Abstract
We aimed to identify neuroanatomical regions associated with deficits to the graphemic buffer, a working memory component of the spelling system that holds the sequence of letter identities during production. We evaluated 331 patients with left hemisphere ischemic stroke with various spelling tests and magnetic resonance diffusion-weighted imaging and perfusion-weighted imaging, within 48 hours of stroke onset. A voxel-wise statistical map showed that ischemia in voxels in posterior and inferior frontal and parietal cortex, subcortical white matter underlying prefrontal cortex, lateral occipital gyrus, or caudate was associated with impairment in maintaining the sequence of letter identities while spelling.
The graphemic buffer is a working memory component of the spelling system that temporarily holds the sequence of graphemes (abstract letters) during production of letter shapes for written spelling or letter names for oral spelling1. Selective damage to the graphemic buffer following brain damage results in a characteristic pattern of errors1. As damage involves a storage component, errors consist of substitutions, additions, deletions, or transpositions of single or multiple letters, resulting in phonologically implausible spelling. In addition, as this storage component is required for all spelling tasks, the same patterns errors are found across written picture naming and oral and written spelling to dictation. Furthermore, as the buffer is a working memory mechanism, errors increase as word length increases. Several patients whose spelling performance appears to reflect selective damage to the graphemic buffer have been reported1–5. However, neuroanatomical regions critical to this mechanism have yet to be identified. Examination of lesions in single case studies of graphemic buffer deficits reveals associated areas of damage in left frontal1,2,4,5 and parietal1,3,5–9 lobes, and less frequently, temporal 10,11 and occipital8,12 cortex and basal ganglia6. However, most of these patients had large strokes, and were studied long after stroke, following the opportunity for extensive reorganization of structure/function relationships or rehabilitation that modified spelling performance. Previous studies also did not evaluate patients without graphemic buffer deficits to evaluate the probability of the lesion causing the deficit.
The current study aimed to identify brain regions where ischemia resulting in tissue dysfunction is associated with impairments to the graphemic buffer in a relatively large number of patients whose spelling performance was indicative of damage or preservation of the graphemic buffer. Unlike previous studies, we tested patients early after stroke to determine the status of the buffer before extensive reorganization. We also identified areas rendered dysfunctional due to hypoperfusion, as well as infarct, because both contribute to deficits in acute stroke12,13.
Methods
Participants
A consecutive series of 331 right-handed English-speaking patients with acute, left hemisphere ischemic stroke, without exclusion criteria, were tested within 48 hours of stroke onset. Exclusion criteria were: known hearing loss or uncorrected visual impairment; history of dementia, previous symptomatic stroke or other neurological disease; and hemorrhage.
Language Tests
Spelling tests included 58 item oral and written spelling to dictation tasks, involving words and pseudowords (e.g. torp) and written naming of 17 or 30 line drawings. Patients were also tested on oral naming, spoken and written word comprehension, repetition, and oral reading to obtain a broader view of their language processing (see references 12–13 for description of tasks).
Imaging
Within 24 hours of language testing, patients underwent MRI, including diffusion weighted imaging (DWI) with apparent diffusion coefficient (ADC) maps, which reveal infarct or dense ischemia early after onset, perfusion-weighted imaging (PWI, which reveals areas of hypoperfusion that correspond to dysfunction), Fluid Attenuated Inversion Recovery (to rule out old infarcts), and T2*-weighted gradient-echo (to rule out hemorrhage). Hypoperfusion was defined as > 4 sec delay in time to peak (TTP) arrival of contrast, relative to homologous voxels in the right hemisphere, based on previous studies showing that this degree of hypoperfusion is associated with clinical deficits, even in the absence of infarct13.
Technicians blinded to language test results outlined areas of tissue dysfunction (dense ischemia or infarct defined as bright on DWI and dark on ADC maps and/or hypoperfusion on PWI, as defined above) on the MNI atlas. We did not use ADC or TTP as continuous measures to relate to continuous measures of graphemic buffer impairment, because the absolute ADC depends critically on the precise time since stroke onset, which could not be controlled.
Statistical Analysis
We first identified two groups of subjects: (1) patients with impairment to the graphemic buffer; and (2) patients with preserved graphemic buffer (see Table 1 for criteria). MRIcroN (http://www.sph.sc.edu/comd/rorden/mricron) was used to carry out a whole-brain analysis (by the Liebermeister measure14), and create a voxel-wise statistical map to show voxels where ischemia (DWI and/or PWI abnormality) was associated with graphemic buffer impairment. An alpha level of 0.05 after a whole-brain False Discovery Rate (FDR) correction for multiple comparisons was used to identify significant associations 15.
Table 1. Criteria for Impaired and Preserved Graphemic Buffer.
A technician blinded to the imaging results identified whether or not each of the following criteria was met.
| A graphemic buffer deficit was defined by meeting all of the following criteria: |
|---|
| 1. >75% of errors were phonologically implausible nonwords (e.g. leopard-> leotald) in dictation tasks and written naming |
| 2. No significant difference (by chi-square) in accuracy rates between tasks or stimuli (words vs nonwords), comparing subset of items matched in length; |
| 3. A length effect was present on accuracy rates, defined as total error rates on long words (5+ letters) at least 10% greater than short (3–4 letters) words, coupled with an average error rate per letter in the word that was greater for long than short words. The latter was computed by identifying the number of incorrect or omitted letters and dividing by the number of letters in the target for each stimulus. This measure of length effect is important because longer words have a greater chance of an incorrect letter. |
| An intact graphemic buffer was defined by meeting either of the following criteria: |
| 1. > 90% errors are real words (visually similar words, semantic errors, and/or morphological errors); or |
| 2. Normal performance (<10% total errors, based on norms for our stimuli) in spelling words or pseudowords. |
Results
Twenty-one patients (17 women) had clear evidence of impaired graphemic buffer; 48 (25 men) had evidence for an intact graphemic buffer. In the remaining patients the status of the graphemic buffer was indeterminate because they met neither criteria for impaired graphemic buffer nor criteria for spared graphemic buffer. In most of these cases, there were too few scorable spelling responses to evaluate the status of the graphemic buffer. There were no differences across groups in age, education, or mean accuracy rates in oral picture naming, tactile naming, repetition, or auditory comprehension (Table 2). Those with graphemic buffer deficits and indeterminate status of the graphemic buffer were significantly more impaired in all spelling tasks and reading tasks than those with intact graphemic buffer. This finding is expected because patients with high accuracy in spelling (and perhaps reading of pseudowords7–8) must have an intact graphemic buffer; those whose spelling was so impaired that there were too few legible responses to analyze were considered indeterminate. Some patients with intact graphemic buffer had low accuracy in spelling, but had damage to other components of spelling, as reflected in different types of errors, such as semantic paragraphias (e.g. “canoe” spelled bike) or phonologically plausible errors (e.g. “canoe” spelled kanue). Some patients with impaired graphemic buffer also had other deficits, such as anomia; but most had relatively spared auditory word comprehension.
Table 2.
Mean (SD) Demographics and Error Rates on Language Tests
| Preserved Graphemic Buffer (n=48) |
Impaired Graphemic Buffer (n=21) |
Indeterminate Status of the Graphemic Buffer |
|
|---|---|---|---|
| Age in Years | 56.7 (15.2) | 61.8 (14.0) | 59.1 (21.5) |
| Education in Years | 14.0 (2.9) | 12.8 (2.3) | 11.7 (3.2) |
| Oral Naming Pictures | 11.5 (23.7) | 41.7 (40.9) | 18.1 (26.2) |
| Oral Naming: Tactile | 9.7(19.3) | 35.7 (37.2) | 13.6 (23.1) |
| Oral Reading* | 9.2 (17.8) | 31.9 (33.6) | 27.2 (32.1) |
| Repetition | 9.0 (19.7) | 14.1 (19.8) | 10.3 (14.6) |
| Auditory Comprehension | 6.1 (12.3) | 10.1 (14.5) | 13.5 (14.9) |
| Reading Comprehension* |
5.1 (9.7) | 21.6 (30.2) | 30.6 (33.8) |
| Written Naming Pictures* |
18.1 (23.8) | 47.1 (28.1) | 62.0 (53.7) |
| Written Spelling to Dictation* |
14.5 (29.1) | 54.5 (29.1) | 40.0 (33.4) |
| Oral Spelling to Dictation** |
21.2 (28.2) | 49.6 (19.9) | 52.1 (25.7) |
differences between groups by ANOVA: p <0.02;
< 0.01
Figure 1a shows the overlap of all regions of tissue dysfunction in all 69 patients with or without deficits. Figure 1b shows the voxels of the MNI atlas where tissue dysfunction (hypoperfusion and/or dense ischemia or infarct) was associated with a graphemic buffer deficit compared to no buffer deficit. Specifically, the most strongly associated voxels (Z > 2.34, p < 0.01 FDR), were in precentral and premotor cortex involving Brodmann’s Areas (BA) 4 and BA 6, and postcentral gyrus involving BAs 2 and 3. Other associated voxels were in deep subcortical white matter underlying prefrontal cortex (BA 48) and caudate nucleus. However, no patient with a graphemic buffer deficit had ischemia restricted to subcortical gray or white matter. Voxels in posterior inferior frontal (BAs 45 and 47), and lateral occipital (BA 19) gyri were also implicated in graphemic buffer impairments, but less reliably (Z > 1.73, p < 0.05 FDR).
Figure 1.
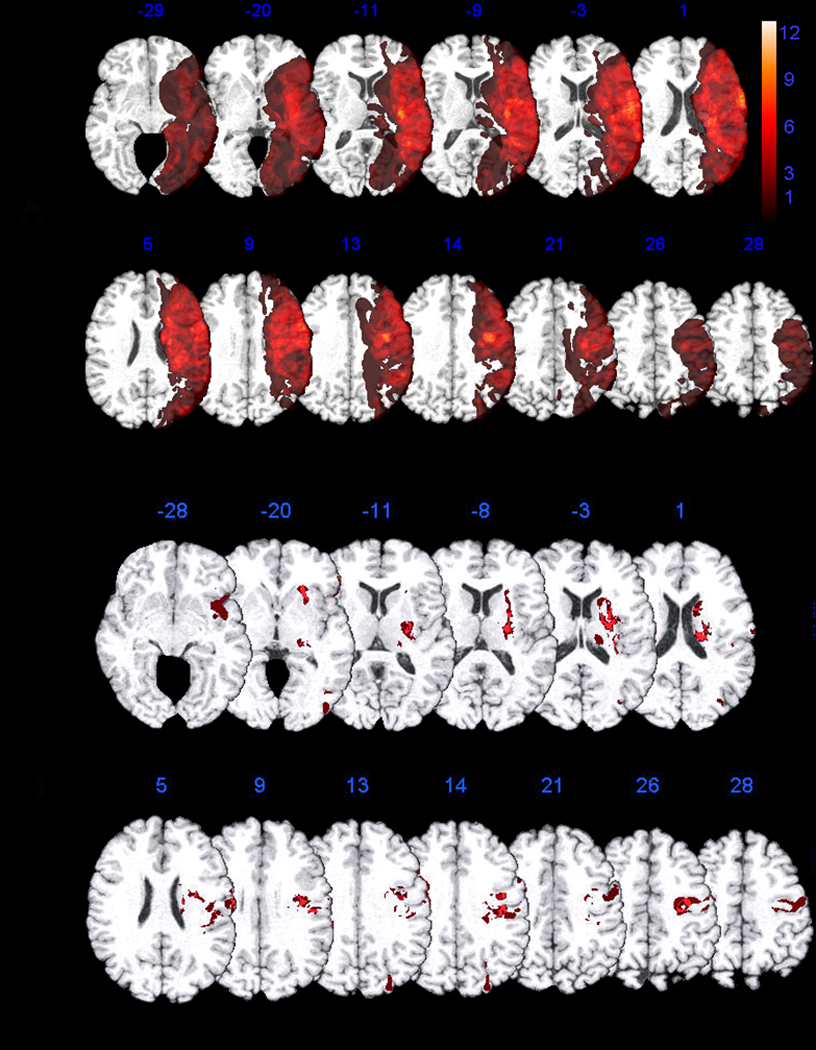
Discussion
We aimed to identify brain regions where ischemia is associated with a deficit to the graphemic buffer, a working memory system that holds the sequence of graphemes while they are processed into letter shapes for written spelling or letter names for oral spelling. Consistent with regions identified in case studies of patients with selective deficits to the buffer1–11, the current study identified a network of regions associated with graphemic buffer deficits including left frontal, parietal, and occipital cortex, in addition to extensive areas of subcortical white matter and caudate nucleus.
The areas we identified as critical for this component of spelling have also been found to be engaged in functional neuroimaging studies of spelling, although the specific areas within frontal and parietal lobes have varied by study and task16–18. Importantly, consistent with the conceptualization of the buffer as a working memory mechanism, functional neuroimaging studies have also identified areas within left frontal cortex (inferior frontal gyrus, precentral gyrus, and dorsolateral prefrontal cortex) and parietal cortex in tasks involving verbal working memory (see reference 19 for review). Some investigators have reported activation in occipital cortex associated with visual working memory 20 or a “visuo-spatial sketchpad”, which might be essential for holding the sequence of graphemes.
Limitations of this study include the relatively small number of spelling tasks and stimuli that were presented, due to the limited time available for testing in the first 48 hours after stroke. We were not able to evaluate for effects of regularity, concreteness, and word class that might signal deficits to additional components of spelling. However, even if our graphemic buffer patients had additional deficits, they each showed evidence graphemic buffer impairment. It was necessary to evaluate patients soon after stroke onset, to avoid including patients in the “spared graphemic buffer” group who initially had the deficit, but recovered.
In conclusion, we identified a cortical-subcortical network of areas in left posterior frontal, parietal, and lateral occipital lobes that are implicated in the graphemic buffer function. We cannot claim that all of these regions are critical to the network; some areas of ischemia may be associated with the deficit only because they are frequently ischemic when other regions that cause the deficit are ischemic. For example, post-central gyrus is not typically engaged in working memory tasks, and seems less likely than other parietal regions to directly cause graphemic buffer deficits. Future studies with more patients with and without dysfunction in each of these regions may reveal which areas are critical. Lesions to at least some of these regions are likely to result in impairments in maintaining the sequence of letter identities while spelling.
Acknowledgments
The research reported in this paper was supported by NIH (NIDCD), through RO1 DC 05375. We gratefully acknowledge this support and the participation of the patients.
References
- 1.Caramazza A, Miceli G, Villa G, Romani C. The role of the graphemic buffer in spelling: Evidence from a case of acquired dysgraphia. Cognition. 1987;26:59–85. doi: 10.1016/0010-0277(87)90014-x. [DOI] [PubMed] [Google Scholar]
- 2.Posteraro L, Zinelli P, Mazzucchi A. Selective impairment of the graphemic buffer in acquired dysgraphia: A case study. Brain Lang. 1988;35:274–286. doi: 10.1016/0093-934x(88)90112-5. [DOI] [PubMed] [Google Scholar]
- 3.Sage K, Ellis AW. Lexical influences in graphemic buffer disorder. Cogn Neuropsychol. 2004;21:381–400. doi: 10.1080/02643290342000438. [DOI] [PubMed] [Google Scholar]
- 4.Hillis AE, Caramazza A. The graphemic buffer and attentional mechanisms. Brain Lang. 1989;36:208–235. doi: 10.1016/0093-934x(89)90062-x. [DOI] [PubMed] [Google Scholar]
- 5.Tainturier MJ, Rapp BC. Complex graphemes as functional spelling units: Evidence from acquired dysgraphia. Neurocase. 2004;10:122–131. doi: 10.1080/13554790409609943. [DOI] [PubMed] [Google Scholar]
- 6.Cardell EA, Chenery HJ. A cognitive neuropsychological approach to the assessment and remediation of acquired dysgraphia. Lang Test. 1999;16:353–388. [Google Scholar]
- 7.Tainturier MJ, Rapp B. Is a single graphemic buffer used in reading and spelling? Aphasiology. 2003;17:537–562. [Google Scholar]
- 8.Hanley JR, Kay J. Does the graphemic buffer play a role in reading? Cogn Neuropsychol. 1998;15:313–318. doi: 10.1080/026432998381195. [DOI] [PubMed] [Google Scholar]
- 9.Miceli G, Silveri MC, Caramazza A. Cognitive analysis of a case of pure dysgraphia. Brain Lang. 1985;25:187–212. doi: 10.1016/0093-934x(85)90080-x. [DOI] [PubMed] [Google Scholar]
- 10.Cotelli M, Abutalebi J, Zorzi M, Cappa SF. Vowels in the buffer: A case study of acquired dysgraphia with selective vowel substitutions. Cogn Neuropsychol. 2003;20:99–114. doi: 10.1080/02643290244000158. [DOI] [PubMed] [Google Scholar]
- 11.Kan IP, Biran I, Thompson-Schill SL, Chatterjee A. Letter selection and letter assembly in acquired dysgraphia. Cogn Behav Neurol. 2006;19:225–236. doi: 10.1097/01.wnn.0000213918.18138.f2. [DOI] [PubMed] [Google Scholar]
- 12.Hillis AE, Kane A, Tuffiash E, et al. Neural substrates of the cognitive processes underlying spelling: Evidence from MR diffusion and perfusion imaging. Aphasiology. 2002;16:425–438. [Google Scholar]
- 13.Hillis AE, Wityk RJ, Tuffiash E, et al. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann Neurol. 2001;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- 14.Rorden C, Karnath H-O, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 15.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 16.Booth JR, Burman DD, Meyer JR, et al. Modality-specific and -independent developmental differences in the neural substrate for lexical processing. J Neurolinguist. 2003;16:383–405. [Google Scholar]
- 17.Harrington GS, Farias D, Davis CH, Buonocore MH. Comparison of the neural basis for imagined writing and drawing. Hum Brain Mapp. 2007;28:450–459. doi: 10.1002/hbm.20286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James KH, Gauthier I. Letter processing automatically recruits a sensory-motor brain network. Neuropsychologia. 2006;44:2937–2949. doi: 10.1016/j.neuropsychologia.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Menon V, Desmond JE. Left superior parietal cortex involvement in writing: Integrating fMRI with lesion evidence. Cogn Brain Res. 2001;12:337–340. doi: 10.1016/s0926-6410(01)00063-5. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]


