Abstract
We investigated potential therapeutic effects of sphingosine-1-phosphate (S1P) receptor modulators FTY720 (fingolimod) and selective S1P1 agonist SEW2871 on a spontaneous autoimmune polyneuropathy (SAP) when given orally at 7 mo (anticipated disease onset) for 4 weeks. Clinical severity, electrophysiologic and histological findings were ameliorated in mice treated with 1 mg/kg of FTY720. Subsequent studies showed that SEW2871 was also effective in halting the progression of SAP, which was accompanied by decreased proliferative and cytokine responses to myelin protein zero (P0), and an increase in regulatory T cells. We conclude that S1P receptor modulators may play a therapeutic role in autoimmune neuropathies.
Keywords: CIDP, Guillain-Barré syndrome, S1P receptors, NOD mice, FTY720, SEW2871
1. INTRODUCTION
Fingolimod (FTY720) is an oral S1P receptor modulator in phase III clinical trials for the treatment of multiple sclerosis (MS). A decrease in MRI and clinical disease activity was observed in a phase II study of FTY720 in MS (Kappos et al., 2006). FTY720 is highly efficacious in the MS model of experimental autoimmune encephalomyelitis (EAE) (Balatoni et al., 2007; Fujino et al., 2003; Webb et al., 2004). The beneficial effect of FTY720 in EAE is attributed to lymphocyte sequestration in secondary lymphoid organs, although it also enhances the expression of neuroregenerative genes in the CNS (Chiba et al., 1998; Foster et al., 2008; Graler and Goetzl, 2004; Mandala et al., 2002; Pinschewer et al., 2000). In addition, dynamic effects of FTY720 and related compounds in oligodendroglial lineage cells have been demonstrated (Coelho et al., 2007; Jaillard et al., 2005; Jung et al., 2007; Miron et al., 2008).
Not all treatments effective in MS also work consistently for autoimmune neuropathies such as chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). For example, interferon beta (IFN-β) while effective in MS, has had contradictory outcome in CIDP (Hadden et al., 1999; Vallat et al., 2003). There is one report describing the beneficial effect of FTY720 (intraperitoneal injection) in P2-induced experimental allergic neuritis (EAN) in Lewis rats (Zhang et al., 2008). FTY720 is converted in vivo to FTY720-phosphate (FTY720P), which is a potent agonist of four of the five known S1P receptors (S1P1, S1P3, S1P4 and S1P5) (Brinkmann et al., 2002). It is unclear whether selective S1P1 modulators such as SEW2871 will have a similar therapeutic effect as FTY720 on EAN or EAE. T lymphocyte migration towards S1P gradient is highly dependent on S1P1 receptors, whereas dendritic cell migration is mediated primarily by S1P3 receptors (Allende et al., 2004; Maeda et al., 2007; Matloubian et al., 2004).
The goal of this study is to investigate whether FTY720 and selective S1P1 agonist SEW2871 exert a therapeutic effect in a model of spontaneous autoimmune polyneuropathy (SAP), the B7-2 knockout (KO) non-obese diabetic (NOD) mice. NOD mice are susceptible to the development of autoimmune diseases such as type 1 diabetes, thyroiditis, sialitis, or neuropathy, but disease manifestations are strongly influenced by co-stimulatory molecules and cytokine milieu (Salomon et al, 2001; Setoguchi et al., 2005). Elimination of a co-stimulatory molecule B7-2 in female NOD mice prevents type 1 diabetes and sialitis, but triggers the development of limb weakness at 6–7 mo, which progresses to quadriparesis by 8–9 mo. The incidence of neuropathy is lower in males compared to female B7-2 KO NOD mice. Unlike EAN, SAP mice do not undergo spontaneous recovery. Similar to human CIDP, electrophysiological findings in SAP are classical for a demyelinating process with superimposed axonal loss. Histological evaluation reveals the presence of inflammatory infiltrates (CD4+, CD8+ T cells, dendritic cells) in dorsal root ganglia and sciatic nerves but not in the CNS (Salomon et al., 2001). We and other investigators found that SAP is a Th1-mediated disease and that myelin protein zero (P0) is one of the autoantigens targeted by T cells in this model (Bour-Jordan et al., 2005; Kim et al., 2008; Louvet et al., 2009). In this study, we examined the effect of S1P receptor modulators on disease severity in SAP as determined by clinical assessment, electrophysiological, and histological evaluations. We also investigated the effect of these compounds at the level of the blood nerve barrier (BNB), on the autoreactivity to myelin P0, and on Schwann cell viability.
2. MATERIALS AND METHODS
Animals and Reagents
All animal use procedures were conducted in strict accordance to the National Institutes of Health and University of Chicago institutional guidelines. Female B7-2 knockout (KO) NOD mice (7 mo old) were used for our studies. FTY720 (Novartis Pharma AG, Basel, Switzerland) was dissolved in distilled water. The drug was freshly prepared and given orally once daily by gavage at 0.3 to 1 mg/kg body weight. SEW2871 (Cayman chemical, MI, USA) was dissolved in dimethyl sulfoxide (DMSO) and diluted with water. SEW2871 was given orally twice a day by gavage at 10 mg/kg body weight.
Clinical and electrophysiology assessment
For clinical assessment, the following nominal scale was used: 0- normal; 1- flaccid tail; 2- mild paraparesis; 3- severe paraparesis; 4- tetraparesis; 5- death. Grip strength testing consisted of five separate measurements in each of two trials per session with a grip strength meter (Columbus Instruments, OH). Results of two trials were averaged for each mouse per session. After the last grip strength measurement, electrophysiologic studies of sciatic nerves were performed as described previously (Kim et al.; Salomon et al., 2001). Briefly, recording needle electrodes were placed subcutaneously in the footpad. Right and left sciatic nerves were stimulated distally at the ankle and proximally at the sciatic notch using needle electrodes. Latencies, conduction velocities and peak to peak amplitudes were measured. Temperature was maintained at ≥ 30 °C during the recording. Values obtained from right and left sciatic nerves were averaged for each animal, which was then used to calculate mean ± SEM (n = number of animals).
Histological studies
Segments of the sciatic nerves were fixed in 0.5–4% paraformaldehyde, embedded in OCT compound, and snap frozen. Longitudinal sections (10 µm slices) of sciatic nerves were stained with haematoxylin–eosin (H&E), and inflammatory cells were counted at 20x magnification with tissue areas measured by image analysis. At least 3 sections were analyzed per nerve. For semithin /epon sections, nerve tissues were obtained at high sciatic levels prior to bifurcation, fixed in 4% PFA, postfixed in 1% osmium tetroxide for 1–2 hr, embedded in plastic resin and stained with toluidine blue. The number of myelinated fibers over a total area of 0.09 mm2 was counted with results expressed as % loss of myelinated fibers compared to unaffected nerves from preclinical mice. Sections were also scored using a semi-quantitative grading system: 0, normal; 1, <25%; 2, 25–50%; 3, 51–75%; and 4 with >75% demyelinated or thinly myelinated fibers. All histological analysis was performed in a blinded fashion.
Blood nerve barrier permeability
Evans blue albumin (EBA) solution was prepared as a mixture of 5% bovine albumin (Sigma, MO, USA) and 1 % Evans blue dye (Sigma, MO, USA) in distilled water that was filtered in a PD-10 desalting column (GE healthcare, NJ, USA). B7-2 KO NOD mice were given FTY720, SEW2871 or PBS via tail vein injection. Six hours post-injection, anesthetized mice were infused with 100 µl of EBA solution through the tail vein. After 30 min, sciatic nerves were harvested and snap frozen. Longitudinal sections (10 µm) of sciatic nerves were placed on microscope slides with coverslips mounted using 50 % glycerin in water. Sections were visualized using a fluorescence microscope at 10 × magnification. Integrated fluorescence intensity was analyzed using the NIH Image J program. For each nerve, at least 2 areas / section for 3 sections were examined.
Splenocyte culture, proliferation and cytokine production assays
For proliferation assay, splenocytes were cultured at a density of 5 × 105 cells/well in HL-1™ medium plus non-essential amino acids (Biowhittaker), L-glutamine (2 mM), sodium pyruvate (1 mM) and β-mercaptopurine (55 µM) in flat-bottom 96 well plates, then cells were stimulated with OVA peptide (20 µg/ml), P0 peptide (20 µg/ml) or P2 peptide (20 µg/ml). On day 3, these cultures were added for 16 h with 1 µCi methyl-[3H] thymidine. A stimulation index was defined by cpm in the presence of antigen divided by cpm in the absence of the antigen. Supernatants collected at 48 hr from replicate cultures were assayed for IFN-γ (Endogen, Rockford, IL), IL-2, IL-10 (BD Biosciences, San Diego, CA) and IL-17 (eBioscience, San Diego, CA) using ELISA Minikits. The following peptides were used as antigens: 1) P0 (180–199) (SSKRGRQTPVLYAMLDHSRS); 2) P2 (53–78) (TESPFKNTEISFKLGQEFEETTADNR); 3) OVA (323–339) (ISQAVHAAHAEINEAGR) (GenScript Corporation, Piscataway, NJ).
Schwann cell (SC) culture and cell viability assay
Primary SCs were prepared from P3–4 rats as previously described (Iwase et al., 2005; Nagano et al., 2001). After treatment with 10 µM cytosine arabinoside (Ara C) for 48 h to eliminate proliferating fibroblasts, SCs on polylysine-coated dishes were incubated with proliferating medium: DMEM containing 10% FBS, 2 µM forskolin (fsk) and 20 µg/ml bovine pituitary extract (BPE). SCs in passages 2 and 3 were used for experiments, after removal of fsk and BPE from culture medium for at least 3 days prior to experiments. For phosphorylation studies, SCs were incubated in serum-free medium (SFM) plus insulin (5 µg/ml), transferrin (5 µg/ml) and selenium (5 ng/ml) for 24 hr prior to exposure to FTY720P for 15 min. For cell viability studies, trypan blue exclusion test was used to evaluate cell death upon serum withdrawal for 3 d in the presence or absence of test agents. Detached SCs (pre and post-trypsin treatment) were centrifuged, resuspended in PBS, stained with 0.4% trypan blue and counted using a hematocytometer.
Real-time PCR
The total RNA was isolated using a Trizol reagent (Invitrogen), followed by Qiagen column purification. Real-time PCR studies were performed as described previously (Jung et al., 2007). The following primers were used: S1P1- forward (5’-CTGACCTTCCGCAAGAACATCT -3’) and reverse (5’-CTTCAGCAAGGCCAGAGACTTC-3’); S1P2- forward (5’-ACATTTCTGGAGGGCAACAC-3’) and reverse (5’-TGGTCCCCA CAGTCACAGTA-3’); S1P3- forward (5’-AGAACGAGAGCCTGTTTCCA-3’) and reverse (5’-CAG CTTCCCCACGTAATCAT-3’); S1P4-forward (5’-GGACTTCGAGGTCACTCAGC-3’) and reverse (5’-CTGCCAAACATTCATCATGG-3’); S1P5-forward (5’- CTCTAGAGCGCCACCTTACCA-3’) and reverse (5’-CCCAGCAGCAGCGACAA-3’); GAPDH-forward (5’-TTC ACCACCATGGAGAAGGC-3’) and reverse (5’-GGCATGGACTGTGGTCATGA-3’).
Western blot analysis
Cell lysis, protein count, SDS PAGE and Western blot analysis were performed as described previously (Iwase et al., 2005; Jung et al., 2007). Sample proteins (30 µg per lane) were resolved by 15% Tris-HCl gel electrophoresis (BIO-RAD, CA, USA) and blotted to polyvinylidene difluoride membranes (Millipore, MA, USA). The following primary antibodies were used in this study; anti-pERK1/2 (1:500), anti-pAkt (1:500), anti-ERK2 (1:500) and anti-Akt (1:500). All experiments were repeated at least three times with similar results. Representative data are shown. Results are expressed as percentage of control values by normalization of relative density of pERK1/2 / total ERK2, and pAkt / total Akt to those from untreated conditions of each experiment.
Statistical analysis
Clinical and demyelination scores were analyzed by nonparametric methods Kruskal-Wallis or Mann-Whitney tests, as specified in the text or figure legends. Results from grip strength measurement, quantitative histologic analysis, and electrophysiology are expressed as mean ± SEM. Unless otherwise specified, statistical significance was determined by analysis of variance (ANOVA) followed by Student’s t test and the Bonferroni method for multiple group experiments.
3. RESULTS
Effect of FTY720 on disease severity in SAP
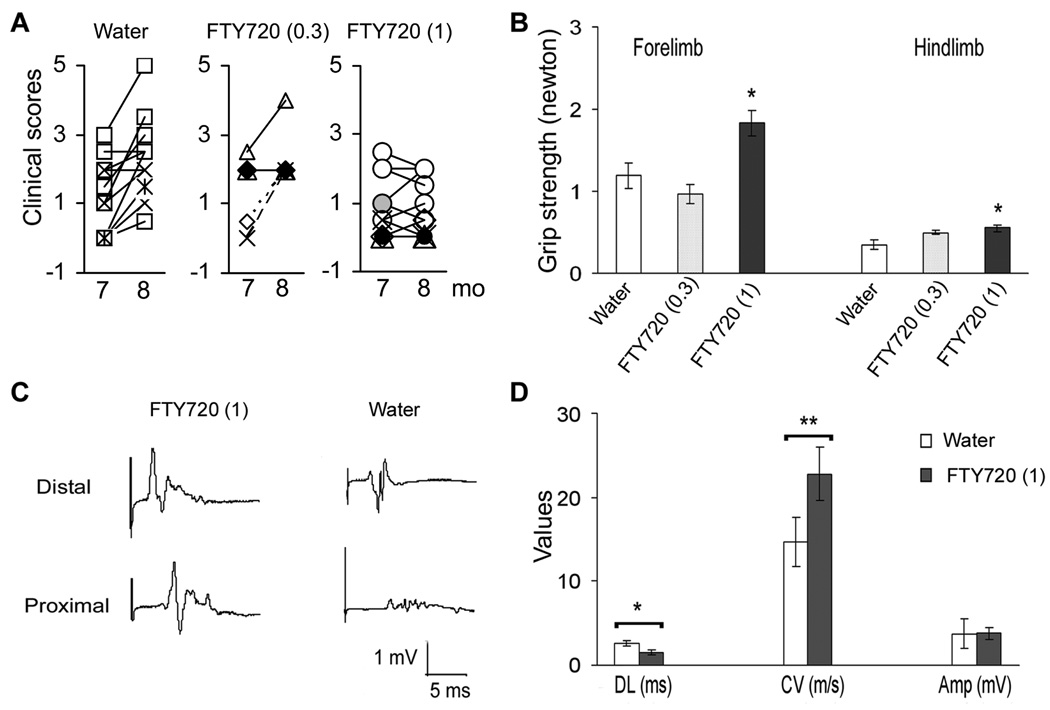
To examine possible therapeutic effects of FTY720 in SAP, animals were treated with FTY720 (0.3 mg/kg) (n = 5), FTY720 (1 mg/kg) (n = 10) or water (control, n = 11) orally once daily at 7 mo of age (anticipated disease onset) for 4 weeks. At 7 mo (before treatment), there was no difference in median clinical scores amongst 3 groups. As shown in Fig. 1A, animals treated with water or FTY720 (0.3 mg/kg) exhibited worsening of clinical scores at 8 mo. By comparison, disease progression was significantly inhibited in animals treated with FTY720 (1 mg/kg), which was also reflected in grip strength measurements (Fig. 1B). Subsequent analysis was focused only in the FTY720 (1 mg/kg) group.
Figure 1.
Suppression of SAP in B7-2 deficient NOD mice by FTY720. Animals were divided into 3 groups: (1) water (n = 11); (2) FTY720 at 0.3 mg/kg (n = 5); and (3) FTY720 at 1.0 mg/kg (n = 10). Daily oral administration was initiated at 7 months of age and continued for 4 weeks. A. Clinical scores. The median clinical score at 8 mo was 2.5 for water-treated group, 2.0 for FTY720 (0.3 mg/kg) and 0.75 for FTY720 (1 mg/kg); * p <0.0006 for FTY720 (1 mg/kg) vs water (Mann-Whitney test). B. Hindlimb and forelimb grip strength measurements. *p <0.01 for FTY720 (1 mg/kg) vs water. C, D. Examples and summary of sciatic nerve electrophysiology. Distal latency (DL), conduction velocity (CV), and amplitude (Amp) of sciatic compound muscle action potentials (CMAPs) were measured [n =7 mice for FTY720 (1 mg/kg) group, and n =6 for water-treated ones; *p <0.02; **p <0.01]. Subsequent analysis was carried out in FTY720 (1 mg/kg) group only.
Electrophysiology and histologic studies were performed in a subset of study animals at 8 mo. Our previous studies in SAP mice (7–8 mo old) revealed that sciatic motor responses had prolonged distal latencies, slowed conduction velocities, and decreased amplitudes compared to those recorded from age-matched wild type NOD mice (Salomon et al., 2001). These observations were confirmed in this study. Distal latency was 3.1 ± 0.5 ms; conduction velocity was 15.5 ± 3.3 m/s; and amplitude was 1.46 ± 0.36 mV in untreated 7 mo old SAP mice (n = 8). Values obtained from the water-treated group at 8 mo were similar to those recorded from untreated mice. As shown in Fig. 1C &D, there was a significant improvement in the distal latency and conduction velocity but not in the amplitude of the motor response in the FTY720 (1 mg/kg) group (n = 7) compared to water-treated group (n = 6).
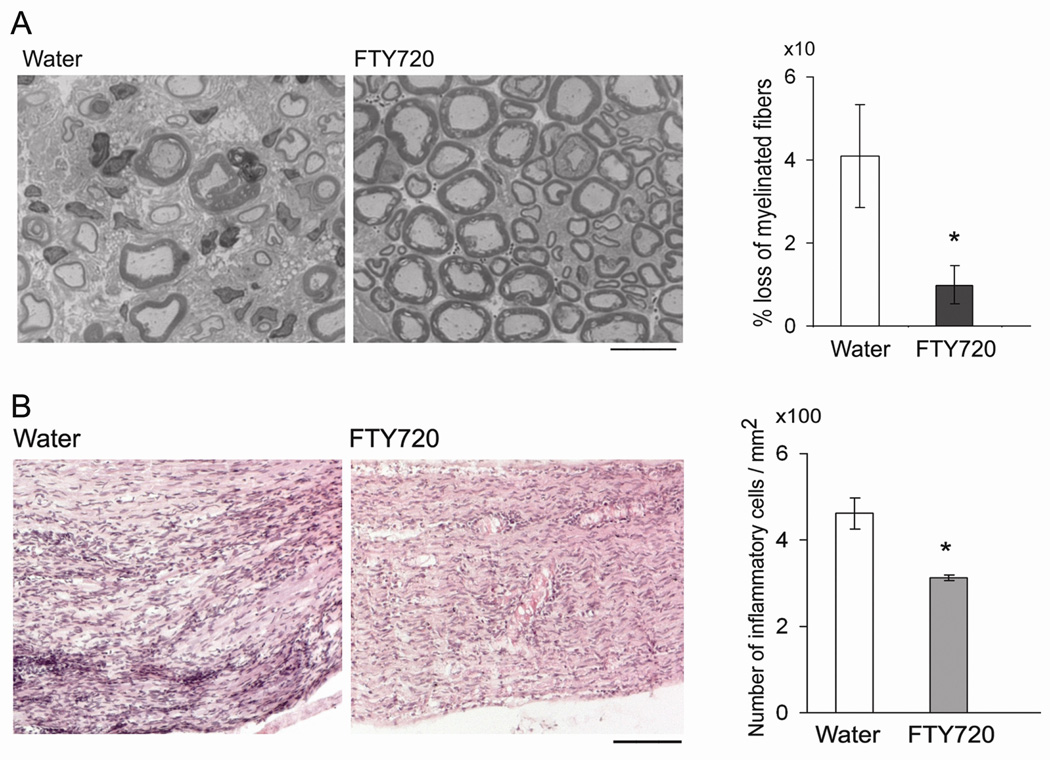
Histological analysis by a blinded observer revealed a milder loss of myelinated fibers and lesser degree of demyelination in epon sections from FTY720-treated mice compared to those from water-treated group (Fig. 2A). The total number of myelinated axons/mm2 was 10533 ± 1992 in sections from water-treated mice, and 14996 ± 1663 in sections from FTY720-treated group. Compared to unaffected nerves from preclinical mice, there was a 40.8 ± 12.8% (n = 6) decrease in myelinated fibers in water-treated mice, and 10 ± 4.7% (n = 7) decrease in FTY720-treated group (p <0.015 by t test). In addition to the loss of myelinated axons, there were more demyelinated or thinly myelinated fibers in water-treated ones compared to FTY720-treated group. The median demyelination score was 2 for water vs 0.5 for FTY720 (p < 0.0.014 by Mann-Whitney test). H & E staining revealed that there was a decrease in the extent of inflammatory infiltrates in nerve sections from FTY720-treated group compared to sections from water-treated ones, as shown in Fig. 2B. Thus, clinical, electrophysiological and histologic findings indicate that FTY720 treatment ameliorates the severity of SAP.
Figure 2.
FTY720 attenuates the severity of sciatic nerve pathology in SAP mice. A. Examples of epon sections from sciatic nerves of SAP mice treated with water or FTY720. Loss of myelinated fibers was attenuated in FTY720-treated mice compared to water-treated mice (*p < 0.015). Scale bar represents 20 µm. B. Examples of H &E sections of sciatic nerves showing decreased inflammatory cell infiltrates in FTY720-treated animals compared to water-treated ones (*p < 0.02). Scale bar represents 100 µm. For A & B, n = 7 for FTY720 group and n = 6 for the water group.
Mechanisms of action of FTY720 and SEW2871 in SAP
A. Effect on the blood nerve barrier (BNB)
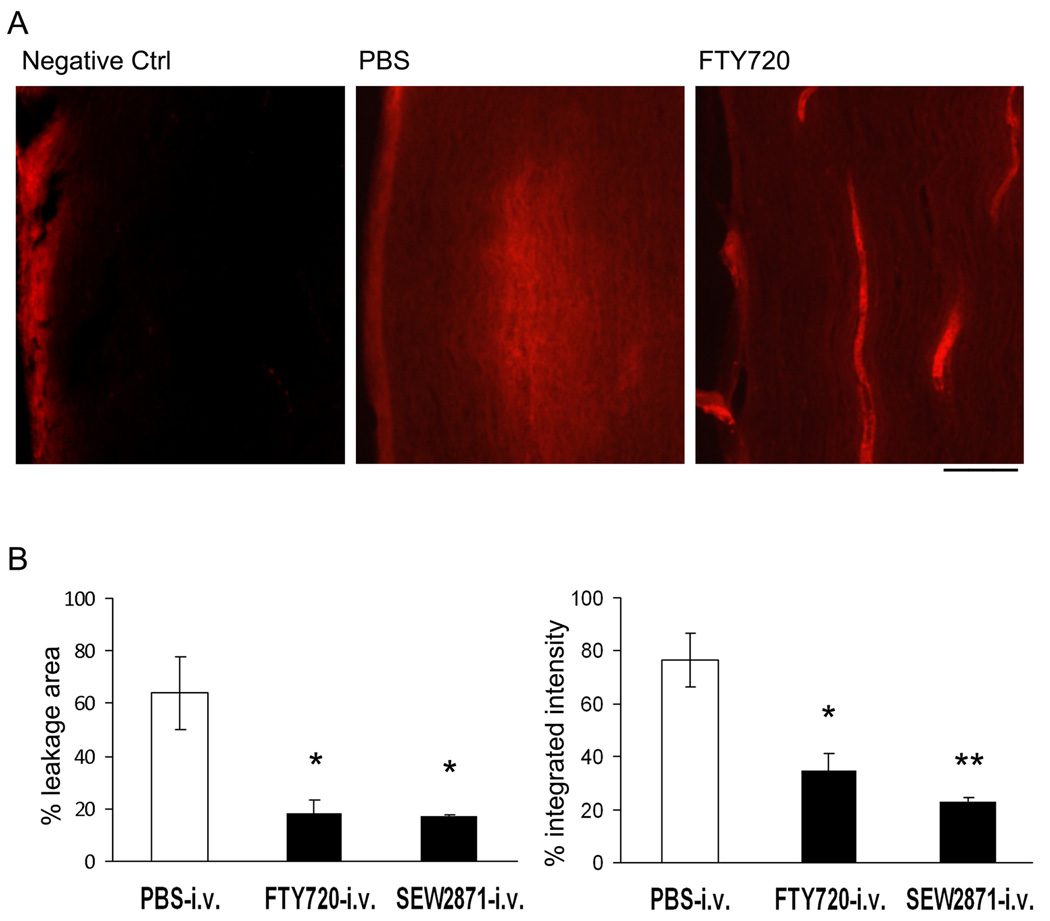
Both functional S1P1 agonism on endothelial cells and functional antagonism at lymphocyte S1P1 receptors play a role in the attenuation of lymphocyte infiltration into target organs (Brinkmann, 2007). We investigated whether a single injection of FTY720 could prevent or attenuate the disruption of the BNB and whether a selective S1P1 agonist SEW2871 could mimic the effect. Sciatic nerve sections of PBS-treated mice showed leakage of EBA from microvessels into the surrounding endoneurium and perineurium; whereas the fluorescence of EBA remained within endoneurial microvessels in nerve sections from FTY720 and SEW2871-treated mice (Fig. 3A, B). These data indicate that S1P receptor modulators rapidly inhibit the disruption of the BNB via S1P1 on endothelial cells.
Figure 3.
Inhibition of blood nerve barrier (BNB) permeability by FTY720 in SAP mice. A. Examples of nerve sections from mice given Evans blue albumin (i.v.) 6 hr after a single dose of intravenous FTY720, SEW2871 or PBS. Animals were sacrificed 30 min after Evans blue. Scale bar represents 100 µm. B. Data summary (n = 3 each). * For % leakage area, p <0.02 for PBS vs FTY720 or SEW2871; for % integrated intensity, * p < 0.02 for PBS vs FTY720, **, p <0.005 for PBS vs SEW2871.
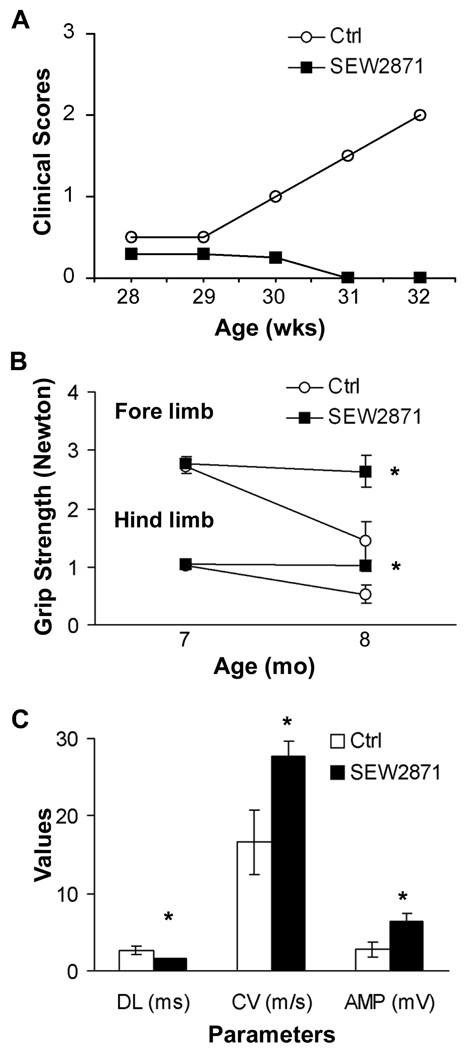
Given the above findings, we examined whether SEW2871 could also ameliorate the clinical severity of SAP. B7-2 KO NOD mice at 7 mo of age were administered SEW2871 or 4 % DMSO (control) by oral gavage for 4 weeks. At 8 mo, clinical scores worsened in the DMSO group (n = 7), but not in SEW2871-treated mice (n = 8) (Fig. 4A). There was no decline in grip strength in SEW2871-treated animals, in contrast to that observed in DMSO-treated mice (Fig. 4B). Furthermore, sciatic nerve conduction studies revealed a significant difference in distal latency, conduction velocity and amplitude of the motor response in SEW2871-treated mice compared with control mice (Fig. 4C).
Figure 4.
Amelioration of SAP by SEW2871. SEW2871 (10 mg / kg) was given orally twice a day at 7 months of age and continued for 4 weeks. A. Median clinical scores (n = 7 for Ctrl; n = 8 for SEW2871). B. Grip strength measurements. * p < 0.007 for SEW2871 vs Ctrl in forelimb at 8 mo, and p < 0.008 in hindlimb. C. Data summary of electrophysiology at 8 mo (n = 6 for Ctrl; n = 8 for SEW2871). * p < 0.02 for DL and CV; p <0.03 in Amp. In B & C, one vehicle-treated mouse died before grip strength measurements and electrophysiologic studies could be performed.
B. Effect on autoreactivity to myelin P0
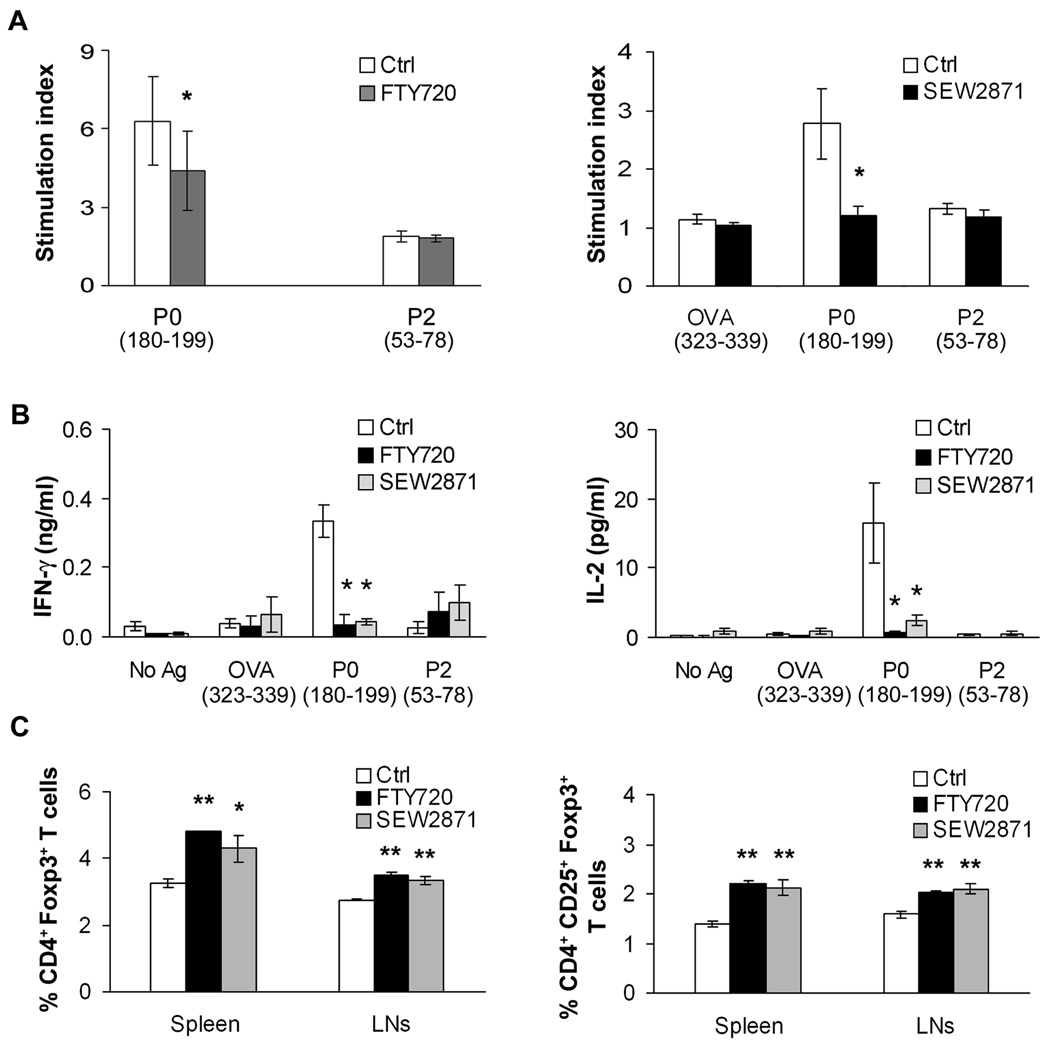
We have recently reported that myelin P0 is one of the autoantigens in SAP, which is Th1-mediated (Kim et al., 2008). We examined the proliferative and cytokine responses of splenocytes isolated from control, FTY720- or SEW2871-gavaged mice. Treatment with FTY720 or SEW2871 led to a decrease in the proliferative response of splenocytes to P0 (180–199) compared to those from control mice. There was no response to OVA (323–339) and P2 (53–78) in all mice (Fig. 5A). IL-2 and IFN-γ levels were also decreased in splenocyte supernatants from FTY720- or SEW2871-gavaged mice compared to control mice (Fig. 5B). No significant effect on IL-10 or IL-17 levels was noted (n = 3–4, not shown). In contrast, exposure of cultured splenocytes for 48 hr to FTY720P (10–100 nM) or SEW2871 (100 nM) did not alter Th1 cytokine responses to P0 (180–199) (n = 3, not shown). Mice treated with FTY720 or SEW2871 had increased percentage of CD4+Foxp3+ T cells and CD4+CD25+Foxp3+ T cells in spleens and lymph nodes (LNs) compared to vehicle-treated mice (Fig. 5C). These results suggest that S1P receptor modulators tilt the balance in favor of regulatory T cells (Tregs) than effector T cells in B7-2 KO NOD mice.
Figure 5.
Effect of FTY720 or SEW2871 treatment on P0 autoreactivity and on Tregs in SAP. A. The proliferative response to P0 (180–199) was decreased in splenocytes from FTY720- or SEW2871-treated mice (n = 3–4 each). * p < 0.01 for FTY720 vs Ctrl; p < 0.02 for SEW2871 vs Ctrl. B. Cytokine responses of splenocytes to the same Ags in replicate culture (n = 7 for Ctrl; n = 3 for FTY720; n = 4 for SEW2871). IFN-γ and IL-2 secretion were decreased in splenocytes from FTY720- or SEW2871 gavaged mice. * p <0.003 for FTY720 or SEW2871 vs Ctrl for IFN-γ; p < 0.05 for FTY720 vs Ctrl or SEW2871 vs Ctrl for IL-2. C. There was an increase in the percentage of Tregs in spleens and LNs of FTY720- or SEW2871-gavaged mice compared to Ctrl (n = 3–5 each). * p<0.01 for SEW2871vs Ctrl in spleen; ** the rest: p<0.001 for FTY720 or SEW2871 vs Ctrl.
C. Potential trophic / protective effect on Schwann cells
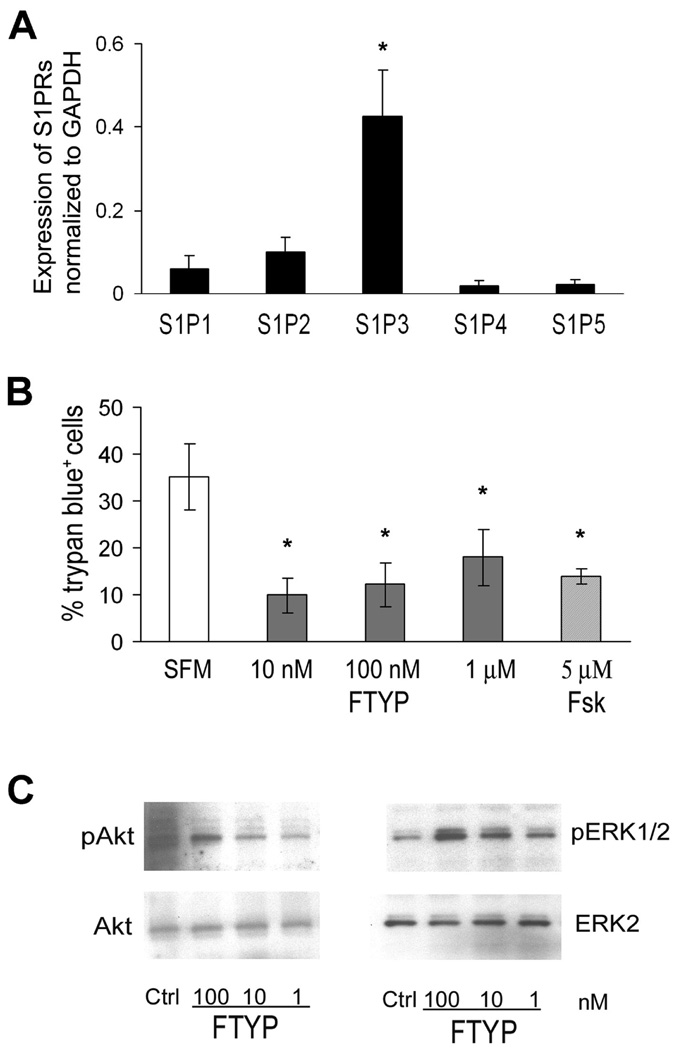
Real-time PCR studies revealed that S1P receptors are expressed by neonatal rat SCs with a relative mRNA level of S1P3 > S1P2> S1P1 > S1P4 = S1P5 (Fig 6A). Given the above, we investigated whether the active form of FTY720 (FTY720P) would exert glioprotective action or promote glial regeneration that could be pertinent in SAP. Treatment of SCs for 3 days with FTY720P in differentiating medium (0.5% FBS + ITS+ forskolin) had no effect on SC differentiation based on P0 protein levels (n = 3, data not shown). When serum withdrawal was used to induce cell death, the percentage of trypan blue+ cells was decreased by FTY720P treatment (Fig. 6B). Western blot analysis showed that exposure of rat SCs to FTY720P (15 min) stimulated the phosphorylation of ERK1/2 (2.8 fold, n = 5) and Akt (1.6 fold, n = 6) compared to controls (Fig. 6C). However, we could not detect a difference in apoptotic (TUNEL+) cells in nerve sections from FTY720-treated mice compared to those from control mice, possibly due to low frequency of TUNEL+ cells at this particular time point (8 mo) (not shown).
Figure 6.
Direct effects of FTY720P on cultured Schwann cells. A. S1P receptor mRNA levels relative to expression of GAPDH mRNA (R = 2−ΔCT) in SCs. *p < 0.005 for S1P3 vs other S1P receptors in rat SCs (n = 3). B. FTY720P rescues SCs from serum withdrawal-induced cell death. SCs were treated with 1nM, 10 nM, 100 nM FTY720P (FTYP) or 5 µM forskolin (Fsk) (n =4–5 each). * p <0.02 for each treatment compared with serum-free medium (SFM). C. Representative examples of Western blot analysis demonstrating that exposure to FTY720P for 15 min stimulates the phosphorylation of ERK1/2 and Akt in rat SCs.
4. DISCUSSION
FTY720, a prototype of S1P receptor modulators, acts acutely as an agonist at S1P receptors when phosphorylated to yield FTY720P, but preferentially desensitizes the S1P1 receptor subtype upon prolonged exposure (functional antagonism). Not all lymphocyte subsets are affected equally. FTY720 induces a preferential depletion of naïve and central memory T cells but not effector memory T cells from the peripheral circulation of MS patients (Mehling et al., 2008). FTY720 also regulates B cell and dendritic cell migration (Cinamon et al., 2008; Lan et al., 2005). S1P1 activation induces an anti-inflammatory phenotype in macrophages and prevents monocyte-endothelial interactions (Hughes et al., 2008; Whetzel et al., 2006). These findings, taken together, suggest that therapeutic actions of FTY720 and related compounds could be extended to inflammatory diseases affecting the peripheral nervous system.
Preclinical studies are usually carried out in two or more animal models of the disease to demonstrate consistent response across species or models. Regarding autoimmune neuropathies, EAN can be induced by myelin proteins such as P0, P2 or by galactocerebroside in rodents and rabbits respectively, as reviewed previously (Hahn, 1996). More recently, we and other investigators found that spontaneous autoimmune neuropathy in B7-2 KO NOD mice is mediated by P0, although possible contributions from other antigens in disease progression cannot be excluded (Kim et al., 2008; Louvet et al., 2009). To our knowledge, there have been no studies of FTY720 or SEW2871 in a spontaneous model of inflammatory neuropathy or multiple sclerosis. We found that FTY720 treatment given at disease onset halted the progression of SAP in B7-2 KO NOD mice, which was confirmed by histological and electrophysiological findings. The rapid inhibition of the BNB by FTY720 and SEW2871 in SAP demonstrates the crucial role of S1P1 receptors on endothelial cells in autoimmune diseases. A two photon imaging study of living T cells also revealed that S1P1 agonist prevents lymphocytes from crossing into medullary sinuses, though it does not distinguish between actions at endothelial S1P1 vs lymphocyte S1P1 (Wei et al., 2005). SEW2871, like FTY720, halted the progression of SAP, though it is less potent as an agonist, has a shorter duration of action, and differs in S1P receptor fate after internalization. FTY720P and AFD-R induce S1P1 receptor degradation, while SEW2871 and the physiologic ligand S1P induce receptor recycling, which correlates with the extent of ubiquitination (Gonzalez-Cabrera et al., 2007; Oo et al., 2007).
It is thought that effector mechanisms may not be affected by S1P receptor modulators (Brinkmann et al., 2002). The effector phase of SAP is mediated by Th1 cytokines, although there is an increase in IL-17 transcripts during the preclinical phase (Bour-Jordan et al., 2005; Kim et al., 2008). We found that FTY720P or SEW2871 had no direct effects on Th1 cytokine responses in vitro, yet splenocytes from FTY720-treated and SEW2871-treated SAP mice exhibited a decreased proliferative and / or Th1 cytokine response to myelin P0. Our observations may simply reflect FTY720-induced T cell depletion from the spleen and the circulation (Hofmann et al., 2006). Another possibility consists of indirect inhibition of pathogenic T cell expansion by S1P receptor modulators via increased number or function of Tregs. Indeed, we found increased number of Tregs in spleens and LNs of FTY720- or SEW2871-gavaged mice. Other investigators have reported that Tregs exhibit a reduced chemotaxis to S1P compared to other T cells, and that Tregs from FTY720-treated mice, but not those from vehicle-treated mice, could inhibit airway inflammation in C57BL/6 mice (Sawicka et al., 2005).
Aside from immune cells, neurons and glial cells also express S1P receptors, albeit differing in the predominating receptor subtypes. We found that Schwann cells express S1P3 > S1P2 > S1P1 > S1P4 = S1P5, in contrast to cells of oligodendroglial lineage where S1P1 and S1P5 predominate (Jaillard et al., 2005; Jung et al., 2007). Other investigators reported that S1P1 is expressed by terminal SCs but not by myelinating SCs, whereas S1P2, S1P3 and S1P4 receptors are expressed by both SC subtypes (Kobashi et al., 2006). S1P activates Rac1 and RhoA and increases SC migration (Barber et al., 2004). We found that FTY720P improves SC survival in vitro as well as activates both ERK2 and Akt pathways, which regulate SC survival, proliferation and differentiation (Hila et al., 2001; Ogata et al., 2004). FTY720P does not enhance SC differentiation as determined by P0 expression. Whether S1P receptor modulators modulate the function of SCs or axons in vivo is unclear and would require investigations in non-autoimmune neuropathies.
In summary, we found that both FTY720 and SEW2871 exert therapeutic actions in SAP, with acute effects mediated by functional agonism at S1P1 receptors of endothelial cells, followed by complex effects on the immune system characterized by T cell depletion with relative sparing of Tregs. Our data indicate that modulation of S1P1 receptors is sufficient to induce a protective effect on autoimmunity, but do not exclude possible contributions from other S1P receptors. One recognizes the limitations of SAP and EAN as animal models of autoimmune neuropathies given that previous attempts found only low frequency of humoral or cellular responses to myelin P0, P2 or glycolipids in human CIDP, perhaps reflecting epitope spreading or the heterogeneity of the disease. Nonetheless, our results suggest a potential therapeutic role for S1P receptor modulators in CIDP and other inflammatory neuropathies based on their actions on BNB permeability and differential sequestration of lymphocyte subsets.
Acknowledgment
We thank Shawna Cook for her assistance in the project. This work was supported by the National Institute of Health Grant R21 NS049014, Miller Group Charitable Trust Fund (Mr. M.P. Miller III), and Jack Miller Center for Peripheral Neuropathy. B7-2 KO NOD mice were generously provided by Dr. Jeffrey A. Bluestone (University of California, San Francisco). FTY720 was generously provided by Novartis (Basel, Switzerland).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflict of interest: nothing to report.
REFERENCES
- Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- Balatoni B, Storch MK, Swoboda EM, Schonborn V, Koziel A, Lambrou GN, Hiestand PC, Weissert R, Foster CA. FTY720 sustains and restores neuronal function in the DA rat model of MOG-induced experimental autoimmune encephalomyelitis. Brain Res Bull. 2007;74:307–316. doi: 10.1016/j.brainresbull.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Barber SC, Mellor H, Gampel A, Scolding NJ. S1P and LPA trigger Schwann cell actin changes and migration. Eur J Neurosci. 2004;19:3142–3150. doi: 10.1111/j.0953-816X.2004.03424.x. [DOI] [PubMed] [Google Scholar]
- Bour-Jordan H, Thompson HL, Bluestone JA. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice. J Immunol. 2005;175:5649–5655. doi: 10.4049/jimmunol.175.9.5649. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–5044. [PubMed] [Google Scholar]
- Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho RP, Payne SG, Bittman R, Spiegel S, Sato-Bigbee C. The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J Pharmacol Exp Ther. 2007;323:626–635. doi: 10.1124/jpet.107.123927. [DOI] [PubMed] [Google Scholar]
- Foster CA, Mechtcheriakova D, Storch MK, Balatoni B, Howard LM, Bornancin F, Wlachos A, Sobanov J, Kinnunen A, Baumruker T. FTY720 Rescue Therapy in the Dark Agouti Rat Model of Experimental Autoimmune Encephalomyelitis: Expression of Central Nervous System Genes and Reversal of Blood-Brain-Barrier Damage. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, Li XK. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305:70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabrera PJ, Hla T, Rosen H. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J Biol Chem. 2007;282:7254–7264. doi: 10.1074/jbc.M610581200. [DOI] [PubMed] [Google Scholar]
- Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. Faseb J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- Hadden RD, Sharrack B, Bensa S, Soudain SE, Hughes RA. Randomized trial of interferon beta-1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 1999;53:57–61. doi: 10.1212/wnl.53.1.57. [DOI] [PubMed] [Google Scholar]
- Hahn AF. Experimental allergic neuritis (EAN) as a model for the immune-mediated demyelinating neuropathies. Rev Neurol (Paris) 1996;152:328–332. [PubMed] [Google Scholar]
- Hila S, Soane L, Koski CL. Sublytic C5b-9-stimulated Schwann cell survival through PI 3-kinase-mediated phosphorylation of BAD. Glia. 2001;36:58–67. doi: 10.1002/glia.1095. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Brinkmann V, Zerwes HG. FTY720 preferentially depletes naive T cells from peripheral and lymphoid organs. Int Immunopharmacol. 2006;6:1902–1910. doi: 10.1016/j.intimp.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase T, Jung CG, Bae H, Zhang M, Soliven B. Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. J Neurochem. 2005;94:1488–1499. doi: 10.1111/j.1471-4159.2005.03290.x. [DOI] [PubMed] [Google Scholar]
- Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K, Zalc B, Lubetzki C. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CG, Kim HJ, Miron VE, Cook S, Kennedy TE, Foster CA, Antel JP, Soliven B. Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia. 2007;55:1656–1667. doi: 10.1002/glia.20576. [DOI] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Jung CG, Jensen MA, Dukala D, Soliven B. Targeting of myelin protein zero in a spontaneous autoimmune polyneuropathy. J Immunol. 2008;181:8753–8760. doi: 10.4049/jimmunol.181.12.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobashi H, Yaoi T, Oda R, Okajima S, Fujiwara H, Kubo T, Fushiki S. Lysophospholipid receptors are differentially expressed in rat terminal Schwann cells, as revealed by a single cell rt-PCR and in situ hybridization. Acta Histochem Cytochem. 2006;39:55–60. doi: 10.1267/ahc.06002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan YY, De Creus A, Colvin BL, Abe M, Brinkmann V, Coates PT, Thomson AW. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant. 2005;5:2649–2659. doi: 10.1111/j.1600-6143.2005.01085.x. [DOI] [PubMed] [Google Scholar]
- Louvet C, Kabre BG, Davini DW, Martinier N, Su MA, DeVoss JJ, Rosenthal WL, Anderson MS, Bour-Jordan H, Bluestone JA. A novel myelin P0-specific T cell receptor transgenic mouse develops a fulminant autoimmune peripheral neuropathy. J Exp Med. 2009;206:507–514. doi: 10.1084/jem.20082113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Matsuyuki H, Shimano K, Kataoka H, Sugahara K, Chiba K. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J Immunol. 2007;178:3437–3446. doi: 10.4049/jimmunol.178.6.3437. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, Kristofic C, Kuhle J, Lindberg RL, Kappos L. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71:1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- Miron VE, Hall JA, Kennedy TE, Soliven B, Antel JP. Cyclical and dose-dependent responses of adult human mature oligodendrocytes to fingolimod. Am J Pathol. 2008;173:1143–1152. doi: 10.2353/ajpath.2008.080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano S, Takeda M, Ma L, Soliven B. Cytokine-induced cell death in immortalized Schwann cells: roles of nitric oxide and cyclic AMP. J Neurochem. 2001;77:1486–1495. doi: 10.1046/j.1471-4159.2001.00358.x. [DOI] [PubMed] [Google Scholar]
- Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–5770. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, Arcella J, Girvin AM, Padilla J, Miller SD, Bluestone JA. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J Exp Med. 2001;194:677–684. doi: 10.1084/jem.194.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicka E, Dubois G, Jarai G, Edwards M, Thomas M, Nicholls A, Albert R, Newson C, Brinkmann V, Walker C. The Sphingosine 1-Phosphate Receptor Agonist FTY720 Differentially Affects the Sequestration of CD4+/CD25+ T-Regulatory Cells and Enhances Their Functional Activity. J Immunol. 2005;175:7973–7980. doi: 10.4049/jimmunol.175.12.7973. [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat JM, Hahn AF, Leger JM, Cros DP, Magy L, Tabaraud F, Bouche P, Preux PM. Interferon beta-1a as an investigational treatment for CIDP. Neurology. 2003;60:S23–S28. doi: 10.1212/wnl.60.8_suppl_3.s23. [DOI] [PubMed] [Google Scholar]
- Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, Mandala S, Chun J, Rao TS. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153:108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- Whetzel AM, Bolick DT, Srinivasan S, Macdonald TL, Morris MA, Ley K, Hedrick CC. Sphingosine-1 phosphate prevents monocyte/endothelial interactions in type 1 diabetic NOD mice through activation of the S1P1 receptor. Circ Res. 2006;99:731–739. doi: 10.1161/01.RES.0000244088.33375.52. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang ZY, Fauser U, Schluesener HJ. FTY720 ameliorates experimental autoimmune neuritis by inhibition of lymphocyte and monocyte infiltration into peripheral nerves. Exp Neurol. 2008;210:681–690. doi: 10.1016/j.expneurol.2007.12.025. [DOI] [PubMed] [Google Scholar]