Abstract
Proteins in the H-NOX family act as sensors of NO or O2. This family includes soluble guanylate cyclase, sGC, the NO sensor that is responsible for vasodilation and neurotransmission in mammals. The crystal structures of bacterial H-NOX domains have revealed a highly distorted heme cofactor. This distortion has now been shown, via replacement of a single sterically critical residue, to be associated with a concerted displacement of the entire N-terminal half of the protein. This displacement likely provides the mechanism for transducing the ligand binding event into signaling.
How does biology sense the presence of the gaseous diatomic molecules CO, NO and O2? They are non-polar, or nearly so, and have no handles for charge or H-bond interactions that proteins typically use for small molecule binding. Nature’s answer is the heme prosthetic group, to which these molecules bind strongly, through back-donation of electrons from the heme Fe. The heme group has been recruited to proteins that transduce the gas binding event into an enzymatic reaction or into gene activation or repression, therebyacting as sensors. How does this transduction work? On page xxx of this issue, Olea et al. (1) provide an exciting clue. For at least one important family of heme sensor proteins, the heme seems to signal by doing the twist.
This family has been dubbed H-NOX, for Heme Nitric oxide/Oxygen binding.(2) In eukaryotes, the principal H-NOX protein is soluble guanylate cyclase (sGC), an enzyme of enormous interest because it is the receptor for NO. sGC is responsible for regulation of vasodilation and neurotransmission through production of the secondary messenger, cyclic GMP, when NO binds to its heme.(3) H-NOX domains are also found in prokaryotes, and likely serve in sensory systems for NO or O2.(2) They are homologous with the heme-binding domain of sGC. Crystal structures are available for H-NOX domains from the bacteria Thermoanaerobacter tengcongensis (Tt-HNOX) (4, 5) and Nostoc (Ns-HNOX).(6) A striking feature of these structures is that the hemes are highly distorted from their normal planar geometry. To varying degrees, the four heme pyrrolerings swivel and twist out of the plane.
Is the heme distortion part of the sensing mechanism? To test this idea, Olea et al. (1) mutated a proline residue in (Tt-HNOX) to alanine. P115 butts up against pyrrole D (Figure 1), which experiences the greatest out-of-plane displacement. Olea et al. reasoned that the less sterically encumbered alanine might allow the heme to relax. Indeed they found a significantly flatter heme in the P115A variant (Figure 2). Strikingly, the heme flattening is associated with significant displacement (3.8 Å rms deviation) of the entire N-terminal half of the molecule, comprised of helices A, B and C, and the connecting loops (Figure 2). This displacement is connected to a number of non-bonded interactions, which are seen to be relaxed when the heme flattens in the P115A variant. In particular, the A helix residues Met1 and Ile5 are in contact with the methyl and propionate substituents of pyrrole A, and shift away from the heme as pyrrole A moves into the heme plane.
Figure 1.
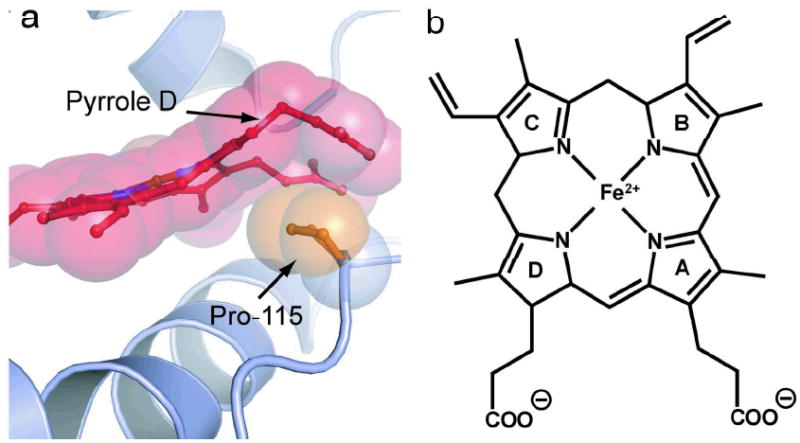
Heme prosthetic group (right) and a view of its distortion (left) in Tt H-NOX, showing the non-bonded contact with Pro115 (from ref 1).
Figure 2.
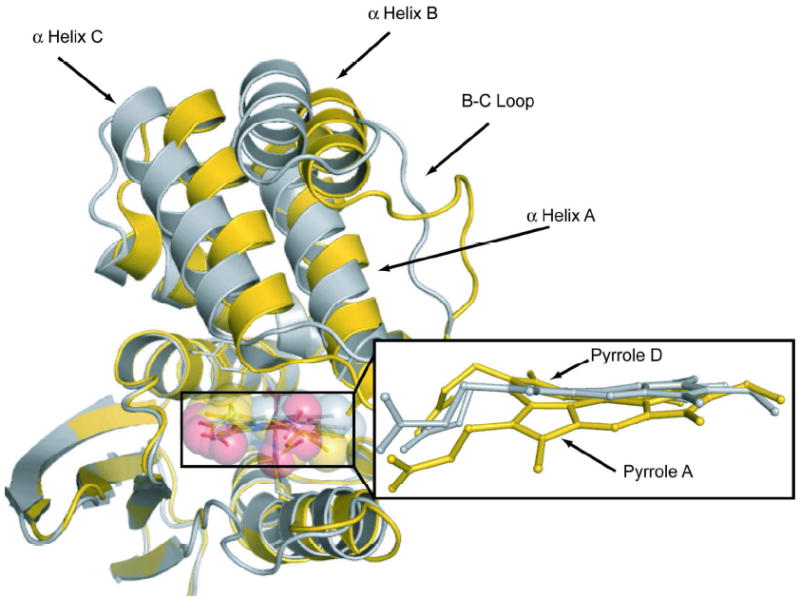
Overlay of wild-type (gold) and P115A (silver) variants of Tt H-NOX molecules, showing the heme flattening and the concertedmovement of the N-terminal half of the structure, associated with theresidue substitution (from ref 1).
The concerted N-terminal displacement suggests an obvious pathway for connecting the ligand binding event to the activation of the sensory apparatus, in which the H-NOX domain is presumably imbedded. Tt H-NOX is a member of the Tar4 family of receptors and is fused to a predicted methyl-accepting chemotaxis protein (MCP).(2) There are two predicted membrane-spanning regions between the H-NOX and MCP domains, which would then be on the same side of the membrane. Ligation induced displacement at the inter-domain contact is therefore aplausible mechanism for signaltransduction.
Olea et al also detected functional consequences at the heme.(1) The Fe(III/II) reduction potential decreased significantly in the P115A variant, by 170 mV. This effect is opposite to what might have been expected (7) from studies of model compounds, which suggest that heme flattening favors Fe(II) over Fe(III). Thus other factors may override the heme distortion effect on redox potential.
In addition, the O2 affinity increased in P115A, mainly due to a 5-fold reduction in the rate of O2 dissociation. No significant changes in bond length or H-bond distances (the bound O2 is H-bonded by a distal tyrosine residue) could be seen between native and P115A adducts. However, the authors noted a tilt in the bond connecting the heme Fe with the proximal histidine ligand (78° bond angle), in the native protein, whereas this bond is perpendicular to the heme plane in the P115A variant. They suggested that the perpendicularorientation improves the imidazole-Fe bond strength, thereby stabilizing the Fe-O2 complex.
These intriguing findings raise many questions. Is the heme distortion directly responsible for signaling, or are other interactions involved? One structural feature to consider is the tight network of H-bonds connecting the heme propionate substituents on pyrroles A and D, (4) the rings that figure prominently in the heme distortion. As these pyrroles swivel in and out of the plane, the propionate interactions must be affected. The H-bonds are donated by a triad of residues, Tyr-Ser-Arg, a triad that is common to all H-NOX sequences. Computational modeling (8) has suggested that the heme distortion itself requires little energy, but distortion-coupled changes in propionate H-bonds could involve significant energy. It has been suggested that these H-bonds can account for anomalous vibrational spectroscopic signatures of H-NOX CO adducts.
Another issue is the relationship of ligand affinity to the heme distortion. To function in sensing, the heme distortion must be caused by ligand binding. Indeed high-resolution crystal structures of the NO-binding protein nitrophorin, (9) which also features significantly distorted heme, have revealed that the distortion increases significantly when NO replaces water as a heme ligand. However, if ligand binding favors heme distortion, then conversely heme distortion must favor ligand binding. Yet Olea et al found stronger O2 binding for the flatter P115A variant. Clearly other factors than heme flattening must be involved (the Fe-histidine tilt noted by the authors may be one of them), but the apparent paradox does warn of complexities that vitiate a simple correlation of ligation and heme distortion.
Finally, there is the question of how these new results can illuminate the mechanism of sGC activation. Although sGC is not membrane-bound it also is constructed of a heme domain at one end and a catalytic (cyclase) domain at the other, with a connecting domain (PAS-like domain) in between. (6) If ligand binding induces concerted displacements in sGC, as in Tt H-NOX, then coupling at the interface between heme and cyclase domains may be the pathway for activation. However, binding of NO to sGC induces release of the proximal histidine from the heme, after transient formation of a six-coordinate adduct. The histidine release is synchronous with cyclase activation, (10) and has been inferred to be a central event in signaling. Is this release connected to heme distortion, or does it involve a separate mechanism? A linkage between the two is suggested by the finding that heme out-of-plane vibrational modes are induced in the resonance Raman spectrum of the sGC-CO adduct upon addition of synthetic molecule activators, YC-1 (11) or BAY-41-2272.(12) The normally low activity of the CO adduct is boosted by these activators to levels comparable to that induced by NO. These out-of-plane modes are indicators of heme distortion. In addition, the Raman spectra reveal that the activators induce release of the proximal histidine in a fraction of the sGC-CO molecules. Thus these activators connect heme distortion with a tendency for histidine release. These observations reinforce the relevance of the new H-NOX structures for our understanding of sGC.
Acknowledgments
I thank Drs. Mohammed Ibrahim and Alexandra Soldatova for help in preparing this article.
References
- 1.Olea C, Boon E, Pellicena P, Kuriyan J, Marletta M. Probing function of heme distortion in the H-NOX family. ACS Chemical Biology. 2008 doi: 10.1021/cb800185h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karow DS, Pan D, Tran R, Pellicena P, Presley A, Mathies RA, Marletta MA. Spectroscopic Characterization of the Soluble Guanylate Cyclase-like Heme Domains from Vibrio cholerae and Thermoanaerobacter tengcongensis. Biochemistry. 2004;43:10203–10211. doi: 10.1021/bi049374l. [DOI] [PubMed] [Google Scholar]
- 3.Ignarro LJ. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacology & Toxicology. 1990;67:1–7. doi: 10.1111/j.1600-0773.1990.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 4.Pellicena P, Karow DS, Boon EM, Marletta MA, Kuriyan J. Crystal structure of an oxygen-binding heme domain related to soluble guanylate cyclases. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12854–12859. doi: 10.1073/pnas.0405188101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nioche P, Berka V, Vipond J, Minton N, Tsai AL, Raman CS. Femtomolar Sensitivity of a NO Sensor from Clostridium botulinum. Science. 2004;306:1550–1553. doi: 10.1126/science.1103596. [DOI] [PubMed] [Google Scholar]
- 6.Ma X, Sayed N, Beuve A, van den Akker F. NO and CO differentially activate soluble guanylyl cyclase via a heme pivot-bend mechanism. Embo Journal. 2007;26:578–588. doi: 10.1038/sj.emboj.7601521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker FA. Nitric oxide interaction with insect nitrophorins and thoughts on the electron configuration of the {FeNO}6 complex. Journal of Inorganic Biochemistry. 2005;99:216–236. doi: 10.1016/j.jinorgbio.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Xu C, Ibrahim M, Spiro TG. DFT Analysis of Axial and Equatorial Effects on Heme-CO Vibrational Modes : Applications to CooA and H-NOX Heme Sensor Proteins. Biochemistry. 2008;47:2379–2387. doi: 10.1021/bi702254y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maes EM, Roberts SA, Weichsel A, Montfort WR. Ultrahigh Resolution Structures of Nitrophorin 4: Heme Distortion in Ferrous CO and NO Complexes. Biochemistry. 2005;44:12690–12699. doi: 10.1021/bi0506573. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Brandish PE, Ballou DP, Marletta MA. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:14753–14758. doi: 10.1073/pnas.96.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal B, Kitagawa T. Interactions of soluble guanylate cyclase with diatomics as probed by resonance Raman spectroscopy. Journal of Inorganic Biochemistry. 2005;99:267–279. doi: 10.1016/j.jinorgbio.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Martin E, Czarnecki K, Jayaraman V, Murad F, Kincaid J. Resonance Raman and Infrared Spectroscopic Studies of High-Output Forms of Human Soluble Guanylyl Cyclase. Journal of the American Chemical Society. 2005;127:4625–4631. doi: 10.1021/ja0440912. [DOI] [PubMed] [Google Scholar]


