Abstract
Abstract Gynecologic cancer patients are at high risk for emotional distress and sexual dysfunction. The present study tested sexual self schema as an individual difference variable that might be useful in identifying those at risk for unfavorable outcomes. First, we tested schema as a predictor of sexual outcomes,including bodychangestress. Second,we examined schema as a contributor to broader quality of life outcomes, specifically as a moderator of the relationship between sexual satisfaction and psychological statue (depressive symptoms and quality of life). A cross-sectional design was used. Gynecologic cancer survivors (N = 175) 2−10 years post treatment were assessed during routine follow up. In regression analyses controlling for sociodemographic variables, patients' physical symptoms/signs as evaluated by nurses, health status, and extent of partner sexual difficulties, sexual self schema accounted for significant variance in the prediction of current sexual behavior, responsiveness, and satisfaction. Moreover, schema moderated the relationship between sexual satisfaction and psychological outcomes, suggesting that a positive sexual self schema might “buffer” patients from depressive symptoms when their sexual satisfaction is low. Furthermore, the combination of a negative sexual self schema and low sexual satisfaction might heighten survivors' risk for psychological distress, including depressive symptomatology. These data support the consideration of sexual self schema as a predictor of sexual morbidity among gynecologic cancer survivors.
Keywords: Schema, Sexual dysfunction, Gynecologic cancer, Depression, Quality of life
Introduction
Women treated for gynecologic cancer have received relatively little psychological study despite the prevalence of the disease, which accounts for 12% of all new U.S. cancer diagnoses in women annually (Jemal et al., 2007). This is particularly troublesome because psychosocial morbidity for these women is high. They are at risk for significant emotional distress; prevalence studies have estimated that 23% of women experience psychological symptoms of sufficient degree to merit a diagnosis of major depressive disorder (Thompson & Shear, 1998). In fact, women with gynecologic cancers might be at higher risk for psychological distress than other cancer samples. Parker, Baile, De Moor, and Cohen (2003) interviewed breast, gastrointestinal, gynecologic, and urologic cancer survivors (N = 351), and gynecologic patients reported the highest levels of depressive symptoms.
The elevated prevalence of psychological distress may be due, in part, to the significant sexual functioning morbidity. While many cancer patients experience some degree of sexual difficulty (Andersen, 1985), prevalence studies have demonstrated that gynecologic cancer patients, much like men treated for prostate cancer (Bertero, 2001; Jenkins et al., 2004; Schover et al., 2002a, b), undergo early reductions in sexual activity and disrupted responsiveness that can be permanent (Gershenson et al., 2007; Hawighorst-Knapstein et al., 2004; Lindau, Gavrilova, & Anderson, 2007). Studies comparing gynecologic cancer patients to healthy controls/norms have shown that women with gynecologic cancer may resume intercourse, but report diminished sexual responsiveness (Weijmar Schultz, van De Wiel, & Bouma, 1991) and lower sexual satisfaction (Gershenson et al., 2007; Lindau et al., 2007), and are found to have higher rates of sexual dysfunction than healthy women or women with benign gynecologic disease (Andersen, Anderson, & deProsse, 1989a). In sum, the prevalence of sexual difficulties among these patients is well known (Andersen, 1994b); these studies provide an estimate of the magnitude of need for support services in this population, but offer little insight into treatment or prevention of sexual problems or emotional distress. Research that identifies variables that confer risk or (or are protective) represents a needed contribution, as it could facilitate early identification of vulnerable patients and guide intervention development.
One characteristic of women that may have particular relevance in the context of gynecologic cancer is a woman's view of herself as a sexual person—her sexual self schema. Self schemas are cognitive generalizations about the self (Markus, 1977, 1987; Markus & Kunda, 1986) and, in this case, generalizations about sexual aspects of oneself. As conceptualized (Andersen & Cyranowski, 1994; Andersen, Cyranowski, & Espindle, 1999), sexual self schemas are manifest in current experience; they guide sexual behavior (past, present, and future) and influence the processing of sexually-relevant information. Individuals who differ in the valence of their schema—positive versus negative—evidence numerous experiential, behavioral, attitudinal, affective, and cognitive differences in the sexual domain (Andersen & Cyranowski, 1994; Cyranowski & Andersen, 1998, 2000). Stated simply, a woman with positive sexual self schema reports positive attitudes regarding sexual expression, high frequencies of sexual behaviors, low levels of negative sexual affect (such as sexual anxiety), and with regard to relationships, greater feelings of passionate love and secure romantic attachments. Conversely, when the self schema is negative, conflicted, or weak, an individual expresses negative attitudes towards sex, low levels of sexual desire and arousal, high levels of sexual anxiety, a tendency to avoid sexual interactions, and anxiety about abandonment and avoidance of intimacy within romantic relationships.
We have suggested that individual differences in sexual self schema constitute a cognitive diathesis in a diathesis-stress model of sexual dysfunction (Andersen, 1999; Cyranowski, Aarestad, & Andersen, 1999), specifically that individuals with positive, non-conflicting sexual self views would be better “immunized” to cope with stressors relevant to their sexuality. Conversely, individuals with negative sexual self views would be more likely to attribute sexual problems to stable, internal attributes, which would, in turn, affect mood (e.g., depression, anxiety) and alter attentional processes, thereby exacerbating sexual difficulties. Thus, in the face of a challenge, such as gynecologic cancer, that directly compromises sexuality with ensuing treatments of surgery, radiation, and chemotherapy, we would anticipate that women with a negative sexual self schema would be vulnerable not only to poorer sexual outcomes, but perhaps a more difficult psychological trajectory as well. In contrast, women with a positive schema would fair better in both the sexual and emotional domains, even in the face of sexual disruptions and low sexual satisfaction.
There were two aims of the present study. First, following a clinical description of the gynecologic cancer survivor sample, we tested the co-variation of sexual self schema and current sexual functioning. We used a multifaceted sexuality assessment that included behavioral (frequency of intercourse), functional (sexual responsiveness), and subjective (global sexual satisfaction) indicators. We also tested the relevance of sexual self schema to body change stress–intrusive and avoidant thoughts and behaviors related to body changes following gynecologic cancer treatment. We anticipated that women coming to the gynecologic cancer stressor with a negative sexual self view might also report traumatic-like stress in viewing their body changes, but that a woman with a positive schema might be more resilient to such changes.
Second, we examined sexual self schema as a contributor to broader quality of life outcomes. Specifically, we tested sexual self schema as a moderator of the relationship between current sexual satisfaction and psychological status. Sexual satisfaction suffers for many following gynecologic cancer diagnosis and treatment, with reported rates of sexual dissatisfaction ranging from 22% (Thranov & Klee, 1994) to 30% (Jensen, Klee, Thranov, & Groenvold, 2004) to 75% (Stewart, Wong, Duff, Melancon, & Cheung, 2001). As many gynecologic cancer survivors do not resume sexual activity following treatment, we reasoned that a subjective measure, such as satisfaction, would be a better metric than sexual activity or reported sexual responsiveness. As discussed above, we reasoned that the lower sexual satisfaction anticipated for the woman with a negative schema might heighten risk for depressive symptoms and disruption of quality of life, both general and gynecologically relevant, whereas a positive schema might instead “buffer” women from added distress. Confirmation of these results would suggest that women diagnosed with gynecologic cancer and reporting a negative sexual self schema would be at heightened risk not only for sexual problems, but also psychological distress. Because quality of life is a multidimensional construct, we included separate measures of depressive symptoms, global quality of life (general health perceptions), and disease-specific quality of life, as each captured a distinct dimension of health status important to the experience of the gynecologic cancer survivor.
We were interested in providing a robust test of sexual self schema, and so we considered four classes of control variables known to be associated with sexual and psychological outcomes in gynecologic cancer samples. Included first were sociodemographic characteristics. In prior research, younger patients reported more distress than older patients (Leake, Gurrin, & Hammond, 2001) and fewer years of education has been associated with poorer quality of life (Miller, Pittman, Case, & McQuellon, 2002). Extent of cancer treatment was the second class of variables considered because some research has suggested an association between treatment modality and sexual outcomes (Greimel, Thiel, Peintinger, Cegnar, & Pongratz, 2002; Schover, Fife, & Gershenson, 1989; Vincent, Vincent, Greiss, & Linton, 1975). Third, a nurse evaluated symptomatology frequently experienced as late-onset physical sequelae of gynecologic cancer treatment—bladder, urinary tract, bowel, and endocrine changes/dysfunction (Janda, Obermair, Cella, Crandon, & Trimmel, 2004). We also included patient-reported post-treatment vaginal changes and fatigue, which are common, significantly affect quality of life (Broeckel, Jacobsen, Horton, Balducci, & Lyman, 1998; Cella, Lai, Chang, Peterman, & Slavin, 2002), and are associated with lower frequency of sexual activity (Cain et al., 2003). Finally, we assessed, from the participant's perspective, her partner's sexual difficulties to control for relative access to a sexual partner, as men with sexual dysfunction are significantly less likely to be sexually active (Blanker et al., 2001).
Method
Participants
Participants (N = 175) were an average of 4 years post-diagnosis (SD = 2 years) and survivors of endometrial (n = 82; 47%), ovarian (n = 47; 27%), cervical (n = 38; 22%), or vulvar (n = 8; 4%) cancers. This distribution of disease sites corresponds closely to that for the U.S. (Jemal et al., 2007) and the state of Ohio (American Cancer Society Ohio Division, 2007). The majority had been diagnosed with stage I (64%) tumors (stage II, 10%; stage III, 23%; and, stage IV, 3%). Consistent with epidemiologic studies, ovarian participants were most likely to present with stage III or IV disease (Jemal et al., 2007). Virtually all were treated with surgery (98%), with most receiving some type of hysterectomy (81%). In addition, 44% received chemotherapy or radiotherapy (23%). The sample was primarily Caucasian (95%; 5% African American), middle aged (M = 55 years, SD = 12, range 23−82), with some college (M = 14 years, SD = 3, range 9−25). The majority was married (91%) with the average duration of relationships being 26 years (SD = 16, range 1−63). While partner gender was not a criterion for study eligibility, all partners were male.
Measures
Sexual Self Schema
The Sexual Self Schema (SSS) Scale for Women (Andersen & Cyranowski, 1994) was used to assess schema. The SSS contains 26 trait adjectives (e.g., cautious, loving, open-minded, experienced) that were self-rated from 0 (not at all descriptive of me) to 6 (very descriptive of me). Previous factor analytic studies have revealed three dimensions: (1) passionate/romantic, (2) open/direct, and (3) embarrassed/conservative. Items from factors 1 and 2 are summed and items from factor 3 subtracted for a total schema score, ranging from −42 to 102. Low scores represent a negative self view and higher scores reflect a more positive self view. Validation studies have demonstrated stability (2-week Pearson r = .89, 2-month r = .88 in Andersen & Cyranowski, 1994). An 18-month test–retest estimate with breast cancer patients was .65, comparable to 18-month data for trait measures (Goldberg, 1992) of neuroticism (.61) and extraversion (.78) (Yang, personal communication). Andersen and colleagues (Andersen & Cyranowski, 1994;Andersenet al., 1999) have also demonstrated that SSS scores do not show social desirability or negative affect biases and that respondents are unaware that a sexual construct is being assessed (see article for a complete discussion). Coefficient α for the present study was .76.
Sexuality
Sexual Activity
Participants reported the frequency of sexual intercourse during the last 2 months, using an 8-point scale ranging from 0 (did not occur at all) to 7 (once/day). Four-month test–retest reliability of r = .75 has been reported in prior research (Andersen & Broffitt, 1988).
Sexual Responsiveness
The Female Sexual Function Index (FSFI) (Rosen et al., 2000) was used. The 19-item self-report measure includes six subscales/domains: desire, arousal, lubrication, orgasm, satisfaction, and pain. Items were rated using 5-point Likert scales ranging from 1 to 5 (response descriptions vary based on item content). Total scores, a weighted sum across the six domains, range from 2 to 36, with higher scores indicating better sexual functioning. Rosen et al. reported 4-week test–retest reliability ranging from .79 to .86 for subscale scores and .88 for the total score. A clinical cut-off score of 26.6 has been suggested for differentiating between women with and without sexual dysfunction (Wiegel, Meston, & Rosen, 2005). Coefficient α for the present study ranged from .89 to .96 for the subscales and was .97 for the total score.
Global Sexual Satisfaction
Participants provided a global evaluation of their current sexual life (Derogatis & Melisaratos, 1979) using a 9-point scale ranging from 0 (could not be worse) to 8 (could not be better). Previous research has demonstrated that this global evaluation is sensitive to pre- to post-cancer treatment effects (Andersen et al., 1989a;Andersen, Woods, & Copeland, 1997).
Body Change Stress
A modified version of the Breast-Impact of Treatment Scale (ITS) (Frierson, Thiel, & Andersen, 2006) assessed intrusive thoughts (“How my body has changed pops into my mind”), avoidant thoughts (“I don't want to deal with how my body looks”), and avoidant behaviors (“I avoid looking at or touching my body”) related to body change stress. A 6-point scale ranging from 0 (not at all) to 5 (often) was used; total scores range from 0 to 65, with higher scores indicating greater body change stress. The measure instructions spec-ified that women were to respond based on their current experience as it related to their cancer treatment. Coefficient α for the present study was .92.
Psychological Status
Depressive Symptoms
The Iowa short-form (Kohout, Berkman, Evans, & Cornoni-Huntley, 1993) of the Center for Epidemiological Studies Depression Scale (CES-D) (Comstock & Helsing, 1976; Radloff, 1977) was used. The CES-D consisted of 11 depressive symptoms rated on a 3-point scale from 0 (hardly ever or never) to 2 (much or most of the time). Total scores ranged from 0 to 22, with higher scores indicating more depressive symptoms. Unlike some depression scales, the CES-D does not include an item assessing loss of sexual desire. Coefficient α for the present study was .83.
Global QoL
The Medical Outcomes Study-Short Form 12 Mental Component Summary (SF-12 MCS) (Ware, Kosinski, & Keller, 1996; Ware, Kosinski, Turner-Bowker, & Gandek, 2002) assessed health-related QoL. This measure has been used with a variety of medical populations and provided a measure of general health perceptions. Mental (e.g., “Have emotional problems interfered with activities with your family?”) and physical (e.g., “Does your health limit you in climbing stairs?”) component summaries were computed by differential weighting of the eight scales. Per author guidelines, the mental component summary (MCS) weighted the mental health, role functioning, social functioning, and vitality scales higher than physical functioning scales. The MCS score was converted to a T-score, with a population mean of 50 and SD of 10; higher scores reflected better QoL. Coefficient α for the present study was .90.
Disease-Specific QoL
Disease-specific subscales of the Functional Assessment of Cancer Therapy (FACT) were used (Cella et al., 1993). These scales were designed to capture disease-specific symptoms that are not captured by more general QoL measures. Scales for cervical, endometrial, ovarian, and vulvar cancer have 4−10 common items (e.g., “I have hot flashes,” “I am bothered by constipation”), with the remainder being site-specific (e.g., cervical: “I am bothered by discharge or bleeding from my vagina;” endometrial: “I have pain or discomfort in my stomach area”). Items were rated on a 5-point scale ranging from 0 (not at all) to 4 (very much). To eliminate overlap with the sexuality measures (see above), items with sexual content were removed. Coefficient α's for the modified scales used in the present study were as follows: FACT-Cx (12 items; .43), FACT-En (15 items; .79), FACT-O (11 items; .65), and FACT-V (13 items; .81). To provide a comparable metric across participants, scales were standardized and mean item scores reported, with higher scores reflecting better QoL.
Control Variables
Health Status
Four measures were used; the first two measures were completed by a research nurse. (1) Functional status. The Karnofsky Performance Status (KPS) rating (Karnofsky & Burchenal, 1949) assessed participants' functional status. The scale ranged from 0 (Dead) to 100 (Normal, no complaints, no evidence of disease) with 10-point intervals. (2) Symptoms/ signs (SymS/Toxicity). Items were derived from the toxicity and status listing used by the Southwest Oncology Collaborative Group (SWOG) (Moinpour et al., 1989) for clinical trials. These ratings occurred following review of the medical chart (including lab and exam results) and participant self-report of specific, subjective symptoms (such as urinary urgency). Like other measures of this sort, items were grouped within body categories. The four most relevant to gynecologic disease—renal/bladder, gastrointestinal, endocrine, and mucosal—were used. Categories had four to six items (e.g., incontinence, dysuria, bladder cramps, increased frequency/urination, creatinine for renal/bladder), each rated on a unique scale; for example, for increased urinary frequency, the scale was 0 = none/no change, 1 = increase 2x normal, nocturia, 2 = increase greater than 2x normal, but less than hourly, and 3 = with urgency and hourly (or more). Items within categories were summed and averaged, and category scores were summed and averaged for an overall score. Higher scores indicated more life threatening symptoms. Internal consistency for the present study was .68. (3) Vaginal changes. Participants were queried about the presence (scored 1) or absence (scored 0) of five common vaginal sequelae of treatment (shortness, tightness, dryness, pain, and numbness). Items were summed to estimate the degree of participant-experienced vaginal change. Internal consistency for the present study was .71. (4) The Fatigue Symptom Inventory-Revised (Hann et al., 1998) assessed the frequency and severity of fatigue. The 7-item total disruption index (TDI) estimated fatigue interference with daily activities on an 11-point scale ranging from 0 (no interference) to 10 (extreme interference). Items were summed for a total score ranging from 0 to 70. The internal consistency for the present study was .94.
Partner Sexual Functioning
Eight items derived from the National Health and Social Life Survey (NHSLS) (Laumann, Gagnon, Michael, & Michaels, 1994) were used to assess partner sexual dysfunction. From the participant's perspective, partner sexual difficulties in the past 12 months were reported. The language of most items was gender neutral. Partner sexual interest, premature or delayed orgasm, pain or lack of pleasure during sexual activity, and ability to achieve/maintain an erection (for male partners) or lubrication response (for female partners) were rated as present or absent. Participants were also queried about medical conditions, such as diabetes, or use of medication that might affect partner sexual functioning. Items were totaled for a score ranging from 0 to 8, with higher scores indicating a greater number of sexual difficulties. Coefficient α for the present study was .79.
Procedure
Patients receiving follow-up care in the Division of Gynecologic Oncology at a university affiliated, National Cancer Institute-designated Comprehensive Cancer Center were accrued. There is not an accepted definition of “cancer survivor,” with some suggesting that survivorship begins when definitive treatment ends and others viewing 5 years post diagnosis as the beginning point. Here, “survivor” was operationalized as a patient who was at least 6 months post any cancer therapy and diagnosed 2−10 years previously as the clinically relevant interval for the study aims. By at least 2 years, the acute stress of diagnosis has ended (Andersen, Anderson, & deProsse, 1989b), patients have returned to their pre-cancer routines (Guidozzi, 1993; Klee, Thranov, & Machin, 2000a, b), and sexual changes have stabilized (Andersen et al., 1989a). By excluding patients treated longer than 10 years previously, we hoped to decrease the likelihood of added, comorbid conditions common in older adulthood that also disrupt sexuality (Lethbridge-Cejku, Schiller, & Bernadel, 2004).
Some patients meeting the follow up criterion were ineligible for reasons of prior non-gynecologic cancer diagnosis (n = 6), and ongoing cancer treatment (n = 3). Other exclusion criteria included: age <20 and >85 years, prior refusal of any cancer treatment, dementia or other condition impairing comprehension, significant visual or hearing deficit, major or untreated mental illness (e.g. schizophrenia), deficient ability to speak/read the English language, and/or current pregnancy, though no participants were excluded based on these criteria.
Two weeks prior to a regularly scheduled follow-up appointment, a letter providing a description of the study (i.e., purpose, time commitment, procedures, risks, and benefits) was sent to potentially eligible patients. Upon their clinic visit, patients were again screened and those remaining eligible were approached for participation. During a 12-month accrual period, 294 patients were found eligible and 260 (88%) were enrolled for a one-time, 60−90 min assessment consisting of interviews and questionnaire completion with a female research assistant and a health assessment with an oncology nurse. Data from the 175 (67%) participants who were married and/or living with a current sexual partner were examined; data from participants without partners (n = 85) are not discussed further.
Analytic Strategy
Preliminary analyses included comparison of the disease groups and clinical description of the sample. Correlations among sociodemographic, health status, partner sexual functioning variables, and sexual and psychological outcomes were also obtained. Only those variables significantly correlated with the outcome variable were included in the respective hierarchical multiple linear regression (HMLR) model. First, HMLR analyses tested the contribution of sexual self schema to current sexuality outcomes: intercourse frequency (sexual behavior), FSFI score (sexual responsiveness), global sexual satisfaction, and ITS score (body change stress). Variables were entered as previously specified (Andersen, 1994a): (1) sociodemographic, disease and treatment (e.g., site, stage of disease), (2) health status, (3) partner sexual functioning, and (4) SSS. The final step tested the association of SSS with each outcome, beyond the contribution of control variables.
Second, SSS was tested as a moderator of the effects of global sexual satisfaction on psychological outcomes: CESD (depressive symptoms), SF-12 MCS (global QoL), and FACT scores (disease-specific QoL). Variables were entered as indicated above for steps 1 thru 3, with the remaining steps as follows: (4) sexual satisfaction, (5) SSS, and the interaction term (6) satisfaction X SSS. The interaction term was computed as the cross product of z-scores of sexual satisfaction and SSS (Cohen, Cohen, West, & Aiken, 2003).
Results
Clinical Description of the Sample
Disease site groups were contrasted and there were no significant between-group differences in the sexuality, body change stress, or schema measures. Descriptive statistics for the sample, collapsed across disease sites, are provided in Table 1. The mean SSS score (sexual self schema) was 59.1, a score similar to that found for other samples, including breast cancer patients (M = 59; Yurek, Farrar & Andersen, 2000), gynecologic cancer patients (M = 57; Andersen et al., 1997; M = 56.1; Scott, Halford, & Ward, 2004), healthy adult women (M = 59; Andersen et al., 1997), and multiple samples of undergraduate women (M = 60.5; Andersen & Cyranowski, 1994 and M = 59.5; Cyranowski & Andersen, 2000).
Table 1.
Descriptive data and intercorrelations among control, sexual self schema, sexuality, body change stress, and quality of life variables (N = 175)
| M (SD) | Correlation coefficient |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Control | |||||||||||||||||
| 1. Age | 54.9 (11.8) | - | |||||||||||||||
| 2. Education | 13.9 (2.5) | -.09 | - | ||||||||||||||
| 3. Annual income ($000s) | 74.9 (57.5) | -.15 | .49** | - | |||||||||||||
| 4. Years since diagnosis | 4.2 (2.1) | .04 | -.05 | .04 | - | ||||||||||||
| 5. Vaginal changes | 1.8 (1.4) | -.09 | -.01 | .04 | -.11 | - | |||||||||||
| 6. KPS | 79.4 (11.2) | -.01 | .23** | .38** | .25** | -.19* | - | ||||||||||
| 7. SymS/Toxicities | 0.5 (0.2) | .01 | -.09 | -.07 | .01 | .38** | -.33** | - | |||||||||
| 8. Fatigue | 14.4 (16.0) | -.09 | -.20** | -.24** | -.14 | .25** | -.64** | .33** | - | ||||||||
| 9. Partner sexual function | 1.6 (2.0) | .23** | -.04 | -.17* | .04 | .14 | -.18* | .12 | .16* | - | |||||||
| Sexual self schema | |||||||||||||||||
| 10. SSS | 59.1 (15.6) | .03 | .02 | .10 | -.08 | .04 | .07 | .08 | -.12 | -.06 | - | ||||||
| Sexual functioning and satisfaction | |||||||||||||||||
| 11. Intercourse frequency | 1.9 (1.7) | -.45** | .03 | .24** | -.02 | -.04 | .27** | -.14 | -.31** | -.28** | .17* | - | |||||
| 12. Sexual responsiveness | 18.4 (11.6) | -.34** | .10 | .19* | -.01 | -.16* | .36** | -.22** | -.42** | -.21** | .23** | .80** | - | ||||
| 13. Global satisfaction | 3.7 (2.1) | -.10 | -.02 | .16* | .15* | -.15 | .28** | .00 | -.24** | -.25** | .19* | .48** | .46** | - | |||
| Body change stress | |||||||||||||||||
| 14. ITS | 17.2 (16.0) | -.27** | .04 | -.08 | -.09 | .35** | -.35** | .20** | .44** | .06 | -.13 | -.12 | -.20* | -.21** | - | ||
| Quality of life | |||||||||||||||||
| 15. CES-D | 4.1 (4.0) | -.17* | -.26** | -.09 | -.14 | .25** | -.48** | .27** | .68** | .02 | -.24** | -.19* | -.30** | -.31** | .45** | - | |
| 16. SF-12 MCS | 52.7 (10.5) | .31** | .06 | -.03 | .13 | -.24** | .22** | -.14 | -.48** | .05 | .14 | .11 | .22** | .27** | -.42** | .35** | - |
| 17. FACT | 3.5 (0.4) | .02 | .12 | .13 | .08 | -.37** | .45** | -.41** | -.50** | -.18* | .19* | .27** | .38** | .29** | -.33** | -.54** | -.62** |
p < .05
p < .01
Average intercourse frequency for the sample corresponded to “1 to 2 times per month.” Thirty percent (n = 53) reported that they were not sexually active; sixty-eight percent (n = 36) of these reported having no interest in sex. The mean FSFI (sexual responsiveness) score was 18.4. By comparison, Rosen et al. (2000) have reported a mean of 30.5 for a large sample of dysfunction-free, healthy controls and Wiegel et al. (2005) have provided evidence for a clinical cut-off score of 26.6. Sixty-four percent of the present sample fell below this cut-off score. The sample mean was also similar to or lower than means reported for clinical samples diagnosed with sexual dysfunction, including otherwise healthy patients with arousal disorders—19.2 (Meston, 2003); orgasm or desire disorders—19.7 (Rosen et al., 2000); or multiple dysfunctions—21.6 (Wiegel et al., 2005).
The mean global sexual satisfaction score corresponded to viewing one's sexual life as “average” in quality, similar to reports from other gynecologic cancer samples (Andersen et al., 1997), as well as healthy women (Laumann et al., 1994). The FSFI satisfaction domain score also provided a reference point for sexual satisfaction in the past 4 weeks. The mean was 3.9 (SD = 1.7), comparable to data from Wiegel et al. (2005) for clinical samples with sexual dysfunction (M range from 3.4 to 4.2) and unlike scores from healthy controls (M = 5.0). The mean for ITS (body change stress, M = 17.2) was similar to that of breast cancer patients treated with segmental mastectomy (lumpectomy; M = 16.1), which involves removal of the tumor and a portion of the surrounding breast tissue and the lining over the chest muscles, and unlike the score from breast patients treated with modified radical mastectomy (M = 29.2), which includes removal of the entire breast and nipple and extensive lymph node dissection (Frierson et al., 2006). Regarding partners' sexual function, more than half (54%) of the sample reported their partners as having at least one sexual problem. In fact, 26% reported 1−2 difficulties, 13% reported 3−4 difficulties, and 15% reported 5 or more sexual difficulties for their partners.
Descriptive statistics for psychological functioning outcomes, collapsed across disease sites, are provided in Table 1. With regard to CES-D score (depressive symptoms), the majority (79%) of the sample had few or no symptoms. However, 10% of the sample met the cutoff for symptom severity suggestive of clinical depression (>10; n = 17) and an additional 9% exceeded the cutoff for subclinical depression (>8; n = 16) (Kohout et al., 1993). By comparison, 12-month prevalence of mood disorders is approximately 8% among adult women in the United States (Kessler, Chiu, Demler, & Walters, 2005).
Contrast of the disease site groups revealed a significant difference on the SF-12 MCS (p = .003) only. Post-hoc comparisons revealed that Vulvar patients reported significantly lower mental health QoL (M = 43) than Endometrial patients (M = 55); other means were 51 for cervical and 52 for ovarian patients. Overall, the sample mean on the SF-12 MCS (52.7) was in the range of the normative score of 50. Similarly, the mean item score of 3.5 on the FACT subscales was comparable to cross-sectional data from validation samples (Basen-Engquist et al., 2001; Janda et al., 2005).
Regarding the health measures, the only disease-site difference found was for the vaginal change measure (p = .01). Follow up analyses revealed that the Vulvar patients reported significantly higher vaginal change scores (M = 3.1), indicating greater disruption, than Endometrial (1.6) or Ovarian patients (1.7); the mean for Cervical patients was 2.2. Descriptive statistics for the sample are provided in Table 1. On the KPS, a mean of 79 corresponds to an overall functional status evaluation of “normal activity with effort, some signs/symptoms of disease.” Similar scores have been reported for breast and lung cancer patients receiving radiation therapy (Lindsey, Larson, Dodd, Brecht, & Packer, 1994). The score of 14.4 on the TDI (fatigue) was midway between surveys of breast cancer patients treated with bone marrow transplant and healthy comparisons (19.1 and 10.4, respectively) (Hann et al., 1997). A score of .5 on the SymS/Toxicity measure suggested that, overall, when symptoms were present, they were of mild severity.
SSS, Sexuality, and Body Change Stress
Table 1 provides the correlations among variables considered for entry as controls. Table 2 summarizes the results of the HMLR analyses. All models were significant, accounting for 16−38% of the variance for the sexuality outcomes and 34% of the variance for ITS score (body change stress). After accounting for the effects of participant age, family income, physical functioning, and sexual functioning of the partner, SSS was significantly associated with both frequency of intercourse (β = .134, p = .047) and FSFI (β = .207, p = .002). SSS was also significantly associated with global sexual satisfaction after controlling for family income, time since the diagnosis of cancer, physical functioning, and partner sexual functioning (β = .165, p = .033).
Table 2.
Results of hierarchical multiple regression analyses testing association between sexual self schema and current sexual functioning and body change stress (N = 175)
| Step and predictor | Statistics by step |
Statistics by predictor |
||
|---|---|---|---|---|
| TR2 | ΔR2 | β | t | |
| Outcome: Frequency of intercourse; F(6, 147) = 13.74 | ||||
| 1. Age | .231 | .231** | −.438 | −6.32** |
| Family income | .070 | .96 | ||
| 2. KPS | .329 | .098** | .029 | .33 |
| TDI (fatigue) | −.276 | −3.17** | ||
| 3. Partner sexual functioning | .342 | .013 | −.114 | −1.63 |
| 4. SSS (sexual self schema) | .359 | .017* | .134 | 2.00* |
| Outcome: FSFI (sexual responsiveness); F(8, 145) = 10.99 | ||||
| 1. Age | .138 | .138** | −.376 | −5.45** |
| Family income | −.002 | −.03 | ||
| 2. Vaginal changes | .335 | .197** | −.080 | −1.10 |
| KPS | .104 | 1.14 | ||
| SymS/Toxicities | −.060 | −.81 | ||
| TDI | −.317 | −3.59** | ||
| 3. Partner sexual functioning | .336 | .001 | −.022 | −.32 |
| 4. SSS | .377 | .041** | .207 | 3.10** |
| Outcome: Global sexual satisfaction; F(6, 147) = 4.87 | ||||
| 1. Family income | .027 | .027* | .038 | .46 |
| 2. Years since diagnosis | .048 | .021 | .129 | 1.64 |
| 3. KPS | .097 | .049* | .132 | 1.25 |
| TDI | −.079 | −.80 | ||
| 4. Partner sexual functioning | .138 | .041** | −.204 | −2.63** |
| 5. SSS | .164 | .026* | .165 | 2.16* |
| Outcome: ITS (body change stress); F(6, 162) = 13.94 | ||||
| 1. Age | .077 | .077** | −.228 | −3.53** |
| 2. Vaginal changes | .331 | .254** | .274 | 3.92** |
| KPS | −.121 | −1.43 | ||
| SymS/Toxicities | −.033 | −.45 | ||
| TDI | .282 | 3.29** | ||
| 3. SSS | .340 | .009 | −.098 | −1.50 |
p < .05
p < .01
In addition, SSS was tested as a correlate of ITS. While age, vaginal changes, and fatigue were significant correlates, SSS did not add significant variance (β = −.098, p = .135). Thus, those survivors who were younger, reporting more adverse vaginal changes and greater fatigue were also experiencing higher levels of intrusive thoughts and avoidance with regard to their bodies.
Testing SSS as a Moderator
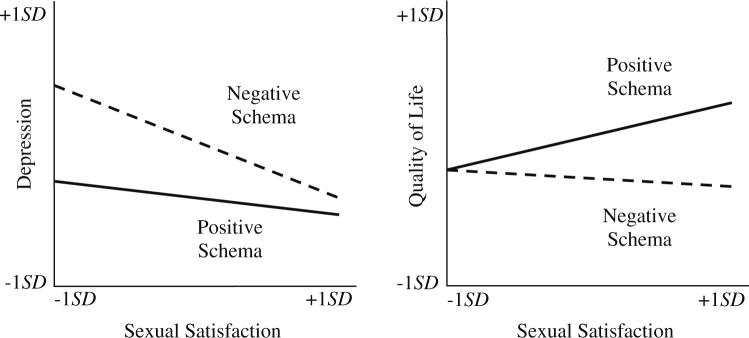
HMLR results are summarized in Table 3. All models were significant, accounting for 38−62% of the variance in the outcomes. The model for CES-D (depressive symptoms) accounted for 56% of the variance and the interaction between sexual satisfaction and schema was significant (β = .116, p = .039). These results suggested that a positive sexual self schema “buffered” participants from depressive symptoms when sexual satisfaction was low. In contrast, the combination of a negative schema and sexual dissatisfaction was associated with heightened depressive symptomatology. This relationship is graphically depicted in Fig. 1 (right panel); although a continuous variable, sexual self schema was dichotomized for this illustration. Per convention in illustrating results of tests of moderation, values one standard deviation above and below the standardized mean were used for positive and negative sexual self-schema lines. Similarly, the predictor (sexual satisfaction) and outcome (CES-D) variables were standardized and values one SD above and below the mean are used to anchor the lines (Cohen et al., 2003).
Table 3.
Results of hierarchical multiple regression analyses testing sexual self schema as a moderator between global sexual satisfaction and quality of life outcomes (N = 175)
| Step and predictor | Statistics by step |
Statistics by predictor |
||
|---|---|---|---|---|
| TR2 | ΔR2 | β | t | |
| Outcome: CES-D (depressive symptoms); F(9, 159) = 22.55 | ||||
| 1. Age | .103 | .103** | −.127 | −2.33* |
| Education | −.142 | −2.57* | ||
| 2. Vaginal changes | .500 | .397** | .070 | 1.19 |
| KPS | −.011 | −.15 | ||
| SymS/Toxicities | .060 | .99 | ||
| TDI | .513 | 7.13** | ||
| 3. Global sexual satisfaction | .529 | .029** | −.177 | −3.04** |
| 4. SSS | .549 | .020** | −.154 | −2.83** |
| 5. Sexual satisfaction × SSS | .561 | .012* | .116 | 2.08* |
| Outcome: SF-12 MCS (global QoL); F(7, 160) = 13.88 | ||||
| 1. Age | .096 | .096** | .265 | 4.14** |
| 2. Vaginal changes | .326 | .229** | −.104 | −1.59 |
| KPS | −.165 | −2.00* | ||
| TDI | −.461 | −5.46** | ||
| 3. Global sexual satisfaction | .366 | .040** | .222 | 3.26** |
| 4. SSS | .368 | .002 | .056 | .87 |
| 5. Sexual satisfaction × SSS | .378 | .010 | −.104 | −1.59 |
| Outcome: FACT (disease-specific QoL); F(8, 154) = 14.38 | ||||
| 1. Vaginal changes | .366 | .366** | −.163 | −2.41* |
| KPS | .142 | 1.74 | ||
| SymS/Toxicities | −.241 | −3.43** | ||
| TDI | −.262 | −3.17** | ||
| 2. Partner sexual functioning | .370 | .004 | −.021 | −.34 |
| 3. Global sexual satisfaction | .391 | .022* | .111 | 1.64 |
| 4. SSS | .413 | .022* | .143 | 2.27* |
| 5. Sexual satisfaction × SSS | .428 | .015* | .127 | 1.99* |
p < .05
p < .01
Fig. 1.
Sexual self schema as a significant moderator between sexual satisfaction and quality of life outcomes. A positive sexual self schema has a buffering effect from depressive symptoms for the patients when sexual satisfaction is low (left panel). Higher sexual satisfaction is related to better disease-specific quality of life only among the patients with a positive sexual self schema (right panel)
For overall SF-12 MCS (global QoL), sexual satisfaction was a significant predictor after controlling for age and physical functioning variables (β = .222, p = .001), although the interaction between sexual satisfaction and schema was not significant (β = −.104, p = .115). Disease site (Vulvar vs. other) was not included as a control step in these analyses because the sample of vulvar participants was small (n = 8). As an alternative strategy, vulvar participants were excluded and the analyses repeated. The effects were replicated with the exception that schema became a significant predictor (p = .038) of SF-12 MCS.
With FACT (disease-specific QoL) as the outcome, the interaction between sexual satisfaction and schema was significant (β = .127, p = .049), with the model accounting for 43% of variance. The significant interaction is illustrated in Fig. 1 (left panel). The interaction suggests that for the positive schema women, low sexual satisfaction and low FACT scores were associated, as were high satisfaction and high FACT scores. Conversely, participants with a negative sexual schema had poorer QoL, regardless of their level of sexual satisfaction.
Discussion
For these gynecologic cancer survivors, sexual morbidity was prevalent, with the majority reporting sexual responsive scores within the range or worse than scores reported by women seeking treatment for sexual dysfunctions. At a time of high health care costs, a rapid, easy strategy for identifying patients and survivors most in need of psychosocial services is important, and these data support consideration of sexual self schema as a relevant individual difference variable. First, sexual self schema was a correlate of current sexual functioning and body change stress, as expected. Second, and more importantly, sexual self schema was confirmed as a moderator of the relationship between participants' sexual satisfaction and psychological status (i.e., depressive symptoms and quality of life).
Schemas, Sexuality, and Psychological Functioning
Despite their sexual difficulties, many gynecologic cancer survivors, including those studied here, resume intercourse (Andersen et al., 1989a). Frequency of intercourse in this sample was comparable to available norms for similarly aged women (Laumann et al., 1994), but these and other longitudinal data have shown sexual satisfaction (Gershenson et al., 2007; Lindau et al., 2007) and responsiveness (Andersen et al., 1989a;Gershensonet al., 2007; Hawighorst-Knapstein et al., 2004; Lindau et al., 2007; Weijmar Schultz et al., 1991) to be significantly impaired following treatment. Thus, gynecologic cancer and its treatment constitute a sexually relevant stressor for women. To establish clinical utility of the sexual self schema construct in a diathesis-stress model for these patients, the diathetic factor must be (1) measurable in a brief and reliable manner; (2) specific to the sexual realm; (3) stable across time; (4) capable of interacting with sexually relevant stressors; and (5) predictive of pertinent outcomes. Previous psychometric studies of the schema measure have provided support for conditions (1) through (3). Here we discuss the interactive and predictive properties of schema using these data in illustration.
We begin by noting that the positive and negative schema patients did not differ in the potential for physical disruption to the pelvis or genitals as they did not differ in the types or combinations of treatments received (surgery, chemotherapy, and/or radiation therapy). Moreover, the analyses controlled for current physical symptomatology, both objective (e.g., signs/symptoms) and subjective (e.g., fatigue). Thus, it is reasonable to consider that women differing in the valence of their sexual self schema did not differ in the threat or the objective disruption that gynecologic cancer and its treatment posed. The finding that a positive sexual self schema was associated with more frequent sexual activity, better sexual responsiveness, and higher global sexual satisfaction for these patients as it is for healthy women (Andersen & Cyranowski, 1994; Wiederman & Hurst, 1997) is important. This suggests that individuals with a positive view were more resilient to the adverse sexual impacts of gynecologic cancer.
We can speculate on why this may be the case. We suggest that women with a positive schema respond differently to sexual disruptions as they arise, consistent with analog studies (Kuffel & Heiman, 2006). For example, they might attribute sexual difficulties to external, treatment-specific circumstances (e.g., vaginal dryness due to radiation therapy effects, fatigue due to chemotherapy) rather than internal causes (e.g., I have even less interest in sex now, I am embarrassed about my incision scar). Women with a positive view would be more comfortable and likely more skilled, in discussing sexual changes and managing sexual difficulties with their partner. These are not the cognitions, emotions, or behavioral patterns that characterize women with negative sexual self schemas. Their sexual repertoire is limited, they are less open to sexual exploration, and, indeed, they are inhibited and embarrassed about all things sexual. This hypothesis is consistent with experimental data showing that women with negative schemas are significantly more likely than women with positive schemas to respond to sexual-romantic cues in a negative manner (Cyranowski & Andersen, 2000).
Beyond noting that women with negative schemas are vulnerable to sexual difficulties, we have also suggested that their sexual disruption would have a negative effect on other emotions (Cyranowski et al., 1999), a relationship not previously tested. As noted above, previous studies have found gynecologic cancer survivors to be at particularly high risk for emotional distress (Parker et al., 2003), with high rates of depressive symptoms (Kornblith et al., 1995). In a review of studies using DSM-IV criteria, Thompson and Shear (1998) reported that as many as 23% had major depressive disorder; our data were consistent, with 10% having symptoms suggestive of major depression and another 9% with subclinical symptomatology. With such a high level of psychological burden, identification of patients at greatest risk becomes vital. Of relevance to the diathetic properties of schema are the data showing an interaction of schema and sexual satisfaction co-varying with the psychosocial outcomes. Interestingly, slightly different patterns were observed for depressive symptoms and quality of life.
To interpret the data in Fig. 1 (left panel), the psychopathology and psychotherapy literatures may be relevant. In Beck's Cognitive Model of Depression (Beck, 1963, 1967; Beck, Brown, Steer, Eidelson, & Riskind, 1987), negative schemas, often referred to as “core beliefs,” about the self, the world, and the future become part of a vicious cycle in which neutral and ambiguous situations are interpreted negatively and result in behavioral and affective responses that build and elaborate schemas (Watson, Clark, & Harkness, 1994). In the circumstances following gynecologic cancer treatment, women with negative, conflicted, or weak sexual self schemas would be reluctant to resume intercourse. Next, as sexual difficulties arose, e.g., absence of lubrication response, it would be stressful and anxiety provoking. With repeated experiences, they may come to avoid sexual contact and/or respond with a negativistic cognitive style (e.g., internal, stable, global attributions about their sexual difficulties). The latter could lower mood and reinforce a negative view of the self. Thus, the combination of low sexual satisfaction and negative schema may have heightened the risk for depressive symptoms. For women with positive schemas who also had low satisfaction, external—rather than internal—attributions for the sexual problems were more likely. Unlike the women with negative schemas, their lowered sexual satisfaction was not associated with more depressive symptoms.
Other important differences between negative and positive schemas were seen in the quality of life data, illustrated in Fig. 1 (right panel). When sexual satisfaction was low, quality of life was also low for women with positive schemas. These data are consistent with the conceptualization that sexuality is an important, central part of one's life for the woman with a positive sexual self schema. Low sexual satisfaction had no such relationship for the women with negative schemas; they did not appear to benefit from a satisfying sexual life in the same way that participants with positive schemas did.
Clinical Implications
The need for interventions to prevent or remediate sexual difficulties for these patients is apparent, but there is a gap between clinical knowledge and practice. Stead, Brown, Fallowfield, and Selby (2003) interviewed 43 physicians and nurses regularly treating women with ovarian cancer. Ninety-eight percent reported that they felt sexual issues should be discussed with patients, but only twenty-one percent reported doing so. When discussed, only 58% of healthcare professionals mentioned the potential for inhibited desire, 48% mentioned the possibility of fears about sexual activity, 42% noted dyspareunia, 30% altered arousal/vaginal dryness, and 7% altered pleasure or frequency of sexual activity. Regarding the psychosocial literature, the majority consists of clinical description (as this study is) and few test models or variables related to heightened risk. Few intervention studies have been conducted, and of them only five have included sexuality as a treatment targets or outcomes (Brotto et al., 2008; Caldwell et al., 2003; Capone, Good, Westie, & Jacobson, 1980; Robinson, Faris, & Scott, 1999; Scott et al., 2004).
Sexual self schema is an easily administered, reliable measure. These and other validity data support its consideration as an individual difference variable capable of making both sexual and psychological distinctions among patients. The data suggest its utility for use in identifying patients at risk. Schemas are generally stable (Markus & Kunda, 1986). That does not imply, however, that negative cognitions arising from a negatively valenced schema cannot be changed (for a discussion, see Padesky, 1994). Indeed, the psychopathology and psychotherapy literatures show that cognitive schema-based therapy is efficacious in the treatment of chronic depression (McCullough, 2000), personality disorders (Nordahl, Holthe, & Haugum, 2005; Nordahl & Nysaeter, 2005), and comorbid addiction (Ball, 2007). Thus, consideration of a cognitive schema component to a comprehensive sexuality intervention would seem important.
Limitations and Strengths
A cross-sectional design provided for efficient recruitment of a large, representative cohort of gynecologic cancer survivors (Jemal et al., 2007). The data were analyzed and discussed with sexuality variables as “predictors” and depressive symptoms and quality of life variables as outcomes, but of course, directionality cannot be established. Data were not obtained at the time of diagnosis or shortly thereafter, instead all variables were assessed, on average, 4 years following the sexual stressor. Yet, the consistency of the mean score of this sample (59.1) and those from multiple other female samples of varying ages, health status, and cancer diagnoses, counters (but does not confirm) the hypothesis that pre-cancer schema scores would have been significantly different than those shown here.
While the literature on sexual outcomes following gynecologic cancer is considerable, there are few data either from partners directly or from patients' reports of their partners' sexual functioning (Andersen et al., 1989a). While partner sexual functioning was correlated with several outcomes (see Table 1), it only contributed significant variance in the analysis predicting sexual satisfaction, with higher levels of partner sexual difficulties associated with participant reports of lower sexual satisfaction.
In contrast, a significant, negative contributor to patients' sexuality (as well as mental health and quality of life) was their health. This was expected, as health worries are the source of greatest concern among survivors (Spencer et al., 1999) and health impairments impact patients' return to normal routines (Bradley et al., 2005), and even the meaning patients derive following the cancer experience (Jim & Andersen, 2007). The variables contributed significant variance—5 to 25% across the sexual outcomes and 23 to 40% across the psychological outcomes. With these data, a new finding was the negative contribution of symptoms—particularly vaginal changes and fatigue—to women's worries and stress regarding body changes. Some quality of life studies have used the KPS or patient symptom reports (Dodd, 1988; Northouse, Kershaw, Mood, & Schafenacker, 2005; Scheier et al., 2005), but the use of the symptom-atology and toxicity listing is novel. These measures are costly, as medical expertise is required of the rater (e.g., a nurse specialist), yet they provide the benefit of objective, symptom-specific scales, unlike patient self reported health which is prone to reporting biases, including co-variation with negative affect (e.g., Denollet, 1991; Geisser, Roth, Theisen, Robinson, & Riley, 2000).
In general, the large sample was representative of the distribution of gynecologic disease sites and patients varied widely in age. Generalizability across ethnicity is unknown, though these data may underestimate outcomes. That is, the available research on African-American cancer patients, for example, shows higher rates of distress, more comorbid medical conditions, and more unmet medical and emotional needs than cancer patients with other ethnic backgrounds (Ashing-Giwa, Ganz, & Petersen, 1999; Ogle, Swanson, Woods, & Azzouz, 2000). Other non-participants were those not returning for follow up, including patients with aggressive, rapidly progressing cancers or those with fewer economic or social resources (Katapodi, Facione, Miaskowski, Dodd, & Waters, 2002) unable to schedule or keep follow up appointments.
Conclusion
Approximately 80,000 women in the United States and 1.8 million worldwide will be diagnosed with gynecologic cancers in 2007 (Ferlay, Bray, Pisani, & Parkin, 2004; Jemal et al., 2007) and increasing numbers of women are surviving (Reis et al., 2004). Despite a substantial cancer survivorship literature, much is yet to be learned about quality of life and sexuality for these patients. The data from the present study suggest that an understanding of patients' sexual self view would enhance our understanding of their sexuality and their quality of life more generally. Additional studies, including prospective, longitudinal research designs testing predictors of sexual and psychological adjustment are needed and would be important in designing tailored, evidence-based interventions.
Acknowledgments
Supported by grants from Henry M. Jackson Foundation for the Military Medicine (Department of Defense; Gynecological Cancer Center for Health Disparities GCC-2004-1), the National Cancer Institute (RO1CA92704, KO5 CA098133), and The Ohio State University Alumni Grants for Graduate Research and Scholarship. We thank the patients for their participation. These individuals also made important contributions: Elisabeth Yost, B.A., Lois Dial, R.N., Laura Peterson, M.P.H., and gynecologic oncologists David E. Cohn, M.D., Larry J. Copeland, M.D., Lynne A. Eaton, M.D., and David O'Malley, M.D.
Contributor Information
Kristen M. Carpenter, Division of Cancer Prevention and Control Research, University of California, Los Angeles, 650 Charles Young Drive South, A2−125, Box 956900, Los Angeles, CA 90095−6900, USA
Barbara L. Andersen, Departments of Psychology and Obstetrics and Gynecology, Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA
Jeffrey M. Fowler, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, College of Medicine, Comprehensive Cancer Center, Ohio State University, Columbus, OH, USA
G. Larry Maxwell, Gynecologic Disease Center, U.S. Military Cancer Institute, Walter Reed Army Medical Center, Washington, DC, USA.
References
- Andersen BL. Sexual functioning morbidity among cancer survivors. Current status and future research directions. Cancer. 1985;55:1835–1842. doi: 10.1002/1097-0142(19850415)55:8<1835::aid-cncr2820550832>3.0.co;2-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL. Surviving cancer. Cancer. 1994a;74(4 Suppl):1484–1495. doi: 10.1002/1097-0142(19940815)74:4+<1484::aid-cncr2820741614>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Andersen BL. Yes, there are sexual problems. Now, what can we do about them? Gynecologic Oncology. 1994b;52:10–13. doi: 10.1006/gyno.1994.1003. [DOI] [PubMed] [Google Scholar]
- Andersen BL. Surviving cancer: The importance of sexual self-concept. Medical and Pediatric Oncology. 1999;33:15–23. doi: 10.1002/(sici)1096-911x(199907)33:1<15::aid-mpo4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Anderson B, deProsse C. Controlled prospective longitudinal study of women with cancer: I. Sexual functioning outcomes. Journal of Consulting and Clinical Psychology. 1989a;57:683–691. doi: 10.1037//0022-006x.57.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Anderson B, deProsse C. Controlled prospective longitudinal study of women with cancer: II. Psychological outcomes. Journal of Consulting and Clinical Psychology. 1989b;57:692–697. doi: 10.1037//0022-006x.57.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Broffitt B. Is there a reliable and valid self-report measure of sexual behavior? Archives of Sexual Behavior. 1988;17:509–525. doi: 10.1007/BF01542339. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Cyranowski JM. Women's sexual self-schema. Journal of Personality and Social Psychology. 1994;67:1079–1100. doi: 10.1037//0022-3514.76.4.645. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Cyranowski JM, Espindle D. Men's sexual self-schema. Journal of Personality and Social Psychology. 1999;76:645–661. doi: 10.1037//0022-3514.76.4.645. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. Journal of Consulting and Clinical Psychology. 1997;65:221–229. doi: 10.1037//0022-006x.65.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African-American and white long term breast carcinoma survivors. Cancer. 1999;85:418–426. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Ball SA. Comparing individual therapies for personality disordered opioid dependent patients. Journal of Personality Disorders. 2007;21:305–321. doi: 10.1521/pedi.2007.21.3.305. [DOI] [PubMed] [Google Scholar]
- Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, Webster K, Cella D, Hu S, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. Journal of Clinical Oncology. 2001;19:1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- Beck AT. Thinking and depression. I. Idiosyncratic content and cognitive distortions. Archives of General Psychiatry. 1963;9:324–333. doi: 10.1001/archpsyc.1963.01720160014002. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Causes and treatment. University of Pennsylvania Press; Philadelphia: 1967. [Google Scholar]
- Beck AT, Brown G, Steer RA, Eidelson JI, Riskind JH. Differentiating anxiety and depression: A test of the cognitive content-specificity hypothesis. Journal of Abnormal Psychology. 1987;96:179–183. doi: 10.1037//0021-843x.96.3.179. [DOI] [PubMed] [Google Scholar]
- Bertero C. Altered sexual patterns after treatment for prostate cancer. Cancer Practice. 2001;9:245–251. doi: 10.1046/j.1523-5394.2001.009005245.x. [DOI] [PubMed] [Google Scholar]
- Blanker MH, Bosch JL, Groeneveld FP, Bohnen AM, Prins A, Thomas S, et al. Erectile and ejaculatory dysfunction in a community-based sample of men 50 to 78 years old: prevalence, concern, and relation to sexual activity. Urology. 2001;57:763–768. doi: 10.1016/s0090-4295(00)01091-8. [DOI] [PubMed] [Google Scholar]
- Bradley EH, Carlson MD, Gallo WT, Scinto J, Campbell MK, Krumholz HM. From adversary to partner: Have quality improvement organizations made the transition? Health Services Research. 2005;40:459–476. doi: 10.1111/j.1475-6773.2005.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. Journal of Clinical Oncology. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- Brotto LA, Heiman JR, Goff B, Greer B, Lentz G, Swisher E, et al. A psychoeducational intervention for sexual dysfunction in women with gynecological cancer. Archives of Sexual Behavior. 2008;37:317–329. doi: 10.1007/s10508-007-9196-x. [DOI] [PubMed] [Google Scholar]
- Cain VS, Johannes CB, Avis NE, Mohr B, Schocken M, Skurnick J, et al. Sexual functioning and practices in a multi-ethnic study of midlife women: Baseline results from SWAN. Journal of Sex Research. 2003;40:266–276. doi: 10.1080/00224490309552191. [DOI] [PubMed] [Google Scholar]
- Caldwell R, Classen C, Lagana L, McGarvey E, Baum L, Duenke SD, et al. Changes in sexual functioning and mood among women treated for gynecological cancer who receive group therapy: A pilot study. Journal of Clinical Psychology in Medical Settings. 2003;10:149–156. [Google Scholar]
- Capone MA, Good RS, Westie KS, Jacobson AF. Psychosocial rehabilitation of gynaecologic oncology patients. Archives of Physical Medicine and Rehabilitation. 1980;61:128–132. [PubMed] [Google Scholar]
- Cella DF, Lai J, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West S, Aiken L. Applied multiple regression/correlation analysis for the behavioral sciences. Lawrence Erlbaum Associates; Mahwah, NJ: 2003. [Google Scholar]
- Comstock GW, Helsing KJ. Symptoms of depression in two communities. Psychological Medicine. 1976;6:551–563. doi: 10.1017/s0033291700018171. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Aarestad SL, Andersen BL. The role of sexual self-schema in a diathesis-stress model of sexual dysfunction. Applied & Preventive Psychology. 1999;8:217–228. doi: 10.1016/S0962-1849(05)80078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Andersen BL. Schemas, sexuality, and romantic attachment. Journal of Personality and Social Psychology. 1998;74:1364–1379. doi: 10.1037//0022-3514.74.5.1364. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Andersen BL. Evidence of self-schematic cognitive processing in women with differing sexual self-views. Journal of Social and Clinical Psychology. 2000;19:519–543. doi: 10.1521/jscp.2000.19.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denollet J. Negative affectivity and repressive coping: Pervasive influence on self-reported mood, health, and coronary-prone behavior. Psychosomatic Medicine. 1991;53:538–556. doi: 10.1097/00006842-199109000-00005. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The DSFI: A multidimensional measure of sexual functioning. Journal of Sex & Marital Therapy. 1979;5:244–281. doi: 10.1080/00926237908403732. [DOI] [PubMed] [Google Scholar]
- Dodd MJ. Efficacy of proactive information on self-care in chemotherapy patients. Patient Education & Counseling. 1988;11:215–225. doi: 10.1016/0738-3991(88)90021-3. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin D. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. IARC CancerBase. 2004 (Electronic Version) [Google Scholar]
- Frierson GM, Thiel DL, Andersen BL. Body change stress for women with breast cancer: The Breast-Impact of Treatment Scale. Annals of Behavioral Medicine. 2006;32:77–81. doi: 10.1207/s15324796abm3201_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisser ME, Roth RS, Theisen ME, Robinson ME, Riley JL. Negative affect, self-report of depressive symptoms, and clinical depression: Relation to the experience of chronic pain. Clinical Journal of Pain. 2000;16:110–120. doi: 10.1097/00002508-200006000-00004. [DOI] [PubMed] [Google Scholar]
- Gershenson DM, Miller AM, Champion VL, Monahan PO, Zhao Q, Cella D, et al. Reproductive and sexual function after platinum-based chemotherapy in long-term ovarian germ cell tumor survivors: A Gynecologic Oncology Group study. Journal of Clinical Oncology. 2007;25:2792–2797. doi: 10.1200/JCO.2006.08.4590. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. The development of markers for the Big-Five factor structure. Psychological Assessment. 1992;4:26–42. [Google Scholar]
- Greimel E, Thiel I, Peintinger F, Cegnar I, Pongratz E. Prospective assessment of quality of life in female cancer patients. Gynecologic Oncology. 2002;85:140–147. doi: 10.1006/gyno.2002.6586. [DOI] [PubMed] [Google Scholar]
- Guidozzi F. Living with ovarian cancer. Gynecologic Oncology. 1993;50:202–207. doi: 10.1006/gyno.1993.1193. [DOI] [PubMed] [Google Scholar]
- Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, et al. Measurement of fatigue in cancer patients: Development and validation of the Fatigue Symptom Inventory. Quality of Life Research. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- Hann DM, Jacobsen P, Martin SC, Kronish LE, Azzarello LM, Fields KK. Fatigue in women treated with bone marrow transplantation for breast cancer: A comparison with women with no history of cancer. Supportive Care in Cancer. 1997;5:44–52. doi: 10.1007/BF01681961. [DOI] [PubMed] [Google Scholar]
- Hawighorst-Knapstein S, Fusshoeller C, Franz C, Trautmann K, Schmidt M, Pilch H, et al. The impact of treatment for genital cancer on quality of life and body image—results of a prospective longitudinal 10-year study. Gynecologic Oncology. 2004;94:398–403. doi: 10.1016/j.ygyno.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Janda M, Obermair A, Cella D, Crandon AJ, Trimmel M. Vulvar cancer patients' quality of life: A qualitative assessment. International Journal of Gynecologic Cancer. 2004;14:875–881. doi: 10.1111/j.1048-891X.2004.14524.x. [DOI] [PubMed] [Google Scholar]
- Janda M, Obermair A, Cella D, Perrin LC, Nicklin JL, Ward BG, et al. The functional assessment of cancer-vulvar: Reliability and validity. Gynecologic Oncology. 2005;97:568–575. doi: 10.1016/j.ygyno.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA: A Cancer Journal for Clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Jenkins R, Schover LR, Fouladi RT, Warneke C, Neese L, Klein EA, et al. Sexuality and health-related quality of life after prostate cancer in African-American and white men treated for localized disease. Journal of Sex & Marital Therapy. 2004;30:79–93. doi: 10.1080/00926230490258884. [DOI] [PubMed] [Google Scholar]
- Jensen PT, Klee MC, Thranov I, Groenvold M. Validation of a questionnaire for self-assessment of sexual function and vaginal changes after gynaecological cancer. Psycho-Oncology. 2004;13:577–592. doi: 10.1002/pon.757. [DOI] [PubMed] [Google Scholar]
- Jim HS, Andersen BL. Meaning in life mediates the relationship between social and physical functioning and distress in cancer survivors. British Journal of Health Psychology. 2007;12:363–381. doi: 10.1348/135910706X128278. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of chemotherapeutic agents in cancer. Columbia University Press; New York: 1949. pp. 199–105. [Google Scholar]
- Katapodi MC, Facione NC, Miaskowski C, Dodd MJ, Waters C. The influence of social support on breast cancer screening in a multicultural community sample. Oncology Nursing Forum. 2002;29:845–852. doi: 10.1188/02.ONF.845-852. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee M, Thranov I, Machin D. Life after radiotherapy: The psychological and social effects experienced by women treated for advanced stages of cervical cancer. Gynecologic Oncology. 2000a;76:5–13. doi: 10.1006/gyno.1999.5644. [DOI] [PubMed] [Google Scholar]
- Klee M, Thranov I, Machin D. The patients' perspective on physical symptoms after radiotherapy for cervical cancer. Gynecologic Oncology. 2000b;76:14–23. doi: 10.1006/gyno.1999.5642. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. Journal of Aging and Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Thaler HT, Wong G, Vlamis V, Lepore JM, Loseth DB, et al. Quality of life of women with ovarian cancer. Gynecologic Oncology. 1995;59:231–242. doi: 10.1006/gyno.1995.0014. [DOI] [PubMed] [Google Scholar]
- Kuffel SW, Heiman JR. Effects of depressive symptoms and experimentally adopted schemas on sexual arousal and affect in sexually healthy women. Archives of Sexual Behavior. 2006;35:163–177. doi: 10.1007/s10508-005-9015-1. [DOI] [PubMed] [Google Scholar]
- Laumann EO, Gagnon JH, Michael RT, Michaels S. The social organization of sexuality: Sexual practices in the United States. University of Chicago Press; Chicago: 1994. [Google Scholar]
- Leake RL, Gurrin LC, Hammond IG. Quality of life in patients attending a low-risk gynaecological oncology follow-up clinic. Psycho-Oncology. 2001;10:428–435. doi: 10.1002/pon.539. [DOI] [PubMed] [Google Scholar]
- Lethbridge-Cejku M, Schiller JS, Bernadel L. [February 2, 2007];Summary health statistics for U.S. adults: National Health Interview Survey, 2002. 2004 from ncbi.nlm.nih.gov. [PubMed]
- Lindau ST, Gavrilova N, Anderson D. Sexual morbidity in very long term survivors of vaginal and cervical cancer: A comparison to national norms. Gynecologic Oncology. 2007;106:413–418. doi: 10.1016/j.ygyno.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey AM, Larson PJ, Dodd MJ, Brecht ML, Packer A. Comorbidity, nutritional intake, social support, weight, and functional status over time in older cancer patients receiving radiotherapy. Cancer Nursing. 1994;17:113–124. [PubMed] [Google Scholar]
- Markus HR. Self-schemata and processing information about the self. Journal of Personality and Social Psychology. 1977;35:63–78. [Google Scholar]
- Markus HR. The dynamic self-concept: A social psychological perspective. Annual Review of Psychology. 1987;38:299–337. [Google Scholar]
- Markus HR, Kunda Z. Stability and malleability of the self-concept. Journal of Personality and Social Psychology. 1986;51:858–866. doi: 10.1037//0022-3514.51.4.858. [DOI] [PubMed] [Google Scholar]
- McCullough JP. Treatment for chronic depression. Guilford Press; New York: 2000. [Google Scholar]
- Meston CM. Validation of the Female Sexual Function Index (FSFI) in women with female orgasmic disorder and in women with hypoactive sexual desire disorder. Journal of Sex & Marital Therapy. 2003;29:39–46. doi: 10.1080/713847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BE, Pittman B, Case D, McQuellon RP. Quality of life after treatment for gynecologic malignancies: A pilot study in an outpatient clinic. Gynecologic Oncology. 2002;87:178–184. doi: 10.1006/gyno.2002.6812. [DOI] [PubMed] [Google Scholar]
- Moinpour CM, Feigl P, Metch B, Hayden KA, Meyskens FL, Crowley J. Quality of life end points in cancer clinical trials: Review and recommendations. Journal of the National Cancer Institute. 1989;81:485–495. doi: 10.1093/jnci/81.7.485. [DOI] [PubMed] [Google Scholar]
- Nordahl HM, Holthe H, Haugum JA. Early maladaptive schemas in patients with or without personality disorders: Does schema modification predict symptomatic relief? Clinical Psychology and Psychotherapy. 2005;12:142–149. [Google Scholar]
- Nordahl HM, Nysaeter TE. Schema therapy for patients with borderline personality disorder: A single case series. Journal of Behavior Therapy and Experimental Psychiatry. 2005;36:254–264. doi: 10.1016/j.jbtep.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Northouse L, Kershaw T, Mood D, Schafenacker A. Effects of a family intervention on the quality of life of women with recurrent breast cancer and their family caregivers. Psycho-Oncology. 2005;14:478–491. doi: 10.1002/pon.871. [DOI] [PubMed] [Google Scholar]
- Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity: Redefining chronic diseases. Cancer. 2000;88:653–663. doi: 10.1002/(sici)1097-0142(20000201)88:3<653::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Padesky C. Schema change processes in cognitive therapy. Clinical Psychology and Psychotherapy. 1994;1:267–278. [Google Scholar]
- Parker PA, Baile WF, De Moor C, Cohen L. Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psycho-Oncology. 2003;12:183–193. doi: 10.1002/pon.635. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reis LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER cancer statistics review, 1975–2002. 2004 (Electronic Version) [Google Scholar]
- Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynaecologic carcinoma treated with radiotherapy. International Journal of Radiation Oncology, Biology, & Physics. 1999;44:497–506. doi: 10.1016/s0360-3016(99)00048-6. [DOI] [PubMed] [Google Scholar]
- Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. Journal of Sex & Marital Therapy. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Helgeson VS, Schulz R, Colvin S, Berga S, Bridges MW, et al. Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early-stage breast cancer. Journal of Clinical Oncology. 2005;23:4298–4311. doi: 10.1200/JCO.2005.05.362. [DOI] [PubMed] [Google Scholar]
- Schover LR, Fife M, Gershenson DM. Sexual dysfunction and treatment for early stage cervical cancer. Cancer Detection and Prevention. 1989;63:204–212. doi: 10.1002/1097-0142(19890101)63:1<204::aid-cncr2820630133>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schover LR, Fouladi RT, Warneke CL, Neese L, Klein EA, Zippe C, et al. Defining sexual outcomes after treatment for localized prostate carcinoma. Cancer. 2002a;95:1773–1785. doi: 10.1002/cncr.10848. [DOI] [PubMed] [Google Scholar]
- Schover LR, Fouladi RT, Warneke CL, Neese L, Klein EA, Zippe C, et al. The use of treatments for erectile dysfunction among survivors of prostate carcinoma. Cancer. 2002b;95:2397–2407. doi: 10.1002/cncr.10970. [DOI] [PubMed] [Google Scholar]
- Scott JL, Halford WK, Ward BG. United we stand? The effects of a couple-coping intervention on adjustment to early stage breast or gynecological cancer. Journal of Consulting and Clinical Psychology. 2004;72:1122–1135. doi: 10.1037/0022-006X.72.6.1122. [DOI] [PubMed] [Google Scholar]
- Spencer SM, Lehman JM, Wynings C, Arena PL, Carver CS, Antoni MH, et al. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychology. 1999;18:159–168. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]
- Stead ML, Brown JM, Fallowfield L, Selby P. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. British Journal of Cancer. 2003;88:666–671. doi: 10.1038/sj.bjc.6600799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DE, Wong F, Duff S, Melancon CH, Cheung AM. What doesn't kill you makes you stronger:” An ovarian cancer survivor survey. Gynecologic Oncology. 2001;83:537–542. doi: 10.1006/gyno.2001.6437. [DOI] [PubMed] [Google Scholar]
- Thompson DS, Shear MK. Psychiatric disorders and gynecological oncology: A review of the literature. General Hospital Psychiatry. 1998;20:241–247. doi: 10.1016/s0163-8343(98)00030-9. [DOI] [PubMed] [Google Scholar]
- Thranov I, Klee M. Sexuality among gynecologic cancer patients—a cross-sectional study. Gynecologic Oncology. 1994;52:14–19. doi: 10.1006/gyno.1994.1004. [DOI] [PubMed] [Google Scholar]
- Vincent CE, Vincent B, Greiss FC, Linton EB. Some marital-sexual concomitants of carcinoma of the cervix. Southern Medical Journal. 1975;68:552–558. doi: 10.1097/00007611-197505000-00009. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. SF-12v2: How to score version 2 of the SF-12 health survey. QualityMetric Inc; Lincoln, RI: 2002. [Google Scholar]
- Watson D, Clark LA, Harkness AR. Structures of personality and their relevance to psychopathology. Journal of Abnormal Psychology. 1994;103:18–31. [PubMed] [Google Scholar]
- Weijmar Schultz WC, van De Wiel HB, Bouma J. Psychosexual functioning after treatment for cancer of the cervix: A comparative and longitudinal study. International Journal of Gynaecologic Cancer. 1991;1:37–46. [Google Scholar]
- Wiederman MW, Hurst SR. Physical attractiveness, body image, and women's sexual self-schema. Psychology of Women Quarterly. 1997;21:567–580. [Google Scholar]
- Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): Cross-validation and development of clinical cutoff scores. Journal of Sex & Marital Therapy. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- Yurek D, Farrar W, Andersen BL. Breast cancer surgery: Comparing surgical groups and determining individual differences in postoperative sexuality and body change stress. Journal of Consulting and Clinical Psychology. 2000;68:697–709. doi: 10.1037//0022-006X.68.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]