Abstract
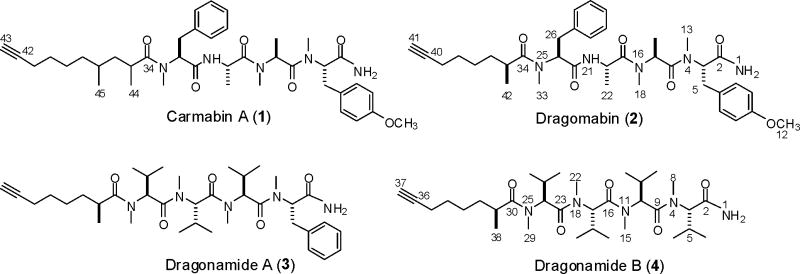
As part of the Panama International Cooperative Biodiversity Groups (ICBG) project, two new (2, 4) and two known (1, 3) linear alkynoic lipopeptides have been isolated from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula. Carmabin A (1), dragomabin (2), and dragonamide A (3) showed good antimalarial activity (IC50 4.3, 6.0, and 7.7 μM, respectively) whereas the non-aromatic analog, dragonamide B (4), was inactive. The planar structures of all four compounds were determined by NMR spectroscopy in combination with mass spectrometry, and their stereoconfigurations were established by chiral HPLC and by comparison of their optical rotations and NMR data with literature values.
Artemisinin Combination Treatments (ACTs) for falciparum malaria are currently the only first-line antimalarial drugs amenable to widespread use against all chloroquine-resistant malaria parasites.1 However, their effective distribution to combat malaria in economically disadvantaged regions could require an annual global subsidy of $300-500 million.2 Furthermore, in the event of successful widespread use of the artemisinins, the development of resistance to these drugs before effective replacements or alternatives are at hand is a cause for profound concern. Therefore, the development of new classes of antimalarial drugs remains an enormous challenge and is a focus of many collaborative research efforts, including the International Cooperative Biodiversity Groups project in Panama, which investigates Panamanian terrestrial plants, endophytes and marine organisms as sources of tropical disease treatments.3 As part of this program, we have been investigating marine cyanobacteria as a source of antimalarial agents, and found that the organic extracts of a red Panamanian strain of the marine cyanobacterium Lyngbya majuscula were active against chloroquine-resistant Plasmodium falciparum. To the best of our knowledge, there are only three reports of marine cyanobacterial metabolites isolated with antimalarial activity. Most recently, members of our group isolated antimalarial venturamides A and B (IC50 8.2 and 5.6 μM, respectively) from a Panamanian Oscillatoria sp.4 These tetrapeptides showed good differential toxicity to parasite versus mammalian host cells. An inseparable mixture of phenolic hierridins A and B from Phormidium ectocarpi inhibited chloroquine-resistant P. falciparum with an IC50 of 5.2 μg/mL,5 and the indolophenanthridine alkaloids calothrixins A and B showed nanomolar potency (IC50 58 and 180 nM, respectively) against the same strain.6 Interestingly, the anticancer microtubule inhibitors dolastatins 10 and 15,7 which have been shown to be of cyanobacterial origin,8 were tested and shown to be potent antimalarials (IC50 100 pM and 200 nM, respectively),9 consistent with the proposal that parasite tubulin is a valid target for antiprotozoal agents.
Herein, we report the antimalarial bioassay-guided isolation of two new lipopeptides, dragomabin (2) and dragonamide B (4), and the related known metabolites carmabin A (1) and dragonamide (3, designated here as dragonamide A), from four separate Panamanian collections of Lyngbya majuscula Gomont (Oscillatoriaceae). Compounds 1, 2, and 3 were responsible for the antimalarial activity of the crude extract. Jamaicamides A and B,10 previously isolated in our laboratories, were also tested for antimalarial activity given their somewhat related chemical structures to these new L. majuscula metabolites. Linear metabolites derived from a putative mixed polyketide synthase and non-ribosomal peptide synthetase biogenesis represent a familiar motif in other cyanobacterial natural products, such as the microcolins,11 apramides,12 and majusculamides A, B13 and D.14
Results and Discussion
The organic extracts of two collections of L. majuscula from different sites in Bocas del Toro, Panama (2002 and 2003) showed significant activity against chloroquine-resistant Plasmodium falciparum (IC50 = 6 and 26 μg/mL). Crude fractionation by normal-phase vacuum-liquid chromatography (NP-VLC) of the most active collection from Isla Bastimentos (Bocas del Toro, 2002) produced two relatively polar fractions (100% EtOAc and 25% MeOH-EtOAc) with good antimalarial activity (<2 and 1 μg/mL, respectively). Solid-phase extraction (SPE) and reversed-phase HPLC of the 25% MeOH-EtOAc NP-VLC fraction yielded carmabin A (1) and dragomabin (2) as the active components.
Structure elucidation of linear lipopeptides such as compounds 1 and 2 is complicated due to the presence of N-methyl amide functionalities, which results in several geometrical isomers exisiting in solution, as noted in the original isolation and structure elucidation work on carmabin A (1).15 Ready identification of carmabin A in our extracts was further hampered because the original structure work used only DMSO-d6 as the NMR solvent, while the work detailed below was derived from samples dissolved in CDCl3. Nevertheless, the following deduction of the molecular formula and several partial structures from NMR analysis identified compound 1 as carmabin A. Carmabin A (1) analyzed for C40H57N5O6 by a combination of HRFABMS (obsd. [M-NH2]+ at m/z 687.4174 for C40H55N4O6) and NMR analysis. 1H NMR data for compound 1 revealed the presence of two major N-methyl peptide conformers (approximate ratio = 3:2) with signals for three N-methyl substituents (δ 2.77-3.03), an O-methyl substituent (δ 3.78), four alpha protons (δ 4.79-5.36), four overlapped high field methyl doublets, and aromatic protons consistent with both para-disubstituted and monosubstituted phenyl moieties. Spin systems delineating tyrosine, phenylalanine, and two alanine residues were confirmed from COSY data for carmabin A (1), and the tetrapeptide segment of 1 could be specifically assigned as N,O-dimethyltyrosyl-N-methylalanyl-alanyl-N-methylphenylalanine (Table 1) from HSQC and HMBC data. The LRFABMS fragmentation pattern for compound 1 was consistent with a sequential loss of N,O-dimethyltyrosine (191), N-methylalanine (85.5) and alanine (71) from the [M-NH2]+ peak at m/z 688, and confirmed the assignment of 1 as carmabin A.15 The identity of carmabin A in this extract assisted structure elucidation of the new compounds dragomabin (2) and dragonamide B (4), as described below.
Table 1.
| carmabin A (1) | dragomabin (2) | ||||
|---|---|---|---|---|---|
| unitc | position | δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) |
| NH2 | 1 | - | 5.54 / 5.40, br s | - | 5.44, br s |
| N,O-diMeTyr | 2 | 171.7 / 171.4, qC | - | 171.7 / 171.5, qC | - |
| 3 | 62.3 / 57.9, CH | 4.73, dd (11, 2) / 5.32, ob | 62.3 / 57.9, CH | 4.74, ob / 5.32, ob | |
| 4 | N | N | |||
| 5 | 33.5 / 32.5, CH2 | 3.19 / 3.16, ob | 33.3 / 32.4, CH2 | 3.17, m | |
| 6 | 129.6, qC | - | 129.6, qC | - | |
| 7 | 130.4 / 129.8, CH | 7.10 / 7.05, d (8) | 130.4 / 129.8, CH | 7.10 / 7.06, d (8) | |
| 8 | 114.4 / 114.1, CH | 6.83 / 6.80, d (8) | 114.4 / 114.1, CH | 6.83 / 6.80, d (8) | |
| 9 | 158.7 / 158.6, qC | - | 158.7 / 158.6, qC | - | |
| 10 | 114.4 / 114.1, CH | 6.83 / 6.80, d (8) | 114.4 / 114.1, CH | 6.83 / 6.80, d (8) | |
| 11 | 130.4 / 129.8, CH | 7.10 / 7.05, d (8) | 130.4 / 129.8, CH | 7.10 / 7.06, d (8) | |
| 12 | 55.3, CH3 | 3.77, s | 55.4, CH3 | 3.77, s | |
| 13 | 31.0 / 29.0, CH3 | 2.76 / 2.90, s | 31.1 / 29.1, CH3 | 2.76 / 2.90, s | |
| N-MeAla | 14 | 172.98 / 172.4, qC | - | 172.9 / 172.4, qC | - |
| 15 | 50.2 / 48.8, CH | 5.35 / 4.81, ob | 50.2 / 48.8, CH | 5.35 / 4.81, ob | |
| 16 | N | N | |||
| 17 | 13.9 / 14.0, CH3 | 1.18 / 0.48, d (7) | 13.9 / 14.0, CH3 | 1.18 / 0.51, d (7) | |
| 18 | 29.2, CH3 | 2.24, s | 29.2, CH3 | 2.28, s | |
| Ala | 19 | 171.3, qC | - | 171.3, qC | - |
| 20 | 45.7 / 45.4, CH | 4.78 / 4.58, p (7) | 45.7 / 45.5, CH | 4.78 / 4.60, p (7) | |
| 21 | NH | 6.94, t (7) | NH | 6.90, ob | |
| 22 | 18.1 / 17.7, CH3 | 1.08 / 1.20, d (7) | 18.2 / 17.8, CH3 | 1.08 / 1.20, ob | |
| N-MePhe | 23 | 169.5, qC | - | 169.5, qC | - |
| 24 | 56.7 / 56.3, CH | 5.52, ob | 57.0 / 56.5, CH | 5.50, m | |
| 25 | N | - | N | - | |
| 26 | 33.7, CH2 | 3.28 / 3.25, ob | 33.6, CH2 | 3.28 / 3.25, m | |
| 27 | 137.0 / 136.9, qC | - | 137.1 / 137.0, qC | - | |
| 28 | 128.86 / 128.80, CH | 7.18, ob | 128.85 / 128.80, CH | 7.18, d (ob) | |
| 29 | 126.60 / 126.58, CH | 7.17, ob | 126.6, CH | 7.17, ob | |
| 30 | 128.4, CH | 7.24, m | 128.5, CH | 7.24, m | |
| 31 | 126.60 / 126.58, CH | 7.17, ob | 126.6, CH | 7.17, ob | |
| 32 | 128.86 / 128.80, CH | 7.18, d (ob) | 128.85 / 128.80, CH | 7.18, d (ob) | |
| 33 | 30.8, CH3 | 2.88, s | 30.8, CH3 | 2.89, s | |
| Moya/Mdya | 34 | 178.3 / 178.1, qC | - | 177.8 / 177.6, qC | - |
| 35 | 33.60 / 33.55, CH | 2.60, sextet (6) | 35.9, CH | 2.53, sextet (6) | |
| 36 | 40.8 / 40.7, CH2 | 1.10 / 1.13, m | 33.33 / 33.25, CH2 | 1.46, m 1.20, m |
|
| 37 | 30.2, CH | 1.12, m | 26.3, CH2 | 1.00, m | |
| 38 | 36.4 / 36.3, CH2 | 0.93, m | 28.40 / 28.36, CH2 | 1.34, m | |
| 39 | 25.7, CH2 | 1.21, m 1.29 m | 18.2 / 17.8, CH2 | 2.06, m | |
| 40 | 28.7, CH2 | 1.42 m | 84.49 / 84.45, qC | - | |
| 41 | 18.3, CH2 | 2.15, t (6.6) | 68.24 / 68.18, CH | 1.93, br s | |
| 42 | 84.6, qC | - | 17.4, CH3 | 1.04, d (7) | |
| 43 | 68.2, CH | 1.95, br s | |||
| 44 | 17.0 / 17.1, CH3 | 1.02 / 1.07, d (6.8) | |||
| 45 | 19.45 / 19.50, CH3 | 0.68, d (6.1) | |||
Measured at 100 Mhz (13C) and 600 MHz (1H), ratio of conformers = 3:2.
Measured at 75 MHz (13C) and 400 (1H) MHz, ratio of conformers = 3:2.
Moya = 2-methyloct-7-ynoic acid, Mdya = 2,4-dimethyldec-9-ynoic acid. ob = obscured
Dragomabin (2) also gave a prominent [M-NH2]+ peak by HRFABMS for C37H49N4O6 (m/z 645.3677), which is 42 mass units (C3H6) less than the major ion for carmabin A (1), consistent with the loss of a propyl unit in 2 relative to 1. Furthermore, the 1H NMR spectrum for 2 lacked the distinctive upfield methyl doublet at δ 0.68 that was present in the spectrum for 1. This signal was assigned to the 4-methyl group of the 2,4-dimethyldec-9-ynoic unit in 1. The identical tetrapeptide segments in dragomabin (2) and carmabin A (1), the lack of the second methyl group, and the reduced molecular weight of 2 compared to 1, implied the presence of the 2-methyloct-7-ynoic acid in dragomabin. Indeed, close examination of a 2D HSQC-TOCSY experiment for dragomabin delineated a -CH(CH3)CH2(CH2)3- spin system consistent with this proposal. HMBC correlations positioned the aliphatic methyl and methine adjacent to the C-34 carbonyl carbon (δ 177.8/177.6). The distal methylene group of this partial structure was placed adjacent to the terminal acetylene by an HMBC correlation from the methylene protons (H2-39, δ 2.06) to the quaternary acetylene carbon (δ 84.45/84.49), thus confirming the nature and location of the aliphatic group in 2. Hence, dragomabin (2) was defined as the 2-methyloct-7-ynoyl amide of the same tetrapeptide unit present in carmabin A (1).
SPE and reversed-phase HPLC of the second antimalarial NP-VLC fraction (100% EtOAc) yielded one major component. The 1H and 13C NMR data again indicated a lipopeptide metabolite, with signals suggestive of phenylalanine and valine residues, as well as a fatty acid unit (Supporting Information, Table S10.). Comparison of these data with literature values, as well as characteristic FAB mass spectrometric fragments at m/z 638 ([M-NH2]+), 476 (638 − N-MePhe), 363 (476 – N-MeVal), 250 (476 − 2 × N-MeVal) and 137 (476 − 3 × N-MeVal), identified the compound as dragonamide (3).16
A parallel investigation was undertaken of a small, biologically inactive 2003 Isla Bastimentos collection of red L. majuscula, which was collected separately due to its “fuzzy” appearance. Upon microscopic investigation, this “fuzzy” facade resulted from a thick coating of epiphytic chain diatoms on the cyanobacterial filaments. 1H NMR profiling of the 100% EtOAc NP-VLC fraction of the crude extract once again revealed N-methyl amide singlets, upfield methyl doublets, and midfield multiplets indicative of peptide metabolites. Therefore, SPE and reversed-phase HPLC were carried out to afford pure compound 4, which exhibited 1H NMR signals for four N-methyl amide substituents, nine overlapped methyl doublets coupled to 1H multiplets, and no aromatic signals. Examination of the 13C NMR and multiplicity-edited HSQC spectra for this metabolite revealed quaternary and methine 13C NMR signals at δ 84.2 and 68.4, respectively, consistent with a terminal acetylene as present in compounds 1-3. Together with five carbonyl signals, this accounted for the seven degrees of unsaturation inherent in the formula of C33H59N5O5 calculated from HRESIMS data ([M+Na]+, m/z 628.4420) for 4. Moreover, these data suggested a similar tetrapeptide-coupled fatty acid motif as observed in compounds 1-3. Isopropyl spin systems of four separate valine residues (Table 2) were delineated by COSY and multiplicity-edited HSQC correlations, and the order of these residues was assigned by careful analysis of the HMBC data. The presence of a 2-methyloct-7-ynoic acid residue attached to this tetravaline partial structure was evident from COSY data and chemical shift comparisons with dragonamide (3). Based on this otherwise close similarity to dragonamide (3), we have designated this non-aromatic analog as dragonamide B (4), and therefore reassign dragonamide (3) as dragonamide A in the present report.
Table 2.
13C and 1H NMR Data in CDCl3 for Dragonamide B (4).d
| dragonamide B (4) | |||
|---|---|---|---|
| unit | position | δC, mult. | δH (J in Hz) |
| NH2 | 1 | 6.02, s | |
| N-Me Val-1 | 2 | 172.0, qC | - |
| 3 | 62.0, CH | 4.57, d (11) | |
| 4 | N | - | |
| 5 | 25.4, CH | 2.29, m | |
| 6 | 17.8, CH3 | 0.74, d (ob) | |
| 7 | 19.60, CH3 | 0.98, d (6) | |
| 8 | 30.6, CH3 | 3.05, s | |
| N-Me Val-2 | 9 | 171.4, qC | - |
| 10 | 58.0, CH | 5.21, d (ob) | |
| 11 | N | - | |
| 12 | 27.21e, CH | 2.35, m | |
| 13 | 19.47f, CH3 | 0.85, d (7) | |
| 14 | 18.0, CH3 | 0.82, d (7) | |
| 15 | 30.38, CH3 | 3.028, s | |
| N-Me Val-3 | 16 | 170.4, qC | - |
| 17 | 58.1, CH | 5.18, d (11) | |
| 18 | N | - | |
| 19 | 27.4, CH | 2.35, m | |
| 20 | 17.71, CH3 | 0.76, d (ob) | |
| 21 | 19.47f, CH3 | 0.87, d (6) | |
| 22 | 30.45, CH3 | 3.033, s | |
| N-Me Val-4 | 23 | 171.0, qC | - |
| 24 | 57.8, CH | 5.24, d (11) | |
| 25 | N | - | |
| 26 | 27.17e, CH | 2.35, m | |
| 27 | 19.55f, CH3 | 0.89, d (7) | |
| 28 | 18.0, CH3 | 0.74, d (ob) | |
| 29 | 30.2, CH3 | 2.99, s | |
| Moya | 30 | 176.9, qC | - |
| 31 | 36.2, CH | 2.72, m | |
| 32 | 33.6, CH2 | 1.35, m 1.70, m |
|
| 33 | 26.8, CH2 | 1.34, m | |
| 34 | 28.4, CH2 | 1.49, m | |
| 35 | 18.3, CH2 | 2.16, 2.15, dd (7) | |
| 36 | 84.2, qC | - | |
| 37 | 68.4, CH | 1.92, t (2) | |
| 38 | 17.67, CH3 | 1.12, d (7) | |
Measured at 75 MHz (13C) and 300 MHz (1H).
Chemical shift assignments to atom number are interchangeable. ob = obscured signal.
Stereoconfiguration of the amino acid residues in lipopeptides 1-4 was assigned by chiral HPLC comparison of their acid hydrolysates with d and l amino acid standards. All standards could be obtained commercially except for N,O-dimethyl-d-tyrosine which was synthesized by methylation of Cbz-protected d-tyrosine following a literature procedure.17 Carmabin A (1) and dragomabin (2) contained N-methyl-l-phenylalanine, N-methyl-l-alanine, l-alanine and N,O-dimethyl-l-tyrosine, while dragonamides A (3) and B (4) contained N-methyl-l-phenylalanine and N-methyl-l-valine residues, and all N-methyl-l-valine residues, respectively. Furthermore, closely similar NMR data for the 2-methyloct-7-ynoic acid unit in dragonamides A and B (see Supporting Information and Table 2, respectively) suggested the same relative stereoconfiguration at the fatty acyl α position in these two compounds, and due to the common source for all four compounds, led us to propose the same stereoconfiguration for dragomabin (2). In the original literature report of dragonamide A (3),16 this fatty acyl α stereocenter (C-35) was assigned an R configuration on the basis of the optical rotation for the 2-methyloctanoic acid liberated following hydrogenation and acid hydrolysis of dragonamide A. This was assigned by comparison to previous literature values for 2-methylalkanoic acids (although no optical rotation values were provided in reference 14). Subsequently, the total synthesis of dragonamide A led to reassignment of the natural product as 35S based on comparisons of its NMR and optical rotation data with both synthetic enantiomers (35R and 35S).18 NMR and optical rotation data for the dragonamide A that we have isolated closely matches those reported for both the natural product16 and the 35S synthetic product, but differs significantly from the 35R synthetic product.18 Therefore, we conclude that dragonamide A (3), dragonamide B (4), and dragomabin (2) all contain 2S-methyloct-7-ynoic acid. Interestingly, this is the opposite configuration to that proposed for the 2-methyloct-7-ynoic acid unit in apramides A and D based on calculation and analysis of the molar optical rotations of these two compounds.19
To the best of our knowledge, carmabin A (1) is the only reported compound containing a 2,4-dimethyldec-9-ynoic acid moiety, the absolute stereochemistry of which has not been assigned. However, the related acyl group, 2,4-dimethyloctanoic acid, is present in the potent immunosuppressant natural products microcolins A and B11 and the closely related metabolites majusculamide D and its deoxy homolog,14 all of which were also isolated from L. majuscula strains. The four 2,4-dimethyloctanoic acid stereoisomers were synthesized from hexanoyl chloride in seven steps as part of the total asymmetric synthesis of microcolin A (36R, 38R) and its three 2,4-dimethylacyl analogs.20 We considered three approaches to the assignment of the absolute configuration of carmabin A (1): (1) reduction, hydrolysis and isolation of the resulting free 2,4-dimethyldecanoic acid from 1, followed by characterization of the free acid by optical rotation and NMR chemical shift comparison with synthetic 2,4-dimethyloctanoic acid stereoisomers; (2) detection, isolation and characterization of free 2,4-dimethyldecanoic acid as a co-metabolite from nonpolar fractions of the L. majuscula extract, which would suggest the same stereoconfiguration in 1; (3) direct comparison of NMR chemical shifts for the acid unit in tetrahydrocarmabin A with those for the four microcolin diastereomers. Unfortunately, we isolated an insufficient amount of material to perform the degradative sequence suggested in the first approach. We were unable to detect free 2,4-dimethyldecanoic acid in the crude lipid extract, thus precluding the second approach. Finally, approach 3 was discounted because the chemical shifts for the acid unit of reduced 1 differed significantly from those for the microcolins, presumably due to the adjacent phenylalanine residue in carmabin A (1) as opposed to the leucine residue in the microcolins. In the ROESY spectrum for 1, methine H-35 (δ2.60) showed strong correlations to CH3-45 (δ0.68) as well as to N-methyl CH3-33 (δ2.88). This implies a configuration of 35S, 37R or 35R, 37S for 1 (Figure S9, Supporting Information) although insufficient spectroscopic resolution precluded observation of any complementary correlation between H-37 (δ1.12) and CH3-44 (δ 1.02/1.07). Thus, the configurations of the two asymmetric methyl groups at C-35 and C-37 remain unassigned in carmabin A (1).
These three collections of red L. majuscula from different sites in the Bocas del Toro region of Panama (approximately 20 square miles) were profiled by LC-MS together with a collection of a morphologically similar L. majuscula from Cacique, near Portobelo (approximately 180 miles directly east of Bocas del Toro). Lipopeptides 1-4 were all present in both the Bastimentos and Bocas del Drago collections, while the Crawl Cay collection yielded dragomabin (2) and dragonamide A (3). Therefore, all L. majuscula collections from Bocas del Toro appear to biosynthesize both the dragomabin/carmabin series as well as the dragonamides. Interestingly, only carmabin A (1) and dragomabin (2) were detected in the Cacique collection (i.e., dragonamides containing valyl residues attached to the acyl group are not present in this population).
Pure compounds 1-3 were tested against the W2 chloroquine-resistant malaria strain, both as they were isolated and subsequently side-by-side, thus providing a good indication of their relative levels of activity in this assay. While initial testing gave IC50 values of 1.4, 21.0, and 10.7 μM for carmabin A (1), dragomabin (2), and dragonamide A (3), respectively, same plate evaluation gave IC50 values of 4.3, 6.0, and 7.7 μM, therefore indicating no significant difference between their levels of antimalarial activity. However, carmabin A was more cytotoxic to Vero cells (IC50 9.8 μM) than dragomabin (IC50 182.3 μM) or dragonamide A (IC50 67.8 μM), and thus, dragomabin possesses the best differential toxicity between parasite and mammalian cells. It appears that the presence of three extra carbons in the aliphatic chain leads to the increase in cytotoxicity of carmabin A (1) over that of dragomabin (2). Dragonamide B (4) was isolated and tested by itself at a later date, and its lack of activity suggests that an aromatic amino acid at the carboxy terminus is necessary for antimalarial activity in this compound series. Interestingly, the non-aromatic ring-containing alkynoic lipopeptide jamaicamide B,10 isolated from L. majuscula collected from Hector's Bay, Jamaica, showed weak antimalarial activity (IC50 18.4 μM) and a similar level of cytotoxicity (IC50 16.2 μM) to Vero cells. However, the terminal bromo-acetylene homolog jamaicamide A10 was inactive in this assay (IC50 >50 μM).
Experimental Section
General Experimental Procedures
Optical rotations were recorded on a JASCO P-1010 polarimeter. IR data were obtained on a Nicolet 510 FTIR spectrophotometer. NMR spectra were recorded on Bruker Avance DRX 300 MHz, Bruker Avance DPX 400 MHz, and Bruker Avance DRX 600 MHz spectrometers operating at 1H frequencies of 300.13, 400.13, and 600.01 MHz, respectively, and carbon frequencies of 75.47, 100.61, and 150.90 MHz, respectively, with the solvent used as an internal standard (CDCl3 at δC 77.23, δH 7.26). Mass spectra were recorded on Kratos MS50TC and Waters Micromass LCT mass spectrometers. HPLC separations were performed using either Waters 515 HPLC pumps, a Rheodyne 7725i injector and a Waters 996 Photodiode Array Detector or Shimadzu LC-6AD HPLC pumps, a Rheodyne 7125 injector and an SPD-10A dual wavelength UV detector. NP-VLC was performed with Merck Si gel G for TLC.
Collection, Extraction and Isolation
Lyngbya majuscula was collected in 2002 and 2003 by hand while snorkeling (2-4 ft) at Isla Bastimentos (2 separate collections), Bocas del Drago and Crawl Cay in Bocas del Toro, Panama. The four samples were stored at -20 °C in 70% EtOH until workup. Voucher specimens are available from K.L.M. (collection numbers PAB-09/13/02-1, PAB-05/25/03-1, PAB-05/27/03-3, and PAB-05/27/03-2). The first Bastimentos collection (56.5 g dry wt) was extracted five times with CH2Cl2-MeOH (2:1) to give a crude organic extract (3.6 g). A portion of the extract (3.5 g) was fractionated on Si gel by NP-VLC to give nine fractions using a stepwise gradient of hexanes-EtOAc and EtOAc-MeOH. Fraction 8 (25% MeOH-EtOAc) was further chromatographed to produce five sub-fractions on a Waters RP-18 SPE cartridge (2 g; MeOH-H2O 1:1, 7:3, 9:1, 100% MeOH and CH2Cl2). The sub-fraction eluting in 7:3 MeOH-H2O was subjected to RP-HPLC (Phenomenex RP-18, 5 μm, 10 × 250 mm; 3:1 MeOH-H2O, 3 mL/min) to yield dragomabin (2, 2.2 mg, 0.06% of extract). RP-SPE (2 g) of NP-VLC fraction 7 (100% EtOAc) to give five sub-fractions (MeOH-H2O 6:4, 8:2, 9:1, 100% MeOH and CH2Cl2) was followed by RP-HPLC (Phenomenex RP-18, 5 μm, 10 × 250 mm; 8:2 MeOH-H2O, 3 mL/min) of the 8:2 MeOH-H2O sub-fraction to yield dragonamide A (3, 6.0 mg, 0.17% of extract). Following the same extraction, NP-VLC and RP-SPE protocols, the Bocas del Drago collection yielded 2.2 g crude extract, of which NP-VLC fraction 8 (25% MeOH-EtOAc) yielded 5.5 mg of carmabin A (1, 0.25% of extract) after repeated RP-HPLC (YMC-ODS RP-18, 10 × 250 mm; 65:35 MeCN-H2O, 77:23 MeCN-H2O, 2 mL/min).
Approximately 8.7 g (dry wt) of a diatom-covered red L. majuscula collected from Isla Bastimentos was repeatedly extracted with CH2Cl2-MeOH (2:1) to afford 1.5 g of extract. RP-SPE of a polar EtOAc NP-VLC fraction (100% EtOAc) produced a fraction eluting with 100% MeOH that possessed interesting, peptide-like 1H NMR signals. This fraction was purified by RP-HPLC (Phenomenex Synergi Hydro RP-18, 4.6 × 250 mm, 1:1 MeOH-H2O to 100% MeOH gradient over 50 min, 0.8 mL/min) to afford pure dragonamide B (4, 2.3 mg, 0.15 % of extract).
Carmabin A (1)
white amorphous solid; [α]25d -137 (c = 0.440, CHCl3); IR νmax (film) 3395, 2931, 2859, 1635, 1523, 1466, 1412, 1253, 1088 cm-1; 1H and 13C NMR data (CDCl3), see Table 1; FABMS (3-nba, positive) m/z (%) 726 (5, [M+Na]+), 705 (17, [M+H]+), 687 (80, [M-NH2]+), 496 (22), 411 (5), 340 (33); HRFABMS (3-nba, positive) obsd [M − NH2]+m/z 687.4174 (calcd for C40H55N4O6, 687.4121).
Dragomabin (2)
white amorphous solid; [α]25d -106 (c = 0.500, CHCl3); IR νmax (film) 3308, 2931, 2859, 1640, 1519, 1471, 1413, 1379, 1253, 1084 cm-1; 1H and 13C NMR data (CDCl3), see Table 1; ESIMS (positive) m/z (%) 684 (20, [M+Na]+), 661 (14, M+), 645 (100), 454 (28); HRESIMS obsd [M − NH2]+m/z 645.3677 (calcd for C37H49N4O6, 645.3652).
Dragonamide A (3)
pale yellow oil; [α]25d -244 (c = 0.250, CHCl3); IR νmax (film) 2963, 2931, 2873, 1694, 1633, 1467, 1401, 1257, 1093 cm-1; 1H and 13C NMR data (CDCl3), see Table S10. (Supporting Information); FABMS (3-nba, positive) m/z (%) 638 (100), 476 (20), 363 (35), 250 (80).
Dragonamide B (4)
pale yellow oil; [α]25d -289 (c = 0.700, CHCl3); IR νmax (film) 2965, 2941, 2883, 1693, 1645, 1466, 1408, 1263, 1093 cm-1; 1H and 13C NMR data (CDCl3), see Table 2; ESIMS (positive) m/z (%) 628 (88, [M+Na]+), 589 (7), 476 (29), 363 (78), 250 (100); HRESIMS obsd [M + Na]+m/z 628.4420 (calcd for C33H59N5O5Na, 628.4414).
Absolute Configurations of the Peptide Portions of Compounds 1-4
Each of compounds 1-4 (0.2 mg) was hydrolyzed separately in 6 N HCl (microwave, 48 s), and then dried in vacuo. The residue of each was reconstituted in 200 μL of EtOH for chiral HPLC analysis using two different column/mobile phase combinations: (1) column Phenomenex Chirex phase 3126 (D) (4.6 × 250 mm), mobile phase 2 mM CuSO4, flow rate 0.8 mL/min, detection 254 nm. The retention times for authentic standards (tR min) were: l-Ala (10.6), d-Ala (16.7), N-Me-l-Ala (14.8), N-Me-d-Ala (15.5), N-Me-l-Val (24.4), N-Me-d-Val (40.4); Hydrolysates of 1-4 showed peaks at 10.6 min (l-Ala), 14.8 min (N-Me-l-Ala) and 24.4 min (N-Me-l-Val); (2) column Chirobiotic T (4.6 × 150 mm), mobile phase 50% aq. EtOH, flow rate 1 mL/min, detection 254 nm. The retention times for authentic standards (tR min) were: N-Me-l-Phe (9.1), N-Me-d-Phe (13.0), N,O-diMe-l-Tyr (9.4), N,O-diMe-d-Tyr (14.0). Hydrolysates of 1 and 2 showed peaks at 9.1 and 9.4 min consistent with N-Me-l-Phe and N,O-diMe-l-Tyr, respectively.
Malaria Assay
The antiplasmodial activity was evaluated in a chloroquine-resistant strain (Indochina W2) of Plasmodium falciparum using a fluorometric method based on the detection of parasite DNA with the fluorochrome PicoGreen.3 A modified Trager and Jensen method21 was used to maintain the parasites in vitro.
Cytotoxicity Assay
Vero cells adhering to 96-well plates were used to evaluate the toxicity of the compounds purified from L. majuscula on the basis of reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma).22 After treatment with the test compound and 4 h incubation at 37 °C, cell viability was evaluated in an ELISA reader at 570 nm.
Supplementary Material
Acknowledgments
We thank the Smithsonian Tropical Research Institute research station at Bocas del Toro for help with collection of L. majuscula, and Autoridad Nacional del Ambiente (ANAM), Panama, for permission to make these collections. We also thank the Biochemistry and Chemistry NMR Facilities at OSU for 600 MHz NMR spectrometer time, and J. Morre and the EIHS Center at Oregon State University (OSU) for mass spectroscopic data acquisition (NIEHS grant P30 ES00210). The National Institute of Health (CA52955, NS053398) and the Fogarty International Center-supported International Cooperative Biodiversity Group grant 1-U01 TW006634-01 provided financial support for this work.
Footnotes
Supporting Information Available: 1H and 13C NMR spectra in CDCl3 for carmabin A (1), dragomabin (2), dragonamide A (3) and dragonamide B (4). Figure showing ROESY correlations for the 2,4-dimethyldec-9-ynoyl unit of carmabin A (1). Table of NMR data for dragonamide A (3) in CDCl3. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Barnes K, Folb P. Reducing malaria's burden: evidence of effectiveness for decision makers. Global Health Council; Washington, DC: 2003. pp. 25–32. technical report. [Google Scholar]
- 2.Panosian CB. Clin Infect Dis. 2005;40:713–717. doi: 10.1086/427807. [DOI] [PubMed] [Google Scholar]
- 3.Corbett Y, Herrera L, González J, Cubilla L, Capson TL, Coley PD, Kursar TA, Romero LI, Ortega-Barria E. Am J Trop Med Hyg. 2004;70:119–124. [PubMed] [Google Scholar]
- 4.Linington RG, González J, Ureña LD, Romero LI, Ortega-Barría E, Gerwick WH. J Nat Prod. 2007 doi: 10.1021/np0605790. Web Release Date: 01-Mar-2007; (Article) [DOI] [PubMed] [Google Scholar]
- 5.Papendorf O, Konig GM, Wright AD. Phytochemistry. 1998;49:2383–2386. doi: 10.1016/s0031-9422(98)00440-3. [DOI] [PubMed] [Google Scholar]
- 6.Rickards RW, Rothschild JM, Willis AC, de Chazal NM, Kirk J, Kirk K, Saliba KJ, Smith GD. Tetrahedron. 1999;55:13513–13520. [Google Scholar]
- 7.a Pettit GR, Kamano Y, Herald CL, Tuinman AA, Boettner FE, Kizu H, Schmidt JM, Baczynskyj L, Tomer KB, Bontems RJ. J Am Chem Soc. 1987;109:6883–6885. [Google Scholar]; b Pettit GR, Kamano Y, Dufresne C, Cerny RL, Herald CL, Schmidt JM. J Org Chem. 1989;54:6005–6006. [Google Scholar]
- 8.Luesch H, Moore RE, Paul VJ, Mooberry SL, Corbett TH. J Nat Prod. 2001;64:907–910. doi: 10.1021/np010049y. [DOI] [PubMed] [Google Scholar]
- 9.Fennell BJ, Carolan S, Pettit GR, Bell A. J Antimicrob Chemother. 2003;51:833–841. doi: 10.1093/jac/dkg151. [DOI] [PubMed] [Google Scholar]
- 10.Edwards DE, Marquez BL, Nogle LM, McPhail K, Goeger D, Gerwick WH. Chem Biol. 2004;11:817–833. doi: 10.1016/j.chembiol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Koehn FE, Longley RE, Reed JK. J Nat Prod. 1992;55:613–619. doi: 10.1021/np50083a009. [DOI] [PubMed] [Google Scholar]
- 12.Luesch H, Yoshida WY, Moore RE, Paul VJ. J Nat Prod. 2000;63:1106–1112. doi: 10.1021/np000078t. [DOI] [PubMed] [Google Scholar]
- 13.Marner FJ, Moore RE, Hirotsu K, Clardy J. J Org Chem. 1977;42:2815–2819. [Google Scholar]
- 14.Moore RE, Entzeroth M. Phytochemistry. 1988;27:3101–3103. [Google Scholar]
- 15.Hooper GJ, Orjala J, Schatzman RC, Gerwick WH. J Nat Prod. 1998;61:529–533. doi: 10.1021/np970443p. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez JI, Scheuer PJ. J Nat Prod. 2001;64:200–203. doi: 10.1021/np000462q. [DOI] [PubMed] [Google Scholar]
- 17.McDermott J, Benoiton L. Can J Chem. 1973;51:1915–1919. [Google Scholar]
- 18.Chen H, Feng Y, Xu Z, Ye T. Tetrahedron. 2005;61:11132–11140. [Google Scholar]
- 19.Luesch H, Yoshida WY, Moore RE, Paul VP. J Nat Prod. 2000;63:1106–1112. doi: 10.1021/np000078t. [DOI] [PubMed] [Google Scholar]
- 20.Decicco CP, Grover P. J Org Chem. 1996;61:3534–3541. [Google Scholar]
- 21.Trager W, Jensen JB. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 22.Chiari E, Camargo EP. In: Genes and Antigens of Parasites. A Laboratory Manual. Morel CM, editor. Fundacão Oswaldo Cruz; Rio de Janeiro, Brazil: 1984. pp. 23–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.