there are predictable changes in inflammatory gene expression in colon tumors, consistent for a role for these genes in carcinogenesis. We found similar changes in gene expression in colon adenomas. This indicates that changes in the inflammatory state may be an early event in colon carcinogenesis.
Expression of inflammatory genes was associated with miR-21 expression. The association of IL-6 and IL-12a expression was statistically significant in both the tumor and noncancerous tissues, separately. IL-6 is thought to drive the expression of miR-21 in a STAT3 dependent mechanism (31). Our results are consistent with that model and provide evidence that this mechanism may be relevant to colon cancer. There is also a predicted binding site for miR-21 in the 3′ untranslated region of IL-12a as indicated by Targetscan 5.0 (38) and miRanda (39). IL-12a has a negative correlation with miR-21, which is consistent with a pattern for a miR-21 target. Based on this finding, mechanistic studies should be performed to determine if IL-12a is a target of miR-21. The interaction between miR-21 and inflammatory genes may play an important role in colon carcinogenesis. While the associations between miR-21, IL-6, IL-12a were significant, the regression models indicated that much of the variability was explained by these models. This indicates that other mechanisms for gene regulation are contributing the expression of these genes. For example, the miR-21 promoter contains putative binding sites for the transcription factors AP-1, Ets/PU.1, SRF, TP53, C/EBPα, and STAT3 (40) and miR-21 expression can also be influenced by EGFR activity (41). Therefore it is likely that the expression of miR-21 is influenced by many of these and other factors in the context of cancer.
The expression of inflammatory genes is altered in colon adenocarcinoma. While this study doesn’t address the causal relationship between changes in inflammatory gene expression and carcinogenesis, these changes are consistent with published work demonstrating a potential causal relationship between altered expression of inflammatory genes and carcinogenesis. For example, IL-8 demonstrated the highest fold-increase in both colon tumors and adenomas in our study. Previous studies have demonstrated IL-8 to be a pro-inflammatory chemokine that is expressed at elevated levels in tumors (42). IL-8 expression has been shown to enhance cell proliferation, cell survival and angiogenesis through induction of the multiple signaling pathways. Conversely, IL-2 demonstrated the largest reduction in tumors and adenomas. IL-2 expression had been found to inhibit tumor growth in vivo and high dose IL-2 therapy has demonstrated some promise to reduce tumor burden in patients (43) demonstrating a causal role between aberrant expression of IL-2 and cancer.
Expression of inflammatory genes in tumors and the surrounding noncancerous tissues are associated with prognosis in colon adenocarcinoma. This cooperation of tumor and noncancerous expression of inflammatory genes was observed in our previous investigation of lung adenocarcinoma (22). Higher expression of IL-10 in noncancerous tissues was associated with worse survival in that study and the current study. IL-10 is an anti-inflammatory cytokine that can suppress cell-mediated immunity (44). Therefore, elevated IL-10 in noncancerous tissue may create an inflammatory environment primed for metastasis and disease progression.
High levels of IL-23a and IL-12a in the noncancerous tissue were associated with improved survival. Both are members of the IL-12 family of proinflammatory cytokines (45). IL-12 activity is important for host resistance to tumors (46), therefore high levels of IL-12 in the tumor macroenvironment may lead to resistance of tumor progression and metastasis through induction of IFN-γ and activation of Natural Killer cells and cytotoxic T cells. In contrast, elevated IL-23a and IL-17a in cancerous tissues were associated with worse survival and may promote a microenvironment that suppresses any host anti-tumor response. IL-23a can stimulate Th17 cells to increase the production of IL-17a and overexpression of IL-17a in cervical cancer (47), non-small cell lung cancer (48), or fibrosarcoma (49) cell lines increases tumor formation and/or tumor growth in xenograft mouse models. These cytokines are associated with a Th17 response. Therefore, a Th17 response in tumors may create a favorable condition for tumor progression.
There are limitations to the current study. First, patients with mucinous or adenosquamous histologies were excluded from this study; therefore IRS may not be applicable to these patients. Additionally, the IRS was built using relatively few cases (n = 53). Developing molecular signatures of these genes on larger cohorts may strengthen the accuracy and precision of this biomarker, although the validation of this biomarker in two independent cohorts demonstrates its potential clinical utility. It will be important to begin exploring the relationship between IRS and other clinical covariates, such as microsatellite instability status, p53 mutational status or K-ras mutational status, to determine if a combination of these markers can provide more clinically useful information. It will also be useful to perform immuno-histochemistry on patient samples to determine localization patterns of these inflammatory genes to gain insights into the cell types responsible for this gene signature. This will provide mechanistic insights as to how the combination of these genes may contribute to the worse prognosis in IRS high patients.
We found the association between IRS and survival to be strongest in TNM stage II patients. The reason for this association in stage II patients and not stage III/IV patients is unclear. One possibility is that high IRS is associated with a favorable inflammatory environment for metastasis. This is consistent in that we see more advanced staged patients are more likely to be classified as high IRS. In this context, it may be a useful marker only in stage II patients, for which metastasis is not yet detectable by current clinical methods. In stage III or IV patients, metastasis is evident and therefore an IRS score to predict metastasis is not meaningful.
Cancer immunotherapy is a promising field of research for colon cancer (50). As in any therapy, successful stratification of patients into groups that are more or less likely to respond will increase the chances of developing successful immunotherapies. IRS is based on the expression of inflammatory genes and the expression of these genes is likely to be correlated with the current state of the immune system. It is possible that IRS may be associated with a patient’s response to immunotherapy. While future investigation of this is needed, there is potential that IRS, or a similar inflammatory gene biomarker, may be able to identify patients more or less likely to respond to immunotherapy.
There is a need for better ways of diagnosing early stage colon cancer patients with undetectable micrometastases. Therefore, we propose that a subset of stage II patients would benefit from therapeutic intervention as their disease will likely progress; but for others, therapeutic intervention unnecessarily harms quality of life and continued screening would be sufficient. We found IRS was significantly associated with prognosis in stage II patients. Previously, we identified miR-21 as a prognostic biomarker for stage II patients (28). The combination of IRS and miR-21 expression was a better predictor of prognosis than either alone. Therefore, IRS and miR-21, alone or in combination, has potential to help diagnose stage II patients and assist in choosing treatment options. Prospective studies to evaluate this potential are warranted.
Supplementary Material
Acknowledgments
We thank Drs. Howard Young and Giorgio Trinchieri for critical reading of the manuscript and thoughtful suggestions and Drs. Xin Wang and Stefan Ambs (National Cancer Institute) for invaluable discussion. We are grateful to Raymond Jones, Audrey Salabes, and John Cottrell of the University of Maryland Medical Center and the Surgery and Pathology Departments at UMD Hospital, Baltimore Veterans Administration Medical Center, Sinai Hospital, Union Memorial Hospital, St. Agnes Hospital, Northwest Hospital Center, and Mercy Medical Center for contributions to this study. This research was supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health. AJS was supported by the Cancer Prevention Fellowship Program, Office of the Director, National Cancer Institute, National Institutes of Health. GHN and JEH were supported by the Howard Hughes Medical Institute Grant for Graduate Medical Education.
References
- 1.Aaltonen LA, Hamilton SR. Pathology and genetics of tumours of the digestive system. Lyon Oxford: IARC Press; Oxford University Press; 2000. World Health Organization., International Agency for Research on Cancer. (distributor) [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Chua YJ, Zalcberg JR. Progress and challenges in the adjuvant treatment of stage II and III colon cancers. Expert Rev Anticancer Ther. 2008;8:595–604. doi: 10.1586/14737140.8.4.595. [DOI] [PubMed] [Google Scholar]
- 4.Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134:1296–310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–80. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 6.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–8. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 7.Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357–9. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 8.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–52. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 11.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–83. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 13.Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987;1:1303–6. doi: 10.1016/s0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 15.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 16.Landi S, Bottari F, Gemignani F, et al. Interleukin-4 and interleukin-4 receptor polymorphisms and colorectal cancer risk. Eur J Cancer. 2007;43:762–8. doi: 10.1016/j.ejca.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Lurje G, Zhang W, Schultheis AM, et al. Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann Oncol. 2008 doi: 10.1093/annonc/mdn368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkening S, Tavelin B, Canzian F, et al. Interleukin promoter polymorphisms and prognosis in colorectal cancer. Carcinogenesis. 2008;29:1202–6. doi: 10.1093/carcin/bgn101. [DOI] [PubMed] [Google Scholar]
- 19.Pages F, Berger A, Henglein B, et al. Modulation of interleukin-18 expression in human colon carcinoma: consequences for tumor immune surveillance. Int J Cancer. 1999;84:326–30. doi: 10.1002/(sici)1097-0215(19990621)84:3<326::aid-ijc22>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Berghella AM, Contasta I, Pellegrini P, Del Beato T, Adorno D. Are immunological mechanisms involved in colon cancer and are they possible markers for biotherapy improvement? Cancer Biother Radiopharm. 2006;21:468–87. doi: 10.1089/cbr.2006.21.468. [DOI] [PubMed] [Google Scholar]
- 21.Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Seike M, Yanaihara N, Bowman ED, et al. Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J Natl Cancer Inst. 2007;99:1257–69. doi: 10.1093/jnci/djm083. [DOI] [PubMed] [Google Scholar]
- 23.Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 24.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–98. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 27.He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–36. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moschos SA, Williams AE, Perry MM, Birrell MA, Belvisi MG, Lindsay MA. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics. 2007;8:240. doi: 10.1186/1471-2164-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Neilson JR, Kumar P, et al. miRNA profiling of naive, effector and memory CD8 T cells. PLoS ONE. 2007;2:e1020. doi: 10.1371/journal.pone.0001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loffler D, Brocke-Heidrich K, Pfeifer G, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–3. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 32.Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–37. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 33.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 34.Hata K, Andoh A, Shimada M, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035–44. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 35.Lo CH, Lee SC, Wu PY, et al. Antitumor and antimetastatic activity of IL-23. J Immunol. 2003;171:600–7. doi: 10.4049/jimmunol.171.2.600. [DOI] [PubMed] [Google Scholar]
- 36.Hofseth LJ, Saito S, Hussain SP, et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci U S A. 2003;100:143–8. doi: 10.1073/pnas.0237083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Gouvello S, Bastuji-Garin S, Aloulou N, et al. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut. 2008;57:772–9. doi: 10.1136/gut.2007.123794. [DOI] [PubMed] [Google Scholar]
- 38.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–53. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita S, Ito T, Mizutani T, et al. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol. 2008;378:492–504. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Seike M, Goto A, Okano T, et al. MiR-21 is an EGFR-related anti-apoptotic factor in lung cancer from never-smokers. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0905234106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 43.McDermott DF. The application of high-dose interleukin-2 for metastatic renal cell carcinoma. Med Oncol. 2009;26 (Suppl 1):13–7. doi: 10.1007/s12032-008-9152-1. [DOI] [PubMed] [Google Scholar]
- 44.O’Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–31. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 45.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 46.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 47.Tartour E, Fossiez F, Joyeux I, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–704. [PubMed] [Google Scholar]
- 48.Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–89. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 49.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 50.Weiner LM. Cancer immunotherapy--the endgame begins. N Engl J Med. 2008;358:2664–5. doi: 10.1056/NEJMp0803663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.