Abstract
Aurora Kinase A (Aurora-A) belongs to a highly conserved family of mitotis-regulating serine/threonine kinases implicated in epithelial cancers. Initially we examined Aurora-A expression levels at different stages of human skin cancer. Nuclear Aurora-A was detected in benign lesions, and became more diffused but broadly expressed in well and poorly differentiated SCC, indicating that Aurora-A deregulation may contribute to SCC development. To mimic the overexpression of Aurora-A observed in human skin cancers, we established a gene-switch (GS) mouse model in which the human variant of Aurora-A (Phe31Ile) was expressed in the epidermis upon topical application of the inducer, RU486 (Aurora-AGS). Overexpression of Aurora-A alone or in combination with the tumor promoter, TPA, did not result in SCC formation in Aurora-AGS mice. Moreover, Aurora-A overexpression in naive keratinocytes resulted in spindle defects in vitro and marked cell death in vivo, suggesting that the failure of Aurora-A to initiate tumorigenesis was due to induction of catastrophic cell death. However, Aurora-A overexpression combined with exposure to TPA and the mutagen, DMBA, accelerated SCC development with greater metastastic activity than control mice, indicating that Aurora-A cannot initiate skin carcinogenesis, but rather promotes the malignant conversion of skin papillomas. Further characterization of SCCs revealed centrosome amplification and genomic alterations by array CGH analysis, indicating that Aurora-A overexpression induces a high level of genomic instability that favors the development of aggressive and metastatic tumors. Our findings strongly implicate Aurora-A overexpression in the malignant progression of skin tumors and suggest that Aurora-Amay be an important therapeutic target.
Keywords: Aurora-A, Genomic Instability, SCC, tumorigenesis
Introduction
Replication of mammalian cells is a very precise and ordered process that results in the faithful duplication of chromosomes and equal distribution of genetic material between daughter cells. De-regulation of kinases that control cell cycle progression is commonly observed in human tumors and may promote aberrant mitosis leading to genomic instability. Many malignant tumors display genetic alterations that result in the activation of oncogenes and/or loss of tumor suppressors, which suggest that genomic instability may be an important factor in the development of highly malignant tumors. Nevertheless, the exact role of genomic instability in tumorigenesis remains poorly understood, if not controversial.
The Aurora-A serine-threonine kinase is one of three members of a highly conserved family of mitotic kinases that have fundamental roles in regulating cell division. Previous studies have shown that Aurora-Ais necessary for the timely entry into mitosis, maturation of centrosomes and assembly of bipolar spindles (1–3). Aurora-A interacts with Polo like Kinase-1 (Plk-1) to indirectly regulate Cyclin-B/Cdk1 complex activity, and thereby, to promote entry into mitosis (4). In addition, Aurora-A also localizes to the centrosomes during the G2 phase of the cell cycle through interactions with various factors, including Plk-1. Two recent independent studies have demonstrated that Plk-1 activation by Aurora-A, mediated by its interaction with a G2-M expressed protein Bora, are essential for mitotic entry (5) and recovery from checkpoint arrest (6). Aurora-A is also essential for the function and localization of centrosomal component proteins (1), and promotes centrosome separation and spindle assembly, helping to establish the two poles of the bipolar mitotic spindle (1). Protein levels of Aurora-A fluctuate during the cell cycle reaching their peak early in mitosis and dropping quickly by the end of anaphase/G1 phase, when it is targeted for degradation by the anaphase promoting complex (7). Disruption of Aurora-A function leads to severe mitotic defects such as delayed entry into mitosis, monopolar spindles, defective centrosome maturation, and misalignment of chromosomes during metaphase (7). Interestingly, ectopic expression of Aurora-A results in centrosome duplication, multipolar spindle, tetraploidization, and aneuploidy (2), indicating that optimal levels of Aurora-Aare necessary for the faithful replication of cells.
Aurora-A, which resides on human chromosome 20q13, is frequently amplified in human breast, bladder, ovarian, gastric and colon carcinomas (8), and it is considered an oncogene implicated in the pathology of these malignancies. Additionally, Aurora-A was also identified as a low penetrance cancer susceptibility gene in both human and mice (9, 10) and shown to be overexpressed at the mRNA or protein level in several human tumor types (8). Aurora-A overexpression transforms immortalized rodent cell lines in vitro, giving rise to tumors when these cells are implanted in nude mice (2, 11). Moreover, a genetic variant of Aurora-A (Phe31Ile) has been identified as a cancer susceptibility gene in multiple types of human cancer (9, 10, 12), and also enhances transforming activity in vitro (9).
There is currently great interest in using small molecule inhibitors to target Aurora-A function in cancer (13). However, the mechanism by which Aurora-A contributes to tumorigenesis in vivo remains unclear and there are currently no tumor models that recapitulate the characteristics of human cancers associated with Aurora-A amplification or overexpression. Several groups have used transgenic approaches to overexpress Aurora-A in mouse mammary glands with conflicting results (14–16). Only one of these groups was able to demonstrate formation of malignant mammary tumors after a long latency (16), while two other groups reported either benign tumor formation or no tumors in mice overexpressing Aurora-A (15, 16). Moreover, metastasis was not reported in any of these studies. To better understand the role of Aurora-A at different stages of carcinogenesis, we developed an inducible model in which the expression of Aurora-A was specifically directed to epithelial cells in the skin using the gene-switch system (17). In this study we show that overexpression of Aurora-A cannot initiate tumorigenesis, but can accelerate the conversion of DMBA-initiated papillomas to squamous cell carcinomas (SCC) that were highly metastatic and displayed a high level of genomic instability.
Material and Methods
Construction of Aurora-A Gene-switch transgenic mice
To engineer mice expressing an inducible form of Aurora Kinase A (Aurora-A), a cDNA encoding the Phe31Ile Aurora-A variant, previously shown to be a low penetrance tumor-susceptability gene associated with the degree of aneuploidy in human colon tumors (9), was cloned into the UAS-TK vector (18). Transgenic founders were established by standard procedures and mice carrying the Aurora-A transgene were routinely genotyped by PCR using the primers: 5′-AGC CGG TTC AGA ATC AGA AG-3′ and 5′-AAG CTC TCC AGC TGA TCC AAG A-3′. Aurora-A monogenic mice were crossed with RU486 inducible K14.GLP65 transgenic mice (19). For ease of reading, Aurora-A; GLP65 bigenic mice will be referred as Aurora-AGS mice.
Molecular and biochemical analyses
Total RNA was extracted from frozen or RNALater (Ambion) stored tissue using Triazol reagent (Invitrogen) and reverse transcription of total RNA was performed as previously described (20). Aurora-A expression was detected in 7 ng of reverse transcribed total RNA by qPCR analysis using a Light cycler 480 system (Roche Diagnostics) and the amplification primers: 5′-GCAGGCAACCAGTGTACCT-3′ and 5′-TGCCAGTTCCTCCTCAGGATTA-3′ and probe: 5′-ATCCTGTCTCCAGGCCACTGA -3′. Aurora-A levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) RNA abundance (AppliedBiosystems). Codon 61 mutations in the Hras and Kras gene were determined as previously described (21, 22). For immunoblotting, tissues were first lyzed in 50mM Tris, 120mM NaCl, 1mM EDTA, 1% NP-40, and 10% glycerol containing protease inhibitors (Roche Diagnostics). Lysates were then resolved via SDS-PAGE, transferred and incubated with antisera against human Aurora-A (generated by S. Sen) or β-Actin (Abcam). Blots were then incubated with appropriate HRP conjugated secondary (Sigma) and the signal visualized using the Supersignal detection system (Pierce Biotech).
Animal, carcinogenesis and cell culture protocols
To activate expression of Aurora-A in Aurora-AGS mice, animals were topically treated with either 1mg/mL (newborn) or 2.5 mg/mL (adult) of RU486 dissolved in ethanol. For two-stage chemical carcinogenesis experiments, newborn mice were first initiated with a single treatment of 50μg 7,12-Dimethylbenz[a]anthracene (DMBA) (Sigma) and promoted with 20 μg of Phorbol 12-myristate 13-acetate (TPA) (Sigma) weekly. The expression of Aurora-A was maintained topically with 200 μg RU486 thrice per week. For Bromodeoxyuridine (BrdU) incorporation experiments, mice were injected with 1mL/100g of body weight dose of Zymed BrdU labeling reagent (Invitrogen). Tissues were harvested 2 hours afterward, fixed and processed for paraffin or OCT embedding, and sections were stained with hematoxylin and eosin or processed for imunohistochemistry (IHC). Mouse keratinocytes were isolated as previously described (23) and grown in 100nM RU486 to induce Aurora-A expression.
Centrosome staining
Immunodetection of centrosomes was performed on 30 μM thick OCT embedded sections. Post-fixed sections were permeabilized, blocked, and incubated with γ–tubulin anteserum (Sigma). Sections were then incubated with FITC-conjugated secondary antibodies (Jackson Labs) and counterstained with propidium iodide (PI) (Sigma). Stained sections were analyzed with a Zeiss LSM 510 META Confocal Imaging Microscope. For centrosome counting, at least five randomly selected regions in each section were scanned. The number of centrosomes per nucleus was counted and a minimum of 300 nuclei were analyzed for each section.
Immunofluorescence and immunohistochemical analyses
Sections processed for immunofluorescence (IF) were blocked, and incubated with the following primary antibodies: anti-K14 (24), α-human Aurora-A (Abcam or from S. Sen), α-γ– tubulin (Sigma), α-TTF1 (kindly provided by Dr. Francesco DeMayo, Baylor College of Medicine, Houston, TX), and α-BrdU (BDBioscience). Slides were then incubated with Alexa Fluor-conjugated secondary antibodies (Invitrogen), mounted with DAPI mounting solution, and analyzed by fluorescent microscopy. Primary keratinocytes stained for IF were initially fixed, permeabilized with 0.2% Triton X100, blocked, and incubated with primary and secondary antibodies as stated above. Immunohistochemical analysis was performed as previously described (25). Antigen retrieval was performed in 0.1M Sodium Citrate using a commercial microwave. Sections were then incubated with Ki67 (Vector Laboratories), active Caspase 3 (Cell Signalling), and p53 (Vector laboratories) antiserum. For detection of Aurora-A in human tumor sections (archival tumors in the Department of Dermatology, University of Colorado Denver, Denver, CO) and a human skin tumor array (Proteinbiotechnologies), were subjected to antigen retrieval in 198 mM Tris, 6 mM EDTA and 1.7% Tween-20 using a commercial pressure cooker. Sections were then incubated with Aurora-A antiserum (Novocastra Laboratory). Signals were then amplified with VECTASTAIN® Elite ABC Kit (Vector Laboratories) and visualized using Vector NovaRed substrate kit (Vector laboratories). Terminal uridine deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays were performed using the DeadEnd™ Fluorometric TUNEL System (Promega).
Array CGH analysis
Genomic DNA (gDNA) was isolated from frozen tumor biopsies using a DNeasy kit (Qiagen Inc.) and 1 μg of tumor and C57BL/6J reference gDNA (Jackson Laboratories) was digested with AluI and RsaI(Promega). Digested gDNA was then fluorescently labeled using Agilent Genomic DNA Labeling Kit Plus (Agilent Technologies). Cy3-labeled tumor DNA and Cy5-labeled reference DNA were combined and hybridized to Agilent 244K oligo array CGH slides using an Agilent CGH hybridization kit and oven (Agilent Technologies). Washed slides were then scanned with Agilent Scanner G2565BA using Agilent Feature Extraction Software V. 9.5.3.1 (Agilent Technologies). Data analysis was performed using Agilent CGH Analytics V3.4 software as previously described (26). Z-score and ADM-2 aberration methods were used with a threshold of 2.5 and 6, respectively, to determine regions of amplification or deletion in tumors.
Statistical analysis
Values are presented as mean±SD. Statistical analysis was determined using curve fitting, Student’s t-, Chi-square, or Fisher’s exact test using Graphpad Prism software.
Results
Aurora-A Overexpression in human Squamous Cell Carcinomas
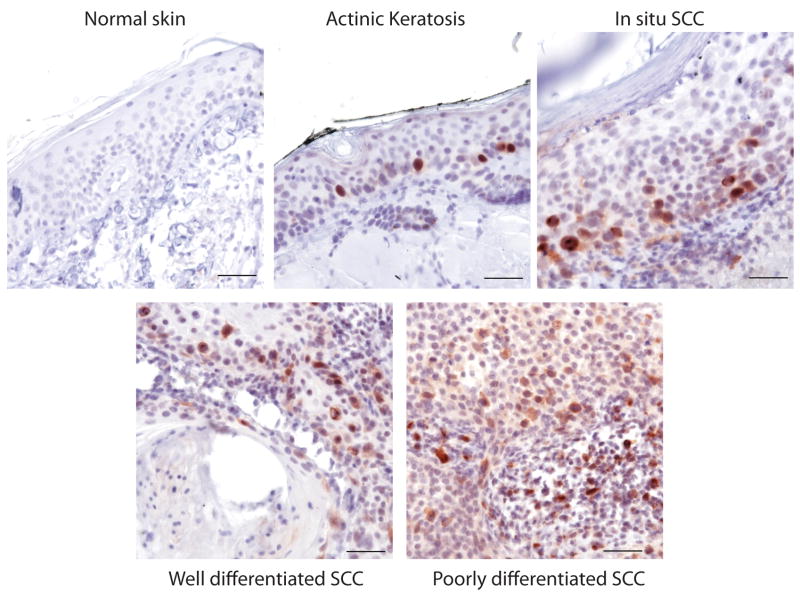
To determine if Aurora-A was overexpressed in human skin cancers, we screened normal skin, pre-benign and benign lesions, and SCC tumor samples for Aurora-A expression. Aurora-A was rarely detected in normal adult skin (Fig 1). However, strong nuclear staining of Aurora was detected in pre-benign actinic keratosis lesions (n=3) near regions of active proliferation (Fig. 1). We observed localization of Aurora staining at both poles and spindles of cells undergoing early mitosis (Supplementary Fig. S1). Nuclear staining of Aurora was also evident in benign (n=5) and well differentiated SCC (n=11). Poorly differentiated SCC (n=9) showed a more diffused, but widespread, pattern of Aurora-A expression (Fig. 1), suggesting that Aurora-A levels correlated with tumor malignancy. In addition, abnormal staining patterns were detected in small numbers of SCC cells in both benign and poorly differentiated tumors (Supplementary Fig. S1). We further screened a commercially available human skin cancer tissue array representing forty SCC and other skin tumor types. We observed a staining pattern similar to our archival tumor samples, indicating that Aurora-A expression may be an important marker of SCC progression.
Figure 1.
Aurora-A is overexpressed in human pre-benign, benign and skin SCC.. Human sections were immunochemically stained for Aurora-A. Non-affected skin was taken from scalp. Representative fields are shown. Bar=100 μm.
Generation of Aurora-AGS mice
To mimic overexpression of Aurora-A observed in human skin tumors, we utilized the gene-switch system (17) to preferentially express the Phe31Ile variant of the human Aurora-A in murine epithelial cells after the topical application of the anti-progesterone drug RU486. This variant of Aurora-A was shown to be associated with increased risk and poor patient prognosis in esophageal carcinoma (9, 10, 12). The gene-switch (GS) system has two components: the activator and the target transgene (Fig. 2A, Inset). The activator transgene, a human keratin14 (K14)-driven fusion protein composed of the GAL4 DNA binding domain, the truncated progesterone receptor, and the transactivating domain of NF-κB p65 (K14.GLP65), translocates to the nucleus in the presence of RU486, but not endogenous progesterone, to activate target genes containing GAL4 binding motifs (19). Although the K14 promoter directs expression of the activator to basal keratinocytes in stratified epithelial tissues including the skin, oral cavity and esophagus, activation of target genes is restricted to the skin by topical application of RU486 (19).
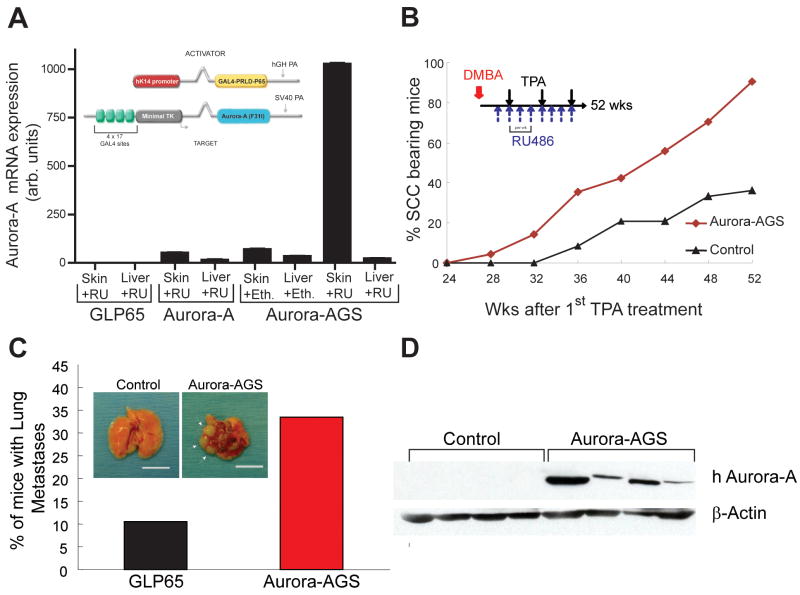
Figure 2.
Aurora-A overexpression promotes development of malignant SCC. A, qPCR assays showing induction of Aurora-A mRNAin skins of control or Aurora-AGS mice topically treated with ethanol or RU486 for 3 days. Inset depicts transgene structures. B, Mice were treated once with DMBA and weekly with TPA/RU486 (Inset). Conversion to SCC was accelerated in Aurora-AGS mice compared to controls. C, A greater number of tumor laden Aurora-AGS mice had lung metastasis compared to control mice with multiple visible nodules (Inset, arrows). Bar=1cm. D, Immunodetection of human Aurora-A in tumor lysates. The membrane was then stripped and reprobed for β-actin.
Several lines of Aurora-A transgenic mice were established and showed no overt pathology. These lines were then bred to K14.GLP65 (GLP65) activator mice to test for Aurora-A inducibility. One line showed robust induction of Aurora-A gene expression after RU486 treatment when co-expressing GLP65 (Fig. 2A) and was used for subsequent experiments. Bigenic mice treated with ethanol showed basal level expression that was comparable to Aurora-A target mice alone. Additionally, there was no induction in liver (i.e. non-squamous epithelial tissue) of RU486 treated bigenic mice, demonstrating skin specific activation of Aurora-Ain Aurora-AGS mice.
The role of Aurora-A in Skin Tumorigenesis
To determine if Aurora-A could initiate skin tumors, we treated Aurora-AGS or control mice (n=24) with RU486 and the tumor promoting drug, TPA, for 6 months. Neither control nor Aurora-AGS mice developed tumors within this period, indicating that Aurora-A overexpression was not sufficient to initiate tumorigenesis in skin. We then performed a two-stage carcinogenesis experiment to determine if Aurora-A overexpression could enhance skin tumors in Aurora-AGS mice after a single treatment of the carcinogen DMBA and weekly application of TPA (Fig. 2, Inset) (n=60 for each group). DMBA/TPA induced skin carcinogenesis is a well-established protocol to generate papillomas in mice that progress infrequently to SCC (27). When combined with RU486 treatment, this protocol yielded papillomas in Aurora-AGS with the same latency and frequency as control mice (data not shown). However, benign tumors in Aurora-AGS mice progressed to SCC with a higher frequency than control mice starting at week 28 (Fig. 2B), reaching 90% of mice by 52 wks in comparison to 36% of mice bearing SCC in the control group (p<0.01). Gross necropsy revealed that 34% of Aurora-AGS mice had visible metastatic lesions in the lungs (Fig. 2C) compared to 9% of control mice. Additionally, Aurora-AGS mice presenting with metastasis had multiple nodules in lungs in contrast to single nodules observed in control tumors with metastasis (Fig. 2C). We confirmed the presence or absence of metastasis by histological examination of lung tissue. Lung tumors stained positive for Keratin-14 and negative for the lung transcription factor TTF1 (data not shown), confirming the epithelial origin of these tumors. These data strongly implicate Aurora-A overexpression in the progression of epithelial skin cancers.
Analysis of primary tumors in Aurora-AGS mice revealed no difference in BrdU incorporation or in the number of TUNEL-positive cells between Aurora-AGS and control tumors (Supplementary Fig. S2). We also detected expression of human Aurora-A protein in tumors from Aurora-AGS mice (Fig. 2D), indicating that overexpression of Aurora-A in tumors did not alter either proliferation or cell death.
Aurora-A Overexpression Induces Genomic Instability in SCC
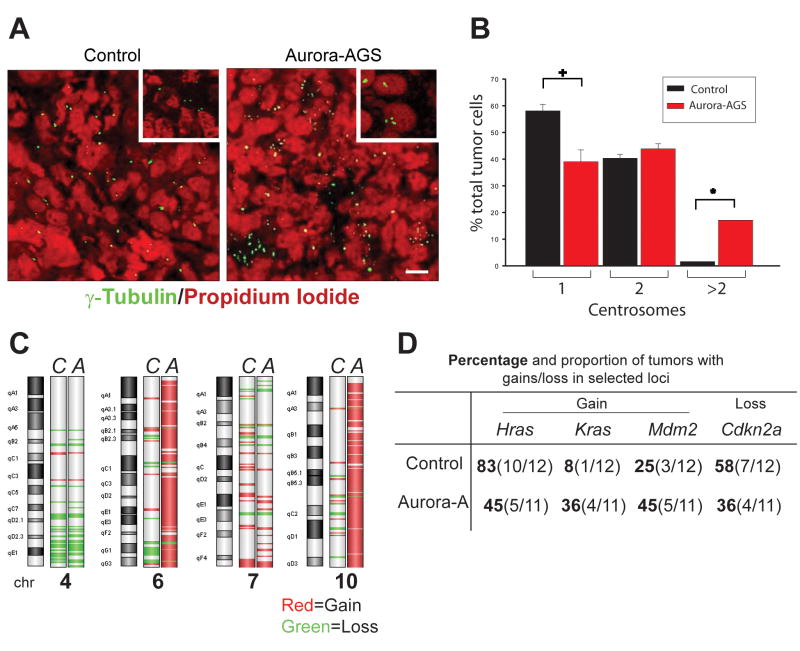
Centrosome amplification has been reported in primary embryonic fibroblasts and cell lines ectopically expressing Aurora-A (2, 28). To determine if centrosome duplication was a prominent feature of SCC overexpressing Aurora-A, we stained tumors forγ-tubulin and with P.I to enumerate centrosomes. Aurora-AGS tumors showed higher content of centrosomes compared to control tumors (Fig. 3A) with 17% of tumors cells having >2 centosomes (Fig 3B). In contrast, a higher number of control tumors cells had single centrosomes than Aurora-AGS tumors (58% vs 39%), while less than 2% of control tumors showed >2 centrosomes (Fig 3B). These results indicate that Aurora-A overexpression promotes centrosome amplification in vivo and may contribute to genomic instability in these tumors.
Figure 3.
Genomic instability in Aurora-A tumors. A, Tumor sections from Aurora-AGS or control mice were stained with γ-tubulin and P.I. B, Centrosomes per tumor cell were tabulated. The data shown were from 3 separate analyses of control and Aurora-A tumors. Bar=5 μm. +,*=p<0.05. C, Aurora-A (A) or control (C) tumors were analyzed by array CGH using the Agilent 244K platform. Probe signal was averaged across groups and genomic aberrations detected by z-score algorithm. Shown are chromosomes most altered in Aurora-A tumors. D, Summary of alterations found in Hras, Kras, Mdm2, and Cdkn2a loci.
To assess global genomic changes, we performed array CGH analysis of tumor DNA using the Agilent 244K array CGH platform. We did not observe common high level chromosomal duplications or deletions in control (n=12) or Aurora-A tumors (n=11). However, Aurora-A tumors displayed amplification of large regions of chromosome 6 (4/11 tumors) and chromosome 10 (5/11 tumors). Most control tumors had amplification of the distal portion of chromosome 7 (10/12 tumors). Analyzed as two groups, there were 29,741 probes amplified in Aurora-A SCC vs. 2,093 probes amplified in control tumors, while there were 14,642 probes showing deletions in Aurora-A tumors and 16,830 probes showing deletions in control tumors (χ2p<0.001). Most amplified regions in Aurora-A tumors were localized to chromosomes 6 (45% of all amplified probes), 7(14%), and 10 (38%) (Fig. 3B), while probes corresponding to chromosome 7 accounted for 53% of all probes amplified in control tumors. The complete list of commonly altered probes can be found in Supplementary file S3.
Closer examination of genetic alterations in chromosome 6 and 7 in SCC of Aurora-AGS mice revealed genomic gains in both the Hras (7qF5) and Kras (6qG3) loci (Fig. 3D), while control tumors predominantly showed gains of the Hras locus (Fisher exact t-test p<0.001). We also detected an A-to-T transversion at codon 61 in exon2 of Hras in all control and most Aurora-AGS tumors (82% of tumors), consistent with previous work showing that alterations in Hras are prominent features of SCC derived from DMBA/TPA treated mice (29, 30). There were no mutations in codons 12, 13, or 61 of Kras in any of the tumors analyzed (data not shown). Analysis of tumor DNA from Aurora-A or control tumors by qPCR showed a similar pattern of genomic alteration in Hras and Kras loci as revealed by array CGH analysis (data not shown).
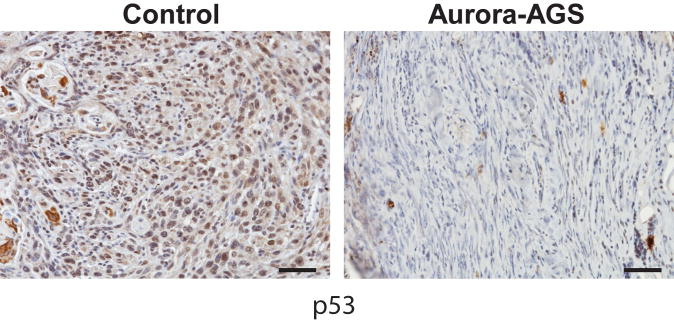
Mutations in the p53 and CDKN2A loci, both of which encodes for tumor suppressors, have been reported in the development of human SCC (31). Numerous studies have shown crosstalk between p53 function and Aurora-A regulation. p53 has been shown to inhibit Aurora A function by direct interaction or via Gadd45a; Aurora-A can phosphorylate p53 leading to Mdm2 mediated degradation or to decreased p53 DNA binding and transactivating activity (1). We therefore examined tumor DNA for loss of tumor suppressor function. Array CGH analysis did not reveal deletions of probes corresponding to the p53 locus (11qB2) in SCC from either control or Aurora-AGS mice. We also sequenced exons 5–6 and 7–8 corresponding to the hot spot regions of p53, and found no mutations in control or Aurora-A tumor DNA (n=10, each). We then examined Cdkn2a (4qC4) and Mdm2 (10qD2) loci for genomic alterations. Mdm2 is a known negative regulator of p53 function (32). Probes corresponding to the mouse Cdkn2a showed deletion in 58% control tumors and 36% of Aurora-AGS tumors (Fig. 3B), suggesting that deletions of p16Ink4a and p19Arf, encoded by this locus, may be important for the development of chemically induced skin tumors. In comparison, Mdm2 was found amplified in 25% control and 45% of Aurora-AGS tumors. To further characterize the p53 status of Aurora-A tumors, we stained tumors for p53 protein by IHC. We observed nuclear p53 staining in 92% of control tumors analyzed, while 55% of Aurora-A tumors showed p53 staining (Fig 4), indicating that downregulation of p53 may be important for Aurora-A’s oncogenic activities.
Figure 4.
Deregulation of p53 protein in Aurora-A tumors. Sections from control and Aurora-A tumors were stained for p53. Bar=50 μm
Aurora-A overexpression in keratinocytes resulted in spindle defects and cell death
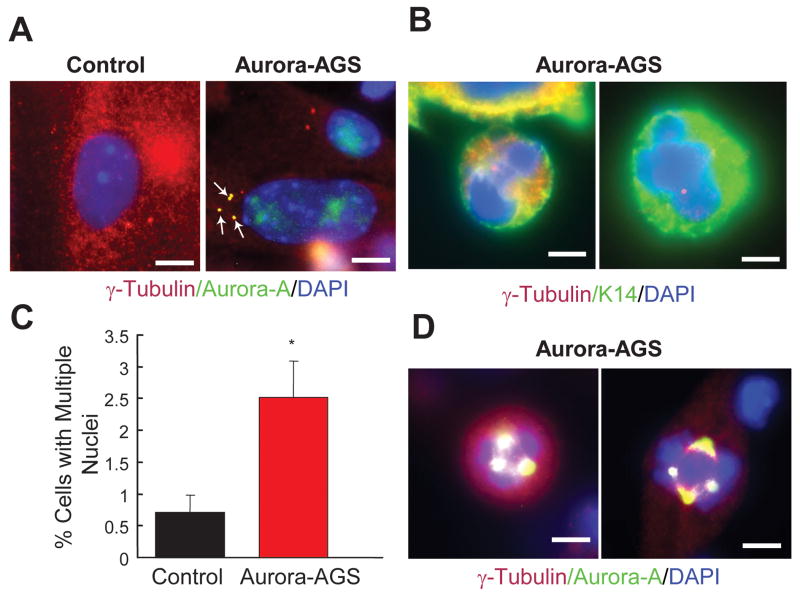
The inability of Aurora-A to initiate tumorigenesis in vivo even when coupled to a strong tumor promoter, such as TPA, suggests that Aurora-A may require co-operation with other oncogenes or loss of tumor surveillance genes to be fully oncogenic. To characterize the acute effects of overexpressing Aurora-A in keratinocytes, we treated primary cultured cells of bigenic or control mice with RU486. Co-staining with a human specific antibody against Aurora-A and the centrosome marker γ-tubulin showed Aurora-A localization to centrosomes of bigenic keratinocytes (Fig. 5A), similar to observations in human tumor cells (Supplementary Fig. S1). Staining for γ-tubulin revealed two centrosomes in control cells undergoing replication (Fig. 5A); however, four centrosomes were a common feature of keratinocytes overexpressing Aurora-A (Fig. 5A). Abnormal centrosome amplification can cause multipolar or disorganized spindles during mitosis, leading to missegregation of chromosomes and the formation of one daughter cell with two or more unequal nuclei (33). These types of abnormal cells were also observed in cultured Aurora-AGS derived keratinocytes following induction with RU486 (Fig. 5B), with significantly increased numbers of multi-nucleated cells (Fig 5C). Cells with multiple spindle poles or abnormal spindle structures were evident, showing a strong concentration of Aurora-A protein in these structures (Fig. 5D). These experiments indicate that Aurora-A overexpression in keratinocytes results in spindle and mitotic defects.
Figure 5.
Aurora-A overexpression in keratinocytes induces spindle defects and centrosome amplification. Immunodetection of γ-tubulin, DAPI, Aurora-A, and Keratin-14 in RU486 treated primary keratinocytes. Yellow depicts co-localization ofγ-tubulin(red) and Aurora-A(green) or Keratin-14(green). A, Localization of Aurora-A in amplified centrosomes (arrows). B, Aurora-A overexpressing cells showed missegregation of chromosomes. C, Tabulation of cells having multiple nuclei. *=p<0.05. D, Co-localization of Aurora-A and γ-tubulin in cells with abnormal spindle structures. Bar=5 μm.
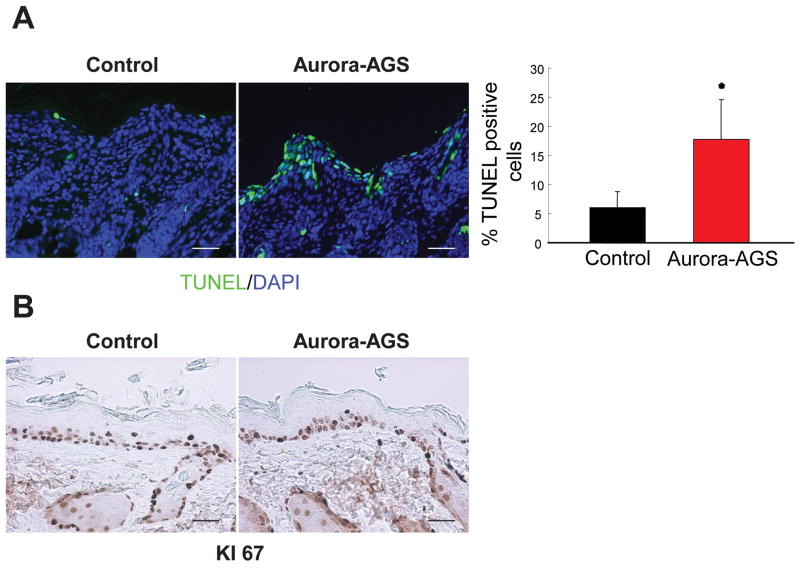
IHC analysis of the skin isolated from newborn or adult mice treated with RU486 revealed elevated levels of TUNEL staining in Aurora-A mice compared to controls (Fig. 6A, pups shown). No obvious changes in proliferation were observed between RU486 treated Aurora-AGS and control animals as demonstrated by Ki67 IHC (Fig. 6B) and BrdU incorporation experiments (data not shown), nor did we observe a change in Loricrin, Keratin-1, or Keratin-14 expression pattern between Aurora-AGS and control animals (data not shown). Additionally, activation of neither caspase 3 nor p53 induction was observed in RU486 treated skins of Aurora-AGS mice (data not shown), indicating that Aurora-A overexpression in keratinocytes led to cell death of a potentially catastrophic nature.
Figure 6.
Aurora-A overexpression leads to cell death of keratinocytes in vivo. Newborn mice were treated with RU486 for 6 days, and skin sections were immunostained for Ki-67 and TUNEL. A, Aurora-A skin demonstrated a marked increase in TUNEL positive cells compared to controls. Bar graph shows tabulation of TUNEL positive cells from 3 control and Aurora-AGS mice. Bar=50 μm. *=p<0.05. B, Ki-67 staining revealed no difference in proliferation between control and Aurora-A expressing keratinocytes. Bar=50 μm.
Discussion
In this study, we established an RU486 dependent system to determine the consequences of overexpressing human Aurora-A and its potential role in skin tumorigenesis. We showed that Aurora-A overexpression in primary keratinocytes resulted in severe mitotic defects, whereas activation of Aurora-A in newborn and adult mouse skin led to apparent cell death of keratinocytes. These observations are consistent with previous studies demonstrating spindle defects when Aurora-A was ectopically expressed in cell lines and primary murine fibroblasts (2, 28, 34), and suggest that deregulation of Aurora-A in skin may trigger cell death caused by delayed or defective mitosis (35). Apparent cell death in keratinocytes may also explain the absence of tumor initiation by Aurora-A overexpression observed in our study, and indicates that Aurora-A requires co-operation with other mutational events leading to faulty G1 checkpoint regulation, to promote tumorigenesis.
We demonstrated that Aurora-A was overexpressed in human pre-benign and benign lesions that precede development of malignant SCC. In Aurora-AGS mice, overexpression of Aurora-A promoted the conversion of benign tumors to malignant SCC without affecting the frequency or onset of papilloma formation, implying a distinct role of genomic instability at the transition between benign and malignant conversion. Additionally, Aurora-A overexpression was reported as an early event in human ovarian tumors and in chemically-induced mammary rat tumors (3, 36) suggesting that deregulation of Aurora-A function has a role during early and late stages of malignant progression of epithelial cancers. The ability to turn on/off Aurora-A by application or withdrawal of RU486 in Aurora-AGS mice may provide the ideal model system to address the importance of Aurora-A deregulation at different stages of skin carcinogenesis.
Aurora-A overexpression with DMBA/TPA-induced carcinogenesis accelerated the conversion of benign tumors to SCC and led to the development of malignant tumors with high metastatic potential. To our knowledge, this is the first mouse model to show enhanced tumor metastasis associated with Aurora-A overexpression in an epithelial tissue. Tumors in Aurora-AGS mice also mirrored previous clinical studies showing a correlation between high Aurora-A levels and metastatic disease with poor patient outcome (12, 37, 38). We are currently investigating the molecular mechanisms by which Aurora-A promotes metastasis in Aurora-AGS mice. Tumors in Aurora-AGS mice displayed supernumerary centrosomes, which have been observed in numerous epithelial human cancers and is considered a marker of genomic instability (39). Whole genome analysis of tumors overexpressing Aurora-A identified gains primarily localized to chromosomes 6, 7 and 10, and strongly implicated the genes on these chromosomes with the aggressive nature of Aurora-A tumors. Amplification of both Hras and Kras loci was a distinguishing feature of tumors from Aurora-AGS mice. In humans, overexpression of ras oncogenes (Hras, Kras, or Nras) are commonly observed in epithelial SCC (40, 41, 42), and ectopic expression of Aurora-A was reported to potentiate the oncogenic properties of ras in vitro (43), suggesting cooperativity between Aurora-A and ras signaling in highly malignant tumors. Future studies combining an activated ras allele with overexpression of Aurora-A in Aurora-AGS mice may help illuminate the potential interactions between the ras and Aurora-A signaling pathways in vivo.
A surprising finding from our CGH analysis was that Aurora-A tumors did not display a high level of chromosomal instability (e.g. chromosomal duplications or deletions). One explanation for our observations may be that excessive chromosomal instability inhibits tumor progression. p53 has been shown to act in genomic surveillance, responding to DNA damage, oncogenic stress, and mitotic defects (32, 44). In addition, p19Arf, encoded by CDKN2a locus, has been shown to respond to oncogenic stress and promote p53 stabilization through inhibition of Mdm2 ubiquitin ligase activity (45). Mdm2 regulates p53 levels by targeting p53 for ubiquitin-mediated degradation (46). Numerous studies have also shown crosstalk between p53 function and Aurora-A regulation (1, 8, 47). We did not observe alterations of the p53 locus by array CGH or mutations in hot spot exons of p53 gene in tumors of Aurora-AGS mice. However, we did observe loss of p53 protein expression in a subset of Aurora-A tumors suggesting that deregulation p53 signaling may be important in malignant tumors formed by Aurora-A overexpression. Our array CGH experiments showed predominant loss of the Cdkn2a locus in control tumors, whereas loss of Cdkn2a and amplification of Mdm2 were mainly found in Aurora-A tumors, suggesting that modulation of p53 levels by Mdm2 activity that allow some tumor suppressor function may be important for development of highly malignant tumors observed in Aurora-AGS mice. Consistent with our data, mutations in CDKN2A are commonly observed in pre-benign skin lesions and in SCC (48, 49), and MDM2 is overexpressed or amplified in head and neck SCC (50).
In summary, we have established a model of Aurora-A overexpression which demonstrates a clear role for Aurora-A in malignant progression of skin SCC. Secondly, this is the first model of metastatic disease associated with Aurora-A overexpression and this model may be useful for testing potential inhibitors prior to clinical trials to determine the stages during tumorigenesis that these inhibitors will be most effective.
Supplementary Material
Acknowledgments
Grant Support: National Cancer Institute grants CA89716 (S.S.), CA52607 and CA105491 (D.R.R.) and the Dan L Duncan Cancer Center at Baylor College of Medicine (BCM) Pilot grant (W.R.B.).
We acknowledge Drs. Thea Goepfert, Tamara Terzian and Yue Wei for technical help, Dr. Lisa White and the BCM Microarray Core Facility, and Drs. Tamara Terzian, Xiao-Jing Wang and Miss Irene Choi for the critical reading of this manuscript.
References
- 1.Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Kuang J, Zhong L, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–93. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 3.Goepfert TM, Adigun YE, Zhong L, Gay J, Medina D, Brinkley WR. Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res. 2002;62:4115–22. [PubMed] [Google Scholar]
- 4.Satinover DL, Brautigan DL, Stukenberg PT. Aurora-A kinase and inhibitor-2 regulate the cyclin threshold for mitotic entry in Xenopus early embryonic cell cycles. Cell Cycle. 2006;5:2268–74. doi: 10.4161/cc.5.19.3316. [DOI] [PubMed] [Google Scholar]
- 5.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–8. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macurek L, Lindqvist A, Lim D, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–23. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 7.Barr AR, Gergely F. Aurora-A: the maker and breaker of spindle poles. J Cell Sci. 2007;120:2987–96. doi: 10.1242/jcs.013136. [DOI] [PubMed] [Google Scholar]
- 8.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 9.Ewart-Toland A, Briassouli P, de Koning JP, et al. Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nat Genet. 2003;34:403–12. doi: 10.1038/ng1220. [DOI] [PubMed] [Google Scholar]
- 10.Ewart-Toland A, Dai Q, Gao YT, et al. Aurora-A/STK15 T+91A is a general low penetrance cancer susceptibility gene: a meta-analysis of multiple cancer types. Carcinogenesis. 2005;26:1368–73. doi: 10.1093/carcin/bgi085. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff JR, Anderson L, Zhu Y, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17:3052–65. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao X, Sun T, Wang Y, Zhang X, Tan W, Lin D. Functional STK15 Phe31Ile polymorphism is associated with the occurrence and advanced disease status of esophageal squamous cell carcinoma. Cancer Res. 2004;64:2680–3. doi: 10.1158/0008-5472.can-04-0651. [DOI] [PubMed] [Google Scholar]
- 13.Warner SL, Stephens BJ, Von Hoff DD. Tubulin-associated proteins: Aurora and Polo-like kinases as therapeutic targets in cancer. Curr Oncol Rep. 2008;10:122–9. doi: 10.1007/s11912-008-0020-0. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda T, Mishina Y, Walker MP, DiAugustine RP. Conditional transgenic system for mouse aurora a kinase: degradation by the ubiquitin proteasome pathway controls the level of the transgenic protein. Mol Cell Biol. 2005;25:5270–81. doi: 10.1128/MCB.25.12.5270-5281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Shimizu T, Araki N, et al. Aurora A overexpression induces cellular senescence in mammary gland hyperplastic tumors developed in p53-deficient mice. Oncogene. 2008;27:4305–14. doi: 10.1038/onc.2008.76. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Zhou YX, Qiao W, et al. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 2006;25:7148–58. doi: 10.1038/sj.onc.1209707. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Kiguchi K, Jiang J, et al. Development of transgenic mice that inducibly express an active form of c-Src in the epidermis. Mol Carcinog. 2004;40:189–200. doi: 10.1002/mc.20027. [DOI] [PubMed] [Google Scholar]
- 18.Wang XJ, Liefer KM, Tsai S, O’Malley BW, Roop DR. Development of gene-switch transgenic mice that inducibly express transforming growth factor beta1 in the epidermis. Proc Natl Acad Sci U S A. 1999;96:8483–8. doi: 10.1073/pnas.96.15.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao T, He W, Roop DR, Wang XJ. K14-GLp65 transactivator induces transgene expression in embryonic epidermis. Genesis. 2002;32:189–90. doi: 10.1002/gene.10063. [DOI] [PubMed] [Google Scholar]
- 20.Torchia EC, Jaishankar S, Baker SJ. Ewing tumor fusion proteins block the differentiation of pluripotent marrow stromal cells. Cancer Res. 2003;63:3464–8. [PubMed] [Google Scholar]
- 21.Ise K, Nakamura K, Nakao K, et al. Targeted deletion of the H-ras gene decreases tumor formation in mouse skin carcinogenesis. Oncogene. 2000;19:2951–6. doi: 10.1038/sj.onc.1203600. [DOI] [PubMed] [Google Scholar]
- 22.Nagase H, Mao JH, Balmain A. Allele-specific Hras mutations and genetic alterations at tumor susceptibility loci in skin carcinomas from interspecific hybrid mice. Cancer Res. 2003;63:4849–53. [PubMed] [Google Scholar]
- 23.Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–17. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koster MI, Dai D, Marinari B, et al. p63 induces key target genes required for epidermal morphogenesis. Proc Natl Acad Sci U S A. 2007;104:3255–60. doi: 10.1073/pnas.0611376104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torchia EC, Boyd K, Rehg JE, Qu C, Baker SJ. EWS/FLI-1 induces rapid onset of myeloid/erythroid leukemia in mice. Mol Cell Biol. 2007;27:7918–34. doi: 10.1128/MCB.00099-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvarajah S, Yoshimoto M, Maire G, et al. Identification of cryptic microaberrations in osteosarcoma by high-definition oligonucleotide array comparative genomic hybridization. Cancer Genet Cytogenet. 2007;179:52–61. doi: 10.1016/j.cancergencyto.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Villanueva J, Greenhalgh D, Wang XJ, et al. Human keratin-1. bcl-2 transgenic mice aberrantly express keratin 6, exhibit reduced sensitivity to keratinocyte cell death induction, and are susceptible to skin tumor formation. Oncogene. 1998;16:853–63. doi: 10.1038/sj.onc.1201610. [DOI] [PubMed] [Google Scholar]
- 28.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. Embo J. 2002;21:483–92. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown K, Buchmann A, Balmain A. Carcinogen-induced mutations in the mouse c-Ha-ras gene provide evidence of multiple pathways for tumor progression. Proc Natl Acad Sci U S A. 1990;87:538–42. doi: 10.1073/pnas.87.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bremner R, Kemp CJ, Balmain A. Induction of different genetic changes by different classes of chemical carcinogens during progression of mouse skin tumors. Mol Carcinog. 1994;11:90–7. doi: 10.1002/mc.2940110206. [DOI] [PubMed] [Google Scholar]
- 31.Pacifico A, Leone G. Role of p53 and CDKN2A Inactivation in Human Squamous Cell Carcinomas. J Biomed Biotechnol. 2007;2007:43418. doi: 10.1155/2007/43418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–23. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 33.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–50. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 35.Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–24. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- 36.Gritsko TM, Coppola D, Paciga JE, et al. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res. 2003;9:1420–6. [PubMed] [Google Scholar]
- 37.Sen S, Zhou H, Zhang RD, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94:1320–9. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka E, Hashimoto Y, Ito T, et al. The clinical significance of Aurora-A/STK15/BTAK expression in human esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:1827–34. doi: 10.1158/1078-0432.CCR-04-1627. [DOI] [PubMed] [Google Scholar]
- 39.Duensing S. A tentative classification of centrosome abnormalities in cancer. Cell Biol Int. 2005;29:352–9. doi: 10.1016/j.cellbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Lu SL, Herrington H, Reh D, et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–42. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paterson IC, Eveson JW, Prime SS. Molecular changes in oral cancer may reflect aetiology and ethnic origin. Eur J Cancer B Oral Oncol. 1996;32B:150–3. doi: 10.1016/0964-1955(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto H, Harada M, Isobe H, et al. Prognostic value of nuclear DNA content and expression of the ras oncogene product in lung cancer. Cancer Res. 1991;51:6346–50. [PubMed] [Google Scholar]
- 43.Tatsuka M, Sato S, Kitajima S, et al. Overexpression of Aurora-A potentiates HRAS-mediated oncogenic transformation and is implicated in oral carcinogenesis. Oncogene. 2005;24:1122–7. doi: 10.1038/sj.onc.1208293. [DOI] [PubMed] [Google Scholar]
- 44.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–51. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–77. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 46.Marine JC, Francoz S, Maetens M, Wahl G, Toledo F, Lozano G. Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ. 2006;13:927–34. doi: 10.1038/sj.cdd.4401912. [DOI] [PubMed] [Google Scholar]
- 47.Mao JH, Wu D, Perez-Losada J, et al. Crosstalk between Aurora-A and p53: frequent deletion or downregulation of Aurora-A in tumors from p53 null mice. Cancer Cell. 2007;11:161–73. doi: 10.1016/j.ccr.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanellou P, Zaravinos A, Zioga M, et al. Genomic instability, mutations and expression analysis of the tumour suppressor genes p14(ARF), p15(INK4b), p16(INK4a) and p53 in actinic keratosis. Cancer Lett. 2008;264:145–61. doi: 10.1016/j.canlet.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 49.Pacifico A, Goldberg LH, Peris K, Chimenti S, Leone G, Ananthaswamy HN. Loss of CDKN2A and p14ARF expression occurs frequently in human nonmelanoma skin cancers. Br J Dermatol. 2008;158:291–7. doi: 10.1111/j.1365-2133.2007.08360.x. [DOI] [PubMed] [Google Scholar]
- 50.Valentin-Vega YA, Barboza JA, Chau GP, El-Naggar AK, Lozano G. High levels of the p53 inhibitor MDM4 in head and neck squamous carcinomas. Hum Pathol. 2007;38:1553–62. doi: 10.1016/j.humpath.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.