Abstract
Background
The spectrum approach was used to examine contributions of comorbid symptom dimensions of substance abuse and eating disorder to abnormal prefrontal-cortical and subcortical-striatal activity to happy and fear faces previously demonstrated in bipolar disorder (BD).
Method
Fourteen remitted BD-type I and sixteen healthy individuals viewed neutral, mild and intense happy and fear faces in two event-related fMRI experiments. All individuals completed Substance-Use and Eating-Disorder Spectrum measures. Region-of-Interest analyses for bilateral prefrontal and subcortical-striatal regions were performed.
Results
BD individuals scored significantly higher on these spectrum measures than healthy individuals (p < 0.05), and were distinguished by activity in prefrontal and subcortical-striatal regions. BD relative to healthy individuals showed reduced dorsal prefrontal-cortical activity to all faces. Only BD individuals showed greater subcortical-striatal activity to happy and neutral faces. In BD individuals, negative correlations were shown between substance use severity and right PFC activity to intense happy faces (p < 0.04), and between substance use severity and right caudate nucleus activity to neutral faces (p < 0.03). Positive correlations were shown between eating disorder and right ventral putamen activity to intense happy (p < 0.02) and neutral faces (p < 0.03). Exploratory analyses revealed few significant relationships between illness variables and medication upon neural activity in BD individuals.
Limitations
Small sample size of predominantly medicated BD individuals.
Conclusion
This study is the first to report relationships between comorbid symptom dimensions of substance abuse and eating disorder and prefrontal-cortical and subcortical-striatal activity to facial expressions in BD. Our findings suggest that these comorbid features may contribute to observed patterns of functional abnormalities in neural systems underlying mood regulation in BD.
Keywords: Bipolar disorder, Functional MRI, Emotion processing, Spectrum approach
1. Introduction
Bipolar disorder (BD) is a most debilitating psychiatric disorder, with a high rate of late or mis-diagnoses, often as unipolar depression (Bowden, 2001). The research agenda for DSM-V emphasizes the need for a new classification system for all psychiatric disorders based upon pathophysiologic and etiological processes (e.g. Phillips and Frank, 2006), that may represent biomarkers of a disorder (e.g. Kraemer et al., 2002). For disorders such as BD, the identification of these biomarkers would be a major step toward improving diagnostic accuracy (e.g. Hasler et al., 2006). Functional neuroimaging can examine abnormalities that may reflect pathophysiological neural mechanisms underlying core clinical features of psychiatric illnesses. Several recent neuroimaging studies in adult BD reported inconsistent findings of dysfunctional dorsal and ventral prefrontal-cortical and subcortical-striatal neural systems implicated in mood-regulation. Specifically, patterns of increased amygdala and striatal, and decreased dorsal pre-frontal-cortical (DPFC) activity to emotionally-salient stimuli, and during cognitive control tasks have been shown (e.g. Green et al., 2007; Phillips et al., 2008a).
Although present studies in BD have focused on neural systems associated with mood-regulation, they have largely ignored common disabling comorbid features that characterize the disorder (Kupfer, 2005) and contribute to its psychopathology. The spectrum approach to BD includes measures of lifetime experiences of symptoms, clinical features and temperamental traits that aim to improve assessing the clinical phenotype of BD, and contains an integrated view of common comorbidities (e.g. Cassano et al., 1999). Comorbid symptom dimensions of BD are associated with more severe illness course, poorer treatment compliance and worse outcomes related to suicide (Krishnan, 2005). They include substance abuse, eating disorders, psychosis, obsessive-compulsive and anxiety symptoms (Krishnan, 2005). For example, an increased risk for substance abuse has been observed in BD (e.g. Regier et al., 1990), and is associated with more severe mood episodes and worse illness course (Nolen et al., 2004). It may be explained in terms of self-medication, improving self-image, or alleviating symptoms of anxiety (Sonne et al., 1994). Eating disorders, particularly binge eating or weight gain, other than those associated with medication effects, are common in BD (e.g. Angst et al., 2002).
There is increasing evidence of overlap between functional abnormalities in neural systems implicated in BD and those associated with the above comorbid symptom dimensions. For example, BD-like reduced activity in right dorsolateral prefrontal-cortical (DLPFC) regions during decision making tasks have been demonstrated in currently dependant amphetamine and opiate users (Ersche et al., 2005a,b), and in abstinent cocaine users (Bolla et al., 2003; Yucel and Lubman, 2007). In contrast to findings in BD, decreased rather than increased ventral striatal activity has been reported to formerly rewarding cues in detoxified alcoholics (Wrase et al., 2007). These findings support the growing literature in substance abuse showing patterns of decreased ventral striatal dopamine release also during pharmacologic challenge with amphetamine (Martinez et al., 2005; Volkow et al., 2007a,b).
The overlap between neural systems underlying mood regulation and appetitive behaviors is well documented (Beaver et al., 2006; Kaye, 2007). Abnormally increased striatal activity during reward processing has been reported in eating-disordered individuals (Wagner et al., 2007), similar to the patterns of elevated ventral striatal and amygdala activity observed in BD adults (Green et al., 2007; Phillips et al., 2008a).
The above findings suggest that dysfunctional prefrontal-cortical and subcortical-striatal neural regions previously observed in BD may be a reflection, at least in part, of comorbid symptom dimensions that characterize the illness. Using an implicit emotion labeling task associated with subcortical limbic activity, we previously demonstrated, in a group of remitted BD individuals, greater striatal activity to happy faces and decreased dorsal prefrontal-cortical (DPFC) activity to both happy and fear faces (Hassel et al., 2008). In these BD individuals, most common comorbid features were substance use and eating disorders, but the contribution of these to abnormal prefrontal-cortical and striatal activity was unexamined. The present study aimed to assess, in these remitted BD individuals, the relationship between comorbid symptom dimensions of substance abuse and eating disorders, and patterns of abnormal prefrontal-cortical and subcortical-striatal activity. Based on the above findings we hypothesized: (1) Severity of comorbid symptom dimensions of substance abuse would be negatively associated with both, the magnitude of striatal activity to happy faces, and the magnitude of DLPFC activity, to happy and fear faces. (2) Severity of comorbid symptom dimensions of eating disorders would be positively associated with the magnitude of amygdala and striatal activity to happy faces.
2. Methods
2.1. Participants
Fourteen BD-subtype-I individuals (23–52 years; 63% female) from our previous sample (Hassel et al., 2008) were recruited from Western Psychiatric Institute and Clinic, Mood Disorders Treatment and Research Program, University of Pittsburgh. All were in remission (DSM-IV criteria; Structured Clinical Interview for DSM-IV (SCID-I); First et al., 1995). Euthymic status was defined, a-priori, as having been in remission for at least 2 months as assessed by SCID and clinical interview. All BD individuals scored <10 on the Young Mania Rating Scale (Young et al., 1978). Twelve BD individuals scored <7 on the Hamilton Rating Scale for Depression (HRSD-25; Williams, 1988). Two scored <14 but were included because clinical evaluation deemed them eligible based on their SCID interview (Table 1). Eight BD individuals had comorbid diagnoses of eating (binge eating; n = 3) disorder and/or substance abuse disorder (n = 7). Twelve BD individuals were taking medication for at least 1 month prior to the study (Table 1). Additionally, recruited were 16 healthy individuals (18–52 years; 50% female) without current and lifetime personal (SCID-I criteria) or family history of psychiatric disorders. Exclusion criteria included borderline personality disorder (SCID-II-criteria); history of head injury or neurological disease; systemic medication, controlled and uncontrolled medical illnesses, cognitive impairment (score <24 in the Mini-Mental State Examination, Folstein et al., 1975); non-right handedness (per Annett, 1970) and failure to meet MRI screening criteria (pregnancy, metallic fragments, cardiac pacemaker or claustrophobia). Both groups had received a detailed assessment of comorbid symptom dimensions using the spectrum questionnaires in our previous study (Hassel et al., 2008).
Table 1.
Demographic and clinical variables, information on Spectrum Measures and relevant statistics (mean, standard deviation, minimum-maximum scores)
| Bipolar individuals | Healthy individuals | Statistics | ||
|---|---|---|---|---|
| N | 14 | 16 | n/a | |
| Handedness | Right | Right | n/a | |
| Gender (female; male) | 8; 6 | 8; 8 | X2 = 0.14; p = 0.73 | |
| Age in years (Mean±SD; range) | 32.64 ± 9.92; 23–52 | 28.50 ± 9.28; 18–52 | t = −1.18; p = 0.24 | |
| NART (Mean ± SD; range) | 111.77 ± 8.85; 92–121.58 | 118.08 ± 5.39; 101.11–125.14 | t = 2.36*; p = 0.026 | |
| Illness duration in years (Mean ± SD; range) year | 11.69 ± 6.32; 2–26 | n/a | n/a | |
| Age-of-Illness onset (Mean ± SD; range) | 22.00 ±8.99; 12–44 | n/a | n/a | |
| HAM-D (version 25) (Mean ± SD; range) | 3.0 ± 4.843; 0–18 | n/a | n/a | |
| YMRS (Mean ± SD; range) | 1.54 ± 2.60; 0–9 | n/a | n/a | |
| Spectrum measures | ||||
| Eating disorder | 42.57 ± 35.22; | 17.00 ± 15.63; | t = −2.19; | |
| (Mean ± SD; minimum-maximum) | 1–96 | 6–52 | p = 0.04 | |
| Substance use | 34.71 ± 27.20; | 18.27±15.78; | t = −2.17; | |
| (Mean ± SD; minimum-maximum) | 8–97 | 0–54 | p = 0.04 | |
| Medication - BD individuals only | ||||
| Mood-stabilizers | Antipsychotics | Antidepressants | Anxiolytics | |
| Name of medication; number of bipolar individuals taking | 1Lithium; n = 3 | 7Risperidone; n = 2 | 15Bupropion; n = 1 | 10Lorazepam; n = 3 |
| 6Valproate; n = 2 | 2Aripiprazole; n = 6 | 12Sertraline; n = 1 | 4Clonazepam; n = 1 | |
| 5Lamotrigine; n = 1 | 11Quetiapine; n = 1 | 9Paroxetine; n = 1 | ||
| 14Carbamazepine; n = 1 | 3Olanzapine; n = 1 | 13Venlafaxine; n = 2 | ||
| 8Gabapentin; n = 1 | ||||
| Monotherapy | N = 1 (1) | N = 3 (2) | N = 0 | N = 0 |
| Combination treatment | ||||
| Mood-stabilizers and antipsychotic | N = 1 (2 5 6) | |||
| Mood-stabilizers, antipsychotic, antidepressant and anxiolytic | N = 1 (1 2 7 8 9 10) | |||
| Mood-stabilizers and antidepressant | N = 2 (1 6 13) | |||
| Mood-stabilizers, antidepressant and anxiolytic | N = 1 (1 2 7 8 9 10) | |||
| Antipsychotics and antidepressants | N = 1 (11 12) | |||
| Antipsychotics and anxiolytics | N = 2 (2 3 4 7 10) | |||
Illness history information (illness duration; age-of-illness onset; HAM-D and YMRS scores) and medication information for bipolar individuals. Names/types of medication and the number of bipolar patients who took these combinations. Numbers in superscript denote types of medication taken in either mono-therapies or combination treatment.
After complete description of the study, participants gave written informed consent. The University of Pittsburgh's Institutional Review Board approved this study.
2.2. Spectrum questionnaires
Measures to assess lifetime prevalence of Substance Use and Eating Disorder were administered to BD and healthy individuals. Validity and reliability of these measures have been demonstrated (see Mauri et al., 2000; Sbrana et al., 2003, respectively for detail on each measure). Questionnaires can be downloaded from http://www.spectrum-project.net.
2.3. Paradigm
All participants completed two, 6 min event-related paradigms; viewing mild and intense happy and fear faces from a standardized series (Young et al., 2002). Both paradigms included neutral faces and a baseline fixation-cross. Participants judged each face's gender, an implicit emotion processing task reliably associated with activity in subcortical-striatal emotion processing regions (e.g. Surguladze et al., 2003, 2005). To determine overt emotion labeling accuracy,11 of the BD and 14 of the healthy individuals also performed an emotion labeling task outside the scanner. For 45 faces depicting sadness, anger, fear, happiness, disgust or neutral expressions (Lundqvist et al., 1998), participants had to choose corresponding emotion labels from a list of these six options. Problems in accommodating entire scanning and offline procedures in some BD and healthy individuals prevented these data being available for all study participants.
2.4. Data acquisition
We collected neuroimaging data using a 3.0 Tesla Siemens Allegra MRI scanner at the University of Pittsburgh/CMU Brain Imaging Research Center. Structural 3D Sagittal MPRAGE images were acquired as follows:TE:2.48 ms, TR:1630 ms, Flip-angle 8°, FOV:200 mm, Slice thickness:1 mm, Matrix:256 × 256, 192 continuous slices. Mean blood oxygenation-level-dependent (BOLD) images were acquired using a gradient-echo EPI sequence:33 axial slices(3 mm thick, 0 mm gap; TR/TE = 2000/25 ms, FOV = 2400 mm, matrix = 64 × 64).
2.5. Imaging analyses
Data were pre-processed and analyzed using statistical parametric mapping software (SPM5; http://www.fil.ion.ucl.ac.uk/spm). All data were first corrected for differences in acquisition time between slices; realigned using the first slice as a reference and unwarped to correct for static inhomogeneity of the magnetic field and movement by inhomogeneity interactions. They were then co-registered with the subject's anatomical image, segmented, normalized to standard MNI template, resampled to 3 × 3 × 3 mm voxels, and spatially smoothed with a Gaussian kernel of 6 mm full-width at half-maximum.
A first-level fixed-effect model was constructed with three emotion intensities (neutral, mild, intense) in both experiments (happy, fear) entered as separate conditions in an event-related design with fixation-cross as baseline in the design matrix. Movement parameters from the realignment stage were entered as covariates of no interest to control for participant movement. Trials were modeled using the Canonical Haemodynamic Response Function. The three intensities were then entered as separate t-contrasts into second-level analyses (intense > baseline; mild > baseline; neutral > baseline).
A second-level random-effects group analysis was conducted on t-contrasts generated in the previous single-subject analyses in a 2(group)-by-3(intensity)-repeated measures ANOVA for each experiment. Between-group comparisons of activity to each intensity in predetermined Regions-of-Interest (RoI) in each experiment were subsequently performed to examine main effects of group and condition and group-by-condition interactions. RoIs were defined using the Wake Forrest University (WFU)-Pickatlas (Maldjian et al., 2003). Neural regions were included in which abnormally increased activity–bilateral amygdalae, striatum(caudate nucleus, putamen), and abnormally decreased activity–DPFC, have previously been reported in BD (Lawrence et al., 2004; Hassel et al., 2008). Brodmann Areas (BA) 8,9,10,11,24,25,46 and 47 were included in the prefrontal cortex (PFC) RoI. BOLD-signal in each RoI identified in the above ANOVAs was extracted using MarsBar0.40-toolbox (Brett et al., 2002). Posthoc analyses were performed using matched-pairs and independent t-tests, as appropriate, to further examine main effects of group and condition, or group-by-condition interactions that emerged from above ANOVAs. Bonferroni-adjusted p-values (p = 0.05/(number of comparisons + number of regions) controlled for multiple comparisons.
Relationships between spectrum measures and neural activity in RoIs to emotional expressions were examined in post-hoc correlational analyses. For all above analyses we used the total score generated for each spectrum measure, and an adjusted statistical threshold of p = 0.025(p = 0.05/2), to control for two separate spectrum measure correlational analyses with each RoI showing a significant interaction, main effect of group or condition in each experiment.
To determine the potential impact of medication and illness variables, we first computed total medication load, employing a strategy reported previously (Hassel et al., 2008; Versace et al., 2008; Phillips et al., 2008b). We used Pearson's correlations to examine associations between total medication load, potential effects of illness duration or age-of-illness onset and magnitude of BOLD-signal in each RoI showing a significant interaction, main effect of group or condition. An adjusted statistical threshold p = 0.05/(number of comparisons) controlled for multiple comparisons.
3. Results
3.1. Participants
BD and healthy individuals were gender-ratio-matched. There was no significant between-group difference in age or weight (p > 0.05), although eight BD individuals had comorbid eating disorders. IQ, estimated by North American Reading Test (NART; Blair and Spreen, 1989) differed between BD and healthy individuals, t = 2.364, p = 0.026 (Table 1). However, there were no significant differences between groups for gender labeling accuracy performed during the scan (p < 0.05), and only a trend towards significant differences for emotion labeling accuracy in the offline task (see below). We nonetheless included IQ in exploratory correlational analyses examining its potential effect upon neural activity.
3.2. Emotion labeling accuracy
There was a trend difference in offline facial emotion labeling accuracy between the two groups (Mann-Whitney-U-Test = 45.5, z = −1.75, p = 0.08): healthy individuals (mean-accuracy: 82%) were more accurate than BD individuals (mean-accuracy: 79%). We therefore included emotion labeling accuracy in exploratory analyses examining potential effects of variables other than spectrum measures of comorbid symptoms upon neural activity in BD.
3.3. Spectrum measures
The total score on each spectrum measure was obtained by adding up individual items of each subdomain. For Mean, Standard Deviation, Minimum and Maximum scores for both spectrum measures see Table 1. BD and healthy individuals differed significantly for total scores on Substance Use (t (2,28) = −2.17, p = 0.038) and Eating Disorder Spectrum measures (t(2,28) = −2.09, p = 0.05).
3.4. Neuroimaging data analyses
We first examined main effects of group and condition, and group-by-condition interactions, upon neural activity in our RoIs for both experiments. We then explored relationships between the two spectrum measures and neural activity in RoIs showing main effects and interactions in each experiment.
3.5. Facial expression experiment—happy
3.5.1. Right dorsal PFC
A significant main effect of group (F(1,84) = 12.89, p (uncorrected) = 0.001,Cohen's d = 1.36) revealed significantly decreased right dorsal PFC (middle frontal gyrus, BA8) activity in BD relative to healthy individuals to all three emotional conditions (neutral:t(1,29) = 6.69, p = 0.015,Cohen's d = 0.97; mild:t(1,29) = 6.73, p = 0.015,Cohen's d = 0.98; intense:t(1,29) = 6.37, p = 0.018,Cohen's d = 0.96) (Fig. 1 A, B).
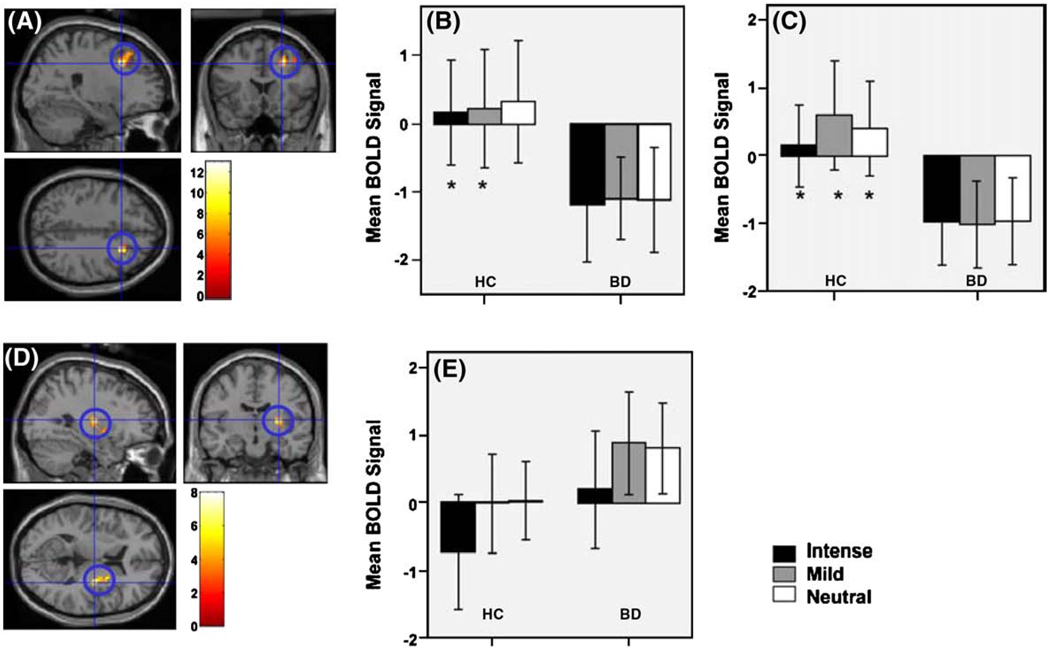
Fig. 1.
(A) Locus of significant activity for main effect of group for the happy and fear face paradigm. Activity is shown for right dorsal PFC, MNI: 24 18 39. Post-hoc analyses revealed that BD relative to healthy individuals showed decreased activity in this region to all three expressions, (B) intense, mild happy and neutral faces, (F(1,84) = 12.89, p(uncorrected)<0.001). (C) intense, mild fear and neutral faces, F(1,84) = 12.11, p(uncorrected)<0.001). (D) Locus of significant activity for main effect of condition for the happy face paradigm, (F(2,84) = 8.01, p(uncorrected)<0.001). Activity is shown for right putamen MNI: 30–129. (E) Post-hoc analyses revealed that BD relative to healthy individuals showed increased activity in this region, particularly to mild happy, but also neutral faces.
3.5.2. Right ventral putamen
There was a main effect of condition (intense versus mild versus neutral faces, F(2,84) = 8.01, p(uncorrected) = 0.001, Cohen's d = 1.07) within the right ventral putamen. Post-hoc analyses showed significant differences in all individuals between intense and mild happy faces (t(1,29) = −4.146, p < 0.001;Cohen's d = 0.48), and between intense happy and neutral faces (t(1,29) = −3.709, p < 0.005;Cohen's d = 0.45). Across both groups, relative to baseline, decreased activity to intense happy (mean BOLD-change:−0.31), but increased activity to mild happy (mean BOLD-change:0.39) and neutral faces (mean BOLD-change:0.38) was observed.
To examine relationships between spectrum measures and neural activity specifically in BD, we analysed neural activity in RoIs in each group separately. Relative to baseline, BD individuals showed patterns of predominant increases in activity, while healthy individuals showed decreases, or minimal increases, in activity to each emotional stimulus. BD individuals showed significantly increased activity to mild happy (mean BOLD-change:0.87) compared to intense happy faces (mean BOLD-change:0.187, t(1,13) = −2.716, p = 0.018; Cohen's d = 0.49),andneutral (meanBOLD-change:0.79)relative to intense happy faces (t(1,13) = −2.867, p = 0.013;Cohen's d = 0.45). Healthy individuals showed significantly decreased activity to intense (mean BOLD-change:−0.738) relative to mild happy (mean BOLD-change: −0.018, t = (1,15) = −3.038, p = 0.008, Cohen's d = 0.49), and neutral faces (mean BOLD change:0.021, t(1,15) = −2.525, p = 0.023;Cohen's d = 0.56). There was no significant difference in activity to mild happy relative to neutral faces (Fig. 1 D, E).
3.5.3. Left caudate nucleus
There was a significant main effect of condition (intense versus mild versus neutral faces, F(2,84) = 7.04, p(uncorrected)<0.001,Cohen's d = 1.01) in the left caudate body. Post-hoc analyses revealed significantly decreased activity to intense happy relative to neutral faces, and mild happy relative to neutral faces in both groups (t(1,29) = −2.794, p = 0.009, t(1,29) = −2.847, p = 0.008), respectively. Considering each group separately, significant differences in activity between intense happy and neutral, and mild happy and neutral faces were observed only in BD individuals. They showed significantly decreased caudate activity to intense happy (mean BOLD-change:−1.29) relative to neutral (mean BOLD-change:−0.58, t(1,13) = −2.257, p = 0.042;Cohen's d = 0.61), and mild happy (mean BOLD-change:−1.2 4), relative to neutral faces (t(1,13) = −3.002, p = 0.01;Cohen's d = 0.75).
3.6. Facial expression experiment—fear
3.6.1. Dorsal PFC
There was a significant main effect of group (F(1,84) = 12.11, p(uncorrected) = 0.001,Cohen's d = 1.32). BD, relative to healthy individuals, showed significantly decreased right dorsal PFC activity to all three emotional intensities (neutral:t(1,29) = 9.39, p = 0.005, Cohen's d = 1.16; mild:t(1,29) = 10.88, p = 0.003, Cohen's d = 1.25; intense:t(1,29) = 7.42, p = 0.011,Cohen's d = 1.16; Fig. 1 A, C).
3.6.2. Putamen
There were no significant main effects of group or emotion-condition or interactions between these.
3.6.3. Bilateral caudate nucleus
There was a significant main effect of emotion-condition within the left (F(2,84) = 13.15, p(uncorrected)<0.001,Cohen's d = 1.37) and the body of right caudate nucleus (F(2,84) = 9.84, p(uncorrected)<0.001;Cohen's d = 1.19).
Post-hoc analyses revealed, within the left caudate nucleus, significantly decreased activity to intense fear (mean BOLD-change:−1.643) versus neutral faces (mean BOLD-change:−0.566, t(1,29) = −3.806, p = 0.001;Cohen's d = 0.65) and mild fear (mean BOLD-change:−1.781) versus neutral faces (t(1,29) = −4.873, p<0.001;Cohen's d = 0.73) across both groups, similar to findings in the happy condition. BD individuals showed significantly decreased activity to intense fear (mean BOLD-change:−1.623) relative to neutral (mean BOLD-change:v0.306, t(1,13) = −2.705, p = 0.018; Cohen's d = 0.74), and mild fear (mean BOLD-change:−1.436) relative to neutral faces (t(1,13) = −2.8, p = 0.013;Cohen's d = 0.63). Healthy individuals showed significantly decreased activity to mild fear (mean BOLD-change:−2.084) relative to neutral faces (mean BOLD-change:−0.795, t = (1,15) = −3.944, p = 0.001, Cohen's d = 0.84), and intense fear (mean BOLD-change:−1.660) relative to neutral faces (t(1,15) = −2.68, p = 0.017;Cohen's d = 0.55).
Within the right caudate body, post-hoc analyses revealed significantly decreased activity to intense fear (mean BOLD-change:−1.21) versus neutral faces (mean BOLD-change: −0.553, t(1,29) = −3.766, p = 0.001;Cohen's d = 0.39), and mild fear (mean BOLD-change: −1.571) versus neutral faces (t(1,29) = −4.774, p<0.001;Cohen's d = 0.55). BD individuals showed significantly decreased activity to intense fear (mean BOLD-change:−1.211) relative to neutral (mean BOLD-change:−0.601, t(1,13) = −2.202, p = 0.046;Cohen's d = 0.3), mild fear (mean BOLD-change:−1.922) relative to neutral faces, (t(1,13) = −3.649, p = 0.003;Cohen's d = 0.61), and mild fear relative to intense fear faces (t(1,13) = +2.38, p = 0.033; Cohen's d = 0.33). Healthy individuals, showed significantly decreased activity to mild fear (mean BOLD-change:−1.2 6) relative to neutral (mean BOLD-change:−0.511, t = (1,15) = −3.201, p = 0.006, Cohen's d = 0.49), and intense fear (mean BOLD-change:−1.214) relative to neutral faces (t(1,15) = −3.061, p = 0.008;Cohen's d = 0.54).
3.6.4. Amygdala
There were no significant main effects of group or condition nor group by condition interactions for happy nor fear faces.
3.7. Relationships between Spectrum Measures and Regions of Interest in BD
Significant and trend correlations between spectrum measures and activity in RoIs are described below. The significance level was set at 0.05/2=0.025 to control for separate analyses.
3.7.1. Happy
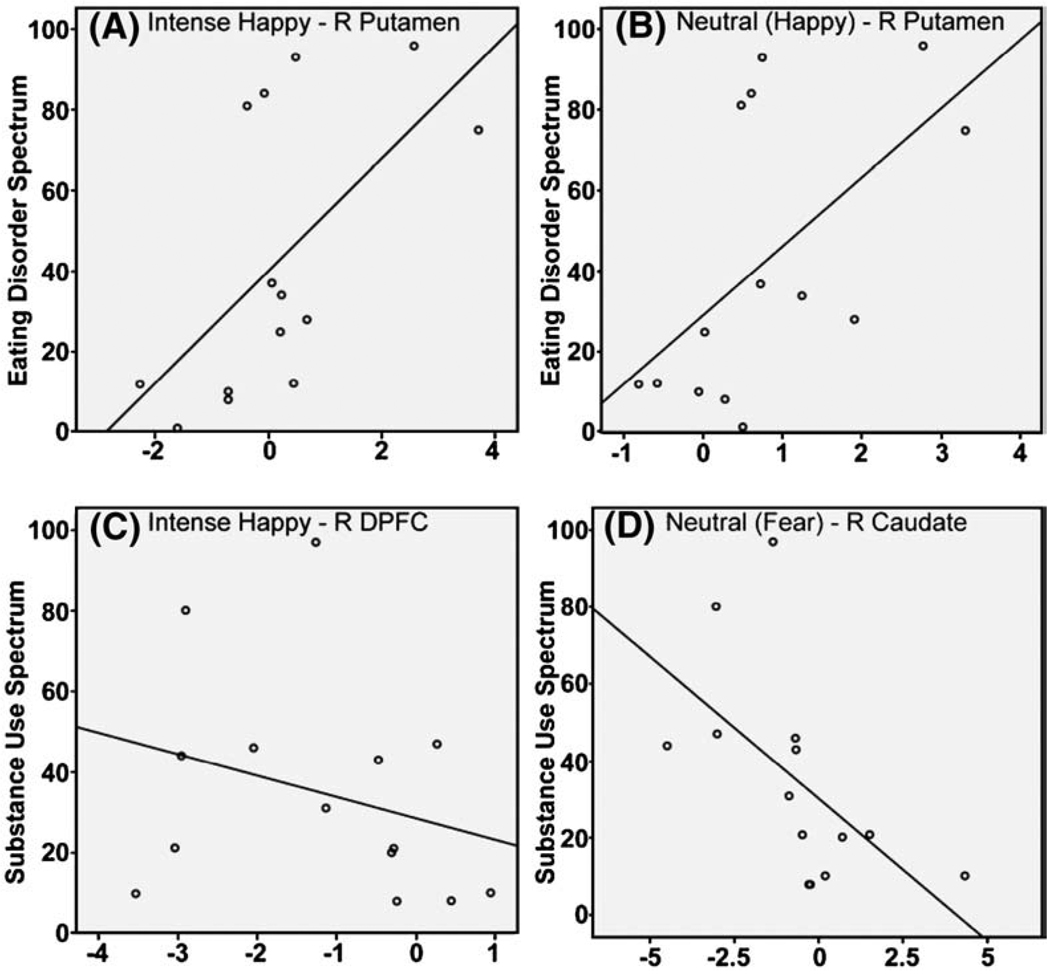
There was a trend negative correlation between total scores on the Substance Use Spectrum and activity to intense happy faces within the right PFC (r = −0.557, p = 0.04). There was a significant positive correlation between total scores on the Eating Disorder Spectrum and activity to intense happy faces within the right ventral putamen (r = 0.603, p = 0.02), and a trend positive correlation between this spectrum measure and activity in this region to neutral faces in the happy experiment (r = 0.572, p = 0.03) (Fig. 2 A–C).
Fig. 2.
Correlational analyses: positive correlations between Eating Disorder Spectrum and activity within right putamen to (A) intense happy faces and (B) neutral faces; negative correlations between Substance Use Spectrum and (C) activity within right dorsal PFC to intense happy faces and (D) activity within right caudate nucleus body to neutral faces in a fear context.
3.7.2. Fear
There was a trend negative correlation between total scores on the Substance Use Spectrum and activity to neutral faces in the fear experiment, within the right caudate body (r = −0.575, p = 0.03; Fig. 2 D).
3.8. Relationships between illness history variables, IQ, task performance and RoIs
Main findings from correlational analyses between RoIs in which significant effects of group and condition were demonstrated in both experiments, illness history measure (illness duration, age-of-illness onset) and medication load index, IQ and facial expression labeling accuracy are described below. Significance levels were set at 0.05/5 = 0.01, to control for five separate analyses including each variable in each RoI. Neither IQ nor task performance correlated with illness history variables or neural activity within RoIs.
3.8.1. Happy
There was a trend positive correlation between illness duration and activity to neutral faces within the left caudate nucleus (r = 0.599, p = 0.03). A trend negative correlation was observed between age-of-illness onset and activity within this region to neutral faces (r = −0.612, p = 0.03). There were trend and significant positive correlations between medication load and activity within this left caudate region to mild happy and neutral faces (r = 0.599, p = 0.04 and r = 0.691, p = 0.006), respectively.
3.8.2. Fear
There was a trend positive correlation between illness duration and activity to neutral faces in the fear experiment within the right caudate region (r = 0.594, p = 0.03). A trend negative correlation between age-of-illness-onset and activity to mild fear and neutral faces within the right dorsal PFC (r = −0.6, p = 0.03, and r = −0.6, p = 0.04), respectively, and significant and trend negative correlations between medication load and activity in this region to mild fear and neutral faces (r = −0.7, p = 0.005; and r = −0.6, p = 0.04), respectively were observed.
4. Discussion
To our knowledge, this is the first study examining the extent to which patterns of abnormal activity in prefrontal-cortical and striatal regions to facial expressions of happiness and fear, previously reported in remitted BD (Hassel et al., 2008; Lawrence et al., 2004; Malhi et al., 2007), are associated with severity of comorbid symptom dimensions of substance abuse and eating disorders. These comorbid illness features were endorsed by most BD individuals in this study. Relative to healthy individuals, BD individuals had significantly higher scores on both spectrum measures. Our findings largely support our main hypotheses regarding abnormal patterns of dorsal prefrontal-cortical and subcortical-striatal (ventral putamen, caudate nucleus) activity to emotional faces in BD. Specifically, in BD, relative to healthy individuals, we showed decreases in activity within prefrontal-cortical regions to happy and fear faces of all intensities, and increased activity in the right putamen to mild happy faces, although this did not reach our stringent threshold for significance in post-hoc analyses. Our findings further suggest that comorbid symptom dimensions of substance abuse and eating disorders may be associated with these patterns of abnormal neural activity in BD.
Observed associations between substance abuse and neural activity in BD supported our hypotheses. A trend negative correlation was demonstrated between mean BOLD signal within the right dorsal PFC to intense happy faces and substance abuse as measured by the Substance Use Spectrum questionnaire. Previous findings in substance abusers have shown decreased DLPFC activity during decision-making tasks (Bolla et al., 2003; Ersche et al., 2005a,b), but also lower activation within PFC regions during a facial matching task (Payer et al., 2008). A negative correlation would indicate that those BD individuals who endorsed more items on the Substance Use Spectrum would show stronger deactivation within PFC regions. This could then be linked to stronger difficulties in integrating socio-emotional information and, subsequently, emotion regulation. There was also a trend negative correlation between mean BOLD signal within the right caudate nucleus to neutral faces in the fear condition and the Substance Use Spectrum in BD. In this context, neutral faces were the more positive expression displayed, and may have been perceived as potentially more rewarding or ambiguous (Somerville et al., 2004). The negative relationship between caudate nucleus activity to these potentially more rewarding stimuli and substance abuse severity in BD individuals may be linked to previous findings of decreased rather than increased ventral striatal activity to formerly rewarding cues in detoxified alcoholics (Wrase et al., 2007). Clearly, these findings require further investigation in larger groups during reward paradigms.
Reported associations between severity of eating disorder and neural activity in BD also support our hypotheses. We showed a significant positive correlation between right putamen activity to intense happy faces, and a trend positive correlation to neutral faces in the happy context, and scores on the Eating Disorder Spectrum. While there was no overall significant effect of group on right ventral putamen activity to faces in the happy experimental condition, only BD individuals showed a pattern of predominant increases in activity, relative to baseline, to happy and neutral faces. The positive correlation between activity in ventral putamen to these faces and eating disorder severity supports previous findings in recovered anorexic individuals, who show greater ventral striatum activity during reward processing (Wagner et al., 2007). In healthy individuals, a network of interconnected brain regions of OFC, ventral striatum, amygdala and midbrain regions have been implicated in food reward processing (Beaver et al., 2006). In our study, happy faces may have been perceived as more rewarding, particularly to BD individuals with eating comorbidities.
In contrast to increased ventral putamen activity during the happy experimental condition, we showed that both BD and healthy individuals had decreased caudate nucleus body activity to emotional, relative to neutral, faces in happy and fear conditions. We reported patterns of deactivation in left-sided and bilateral caudate nuclei, relative to baseline, in happy and fear conditions, respectively. These findings suggest differential roles of caudate nucleus and ventral putamen during emotion processing, and support previous research indicating distinct roles of ventral and dorsal striatum in affective versus cognitive/associative processing (e.g. Haber et al., 2006). Our findings of overall deactivation in caudate nucleus body in happy and fear conditions in both groups may, therefore, support this region's role in cognitive rather than emotional processing. The association between substance abuse and decreased activity in this region to neutral faces in the happy context in BD individuals, and the link with previous findings in substance abusers during reward processing does, however, provide some support for a role of this region in reward processing. Further examination of ventral and dorsal striatal activity during reward and cognitive control paradigms will help clarify the potentially distinct, or overlapping, roles of ventral and more dorsal striatal regions in emotional and cognitive processing.
We explored potential influences of illness history variables and medication upon neural activity. The major finding here pertained to activity in the dorsal PFC and medication. There were significant and trend negative correlations between medication load and right dorsal PFC activity to mild fear and neutral faces. This suggests a more abnormal pattern of activity in this region in those BD individuals with greater medication load and, potentially, more severe illness course. There were trend and significant positive correlations between medication load and left caudate nucleus activity to mild happy and neutral faces, respectively. Again, in those BD individuals taking larger numbers and/or doses of psychotropic medications a more abnormal pattern of activity, i.e. reduced deactivation were observed.
For illness duration variables, trend positive correlations with activity in the left and right caudate body to neutral faces in happy and fear conditions, respectively, were observed. Conversely, a trend negative correlation between activity in the left caudate body and age-of-illness-onset to neutral faces in the happy condition was also reported. Since BD, like healthy individuals, showed left and right caudate body deactivation to all faces in both experimental conditions, these findings suggest that longer illness duration may have been linked to reduced deactivation, i.e. a more abnormal pattern of activity in these regions. Interestingly, earlier age-of-illness onset was associated with greater, not reduced, dorsal PFC activity to neutral and mild fear in BD. This suggests that the reduced PFC activity to emotional facial expressions in BD adults in the present and previous studies (Hassel et al., 2008; Lawrence et al., 2004; Yurgelun-Todd et al., 2000) may be particularly evident in individuals with later rather than earlier age-of-illness onset.
While it is not possible to determine cause and effect between medication load and illness severity and/or duration, these findings, together with those of relationships between illness duration and caudate nucleus activity are consistent with greater illness severity, reflected by longer illness duration and greater medication load, being associated with more abnormal patterns of PFC activity in BD. Our findings regarding effects of illness history variables and medication load should be considered preliminary, given the exploratory nature of these analyses. They indicate, however, the importance of examining relationships between illness-history variables and patterns of neural activity in BD individuals in future studies.
To our knowledge, this is the first functional neuroimaging study to examine relationships between patterns of abnormal activity in prefrontal cortical and striatal regions to facial expressions of happiness and fear and severity of comorbid symptom dimensions of substance abuse and eating disorders in BD. Nonetheless, the following limitations have to be noted. The number of euthymic BD patients in this study is relatively small, so future studies should examine these relationships in larger groups of euthymic BD individuals. Significant differences in IQ were observed between BD and healthy individuals, yet task performances did not differ significantly, nor did correlational analysis show that IQ confounded activity within our predetermined RoIs. Additionally, nearly all BD individuals in this study were taking psychotropic medication. While it remains difficult, and potentially unrepresentative of the BD population at large, to limit studies solely to unmedicated BD individuals (Phillips et al., 2008b), if possible, medicated and unmedicated BD individuals should be included in future studies.
We present first evidence of relationships between comorbid symptom dimensions of substance abuse, eating disorders, and patterns of dorsal-prefrontal and striatal activity to emotional facial expressions in BD. Our findings highlight the importance of including these measures in future neuroimaging studies to inform understanding of the contribution of comorbid symptom dimensions to patterns of functional abnormalities in neural systems in individuals with this debilitating psychiatric illness.
Acknowledgements
All work was carried out within the Department of Psychiatry, University of Pittsburgh. All neuroimaging data was collected at the Brain Imaging Research Center (BIRC), University of Pittsburgh and Carnegie Mellon University. We thank Dr. K.J. Jung, S. Kurdilla and D. Vizslay for their help in acquiring the neuroimaging data.
Role of funding support
Funding for this study was provided in part by a NARSAD Independent Investigator Award to MLP, who is the NARSAD Nellie Blumenthal Investigator, and by NIMH Grant 5R01MH076971-01. Neither funding agency had any further role in study design, data collection, analyses or interpretation, nor in the writing of this manuscript or the decision to submit for publication.
Footnotes
Conflict of Interest
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- Angst J, Gama A, Sellaro R, Zhang H, Merikangas K. Toward validation of atypical depression in the community: results of the Zurich cohort study. Journal of Affective Disorders. 2002;72:125–138. doi: 10.1016/s0165-0327(02)00169-6. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association. British Journal of Psychology. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. Journal of Neurosciences. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clinical Neuropsychologist. 1989;3:29–136. [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction on abstinent cocaine abusers performing a decision making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden CL. Strategies of reduced misdiagnosis of Bipolar Disorder. Psychiatric Services. 2001;52:51–55. doi: 10.1176/appi.ps.52.1.51. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16:S497. [Google Scholar]
- Cassano GB, Dell'Osso L, Frank E, Miniati M, Fagiolini A, Shear K, Pini S, Maser J. The bipolar spectrum: a clinical reality in search of diagnostic criteria and an assessment methodology. Journal of Affective Disorders. 1999:319–328. doi: 10.1016/s0165-0327(98)00158-x. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology. 2005a;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Rosier JP, Clark L, London M, Robbins TW, Sahakian BJ. Punishment induces risky decision-making in methadone-maintained opiate users but not in heroin users or healthy volunteers. Neuropsychopharmacology. 2005b;30:2115–2124. doi: 10.1038/sj.npp.1300812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon ML, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV (SCID-I): User's Guide and Interview, Research Version. New York, NY: Biometrics Research Department, New York Psychiatric Institute; 1995. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State—a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. Journal of Affective Disorders. 2007;103:29–42. doi: 10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neurosciences. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biological Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Hassel S, Almeida JRC, Kerr N, Nau SA, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity to emotional stimuli in bipolar disorder: no association with psychotropic medication load. Bipolar Disorders. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH. Neurobiology of anorexia and bulimia nervosa. Physiology and Behavior. 2007;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H, Schultz S, Arndt S. Biomarkers in psychiatry: methodological issues. American Journal of Geriatric Psychiatry. 2002;10:53–59. [PubMed] [Google Scholar]
- Krishnan KR. Psychiatric and Medical Comorbidities of Bipolar Disorder. Psychosomatic Medicine. 2005:1–8 . doi: 10.1097/01.psy.0000151489.36347.18. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ. The increasing medical burden in Bipolar Disorder. JAMA. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguished patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. The Karolinska Directed Emotional Faces-KDEF. Stockholm: Department of Clinical Neuroscience, P.S., Karolinska Institute; 1998. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Owen AM, Ivanovski B, Shnier R, Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. Journal of Affective Disorders. 2007;97:109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biological Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mauri M, Borri C, Baldassari S, Benvenuti A, Rucci P, Cassano GB, Shear PK, Grochocinski VJ, Maser JD, Frank E. Acceptability and psychometric properties of the Structured Clinical Interview for Anorexic-Bulimic Spectrum (SCI-ABS) International Journal of Methods in Psychiatry Research. 2000;9:68–78. [Google Scholar]
- Nolen WA, Luckenbaugh DA, Altshuler LL, Suppes T, McElroy SL, Frye MA, Kupka RW, Keck PEJ, Leverich GS, Post R. Correlates of 1-year prospective outcome in bipolar disorder: results from the Stanley Foundation Bipolar Network. American Journal of Psychiatry. 2004;161:1447–1454. doi: 10.1176/appi.ajp.161.8.1447. [DOI] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug and Alcohol Dependence. 2008;93:93–102. doi: 10.1016/j.drugalcdep.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Frank E. Redefining Bipolar Disorder: toward DSM-V. Redefining bipolar disorder: toward DSM-V. American Journal of Psychiatry. 2006;163:1135–1136. doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008a;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of Bipolar Disorder. American Journal of Psychiatry. 2008b;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Sbrana A, Dell'Osso L, Gonnelii C, Impagnatiello P, Doria MR, Spagnolli S, Ravani L, Cassano GB, Frank E, Shear MK, Grochocinski VJ, Rucci P, Maser JD, Endicott J. Acceptability, validity and reliability of the structured clinical interview for the spectrum substance use (SCD-SUBS): a pilot study. International Journal of Methods in Psychiatry Research. 2003;12:105–115. doi: 10.1002/mpr.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlation with state anxiety. Biological Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sonne SC, Brady KT, Morton WA. Substance abuse and bipolar affective disorder. Journal of Nervous and Mental Disorders. 1994;182:349–352. doi: 10.1097/00005053-199406000-00007. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Brammer MJ, Young AW, Andrew C, Travis MJ, Williams SCR, Phillips ML. A preferential increase in the extrastriate response to signals of danger. NeuroImage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Versace A, Almeida JRC, Hassel S, Walsh NX, Novelli M, Klein CR, Kupfer DJ, Phillips ML. Elevated left orbitofrontal white matter fractional anisotrophy in bipolar adults revealed by tract-based spatial statistics. Archives of General Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GI, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Archives of Neurology. 2007a;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GI, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Profound decreases in dopamine release in striatum in detoxofied alcoholics: possible orbitofrontal involvement. Journal of Neurosciences. 2007b;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, Frank GK, Bailer UF, Fischer L, Nguyen V, Carter C, Putnam K, Kaye WH. Altered reward processing in women recovered from anorexia nervosa. American Journal of Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wuestenberg T, Bermpohl F, Kahnt T, Beck A, Stroehle A, Juckl G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Young AW, Perrett D, Calder A. Facial Expressions of Emotions: Stimuli and Test (FEEST) Thurstone: Thames Valley Test Company; 2002. [Google Scholar]
- Yucel M, Lubman DI. Neurocognitive and neuroimaging evidence of behavioural dysregulation in human drug addiction: implications for diagnosis, treatment and prevention. Drug and Alcohol Review. 2007;26:33–39. doi: 10.1080/09595230601036978. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disorders. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]