Abstract
Cyclin dependent kinase (Cdk)-5 is ubiquitously expressed in the brain and plays an essential role in CNS development and synaptic plasticity. The p35 kinase is a neuronal specific activator of Cdk5. Here, we show for the first time that Cdk5 activation modulates leptin signaling. P35 and its metabolite p25 were co-localized with the leptin receptor ObR in selective neurons in the hypothalamus. Overexpression of p35 alone was sufficient to induce the transcriptional activation of Signal Transducer and Activator of Transcription 3 (STAT3) in a cellular model. In retinoic acid-differentiated SH-SY5Y neuronal cells where ObRb was induced, leptin increased the expression of Cdk5, p35, and p25 kinases. The time course of induction coincided with that of phosphorylated (p)-STAT3. When Cdk5 activity was inhibited, either by roscovitine or overexpression of dominant negative Cdk5, there was a reduction of pSTAT3 activation. The results show that the activation of Cdk5 by p35 sustained leptin-induced pSTAT3 at 3 – 6 h. Thus, p35 is a novel modulator of leptin-induced STAT3 signaling.
Keywords: Cdk5, p35, p25, leptin, STAT3, signal transduction, neuron
Introduction
It is not uncommon that a polypeptide is named for the effect by which it was discovered, but later found to have a multitude of actions (Kastin, 1983). Cyclin-dependent kinase (Cdk) 5 is a member of the Cdk family but appears to have unique functions in the CNS that are independent of cell cycle regulation. Cdk5 is predominantly expressed in the brain. It participates in cognitive functions, learning, and synaptic plasticity (Cheung, 2006; Tang, 1996). Mice with Cdk5 deletion have defective brain development (Ohshima, 1999). The level of Cdk5 expression is highest in postmitotic neurons in the developing and adult nervous system, as compared with that in the kidney, testis, and ovary (Lew, 1995). Cdk5 is unique in its family in that it is not activated by cyclins and its activity requires association with one of two brain-specific regulatory subunits termed p35 and p39 (Tsai, 1994; Zheng, 1998).
P35 is a neuronal specific activator of Cdk5 that has a short half-life and cell membrane distribution, whereas its caplain-cleaved product p25 has a 5 – 10 fold longer half-life and is localized diffusely in cells (Patrick, 1999). Conversion of p35 and sustained activation of p25 have been shown to contribute to abnormal tau phosphorylation in Alzheimer’s disease (Baumann, 1993; Tseng, 2002). Although recent studies clearly indicate interactions between Cdk5 and the Signal Transducer and Activator of Transcription (STAT3) (Fu, 2004; Lin, 2007; Wang, 2006), there are no studies showing a direct relationship between Cdk5 system and leptin signaling.
Leptin is a 16 kD adipokine produced mainly by adipocytes. It has multiple cellular targets in the CNS after crossing the blood-brain barrier (BBB) (Banks, 1996; Levin, 2004; Pan, 2007a, 2008b) or blood-CSF barrier (Zlokovic, 2000). It may also reach the median eminence of the hypothalamus, a circumventricular region lying outside the BBB (Kastin, 2006). The activation of hypothalamic neurons by leptin is associated with neuronal development and neuroendocrine regulation. Leptin also improves synaptic plasticity and shows anti-epileptic functions (Harvey, 2007; Xu, 2008). Although direct activation of cation channels and phosphoinositol-3 kinase (PI3K)-Akt and other signaling pathways have been shown (Guo, 2008), STAT3 phosphorylation is a predominant means of activation by the long cytoplasmic domain receptor ObRb. Inhibitory signals that counteract this activation include Suppressor of Cytokine Signaling (SOCS)-3 (Bjorbak, 2000; Schwartz, 2000; Waelput, 2000) and protein-tyrosine phosphatase (PTP1B) (Zabolotny, 2002). In obese mice with dysregulation of the leptin system, there are defects in intracellular signaling (Garris, 1989; Vaisse, 1996).
Protein sequence analysis indicates that Ser727 in STAT3 is a typical phosphorylation site for Cdk5. The Cdk5/p35 complex phosphorylates STAT3 at the Ser727 residue in vitro and in vivo. In muscle of Cdk5-deficient mice, both the DNA-binding activity of STAT3 and the transcription of its downstream target genes are reduced. In macrophages, STAT3 phosphorylation at Ser727 is essential for its maximal activation (O'Rourke, 2002). These results suggest a physiological role of Cdk5 in regulating STAT3 phosphorylation and modulating its transcriptional activity that can also be tested after leptin stimulation.
Material and Methods
Cell culture and treatment
SH-SY5Y human neuroblastoma cells and HEK293 cells (American Type Culture Collection, ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (DMEM) with 10 % fetal bovine serum (FBS). The cells were differentiated by treatment with 10 µM of all-trans retinoic acid (Sigma, St. Louis, MO) between 1 – 6 d after plating. Sixteen h after serum-starvation, the cells were treated with the specific Cdk5 inhibitor roscovitine (30 µM for 15 min pretreatment as well as cotreatment for specified intervals; Sigma) and 30 nM leptin (R & D Systems, Minneapolis, MN) for different time intervals (10 min – 6 h), as specified in the Results section. The vehicle control for roscovitine was 0.1 % DMSO. The 0 time group in the roscovitine study was pretreated with roscovitine (30 µM) for 15 min. All cells were plated at the same time, treated according to the time intervals designed for individual experiments, and harvested at the same endpoint. All findings were replicated.
Mouse models of obesity
C57BL/6J male mice (Jackson Laboratories, Bar Harbor, ME) were used to induce DIO following a protocol approved by the Institutional Animal Care and Use Committee. Four-week-old mice were group-housed and randomly assigned to either a high fat diet (HFD, 45 % kcal fat (D12451, Research Diets, New Brunswick, NJ) or regular rodent chow for 16 weeks. Body weight was measured weekly after induction of obesity for both the DIO and control groups. The percentage of body fat was determined with a Bruker minispec Live Mice Analyzer (model mq7.5, LF50; Bruker Optics, Inc., Billerica MA), as described previously (Pan, 2007b). The obesity phenotype of Avy mice (Jackson Laboratories) has been characterized previously, and studied in parallel with the C57BL/6J littermate controls (Pan, 2008a). After the onset of obesity, these mice were studied along with their respective controls for western blotting in hypothalamic tissue, or immunohistochemistry of ObR and p35, by use of methods established in our laboratory.
Immunofluorescent staining
Abiding by animal protocols approved by the Institutional Animal Care and Use Committee, adult male B6 mice or age-matched Avy mice (3 – 5 month-old) were anesthetized by urethane intraperitoneally, and perfused intracardially with 30 ml normal saline followed by 4 % paraformaldehyde. The brain was post-fixed overnight in 4 % paraformaldehyde, and cryoprotected in 15 and then 30 % sucrose. Coronal hypothalamic sections of 20 µm thickness were obtained by use of a cryostat. Cultured cells were fixed with 4 % paraformaldehyde for 10 min at room temperature. The hypothalamic tissue sections or cells were permeabilized with 0.3 % Triton X-100 and blocked with 10 % normal donkey serum, incubated with a primary antibody overnight at 4 °C. The antibodies include rabbit anti-p35 antibody (1:200 - 1:100, Santa Cruz Biotechnology sc-820, Santa Cruz, CA), and goat anti-mouse ObR antibody (1:100, Santa Cruz Biotechnology; sc-1834). The p35 antibody also recognizes the shorter fragment p25 that is cleaved from p35 but has a longer half-life, more diffuse subcellular distribution, and probably higher biological activity (Lew, 1994). The ObR antibody is raised against the membrane juxtapositional cytoplasmic domain of mouse ObR short forms and reacts with all membrane-bound leptin receptor isoforms . After thorough wash, they were incubated with their respective Alexa488-conjugated secondary antibodies for 1 h, washed, and mounted. Negative controls were incubated with a secondary antibody only.
Western blot analysis
The cells were lysed in ice-cold RIPA buffer (100 mM NaCl, 10 mM Tris, pH 7.2, 0.1 % SDS, 1 % Triton X-100, 1 % deoxycholate, 5 mM EDTA) in the presence of protease inhibitor cocktail (Pierce, Rockford, IL). The lysates were sonicated and cleared by ultracentrifugation. The protein content was measured by bicinchoninic acid assay (Pierce). Thirty – 50 µg of protein was electrophoresed on 12 % SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). The membrane was blocked with 5 % non-fat dry milk in Tris-buffered saline (pH 7.6) containing 0.1 % Tween-20, and probed with rabbit anti-p35 (polyclonal, 1:200, Santa Cruz Biotechnology, sc-820), mouse anti-Cdk5 (monoclonal, 1:1000, Upstate; 05–364), rabbit anti-pSer727-STAT3 (polyclonal, 1:500, Biosource, 44–384G ) and rabbit anti-pTyr705-STAT3 (polyclonal, 1:100, Santa Cruz Biotechnology, sc-7993-R), rabbit anti-pSOCS-3 (polyclonal, 1:200, Santa Cruz Biotechnology, sc-9023) and mouse anti-β-actin (monoclonal, 1:10000, Sigma, A2228) overnight at 4 °C. After thorough wash, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The signals were developed with enhanced chemiluminescence (ECL)-plus western blotting detection reagents (Amersham Biosciences, Piscataway, NJ). All findings were confirmed in replicate studies.
Plasmids, transient transfection, and luciferase assay
For luciferase assays, HEK293 cells in 24-well plates were transfected with 0.2 µg/well of pAH-Luc STAT3 luciferase reporter plasmid from Dr. Rosenblum (Rosenblum, 1996) and 0.01 µg of phRL-TK (Renilla luciferase) reference plasmid (Promega, Madison, WI) along with the genes of interest. These include empty vector, WT-Cdk5, DN-Cdk5, ObRb, or p35-pEGFP-C2, according to the group design (n = 4 /group). The construction of WT-Cdk5 and DN-Cdk5 from Dr. Wang had been described previously . At 24 h after transfection, the cells were pelleted and lysed with a component from the Dual-Luciferase Reporter Assay 1000 System kit (Promega). After addition of substrates, the activities of firefly luciferase and Renilla luciferase were measured on a luminometer (20/20n, Turner Biosystems, Sunnyvale, CA). The luminescent intensity of firefly luciferase was normalized as a ratio to that of Renilla luciferase, as previously described (Pan, 2007c).
HEK293 cells grown in 24-well plates at 90 % confluency were transfected with 1.2 µg of plasmids by use of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in serum-free DMEM. Four h later, the medium was replaced with DMEM with 10 % FBS. Differentiated SH-SY5Y cells (90 % confluency) were suspended in electroporation buffer provided in the Nucleofector Kit V (Amaxa, Gaithersburg, MD) to a final concentration of 2 ×106 /100 µl after detachment by trypsin. A mixture of 100 µl of cell suspension and 9 µg of plasmids was electronically transfected by use of the Nucleofector Device (Amaxa) with program G-004. The cells were aliquoted into 24-well plates, and cultured in 5 % CO2 at 37 °C for another 24 h.
RNA Extraction and RT-PCR
Total RNA was obtained with an Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA) and reversely transcribed. PCR was performed beginning with a single cycle of 94 °C for 2 min, followed by 35 cycles (human ObRb) or 26 cycles (β-actin) of 94 °C for 30 sec, 56 °C 30 sec and 72 °C for 30 sec. This was followed by a single cycle of 72 °C for 10 min to facilitate final extension. The primers for human ObRb were: forward 5’-CCT CTT CCA TCT TAT TGC TTG GA, and reverse 5’-CTC AAA CGT TTC TGG CTT CTG AA. The primers for the housekeeping gene β-actin were: forward 5’-GAT CTG GCA CCA CAC CTT CT, and reverse 5’-GGG GTG TTG AAG GTC TCA AA. The PCR products were electrophoresed on 2 % agarose gel containing ethidium bromide, and imaged by the Kodak EDAS290 system.
Statistical analysis
Data are expressed as means ± standard errors. Significant differences were determined by repeated measures (for body weight) or one-way analysis of variance (for NMR measurement of fat composition and for luciferase reporter assay), followed by Tukey’s post-hoc test. This was performed by use of Statistical Analysis for Social Science software (SPSS Inc, Chicago, IL), and graphed by Prism GraphPad software (San Diego, CA).
Results
1. P35 is present in ObR(+) neurons in mouse hypothalamus
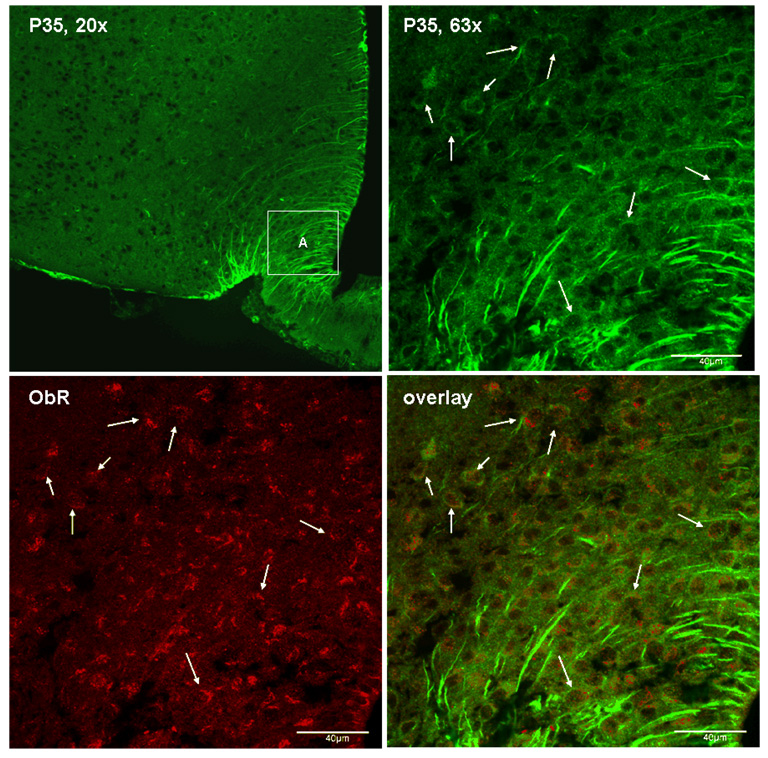
Immunostaining was performed by use of a polyclonal antibody against the common N-terminal domain of p35 and p25 in hypothalamic sections from adult B6 mice. The negative control group showed no fluorescent signal in the absence of the primary antibody. There were at least two distinctive populations of cells that are p35 (+): neurons that showed cytoplasmic immunoreactivity, and tanycytes around the third ventricle and median eminence that showed fibrous staining. Confocal analyses showed that some of the p35 (+) neurons also express the leptin receptor ObR (Fig.1). Since leptin activates STAT3 through ObR in neurons, we further determined the interactions between p35-induced Cdk5 activation and STAT3 signaling in cultured cells.
Fig. 1.
Expression of p35 in ObR (+) neurons, shown by confocal microscopy. Top left: p35/p25 immunoreactivity in the arcuate nucleus of the hypothalamus, present in neuronal cytoplasma and in tanycytes. Top right: enlarged area A from the top left panel. Bottom left: ObR immunoreactivity in the same area A. Bottom right: confocal overlay of p35 (green) and ObR (red) immunoreactivity. Arrows indicate co-localized cells.
2. P35 alone is sufficient to induce transcriptional activation of STAT3
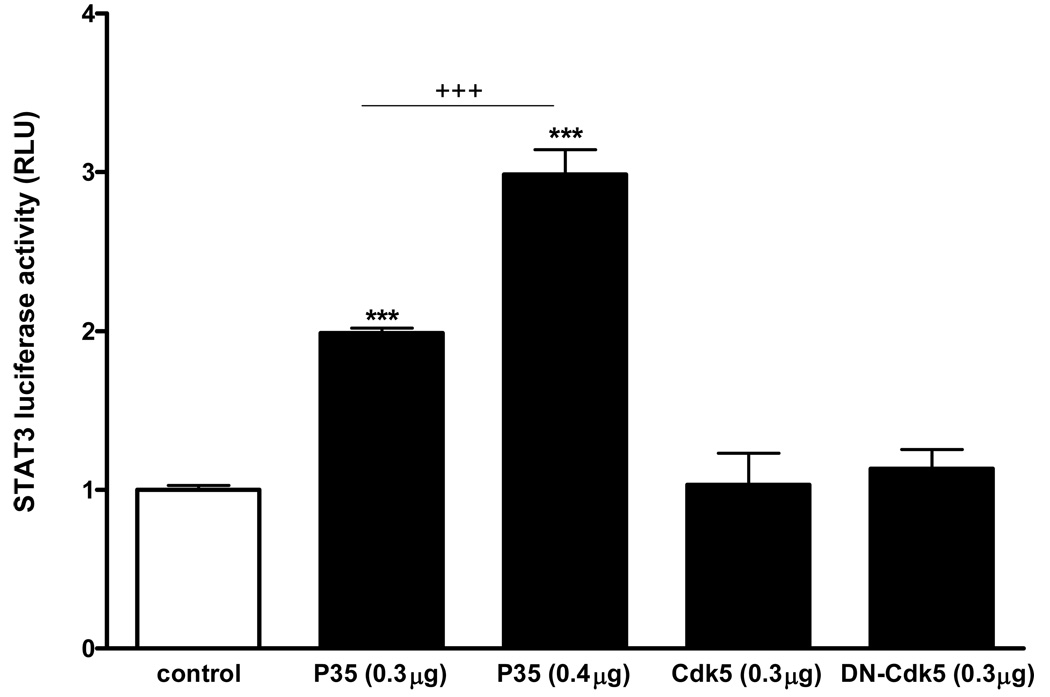
The cDNAs of P35, Cdk5, or dominant-negative (DN)-Cdk5 were overexpressed in HEK293 cells along with luciferase reporter genes (n = 4 /group). A negative control group was transfected with the empty vector along with the luciferase reporters. At 24 h after transfection, the cells were pelleted without treatment, and basal luciferase reporter activity was measured. Despite the absence of ligand stimulation, the groups of cells overexpressing p35 kinase showed a significant elevation of STAT3 luciferase reporter gene activity. This was a dose-dependent effect, as the increase of STAT3 luminescence was significantly higher in the cells transfected with the higher dose (0.4 µg/ml/well) of p35 than with the lower dose (0.3 µg/ml/well). By contrast, neither the wildtype Cdk5 nor DN-Cdk5 caused a significant change in the minimal activation of STAT3 (Fig.2). To determine whether leptin activates p35 current to its induction of STAT3 and thus potentiates STAT3 activity, we treated SH-SY5Y neuroblastoma cells with leptin.
Fig. 2.
Overexpression of p35 alone, in the absence of leptin or other ligands, was sufficient to induce STAT3 transcriptional activity in HEK293 cells shown by luciferase reporter assays. Both 0.3 µg/ml and 0.4 µg/ml of p35 caused significant increases of STAT3 luciferase activity in comparison with the empty vector control (***: p < 0.005). This contrasts with the lack of effect of Cdk5 or DN-Cdk5 in the absence of ligands. The higher dose of p35 caused a greater increase than the lower dose (+++: p < 0.005 when the 0.3 and 0.4 µg groups were compared).
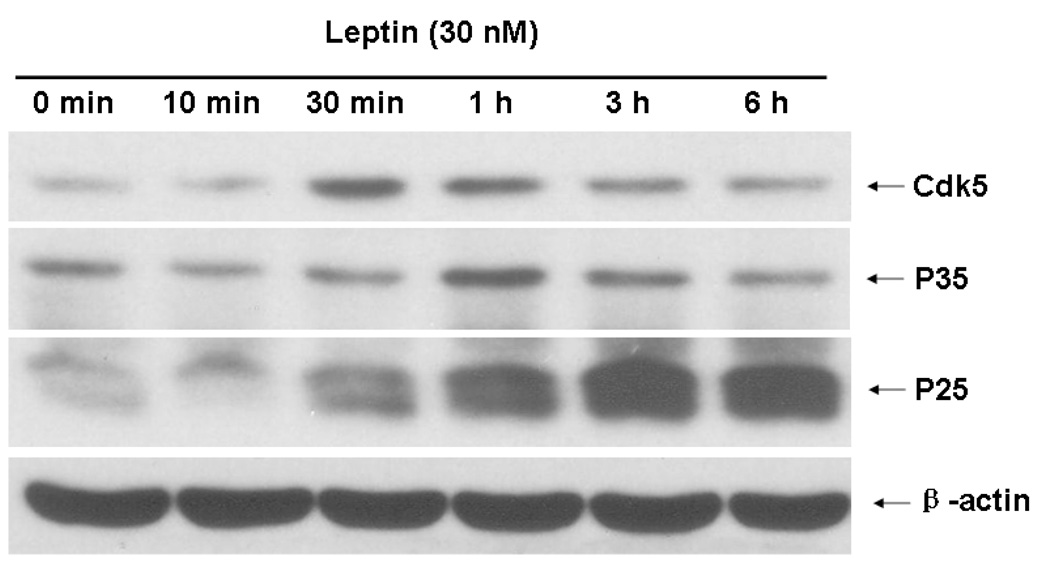
3. Leptin induces Cdk5 and its activators p35 and p25 by increasing their expression and causing subcellular redistribution
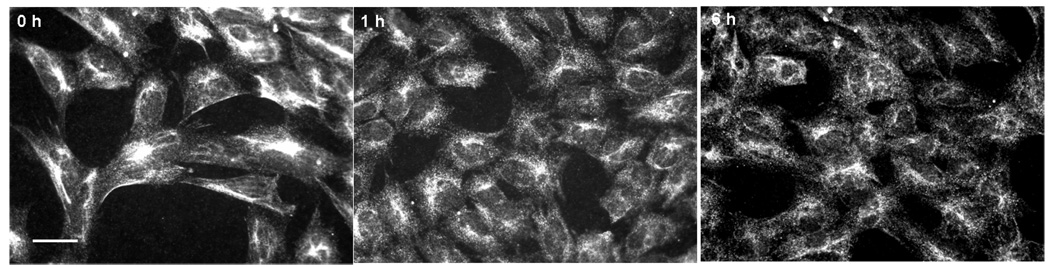
Differentiated SHSY-5Y cells represent a suitable cellular model with induced ObRb expression and time-dependent STAT3 after leptin stimulation (Fig.3A–C). Cellular staining with a shared antibody against p35 and its smaller fragment p25 showed that leptin (30 nM) induced redistribution of the immunofluorescence within the cells. In the basal state, p35/p25 was clustered in cytoplasm. At either 1 or 6 h after leptin treatment, there was no apparent increase of fluorescent intensity, but there was a change of subcellular distribution. A more diffuse pattern of p35/25 immunofluorescence was seen (Fig. 4A). Western blotting further differentiated the p35 and p25 kinases by their sizes. Leptin treatment caused a time-dependent increase of both p35 and p25. Cdk5 itself was also increased. These changes were apparent at 30 min. Cdk5 and p35 reached their peaks by 1 h, while p25 showed a persistent increase at 3 and 6 h (Fig. 4B). Consistent with reports that p25 has a more diffuse distribution pattern as well as a longer half-life, the results suggest that there was an increase of conversion of p35 to p25 by proteases. The major increase in p25 seen in western blotting was thus consistent with a more diffuse subcellular distribution pattern seen in immunostaining.
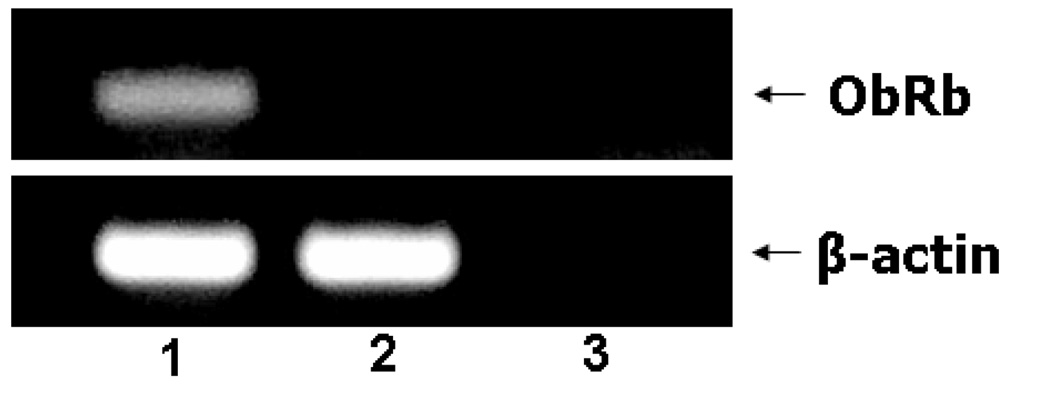
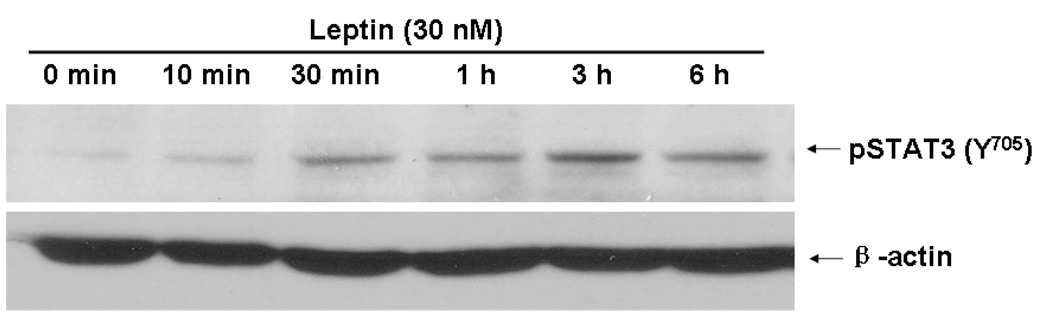
Fig. 3. The cellular model of differentiated SH-SY5Y cells.
3A. Induction of ObRb mRNA in SH-SY5Y cells and the effects of leptin on STAT3 activation. ObRb mRNA was present in retinoic acid-differentiated cells (lane 1), but not in non-differentiated cells (lane 2) or the no-template negative control (lane 3).
3B. Retinoic acid-induced differentiation of SH-SY5Y cells resulted in subcellular redistribution of ObRb. Immunocytochemistry showed that ObRb was present in the cytoplasm of the undifferentiated cells (middle panel), but at the cell surface of differentiated cells (right panel). The specificity of the staining was shown by the lack of signals in the negative control with secondary antibody only (left panel).
3C. Leptin treatment (30 nM) induced pSTAT3 throughout the study period (10 min – 6 h), with a peak at 3 h. This contrasts with the lack of changes of the β-actin signal.
Fig. 4. Effect of leptin treatment (30 nM) on Cdk5 in SH-SY5Y cells.
4A. Western blotting showed that leptin induced Cdk5, p35, and p25 by 30 min, with peaks at 30 min, 1 h, and 3 h, respectively. The housekeeping gene β-actin was unchanged.
4B. Immunocytochemistry showed that leptin treatment altered the subcellular distribution of p35/p25 from the cytoplasmic cluster at the basal state to a more diffuse pattern at 1 and 6 h.
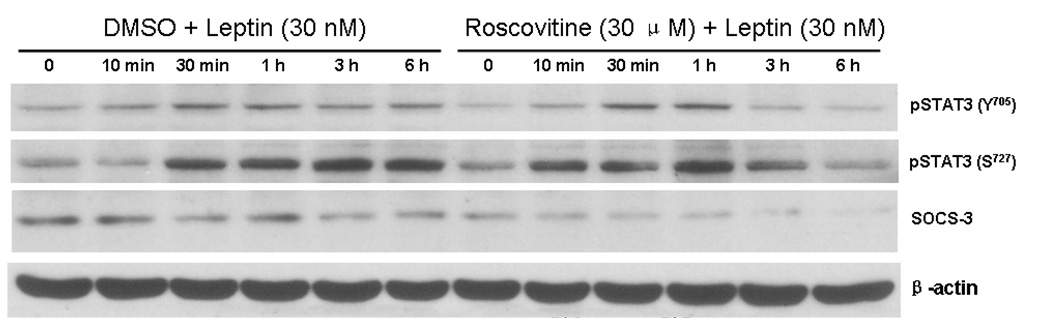
4. Roscovitine, an inhibitor of Cdk5, effectively blocks leptin-induced pSTAT3
Leptin treatment induced STAT3 activation at both the Y705 and S727 sites between 30 min and 6 h, and reduced SOCS-3 expression simultaneously (left panels, Fig.5). When the Cdk5 inhibitor roscovitine (30 µM) was present when the cells were stimulated with leptin, the time course and phosphorylation sites of STAT3 activation both changed. For pSTAT3-Y705, the increase at 3 and 6 h was no longer present (top lane). For pSTAT3-S727, there appeared to be an early potentiation and later depression by roscovitine. This resulted in a shift of activation to earlier times, and reduced pSTAT3 signal at 3 and 6 h (second lane). Moreover, roscovitine induced a persistent reduction of SOCS-3 signal (third lane). The expression of the housekeeping gene β-actin was not affected by the treatment.
Fig. 5. The Cdk5 inhibitor roscovitine modulates leptin-induced pSTAT3 in SH-SY5Y cells.
Leptin (30 nM) increased pSTAT3 (both Y705 and S727) between 30 min – 6 h of treatment; the increase at the later time points (3 and 6 h) was dampened by co-treatment with roscovitine, which also shifted pSTAT3-S727 activation to an earlier time. The induction of SOCS-3 was greatly diminished by roscovitine, which dampened SOCS-3 signal between 10 min and 6 h.
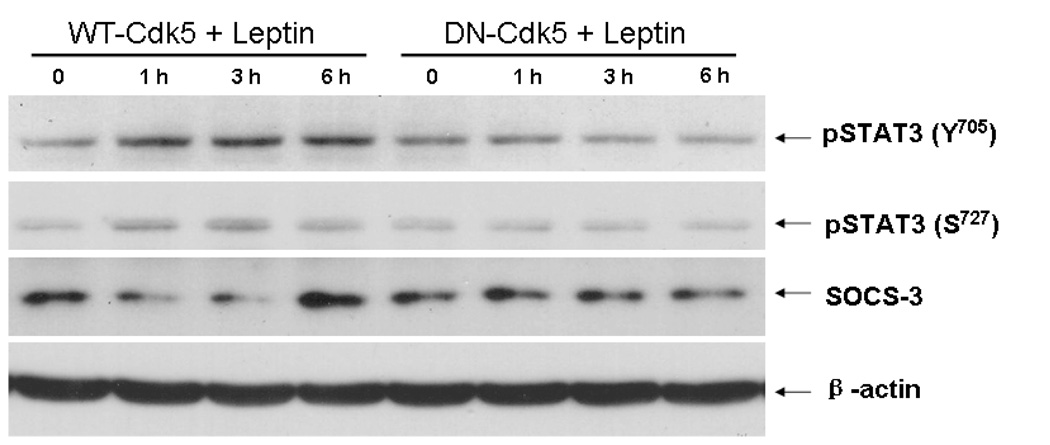
5. Inhibition of endogenous Cdk5 activity by dominant negative Cdk5 (DN-Cdk5) blocks leptin-induced pSTAT3 upregulation
At 16 h after transfection of the differentiated SH-SY5Y cells with DN-Cdk5 or wildtype (WT)-Cdk5 by electroporation, the cells were treated with leptin (30 nM) for 1, 3, or 6 h, in parallel with the non-treated controls (time 0). Figure 6 shows that leptin treatment in cells overexpressing WT-Cdk5 induced pSTAT3 at both the Y705 and S727 sites, without changing the expression of the housekeeping gene β-actin. This increase of pSTAT3 was not seen in the groups of cells overexpressing DN-Cdk5 at any of the time points studied. Surprisingly, WT-Cdk5 reduced SOCS-3 at 1 and 3 h, but increased it at 6 h after leptin treatment.
Fig. 6.
Overexpression of Cdk5 (wildtype, WT-Cdk5) in SH-SY5Y cells increased leptin-induced pSTAT3 production at 1, 3, and 6 h, whereas DN-Cdk5 (dominant negative without kinase activity) suppressed it at all time points. This was seen for both Y705 and S727, and did not affect the housekeeping gene β-actin. By contrast, WT-Cdk5 reduced SOCS-3 at 1 and 3 h but increased it at 6 h.
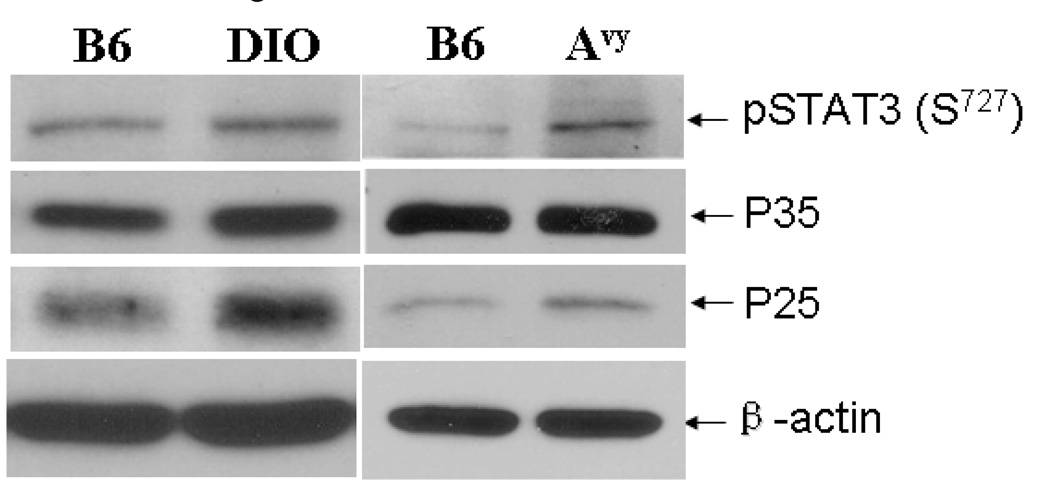
6. Increased pSTAT3 coincides with elevated p35/p25 in DIO and Avy mice
Consistent with the increase in immunofluorescent staining of p35/p25 in the arcuate nucleus, DIO mice had increased protein expression of both p35 and p25 in the hypothalamus, although the total amount of Cdk5 remained constant (Fig.7, left panel). In Avy mice, the protein level of p35 remained the same while the more active p25 kinase was increased (Fig.7, right panel). In both types of obese mice, there was an increase in the level of pSTAT3 in comparison with the lean B6 controls. This leads to the question whether the activation of the Cdk5 system results in STAT3 phosphorylation in neuronal cells.
Fig. 7.
Activities of STAT3 and Cdk5 in the hypothalamus of obese mice. The hypothalamus of a 5-month old DIO mouse and a 3-month old Avy mouse both had higher levels of pSTAT3 (S727) and p25 than their respective controls. The increase of p35 was apparent in DIO but not in the Avy mouse. The house-keeping gene β-actin remained unchanged.
Discussion
Since the discovery of Cdk5 nearly two decades ago (Hellmich, 1992; Meyerson, 1992), much has been revealed about its role in CNS development, migration, synaptic plasticity, pain, addiction, learning, memory, and neurodegenerative disorders. Surprisingly, little attention has been paid to the role of Cdk5 in leptin signaling. Leptin is a hormone that exerts its neuroendocrine effects by multiple signaling pathways downstream to ObR, and many of these may be cell-type specific depending on the co-existence of other receptors (Tu, 2007, 2008). Cdk5 is a ubiquitous kinase in the brain that plays essential roles in CNS development and plasticity, yet it has not been sort after that Cdk5 system may participate in leptin signaling. In this study, we showed for the first time that the Cdk5 activators p35/p25 kinases can be induced by leptin and in turn provide tight controls of leptin signaling manifested by STAT3 and SOCS-3 activation. This regulatory role of Cdk5/p35/p25 may have broad implications in deciphering the phenomenon of leptin resistance, as seen in many forms of obesity where leptin concentration in blood is elevated.
We first showed the co-localization of Cdk5 and ObR in the same cells in the hypothalamus (Fig.1). This illustrates biological relevance of the questions. In fact, adult-onset obesity is associated with increased level of expression of p35 and p25 kinases (Fig. 7). Even in HEK293 cells, overexpression of p35 by transient transfection caused a significant increase of STAT3 transcriptional activity (Fig.2), indicating that the Cdk5 system is a robust regulator of STAT3 signaling. We then determined leptin-induced interactions of Cdk5/p35/p25 and STAT3 in SH-SY5Y neuroblastoma cells, because the induction of ObRb in these cells by differentiation and their STAT3 signaling are well documented (Benomar, 2005; Jang, 2007; Russo, 2004). In these studies, leptin was used at a range of 15 – 100 nM, and cell differentiation increased the level of ObRb expression (Fig.3A–C). This provided a basis for us to choose 30 nM of leptin in our studies.
In differentiated SH-SY5Y cells, leptin activated Cdk5 and its activators p35 and p25 without affecting the housekeeping gene β-actin (Fig.4). The only available antibody for p35/p25 recognizes both; nonetheless, the signals corresponding to their respective molecular size indicated that the p25 kinase showed a greater degree of induction. The findings by western blotting are consistent with redistribution of the immunofluorescence of p35/p25 in SH-SY5Y cells treated with leptin for 1 or 6 h. p25 is associated with phosphorylated tau protein and shows a longer half-life as well as wider intracellular distribution than the full-length p35 kinase. Knowing that Cdk5 induces phosphorylation of STAT3 at the S727 residue, we further determined whether Cdk5 in turn modulates leptin signaling.
Although the Cdk5 inhibitor roscovitine affected leptin-induced STAT3 activation as anticipated, the patterns of modulation were more complicated than expected, and differed at the Y705 and S727 sites of pSTAT3. Roscovitine is a commonly used chemical inhibitor of Cdk5 (He, 2007; Meijer, 1997). Interestingly, the level of S727 pSTAT3 in the DMSO vehicle control group showed a transient reduction 10 min after leptin treatment. The exact function of S727 phosphorylation on STAT3 proteins has been controversial, as an increase, decrease, and absence of change have been reported. Although it is beyond the scope of the current study to determine the inter-relationship between Y705 and S727 STAT3 activation, the different kinetics of basal activation and differential response to roscovitine suggest separate roles of these two sites. The transient increase of pSTAT3 by roscovitine might be related to inhibition of calcium channels (Yan, 2002).
The long-term potentiating effect of Cdk5 on leptin-induced STAT3 activation was further shown by the time-dependent increase of pSTAT3 (both Y705 and S727) in cells overexpressing Cdk5, and by the inhibition of pSTAT3 in cells overexpressing DN-Cdk5. Even in the absence of leptin or other ligands, overexpression of the Cdk5 activator p35 induced dose-related STAT3 transcriptional activity. Since the luciferase reporter assay was performed on HEK293 cells, the lack of effect of Cdk5 and DN-Cdk5 was probably explained by the observation that actively proliferating cells do not have robust Cdk5 activation (Tsai, 1993).
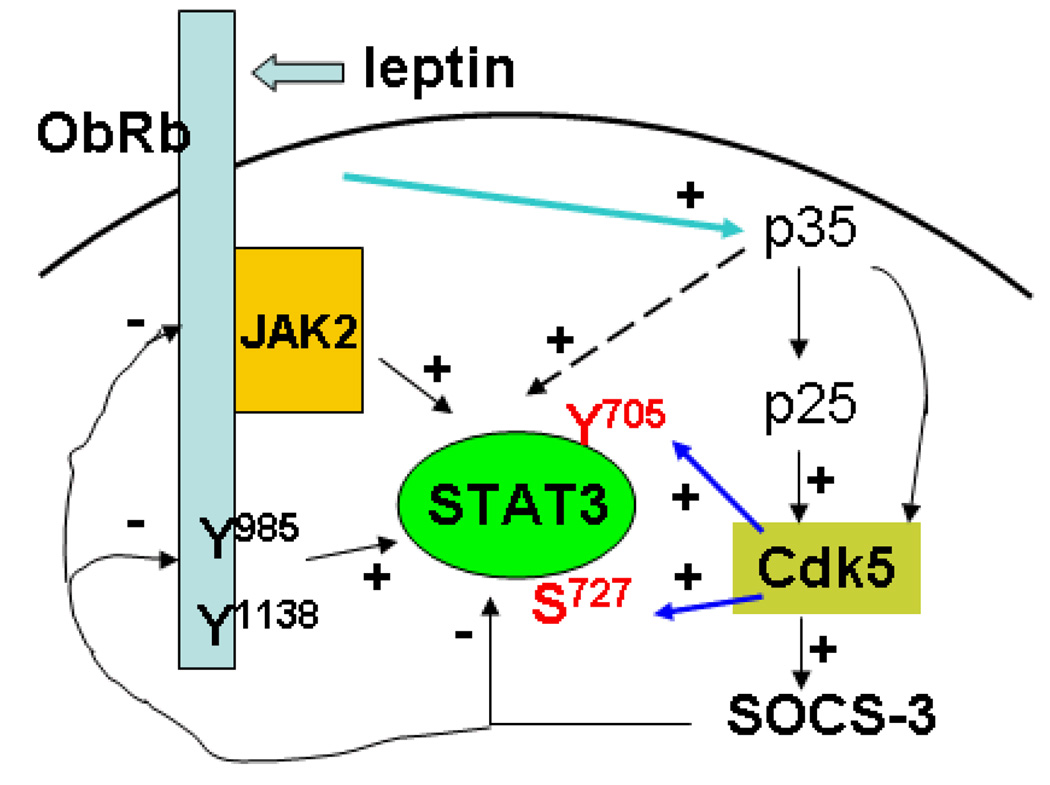
The results show paradoxical activation of SOCS-3 by Cdk5. Roscovitine not only shifted the peak of pSTAT3 activation to earlier times, but also reduced SOCS-3 throughout the study. This suggests a role of Cdk5 in enhancing this prominent negative regulatory pathway to curtail prolonged STAT3 activation after leptin stimulation. Cdk5 is associated with microtubules and implicated in neurodegeneration (Veeranna, 2000). Recently, SOCS3 has also been shown to participate in cell cycle control by promoting p53-dependent p21 expression that inhibits Cdk activity (Sitko, 2008). SOCS proteins modulate the JAK/STAT pathway by several mechanisms: binding to phosphotyrosines through the SH2 domain and inhibiting signal transduction by N-terminal inactivation of JAK; blocking access of STAT to the receptor sites; or by SOCS box-targeting bound proteins to proteasomal degradation (Larsen, 2002). Our finding that Cdk5 acts in conjunction with SOCS-3 further supports the novel dual role of Cdk5 in fine-tuning leptin-induced STAT3 signaling, and indicates the convergence of signaling pathways. The interrelationships of these signaling elements are illustrated in figure 8.
Fig. 8.
Diagram of the induction and consequences of activation of Cdk5. (1) Leptin signaling through ObRb induces activation of p35, p25, and Cdk5. (2) Cdk5 potentiates the activity of pSTAT3 by enhancing phosphorylation at both Y705 and S727 sites. (3) Cdk5 also increases SOCS-3 activity which eventually facilitates termination of STAT3 activation.
This is the first study to show that (a) Cdk5 can modulate the activation patterns of leptin-induced pSTAT3 at both Y705 and S727 sites, and can prolong the resulting activation. (b) Reciprocally, leptin induces Cdk5 activation and increases the protein expression of Cdk5, p35, and p25. (c) While these function to prolong STAT3 activation, Cdk5 also increases SOCS-3 expression at 6 h, thus facilitating the termination of activation. Therefore, we conclude that Cdk5 is a dual player to modulate the long-term effects of leptin.
Acknowledgements
Supported by NIH (DK54880, NS45751, NS46528, and NS62291). The Cdk5-pcDNA3.1 (−) (WT-Cdk5), Dominant negative (kinase inactive) D144N Cdk5-pcDNA3.1 (−) (DN-Cdk5), and p35-pEGFP-C2 plasmids originated from Prof. Yun Wang’s Laboratory (Institute of Neuroscience, Peking University, Beijing, China). The ObRb-pcDNA3.1(−) plasmid was kindly provided by Dr. Christian Bjorbaek (Harvard Medical School, Boston, MA). The pAH-Luc luciferase reporter plasmid for STAT3 activation was kindly provided by Dr. Charles Rosenblum at Merck Research Laboratories (Rahway, NJ). We thank Dr. George Argyropoulos in PBRC for assistance in electroporation, Loula Burton for manuscript submission, and other members of the BBB Group for helpful discussions.
References List
- 1.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 2.Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993;336:417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- 3.Benomar Y, Roy AF, Aubourg A, Djiane J, Taouis M. Cross down-regulation of leptin and insulin receptor expression and signalling in a human neuronal cell line. Biochem.J. 2005;388:929–939. doi: 10.1042/BJ20041621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 5.Cheung ZH, Fu AK, Ip NY. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. doi: 10.1016/j.neuron.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Fu AK, Fu WY, Ng AK, Chien WW, Ng YP, Wang JH, Ip NY. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc Natl Acad Sci U.S.A. 2004;101:6728–6733. doi: 10.1073/pnas.0307606100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garris DR. Morphometric analysis of obesity (ob/ob)- and diabetes (db/db)-associated hypothalamic neuronal degeneration in C57BL/KsJ mice. Brain Res. 1989;501:162–170. doi: 10.1016/0006-8993(89)91037-8. [DOI] [PubMed] [Google Scholar]
- 8.Guo Z, Jiang H, Xu X, Duan W, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem. 2008;283:1754–1763. doi: 10.1074/jbc.M703753200. [DOI] [PubMed] [Google Scholar]
- 9.Harvey J. Leptin: a diverse regulator of neuronal function. J Neurochem. 2007;100:307–313. doi: 10.1111/j.1471-4159.2006.04205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y, Li HL, Xie WY, Yang CZ, Yu AC, Wang Y. The presence of active Cdk5 associated with p35 in astrocytes and its important role in process elongation of scratched astrocyte. Glia. 2007;55:573–583. doi: 10.1002/glia.20485. [DOI] [PubMed] [Google Scholar]
- 11.Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci U.S.A. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang EH, Park CS, Lee SK, Pie JE, Kang JH. Excessive nitric oxide attenuates leptin-mediated signal transducer and activator of transcription 3 activation. Life Sci. 2007;80:609–617. doi: 10.1016/j.lfs.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Kastin AJ, Banks WA, Zadina JE, Graf MV. Brain peptides: the dangers of constricted nomenclatures. Life Sci. 1983;32:295–301. doi: 10.1016/0024-3205(83)90073-5. [DOI] [PubMed] [Google Scholar]
- 14.Kastin AJ, Pan W. Intranasal leptin: blood-brain barrier bypass (BBBB) for obesity? Endocrinol. 2006;147:2086–2087. doi: 10.1210/en.2006-0208. [DOI] [PubMed] [Google Scholar]
- 15.Larsen L, Ropke C. Suppressors of cytokine signalling: SOCS. APMIS. 2002;110:833–844. doi: 10.1034/j.1600-0463.2002.1101201.x. [DOI] [PubMed] [Google Scholar]
- 16.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am.J.Physiol. 2004;286:R143–R150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 17.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 18.Lew J, Qi Z, Huang QQ, Paudel H, Matsuura I, Matsushita M, Zhu X, Wang JH. Structure, function, and regulation of neuronal Cdc2-like protein kinase. Neurobiol Aging. 1995;16:263–268. doi: 10.1016/0197-4580(95)00014-6. [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Chen MC, Chiu CY, Song YM, Lin SY. Cdk5 regulates STAT3 activation and cell proliferation in medullary thyroid carcinoma cells. J Biol Chem. 2007;282:2776–2784. doi: 10.1074/jbc.M607234200. [DOI] [PubMed] [Google Scholar]
- 20.Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 21.Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Rourke L, Shepherd PR. Biphasic regulation of extracellular-signal-regulated protein kinase by leptin in macrophages: role in regulating STAT3 Ser727 phosphorylation and DNA binding. Biochem J. 2002;364:875–879. doi: 10.1042/BJ20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohshima T, Gilmore EC, Longenecker G, Jacobowitz DM, Brady RO, Herrup K, Kulkarni AB. Migration defects of cdk5(−/−) neurons in the developing cerebellum is cell autonomous. J Neurosci. 1999;19:6017–6026. doi: 10.1523/JNEUROSCI.19-14-06017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte Leptin Receptor (ObR) and Leptin Transport in Adult-Onset Obese Mice. Endocrinology. 2008a;149:2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan W, Hsuchou H, Tu H, Kastin AJ. Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology. 2008b;149:877–885. doi: 10.1210/en.2007-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan W, Kastin AJ. Adipokines and the blood-brain barrier. Peptides. 2007a;28:1317–1330. doi: 10.1016/j.peptides.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan W, Kastin AJ. Mahogany, blood-brain barrier, and fat mass surge in A(VY) mice. International Journal of Obesity. 2007b;31:1030–1032. doi: 10.1038/sj.ijo.0803536. [DOI] [PubMed] [Google Scholar]
- 28.Pan W, Tu H, Hsuchou H, Daniel J, Kastin AJ. Unexpected amplification of leptin-induced Stat3 signaling by urocortin: implications for obesity. J Mol Neurosci. 2007c;33:232–238. doi: 10.1007/s12031-007-0071-y. [DOI] [PubMed] [Google Scholar]
- 29.Patrick GN, Zukerberg L, Nikolic M, de la, Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 30.Rosenblum CI, Tota M, Cully D, Smith T, Collum R, Qureshi S, Hess JF, Phillips MS, Hey PJ, Vongs A, Fong TM, Xu L, Chen HY, Smith RG, Schindler C, Van der Ploeg LH. Functional STAT 1 and 3 signaling by the leptin receptor (OB-R); reduced expression of the rat fatty leptin receptor in transfected cells. Endocrinology. 1996;137:5178–5181. doi: 10.1210/endo.137.11.8895396. [DOI] [PubMed] [Google Scholar]
- 31.Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145:4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 33.Sitko JC, Yeh B, Kim M, Zhou H, Takaesu G, Yoshimura A, Jewett A, Jamieson CA, Cacalano NA. SOCS3 regulates p21 expression and cell cycle arrest in response to DNA damage. Cell Signal. 2008 doi: 10.1016/j.cellsig.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang D, Lee KY, Qi Z, Matsuura I, Wang JH. Neuronal Cdc2-like kinase: from cell cycle to neuronal function. Biochem Cell Biol. 1996;74:419–429. doi: 10.1139/o96-046. [DOI] [PubMed] [Google Scholar]
- 35.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 36.Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- 37.Tseng HC, Zhou Y, Shen Y, Tsai LH. A survey of Cdk5 activator p35 and p25 levels in Alzheimer's disease brains. FEBS Lett. 2002;523:58–62. doi: 10.1016/s0014-5793(02)02934-4. [DOI] [PubMed] [Google Scholar]
- 38.Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;214:301–305. doi: 10.1002/jcp.21195. [DOI] [PubMed] [Google Scholar]
- 39.Tu H, Pan W, Feucht L, Kastin AJ. Convergent trafficking pattern of leptin after endocytosis mediated by ObRa - ObRd. J.Cell Physiol. 2007;212:215–222. doi: 10.1002/jcp.21020. [DOI] [PubMed] [Google Scholar]
- 40.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 41.Veeranna, Shetty KT, Takahashi M, Grant P, Pant HC. Cdk5 and MAPK are associated with complexes of cytoskeletal proteins in rat brain. Brain Res Mol Brain Res. 2000;76:229–236. doi: 10.1016/s0169-328x(00)00003-6. [DOI] [PubMed] [Google Scholar]
- 42.Waelput W, Verhee A, Broekaert D, Eyckerman S, Vandekerckhove J, Beattie JH, Tavernier J. Identification and expression analysis of leptin-regulated immediate early response and late target genes. Biochem J. 2000;348(Pt 1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Xie WY, He Y, Wang M, Yang YR, Zhang Y, Yin DM, Jordan-Sciutto KL, Han JS, Wang Y. Role of CDK5 in neuroprotection from serum deprivation by mu-opioid receptor agonist. Exp Neurol. 2006;202:313–323. doi: 10.1016/j.expneurol.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Rensing N, Yang XF, Zhang HX, Thio LL, Rothman SM, Weisenfeld AE, Wong M, Yamada KA. Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. J Clin Invest. 2008;118:272–280. doi: 10.1172/JCI33009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Z, Chi P, Bibb JA, Ryan TA, Greengard P. Roscovitine: a novel regulator of P/Q-type calcium channels and transmitter release in central neurons. J Physiol. 2002;540:761–770. doi: 10.1113/jphysiol.2001.013376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 47.Zheng M, Leung CL, Liem RK. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol. 1998;35:141–159. doi: 10.1002/(sici)1097-4695(199805)35:2<141::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 48.Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinol. 2000;141:1434–1441. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]