Abstract
BACKGROUND:
Because of its over-expression in many human tumors, the folate-receptor (FR) is a promising target for tumor-specific imaging.
OBJECTIVE:
To evaluate the uptake of FR-targeted gadolinium (P866) and iron-oxide (P1048) agents in an ovarian tumor model.
MATERIALS AND METHODS:
FR-positive ovarian cancer cells (IGROV-1) were incubated with FR-targeted agents (P866 or P1048) in the absence or presence of competing free folate. Intracellular gadolinium or iron-oxide concentrations were measured. MR imaging of implanted ovarian tumors in rats was performed following injection of FR-targeted (P866 and P1048) and non-targeted (P1001 and P904) agents. Changes in longitudinal and transverse relaxation rates (ΔR1 and ΔR2), which were proportional to the contrast concentration in the tumors, were compared between tumors injected with FR-targeted and non-targeted agents.
RESULTS:
IGROV-1 cells showed uptake of P866 and P1048, which decreased with competing free folate. The ΔR1 values were higher at 1h following P866 versus P1001 injection (p<0.05), indicating higher amount of contrast retained in the tumor following P866 injection. There was a trend of higher ΔR2 values at 48h following P1048 versus P904 injection, although not statistically significant (p=0.09).
CONCLUSION:
A specific accumulation of the FR-targeted gadolinium agent P866 was suggested in a FR-positive ovarian tumor model.
Introduction:
The development of tumor specific imaging agents is highly desirable, as they may provide earlier and more accurate diagnosis, improve the assessment of the biological aggressiveness of the evaluated tumors, and monitor treatment response. The folate-receptor (FR) has particular characteristics that make it a promising target for tumor specific imaging and therapy.
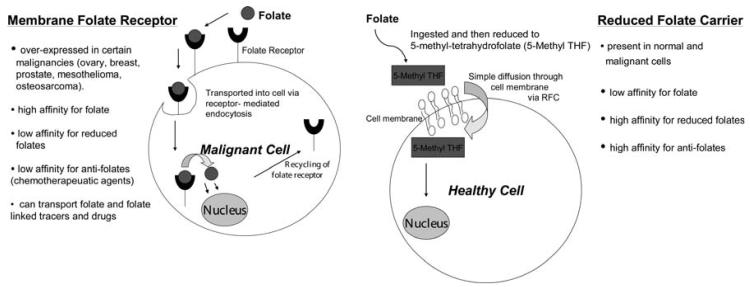
The vitamin folate is required by all cells for metabolism and survival. Eukaryotic cells are unable to produce folate, and therefore must acquire it from the environment. There are two different routes whereby folate can enter into a cell (Fig. 1).
Figure 1.
Mechanism of folate entry into cells.
(1) Reduced folate carrier (RFC) is a transmembrane transporter that is ubiquitously expressed throughout development and in normal adult tissue. It is the major route of entry for the reduced forms of the folate [1, 2]. Upon oral ingestion of folate, the vitamin undergoes intestinal absorption and is rapidly taken up by the liver, where it is either [3]stored in hepatocytes or converted to dihydrofolate, tetrahydrofolate, or methylene tetrahydrofolate. The reduced form of folate is released by the liver and enters cells of normal organ via the reduced folate carrier.
(2) Folate-receptor (FR) is a glycopolypeptide that binds folate with high affinity [4]. FR is over-expressed in various types of human carcinomas including ovarian, breast, colorectal and nasopharyngeal carcinomas in adults [5-7], as well as pediatric tumors such as choroid plexus tumors, ependymomas, osteosarcomas and leukemia [8-10]. The FR is relatively absent in normal tissues [11]. It is important to understand that the FR has a high affinity for folate, but not its reduced forms (Fig. 1). There are several FR isoforms (α, β, and γ). The α isoform is over-expressed in cancer cells and has a high affinity for the folate ligand (Kd ∼10^−10 M) [12]. This makes folate a valuable vehicle for conjugation with specific tracers, thereby allowing the delivery of the tracers to FR-positive cancer cells and providing cancer specific imaging.
Successful tumor selective FR-targeting has been reported both in vitro and in vivo with a variety of radionuclides [13-20] (Table 1). The FR-targeted radionuclides have several advantages, including relative ease and cost-effectiveness in their synthesis, potential broad applications for a large variety of tumors, and favorable tumor-to-background ratios. However, current radiotracer based imaging techniques are limited in anatomic resolution, which necessitates additional imaging modalities to further localize the detected tumor. In addition, the radiotracer techniques cause a considerable radiation exposure. FR-targeted optical imaging probes have subsequently been developed, which provide a similar sensitivity and specificity for cancer diagnosis [21-23] (Table 2). The optical imaging techniques, however, also suffer from limited anatomical resolution, and are not readily available for clinical applications.
Table 1.
Selected references of successful in vivo tumor targeting using radionuclides conjugated with the folate-receptor (FR).
| Authors | References | FR-targeted radionuclides |
Targeted pathology |
Targeted species |
|---|---|---|---|---|
| Mathias et al | J. Nucl. Med. (1998) [16] |
111In-DTPA- folate |
FR-positive KB cells (human nasopharyngeal carcinoma) |
mouse |
| Guo et al | J. Nucl. Med. (1999) [15] |
99mTc-HYNIC- folate |
FR-positive 24JK-FBP cells (mouse sarcoma cell) |
mouse |
| Leamon et al | Bioconj. Chem. (2002) [18] |
99mTc-EC20 | FR-positive M109 cells (murine lung carcinoma) |
mouse |
| Okarvi et al | Cancer Biother Radiopharm. 2006 [20] |
99mTc-MAG3- folate |
MCF-7 (human breast cancer) |
mouse |
| Bettio et al | J. Nucl. Med. (2006) [13] |
18F-FBA-folate | FR-positive K31 cells (epidermal carcinoma) |
mouse |
Table 2.
Selected references of successful in vivo tumor targeting using fluorescent probes conjugated with the folate-receptor (FR).
| Authors | References | FR-targeted fluorescent probes |
Targeted pathology |
Targeted species |
|---|---|---|---|---|
| Moon et al | Bioconjugate Chem 2003 [21] |
folate targeting NIR fluorochrome |
FR-positive KB cells (Human nasopharyngeal Carcinoma) |
mouse |
| Kennedy et al | J. of Biomed Optics 2003 [22] |
folate-fluorescein conjugate |
FR-positive M109 cells (murine lung carcinoma) and L1210 cells (leukemia cells) |
mouse |
| Chen et al | Molecular Imaging 2005 [23] |
fluorescent folate probe (FFP) |
Activated macrophages on dysplastic intestinal adenomas |
mouse |
More recently, efforts have been directed towards the development of imaging probes that are detectable with magnetic resonance (MR) imaging [24-27]. MR imaging provides a noninvasive means for tumor detection with excellent soft tissue contrast and anatomic resolution, without radiation exposure. The aim of our study was to investigate the diagnostic performance of new formulations of FR-targeted MR contrast agents in an ovarian tumor model. We focused our studies on ovarian cancer as a representative tumor with high levels of FR over-expression [6] in order to evaluate the feasibility of FR-targeted MR imaging. The concept of receptor-targeted imaging, however, would be applicable to other FR-positive tumors.
Materials and Methods
Contrast Agents
All contrast agents were provided by the Research Division of Guerbet, Paris, France.
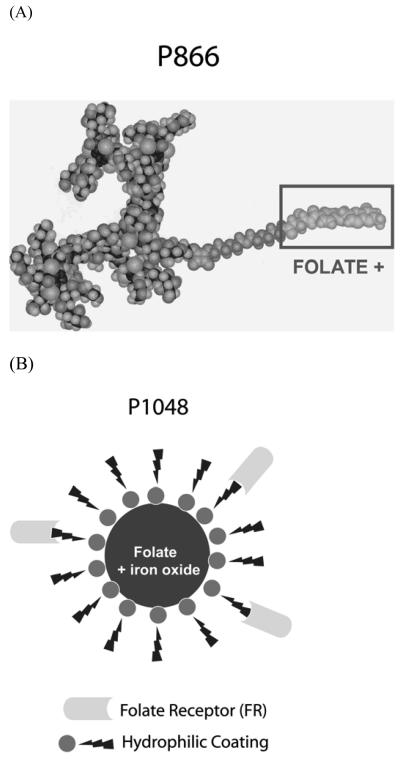
P866 is a high relaxivity dimeric gadolinium chelate, which is surrounded by hydrophilic branches and conjugated to a folate moiety (Fig. 2A). It has a molecular weight of 9.4 kDa. The r1 relaxivity is 42 s−1 mM−1 (i.e. 21 s−1 mM−1 Gd), and the r2 relaxivity is 60 s−1 mM−1 (i.e. 30 s−1 mM−1 Gd) in water at 60MHz and 37°C. The P866 molecule was found to be stable in plasma and was excreted as a whole molecule in urine (no metabolism) (unpublished data, Guerbet Research).
Figure 2.
FR-targeted gadolinium MR contrast agent P866 (A), and FR-targeted iron-oxide MR contrast agent P1048 (B). P866 is a high relaxivity dimeric gadolinium chelate conjugated to folate. P1048 consists of an iron-oxide core conjugated to folate.
P1001 is a non-FR-targeted analog of the P866. It is composed of an identical gadolinium chelate without the folate moiety. It has the same molecular weight, r1 and r2 relaxivities as the P866.
P1048 consists of an iron-oxide core conjugated to folate (Fig. 2B). The iron-oxide core is coated with hydrophilic branches of amino-alcohol, and grafted with 8-10 folate moiety per nanoparticle. The size of P1048 is 25nm. The r1 relaxivity is 14 s−1 mM−1 Fe, and the r2 relaxivity is 92 s−1 mM−1 Fe in water at 60MHz and 37°C. There was no loss of activity after incubation of P1048 in standard cell culture media, which was an indirect proof of stability of the contrast material in biological media (unpublished data, Guerbet Research).
P904 is a non-FR-targeted analog of the P1048. The size is 21 nm. It has the same r1 and r2 relaxivity as the P1048.
Cell Culture
FR-positive human ovarian cancer cells (IGROV-1, from Dr. Joe Gray, UCSF) were grown in folate-free RPMI 1640 medium (Gibco BRL, Grand Isalnd, NY) supplemented with 10% fetal calf serum at 37°C. Ten million (107) cells were incubated for 24 hours with 50 μM of FR-targeted P866 in the absence or presence of 750 μM competing free folate, or with the non-FR-targeted analog P1001. Similarly, ten million (107) cells were incubated for 24 hours with 20 μM of FR-targeted P1048 in the absence or presence of 750 μM competing free folate, or with the non-FR-targeted analog P904. Intracellular gadolinium or iron-oxide concentrations were measured by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (Perkin Elmer, Paris, France).
Animal Model
The study was approved by the Committee for Animal Research at our institution. For tumor implantation and MR imaging, animals were anesthetized with 1.5-2% isoflurane (Narkomed, North American DRAGER) in oxygen administered via a facemask.
IGROV-1 ovarian tumors were implanted in nine 3-4 week old female athymic Harlan rats. Approximately 6 × 106 tumor cells were injected subcutaneously into the right lower flank of each rat. All rats received a folate-deficient diet (Dyets, Bethleham, PA) for 3-4 weeks prior to MR imaging in order to reduce the serum folate concentration to levels that are normally found in humans. The tumors were imaged when they reached a size of approximately 1 cm.
Animal Experimental Procedure
For the evaluation of the FR-targeted gadolinium agent P866, MR imaging was performed in a group of 3 rats before, and up to 1 hour post injection of P866 (0.03 mmol Gd/Kg) on day 1. On day 2, the same group of rats was imaged before, and up to 1 hour post injection of non-FR targeted analog P1001 (0.03 mmol Gd/Kg). We evaluated the gadolinium based contrast agents over one hour, as a previous pharmacokinetic study done by our group showed the elimination half-life of P866 to be 36 minutes based on plasma measurements [28]. The same group of 3 rats was used for both the P866 and P1001 studies, since pilot studies showed no significant residual P866 present in the tumors at 24 hours delay on day 2.
For the evaluation of the FR-targeted iron-oxide agent P1048, MR imaging was performed in one group of 3 rats before, immediately after, and at 24 hours and 48 hours post injection of the FR-targeted P1048 (0.3 mmol Fe / Kg). For comparison, MR imaging was performed in a separate group of 3 rats using the same protocol but with the non-FR-targeted P904 (0.3 mmol Fe / Kg). We evaluated the iron-oxide contrast agents over 48 hours and in two separate but comparable groups of rats because of the much longer elimination half-life (elimination half time ∼ 15 hours, unpublished data).
MR Imaging
MR imaging was performed on a 2-Tesla Omega CSI-II superconducting MR scanner (Bruker Instruments, Fremont, CA). The animals were placed in a birdcage radiofrequency coil on a warming mattress filled with 37°C warm deuterium oxide, in order to keep the body temperature of the animals relatively constant.
For the evaluation of the gadolinium contrast agent P866, the following pulse sequences were used. Pre-contrast images were acquired using a single-slice inversion recovery (IR) snapshot FLASH sequence with the following parameters: TR 3ms, TE 1.5ms, field of view 55 × 55 mm, matrix 64 × 64, slice thickness 3 mm, flip angle of 5 degree, TI (inversion times) of 100-2500ms. Dynamic contrast enhanced MR imaging was performed every 2 minutes for 1 hour using T1-weighted 3D-spoiled gradient recalled (SPGR) sequence with the following parameters: TR 30ms, TE 4.83ms, field of view 55 × 55 × 48mm, matrix 128 × 128 × 16, an effective slice thickness of 3.6 mm and flip angle of 90 degrees. In addition, 1 hour post-contrast inversion recovery (IR) snapshot FLASH sequence was acquired with the same parameters as listed above for the pre-contrast scan.
For the evaluation of the iron-oxide contrast agent P1048, multi-echo (4 echos) T2*-weighted 3D-SPGR sequences, and muti-echo (4 echos) T2-weighted 2D spin-echo (SE) sequence were obtained. The T2*-weighted sequence had the following parameters: TR 200ms, TE 5, 10, 15, 20ms, field of view 50 × 50 mm, matrix 256 × 128, slice thickness 2mm. The T2-weighted SE sequence had the following parameters: TR 2000 ms, TE 20, 40, 60, 80 ms, field of view 50 × 50 mm, matrix 256 × 128, slice thickness 2 mm. Dynamic imaging was not performed because iron-oxide contrast agents are expected to have a much longer blood half life.
Imaging Analysis
MR images were analyzed using the MR-Vision Software (MR-Vision Co, Menlo Park, CA). The relative signal enhancement ΔSI (%) was quantified as ΔSI (%) = {(SIpost – SIpre) / SIpre} × 100%. For the evaluation of the gadolinium contrast agents, the relative signal enhancement of the tumors before and 1 hour after injection of P866 was compared to that injected with P1001. In addition, longitudinal relaxation rates R1 (1/T1) estimates for tumors were obtained by curve fitting based on one set of inversion recovery images. ΔR1, the difference between pre-contrast R1 and 1 hour post-contrast R1, is assumed to be directly proportional to the concentration of the contrast medium in the tumor. ΔR1 values were compared between tumors injected with P866 and those with P1001. For the evaluation of the iron-oxide contrast agents, the relative signal loss of the tumors before, immediately after, and at 24 hours and 48 hours after injection of P1048 was compared to that injected with P904. In addition, transverse relaxation rates R2 (1/T2) estimates for tumors were obtained by curve fitting based on one set of multi-echo T2 SE images. ΔR2, the difference between pre-contrast R2 and 24 hours or 48 hours post contrast R2, which were proportional to the concentration of the ion-oxide in the tumors, were compared between tumors injected with P1048 and those with P904.
Histology
Immediately after the last MR imaging procedure, the animals were sacrificed and the tumors were dissected and frozen at -80°C. Frozen sections were stained with α-FR antibody specific to human (Santa Cruz #sc-16386, 1:100 dilution) and counter-stained with hematoxylin. As a negative control, implanted HT1080 human fibrosarcoma tumors from a different study, were also stained with the same antibody. The IGROV-1 ovarian cancer cells were reported to have high level of FR-α expression [29], while the HT1080 human fibrosarcoma cells were reported to be FR-negative [30].
Statistics
Statistical analysis was performed using Stata software package version 7.0 (Stata Corporation, College Station, TX). Differences in ΔSI (%) of tumor before and after injection of P866 versus P1001 or P1048 versus P904, differences in ΔR1 values between tumors injected with P866 and P1001, and differences in ΔR2 values between tumors injected with P1048 and P904 were tested with 2-tail student t-test. Results were considered statistically significant where p < 0.05.
Results
Cell Culture
Twenty-four hours after the incubation of IGROV-1 cells in P866, 0.668 nmol Gd / 107 cells were measured by ICP-MS in the cell lysate, which decreased by 25% to 0.499 nmol Gd / 107 cells in the presence of competing free folate (Table 3). Similarly, 24 hours after the incubation of IGROV-1 cells in P1048, 18.3 nmol Fe / 107 cells were measured in the cell lysate, which decreased by 32% to 12.3 nmol Fe / 107cells in the presence of competing free folate (Table 3).
Table 3.
Intracellular gadolinium or iron-oxide concentration as measured by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) in the IGROV-1 cell lysate. The IGROV-1 cells were incubated with FR-targeted contrast agents in the presence or absence of free folate, or with the non-FR-targeted analogs.
| P866 | P866 + folate | P1001 | |
|---|---|---|---|
|
Intracellular gadolinium (nmol Gd / 107 cells) |
0.668 | 0.499 | 0.514 |
| P1048 | P1048 + folate | P904 | |
|
Intracellular iron oxide (nmol Fe / 107 cells) |
18.3 | 12.3 | 13.7 |
Animal Studies
Gadolinium agents (P866 and P1001)
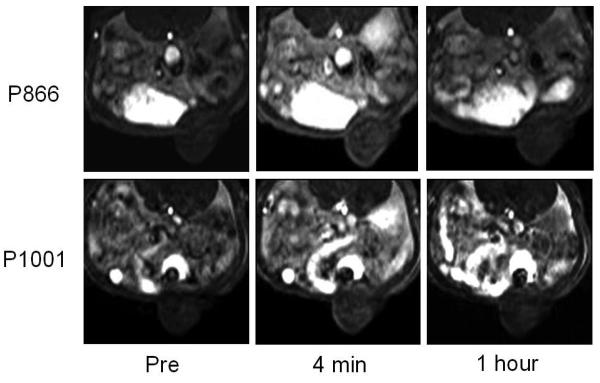
The implanted tumors showed a mild peak enhancement at approximately 4 minutes following injection of either P866 or P1001 on the T1-weighted dynamic SPGR sequences (Fig. 3). This initial peak enhancement of the tumors was apparently a perfusion effect and was followed by a rapid decline, with minimal residual tumor enhancement at 1 hour following injection of either contrast agents (Fig. 3). There was no significant difference in relative signal enhancement between tumors injected with P866 and P1001 (P = 0.70). However, the mean ΔR1 relaxation rates were significantly higher at 1 hour following the injection of P866 (mean ΔR1 = 0.214 s−1) compared to P1001 (mean ΔR1 = 0.112 s−1) (p = 0.03), indicating a higher amount of contrast retention in the tumor following injection of P866 compared to P1001 (Fig. 4).
Figure 3.
Representative axial T1-weighted 3D-SPGR MR images through the implanted ovarian tumor before, at 4 minutes, and at one hour post injection of P866 (top row) and P1001(bottom row). There is mild enhancement of the tumor at 4 minutes post injection of either P866 or P1001, with minimal residual enhancement at 1 hour with either contrast agent.
Figure 4.
The mean ΔR1 relaxation rates were significantly higher at 1 hour following the injection of P866 (mean ΔR1 = 0.214 s−1) compared to P1001 (mean ΔR1 = 0.112 s−1) (p < 0.05), suggesting at least a component of folate-receptor specific uptake of P866 in the tumors.
Iron oxide agents (P1048 and P904)
The iron-oxide sensitive T2*-weighted MR images showed moderate signal loss in the tumors immediately following injection of either P1048 and P904, likely representing a perfusion effect (Fig. 5). There was washout of both contrast agents over 48 hours. No significant difference in relative signal change of the tumors was observed between tumors injected with P1048 and P904 at either 24 or 48 hours (P = 0.85). At 24 hours post injection, the mean ΔR2 relaxation rates, which were proportional to the iron-oxide concentration in the tumors, were also similar between the rats injected with P1048 and P904 (p = 0.76). At 48 hours, however, there was a trend of higher mean ΔR2 values, suggesting higher amount of retained contrast, in the tumor following the injection of P1048 (mean ΔR2 = 0.0027 s−1) compared to P904 (mean ΔR2 = 0.0011 s−1), albeit not statistically significant (p = 0.09) (Fig. 6).
Figure 5.
Representative axial T2*-weighted SPGR MR images through the implanted ovarian tumor before, immediately after, at 24 hours, and at 48 hours post injection of P1048 (top row) and P904 (bottom row). There was moderate signal loss in the tumors immediately following injection of either P1048 or P904, with washout of both contrast agents over 48 hours.
Figure 6.
Mean ΔR2 relaxation rates at 48 hours following injection of either P1048 or P904. There was a trend of higher mean ΔR2 values following the injection of P1048 (mean ΔR2 = 0.0027 s−1) compared to P904 (mean ΔR2 = 0.0011 s−1) (p = 0.09).
Histopathology
FR-α staining showed strong staining of multiple tumor cells from the implanted IGROV-1 ovarian tumors, consistent with reported high level of FR-α expression (Fig. 7). In contrast, no significant staining was noted in the HT1080 human fibrosarcoma cells which were reported to be FR-negative (Fig 7).
Figure 7.
Folate-receptor (FR) stains of FR-positive IGROV-1 ovarian tumor (A) and FR-negative HT1080 human fibrosarcoma (B). The IGROV-1 ovarian tumor cells demonstrate dark, positive cytoplasmic and cytoplasmic membrane staining for the FR (arrows). The HT1080 human fibrosarcoma cells demonstrate negative staining for the FR.
Discussion
The FR-targeted gadolinium chelate P866, but not its non-FR targeted analog P1001, caused a significant increase in R1 relaxation rates of FR-positive ovarian tumors on delayed MR scan (1 hour post injection). Because R1 relaxation rates reflect the amount of contrast in the tumors, our results suggests a component of specific accumulation of FR-targeted P866 in FR-positive tumors. This is also supported by the in vitro study which showed decreased gadolinium concentration in cells incubated with P866 in the presence of competing free folate.
The FR-targeted iron-oxide agent P1048, when compared to its non-targeted analog P904, showed a trend of increase in R2 relaxation rates, corresponding to higher retained contrast, in FR-positive ovarian tumors on delayed MR scan (48 hours post injection), albeit not statistically significant (p = 0.09). The in vitro study showed decreased iron-oxide concentration in cells incubated with P1048 in the presence of competing free folate, suggesting specificity of P1048 for the FR on the ovarian tumor cells in vitro. When the ovarian tumors were imaged in vivo, there may be more nonspecific uptake of the iron-oxide contrast agents due to non-specific leak of the iron-oxide particles into the tumor interstitium and the presence of other cell populations such as macrophages which are known to take up iron-oxide [31]. This may partially explain why we were unable to find a statistically significant difference in contrast uptake between P1048 and P904 in vivo. The detection of nonspecific uptake may be reduced by allowing the unbound iron-oxide particle to washout over time, which was the rationale behind imaging the tumors at 48 hours in our study. This process is dependent on the concentration gradient across the vasculature and the pharmacokinetics of the FR-targeted iron oxide particles. Thus, further modification of the contrast agents that allow higher interstitial accumulation and longer interstitial residence, along with optimization of the imaging efficiency, are needed to improve the FR-specific uptake into tumor cells and to improve sensitivity and specificity of the FR-targeted iron-oxide contrast agents.
Few other investigators have studied FR-targeted MR contrast agents for tumor imaging in vivo. Wiener, Konda and colleagues described uptake of a gadolinium polyamidoamine (PAMAM) folate-dendrimer in FR-α positive ovarian cancers (OVCA 432) [24, 25, 32]. More recently, Choi and colleagues reported iron oxide nanoparticles conjugated to folate as MR contrast agent for imaging of nasopharyngeal carcinomas (KB cells) [27]. Our data are in agreement with those previous studies showing the feasibility of imaging FR-positive tumors in vivo using targeted MR contrast agents. In addition, our data suggest active FR-targeting by P866 because we were able to compare the uptake of contrast in tumors between targeted- (P866) and non-targeted- (P1001) contrast agents. Those previous studies did not have non-targeted contrast agents available for comparison. Corot and colleagues have recently shown a specific uptake of P866 in a nasopharyngeal carcinoma tumor model (KB cells) in mice [33]. The degree of specific uptake of P866 in the KB cells was much higher than what was observed in our ovarian tumor model in rats. The differences in P866 uptake may be attributed to a much higher level of FR-α expression in the KB cells than the IGROV-1 cells [34].
As shown in Figure 1, the specific uptake of FR-targeted contrast agents in vivo was better evaluated using a non-FR-targeted analog as a control rather than performing folate competition experiments. For in vivo folate competition studies, excessive amount of free folate would be required to saturate the metabolic pathways in the liver, therefore resulting in nonphysiological amount of folate in the blood. While our study suggested a specific retention of the FR-targeted contrast agent P866 in ovarian tumors, we did not investigate if the P866 was bound to FR on the cell surface or if some of the contrast agent was internalized into the cells. The mechanism is important with respect to the elimination pathway of this diagnostic agent as well as potential future designs of FR-targeted therapy.
The depiction of the FR-specific uptake in our study was close to the detection limit of MR imaging. The differences between the FR-targeted and non-targeted agents could only be detected by direct measurements of the R1 relaxation rates for the gadolinium agent rather than signal intensity measurements. It is known that the changes in the relaxation rates are proportional to the concentration of contrast agents in the tumors, and that the relaxation rates measurements are more sensitive than actual signal intensity measurements. Compared to the FR-targeted radionuclide and optical imaging techniques, MR imaging of cell surface receptors still faces many challenges mostly because of the relatively low signal yield of MR contrast agents. The gadolinium agent P866 used in our study is composed of folate coupled to a high relaxivity dimeric gadolinium chelate in order to reach a higher MR sensitivity. Similarly, P1048 is composed of folate moiety coupled to an iron oxide core in an attempt to improve the sensitivity of signal detection [35]. Clearly our study represents work-in-progress, and further modification of the contrast agents, improvement in pulse sequences, and use of higher field MR scanners will be needed to improve the sensitivity of FR-specific uptake in tumors. Nonetheless, our current study serves as proof-of-concept and demonstrates the possibility of combining the specificity of receptor targeting with the improved anatomic resolution of MR imaging.
The impetus behind the FR-targeted MR contrast research is the potential clinical applications which include characterization and treatment monitoring of FR-positive tumors. FR-targeted contrast agents may allow more sensitive and specific diagnosis of FR-positive tumors by detecting additional sites of tumor that are missed on conventional imaging, and by better distinguishing between tumor and treatment-related fibrosis or scarring. Assessment of tumor FR level with targeted contrast agents may also lead to improved characterization of tumor aggressiveness, provide rational means of selecting patients who would most benefit from anti-folate therapy, and allow better treatment monitoring. We focused our studies on ovarian tumors because FR is over-expressed in 90% of ovarian tumors [6], which serve as a good model to evaluate the feasibility of FR-targeted MR imaging. Analyses of human ovarian tumors to date have demonstrated considerable variability in the expression levels of FR among patients as well as heterogeneity within the same tumor [36-38]. FR-specific MR contrast agents may estimate the level of over-expression of the receptors, which has been correlated directly with the biological aggressiveness such as the histological grade and S-phase fraction [6, 36]. The FR status has also been documented to reflect the tumor's response to chemotherapy [39]. Although ovarian tumors are relatively rare in the pediatric population, our study is designed to test the feasibility and potential of FR-targeted MR imaging. The concept of FR-targeted MR contrast agents may be applied to various FR-positive tumors, including pediatric tumors such as choroid plexus tumors and ependymomas [8], osteosarcoma [9], and acute myelogenous leukemia [3, 10].
In conclusion, a specific accumulation of the FR-targeted gadolinium agent P866 was suggested in a FR-positive ovarian tumor model. Further development in the FR-targeted contrast agents and improvement in imaging efficiency are needed to improve the sensitivity and specificity of MR imaging of FR-positive tumors.
Acknowledgments
ZJW supported by NIBIB T32 Training Grant 1 T32 EB001631-01A1
References
- 1.Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm. 2003;66:403–456. doi: 10.1016/s0083-6729(03)01012-4. [DOI] [PubMed] [Google Scholar]
- 2.Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26:141–152. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- 3.Sadasivan E, Rothenberg SP, da Costa M, Brink L. Characterization of multiple forms of folate-binding protein from human leukemia cells. Biochim Biophys Acta. 1986;882:311–321. doi: 10.1016/0304-4165(86)90253-9. [DOI] [PubMed] [Google Scholar]
- 4.Antony AC. Folate receptors. Annu Rev Nutr. 1996;16:501–521. doi: 10.1146/annurev.nu.16.070196.002441. [DOI] [PubMed] [Google Scholar]
- 5.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994;73:2432–2443. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997;74:193–198. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Weitman SD, Lark RH, Coney LR, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–3401. [PubMed] [Google Scholar]
- 8.Patrick TA, Kranz DM, van Dyke TA, Roy EJ. Folate receptors as potential therapeutic targets in choroid plexus tumors of SV40 transgenic mice. J Neurooncol. 1997;32:111–123. doi: 10.1023/a:1005713115147. [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Kolb EA, Qin J, et al. The folate receptor alpha is frequently overexpressed in osteosarcoma samples and plays a role in the uptake of the physiologic substrate 5-methyltetrahydrofolate. Clin Cancer Res. 2007;13:2557–2567. doi: 10.1158/1078-0432.CCR-06-1343. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Zheng X, Behm FG, Ratnam M. Differentiation-independent retinoid induction of folate receptor type beta, a potential tumor target in myeloid leukemia. Blood. 2000;96:3529–3536. [PubMed] [Google Scholar]
- 11.Shen F, Ross JF, Wang X, Ratnam M. Identification of a novel folate receptor, a truncated receptor, and receptor type beta in hematopoietic cells: cDNA cloning, expression, immunoreactivity, and tissue specificity. Biochemistry. 1994;33:1209–1215. doi: 10.1021/bi00171a021. [DOI] [PubMed] [Google Scholar]
- 12.Antony AC. The biological chemistry of folate receptors. Blood. 1992;79:2807–2820. [PubMed] [Google Scholar]
- 13.Bettio A, Honer M, Muller C, et al. Synthesis and preclinical evaluation of a folic acid derivative labeled with 18F for PET imaging of folate receptor-positive tumors. J Nucl Med. 2006;47:1153–1160. [PubMed] [Google Scholar]
- 14.Reddy JA, Xu LC, Parker N, Vetzel M, Leamon CP. Preclinical evaluation of (99m)Tc-EC20 for imaging folate receptor-positive tumors. J Nucl Med. 2004;45:857–866. [PubMed] [Google Scholar]
- 15.Guo W, Hinkle GH, Lee RJ. 99mTc-HYNIC-folate: a novel receptor-based targeted radiopharmaceutical for tumor imaging. J Nucl Med. 1999;40:1563–1569. [PubMed] [Google Scholar]
- 16.Mathias CJ, Wang S, Waters DJ, Turek JJ, Low PS, Green MA. Indium-111-DTPA-folate as a potential folate-receptor-targeted radiopharmaceutical. J Nucl Med. 1998;39:1579–1585. [PubMed] [Google Scholar]
- 17.Ke CY, Mathias CJ, Green MA. Targeting the tumor-associated folate receptor with an 111In-DTPA conjugate of pteroic acid. J Am Chem Soc. 2005;127:7421–7426. doi: 10.1021/ja043006n. [DOI] [PubMed] [Google Scholar]
- 18.Leamon CP, Parker MA, Vlahov IR, et al. Synthesis and biological evaluation of EC20: a new folate-derived, (99m)Tc-based radiopharmaceutical. Bioconjug Chem. 2002;13:1200–1210. doi: 10.1021/bc0200430. [DOI] [PubMed] [Google Scholar]
- 19.Muller C, Hohn A, Schubiger PA, Schibli R. Preclinical evaluation of novel organometallic 99mTc-folate and 99mTc-pteroate radiotracers for folate receptor-positive tumour targeting. Eur J Nucl Med Mol Imaging. 2006;33:1007–1016. doi: 10.1007/s00259-006-0111-9. [DOI] [PubMed] [Google Scholar]
- 20.Okarvi SM, Jammaz IA. Preparation and in vitro and in vivo evaluation of technetium-99m-labeled folate and methotrexate conjugates as tumor imaging agents. Cancer Biother Radiopharm. 2006;21:49–60. doi: 10.1089/cbr.2006.21.49. [DOI] [PubMed] [Google Scholar]
- 21.Moon WK, Lin Y, O'Loughlin T, et al. Enhanced tumor detection using a folate receptor-targeted near-infrared fluorochrome conjugate. Bioconjug Chem. 2003;14:539–545. doi: 10.1021/bc0340114. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy MD, Jallad KN, Thompson DH, Ben-Amotz D, Low PS. Optical imaging of metastatic tumors using a folate-targeted fluorescent probe. J Biomed Opt. 2003;8:636–641. doi: 10.1117/1.1609453. [DOI] [PubMed] [Google Scholar]
- 23.Chen WT, Khazaie K, Zhang G, Weissleder R, Tung CH. Detection of dysplastic intestinal adenomas using a fluorescent folate imaging probe. Mol Imaging. 2005;4:67–74. doi: 10.1162/15353500200504199. [DOI] [PubMed] [Google Scholar]
- 24.Konda SD, Aref M, Brechbiel M, Wiener EC. Development of a tumor-targeting MR contrast agent using the high-affinity folate receptor: work in progress. Invest Radiol. 2000;35:50–57. doi: 10.1097/00004424-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Konda SD, Aref M, Wang S, Brechbiel M, Wiener EC. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magma. 2001;12:104–113. doi: 10.1007/BF02668091. [DOI] [PubMed] [Google Scholar]
- 26.Wiener EC, Konda SD, Wang S, Brechbiel M. Imaging folate binding protein expression with MRI. Acad Radiol. 2002;9(Suppl 2):S316–319. doi: 10.1016/s1076-6332(03)80215-5. [DOI] [PubMed] [Google Scholar]
- 27.Choi H, Choi SR, Zhou R, Kung HF, Chen IW. Iron oxide nanoparticles as magnetic resonance contrast agent for tumor imaging via folate receptor-targeted delivery. Acad Radiol. 2004;11:996–1004. doi: 10.1016/j.acra.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Saborowski O, Simon GH, Raatschen HJ, et al. MR imaging of antigen-induced arthritis with a new, folate receptor-targeted contrast agent. Contrast Media Mol Imaging. 2007;2:72–81. doi: 10.1002/cmmi.128. [DOI] [PubMed] [Google Scholar]
- 29.Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS. Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol. 2004;66:1406–1414. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]
- 30.Tung CH, Lin Y, Moon WK, Weissleder R. A receptor-targeted near-infrared fluorescence probe for in vivo tumor imaging. Chembiochem. 2002;3:784–786. doi: 10.1002/1439-7633(20020802)3:8<784::AID-CBIC784>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 31.Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004;39:56–63. doi: 10.1097/01.rli.0000101027.57021.28. [DOI] [PubMed] [Google Scholar]
- 32.Wiener EC, Konda S, Shadron A, Brechbiel M, Gansow O. Targeting dendrimer-chelates to tumors and tumor cells expressing the high-affinity folate receptor. Invest Radiol. 1997;32:748–754. doi: 10.1097/00004424-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Corot C RP, Lancelot E, Prigent P, Port M. Tumor imaging using a high relaxivity gadolinium chelate targeted to the folate receptor; Proceedings of the 13th Scientific Meeting of the International Society of Magnetic Resonance in Medicine; Miami, FL: 2005. p. 2601. [DOI] [PubMed] [Google Scholar]
- 34.Miotti S, Bagnoli M, Ottone F, Tomassetti A, Colnaghi MI, Canevari S. Simultaneous activity of two different mechanisms of folate transport in ovarian carcinoma cell lines. J Cell Biochem. 1997;65:479–491. [PubMed] [Google Scholar]
- 35.Corot CPM, Guilbert I, Robert P, Raynal I, Robic C, Raynaud JS, Prigent P, Dencausse A, Idee JM. Superparamagnetic contrast agents. In: Bulte MMMaJW., editor. Molecular and Cellular MR Imaging. CRC Press; Boca Raton: 2007. pp. 60–83. [Google Scholar]
- 36.Wu M, Gunning W, Ratnam M. Expression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol Biomarkers Prev. 1999;8:775–782. [PubMed] [Google Scholar]
- 37.Buist MR, Molthoff CF, Kenemans P, Meijer CJ. Distribution of OV-TL 3 and MOv18 in normal and malignant ovarian tissue. J Clin Pathol. 1995;48:631–636. doi: 10.1136/jcp.48.7.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figini M, Ferri R, Mezzanzanica D, et al. Reversion of transformed phenotype in ovarian cancer cells by intracellular expression of anti folate receptor antibodies. Gene Ther. 2003;10:1018–1025. doi: 10.1038/sj.gt.3301962. [DOI] [PubMed] [Google Scholar]
- 39.Toffoli G, Russo A, Gallo A, et al. Expression of folate binding protein as a prognostic factor for response to platinum-containing chemotherapy and survival in human ovarian cancer. Int J Cancer. 1998;79:121–126. doi: 10.1002/(sici)1097-0215(19980417)79:2<121::aid-ijc4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]