Abstract
Neuropeptides of the adipokinetic hormone (AKH) family are among the best studied hormone peptides, but its signaling pathways remain to be elucidated. In this study, we molecularly characterized the signaling of Bombyx AKH receptor (AKHR) and its peptide ligands in HEK293 cells. In HEK293 cells stably expressing AKHR, AKH1 stimulation not only led to a ligand concentration dependent mobilization of intracellular Ca2+ and cAMP accumulation, but also elicited transient activation of extracellular signal-regulated kinase 1/2 (ERK1/2) pathway. We observed that AKH receptor was rapidly internalized after AKH1 stimulation. We further demonstrated that AKH2 exhibited high activities in cAMP accumulation and ERK1/2 activation on AKHR comparable to AKH1, whereas AKH3 was much less effective.
Keywords: Adipokinetic hormone, Signal transduction, Receptor, G protein, Internalization, Mitogen-activated protein kinase, Bombyx mori
1. Introduction
Adipokinetic hormones (AKHs) produced by the insect corpora cardiaca are among the most extensively characterized peptide hormones with almost 40 family members from most of the major insect orders [1–7]. AKH is normally 8–10 amino acids long with a pyroglutamate at the N-terminus and an amidated C-terminus. In addition to the essential role of mobilization of metabolites during energy-expensive activities such as flight and locomotion, AKH is involved in the control of carbohydrate homeostasis in the haemolymph of Drosophila and Bombyx larvae [8,9]. As shown in Table 1, in Bombyx, a non-apeptide identical with Manduca AKH (AKH1) has been chemically identified [10], and recently another two cDNAs encoding the prepro-Bombyx AKH2, and 3 have been annotated and identified by combining homology search with cDNA cloning [11].
Table 1.
Primary structure of adipokinetic hormone peptides from Bombyx mori.
The receptor of AKH was first identified as a typical G protein-coupled receptor (GPCR) from the fruitfly Drosophila melanogaster and the silkworm Bombyx mori in 2002 [12], and then from the cockroach Periplaneta americana [13] and African malaria mosquito Anopheles gambiae [14]. Previous biochemical characterization with isolated fat body suggested that AKH binds to its receptor and activates adenylyl cyclase via the Gs protein, which results in an increase of intracellular cAMP levels. In addition, AKH activates phospholipase C (PLC) to induce the release of Ca2+ from intracellular Ca2+ stores [15–17]. However, the mechanistic details of AKHR signaling remain to be further elucidated.
In this present study, we cloned the AKHR from the fat body of the silkworm B. mori and further functionally characterized it and its peptide ligands in HEK293 cells. We conclude that after activation of AKHR, in addition to cAMP accumulation and Ca2+ release from Ca2+ stores, the mitogen-activated protein kinase (MAPK) pathway is subsequently activated and AKHRs are rapidly internalized from the plasma membrane upon agonist stimulation. AKH1 and AKH2 activated AKHR with similar affinity, but AKH3 exhibits almost much less activity on AKHR. These findings provide a foundation for future studies of the physiological role of AKH/AKHR signaling in the diapauses, development and reproduction of Bombyx.
2. Materials and methods
2.1. Materials
Larvae and pupae of the silkworm strain Feng-Yi were kindly provided by Dr. Kerong He (Zhejiang Agricultural Institute). Cell culture media and G418 were purchased from Invitrogen (Carlsbad, CA). The pEGFP-N1 and pCMV-Flag vectors were purchased from Clontech Laboratories Inc. (Palo Alto, CA) and Sigma (St. Louis, MO), respectively. The membrane probe DiI and nuclear dye Hoechst33258 were purchased from Beyotime (Haimen, China). Pertussis toxin (PTX) and cholera toxin (CTX) were purchased from Sigma and Calbiochem (Cambridge, MA), respectively. Primary antibodies for Western blotting were purchased from Cell Signaling (Danvers, MA) and Beyotime.
2.2. Cell culture and transfection
The human embryonic kidney cell line (HEK293) was maintained in Dulbecco’s Modified Eagles Medium (DMEM, Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone) and 2 mM l-glutamine (Invitrogen). The AKHR cDNA plasmid constructs were transfected or co-transfected into HEK293 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours after transfection, selection for stable expression was initiated by the addition of G418 (800 µg/ml). Transfected cells were evaluated for expression of AKHR at the cell surface by flow cytometry.
2.3. Cloning of Bombyx AKHR cDNA and construction of mammalian expression vectors
Total RNA was isolated from the fat body of pupae of B. mori using the TRIzol reagent (Keygen, Nanjing, China) following the manufacturer’s instructions. The cDNA was prepared with an AMV First Strand cDNA Synthesis Kit (Sangon, Shanghai, China) according to the manufacturer’s instructions. To amplify the full-length sequence encoding Bombyx AKHR, two pairs of primers were designed based on the sequence of GenBank Accession No. AF403542 and are as follows: forward primer 5′-AAGCTTATGGATATAGACGAGAAAGTGTCC-3′; reverse primer 5′-TCTAGATTAAACCATACCGTTCGTTACGTG-3′ for pCMV-Flag; and forward primer 5′-AAGCTTGCCACCATGGATATAGACGAGAAAGTGTCC-3′; reverse primer 5′-GGTACCGTAACCATACCGTTCGTTACGTGGTT-3′ for pEGFP-N1. The corresponding PCR products were inserted into the HindIII and XbaI sites of the pCMV-Flag vector and the HindIII and KpnI sites of the pEGFP-N1 vector, named these two vectors Flag-AKHR and AKHR–EGFP, respectively. All constructs were sequenced to verify the correct sequences and orientations.
2.4. cAMP accumulation measurement
After seeding in a 24-well plate overnight, 293 cells stably co-transfected with Flag-AKHR and pCRE-Luc were grown to 90–95% confluence, stimulated with the indicated concentration of AKH in DMEM without FBS and incubated for 4 h at 37 °C. Luciferase activity was detected by use of a firefly luciferase assay kit (Ken-real, Shanghai, China). When required, cells were treated overnight with PTX (100 ng/ml) or CTX (300 ng/ml) in serum-free DMEM before the start of the experiment.
2.5. Intracellular calcium measurement
Calcium mobilization was performed as described previously with slight modifications [18]. The stable Flag-AKHR-expressing 293 cells were harvested with Cell Stripper (Mediatech, Herndon, VA), washed twice with phosphate-buffered saline, and resuspended at 5 × 106 cells/ml in Hanks’ balanced salt solution containing 0.025% bovine serum albumin. The cells were then loaded with 3 µM fura-2/AM (Molecular Probes, Eugene, OR) for 30 min at 37 °C. Calcium flux was measured using excitation wavelengths of 340 and 380 nm in a fluorescence spectrometer (LS55, Perkin–Elmer Life Sciences).
2.6. Immunoblot analysis
The 293 cells stably expressing Flag-AKHR seeded in six-well plates were starved by growth in serum-free media overnight. After stimulation with AKH, cells were lysed. Equal amounts of total cell lysates were size-fractionated by Tris–glycine SDS–PAGE (10%) and transferred to a PVDF membrane (Millipore). Membranes were blocked in blocking buffer TBST containing 5% nonfat dry milk for 1 h at room temperature (RT) and then probed with rabbit monoclonal anti-p-extracellular signal-regulated kinase 1/2 (ERK1/2) antibody (Cell signaling, Danvers, MA) and next probed with anti-rabbit HRP-conjugated second antibody (CHEMICON, Temecula, CA) according to protocol of the products. β-Actin (Beyotime, Haimen, China) and total ERK1/2 (Cell signaling, Danvers, MA) was assessed as a loading control after p-ERK1/2 chemiluminescence detection using HRP-substrate purchased from Cell signaling.
2.7. Internalization assay and fluorescence microscopy
For the internalization assay, 293 cells stably expressing AKHR–EGFP were seeded in cover glass-bottomed six-well plates. After treatment with AKH peptides at 37 °C for 60 min, 293 cells were stained with the membrane probe DiI (Beyotime, Haimen, China) at 37 °C for 5–10 min, fixed with 2% paraformaldehyde for 15 min, and finally incubated with Hoechst 33258 (Beyotime) for cell nuclei staining for 10 min. The cells were mounted in mounting reagent (DTT/PBS/glycerol). Fluorescence microscopy was performed on a Zeiss LSM510 laser scanning confocal microscope attached to a Zeiss Axiovert 200 microscope using a Zeiss Plan-Apo 63 × 1.40 NA oil immersion lens.
2.8. Flow cytometry analysis
Cells (5 × 105) were washed with PBS supplemented with 0.5% BSA and incubated with 10 µg/ml FITC-labeled anti-Flag M2 monoclonal antibody (Sigma, St. Louis, MO) in a total volume of 100 µl. After incubating for 30 min at 4 °C, cells were fixed with 2% paraformaldehyde in PBS and subjected to flow cytometry analysis on a FACScan flow cytometer (Coulter EPICS Elite, Coolten Corp., Hialeah, FL).
2.9. Peptide synthesis
The AKH peptides (Table 1) were prepared by solid-phase synthesis using the Fmoc strategy on a 430A peptide synthesizer (Applied Biosystems, Foster City, CA) and a 9050 Pepsynthesizer Plus (Perceptive Biosystems, Cambridge, MA) and purified by preparative reverse-phase high-performance liquid chromatography using a Dynamax-300 Å C18 25 cm × 21.4 mm ID column with a flow rate of 9 ml/min and two solvent systems of 0.1% TFA/H2O and 0.1% TFA/acetonitrile.
3. Results
3.1. Expression and cellular localization of AKHR
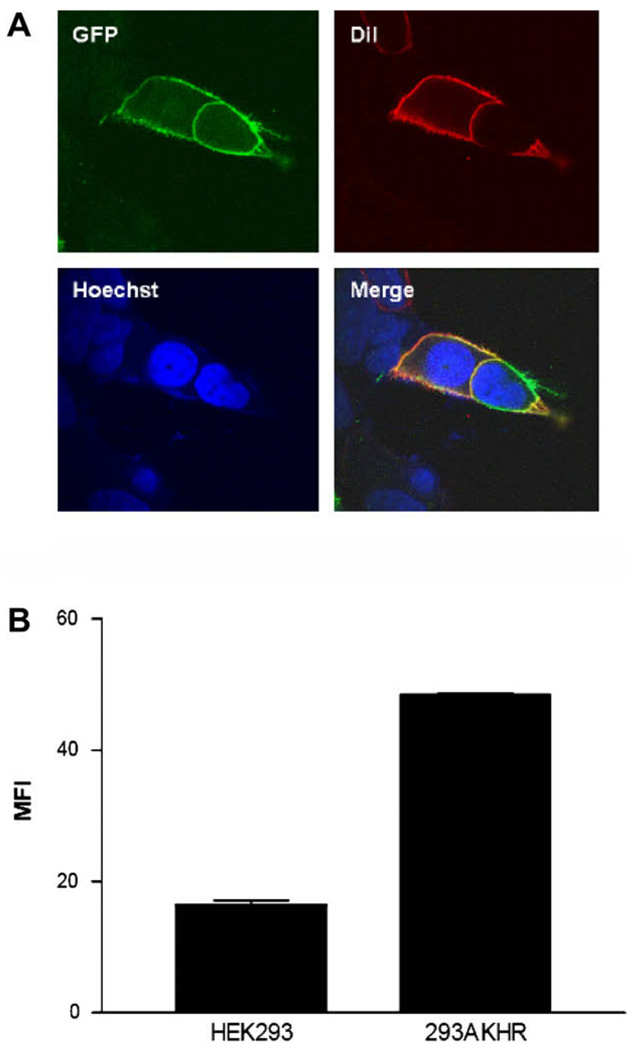
We cloned the adipokinetic hormone receptor (AKHR) cDNA from the fat body of pupae of the silkworm B. mori by RT-PCR, and constructed two vectors to express AKHR with either a Flag-tag at the N-terminus or enhanced green fluorescent protein (EGFP) at the C-terminus. After transfection of HEK293 cells with Flag-AKHR and AKHR–EGFP, stably expressing cells were selected by the addition of 800 µg/ml G418, and confirmed by FACS analysis and fluorescent microscopy (Fig. 1A and B). As shown in Fig. 1A, significant cell surface expression was detected by fluorescent microscopy with minimal intracellular accumulation in the absence of AKH1.
Fig. 1.
Expression of AKHR in stably transfected HEK293 cells. (A) HEK293 cells stably expressing AKHR–EGFP (GFP) were stained with a membrane plasma probe (DiI) and a nuclei probe (Hoechst 33258). (B) The cell surface expression of the stably transfected HEK293 cells was analyzed by FACS. Stable 293 cells were analyzed for cell surface expression of Flag-AKHR by flow cytometry using the anti-Flag mAb M2. Bars represent the mean fluorescence intensity for cells expressing Flag-AKHR. All data are shown as means ± S.E. from at least three independent experiments.
3.2. cAMP accumulation and intracellular calcium mobilization in AKHR expressing cells stimulated by AKH peptides
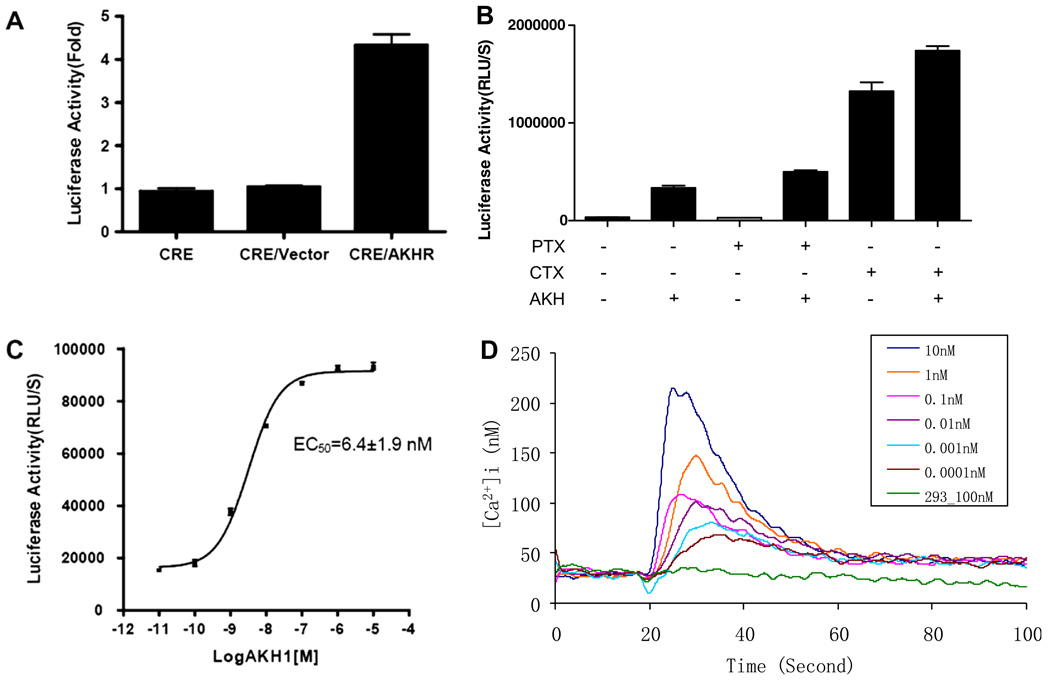
cAMP accumulation depends upon the coupling of AKHR to G proteins. To evaluate the role of AKHR in stimulating cAMP production, a stable cell line co-transfected with Flag-AKHR and pCRE-Luc was established. Upon stimulation with different concentrations of AKH1 peptides, the cAMP inside of the cells accumulated in a dose-dependent manner with an EC50 of 6.4 nM. As a control, no change in the cAMP level was detected in parental HEK293 cells (Fig. 2A and C). Pre-treatment with 100 ng/ml PTX was found to have no effect on cAMP generation in Flag-AKHR-expressing cells stimulated by AKH1, whereas stimulation with CTX led to a remarkable increase in the cellular levels of cAMP (Fig. 2B), suggesting that coupling of the Gs protein was involved in the AKHR signaling pathway in 293 cells.
Fig. 2.
AKH-induced cAMP accumulation and intracellular Ca2+ mobilization in HEK293 cells stably expressing Flag-AKHR. (A) cAMP accumulation in HEK293 cells transiently co-transfected with CRE-Luc and Flag-AKHR was determined in response to AKH1 treatment (2 µM). (B) Effects of PTX or CTX on AKH-mediated stimulation of cAMP accumulation in Flag-AKHR/CRE/HEK293 cells. HEK293 cells were transiently co-transfected with Flag-AKHR and CRE-Luc, and pre-treated with PTX (100 ng/ml) or CTX (300 ng/ml) overnight prior to incubation with AKH (2 µM) for 4 h. (C) cAMP accumulation in HEK293 cells stably expressing Flag-AKHR/CRE-Luc was assayed in response to different doses of AKH1. Data are expressed as the means ± S.E. (n = 3). (D) Intracellular Ca2+ influx in non-transfected 293 cells or 293 cells stably expressing Flag-AKHR was measured in response to different concentrations of AKH1 peptide using the fluorescent Ca2+ indicator fura-2. The figures are representative of more than three independent experiments.
Bombyx AKHR was further evaluated by an assay that is dependent upon ligand activation of the phospholipase C signaling pathway resulting in mobilization of intracellular Ca2+ from the ER pool to the cytoplasm. We then examined the effects of AKH1 peptides on the intracellular Ca2+ change in the AKHR-expressing cells using the calcium probe fura-2. As indicated in Fig. 2D, AKH1 peptides did not affect the Ca2+ fluxes in the parental HEK293 cells, but, in parallel, elicited a rapid increase of Ca2+ in the Flag-AKHR-expressing cells in a dose-dependent manner, as demonstrated by previous reports [15,19].
3.3. AKH1 mediates activation of MAPK pathway in AKHR-expressing cells
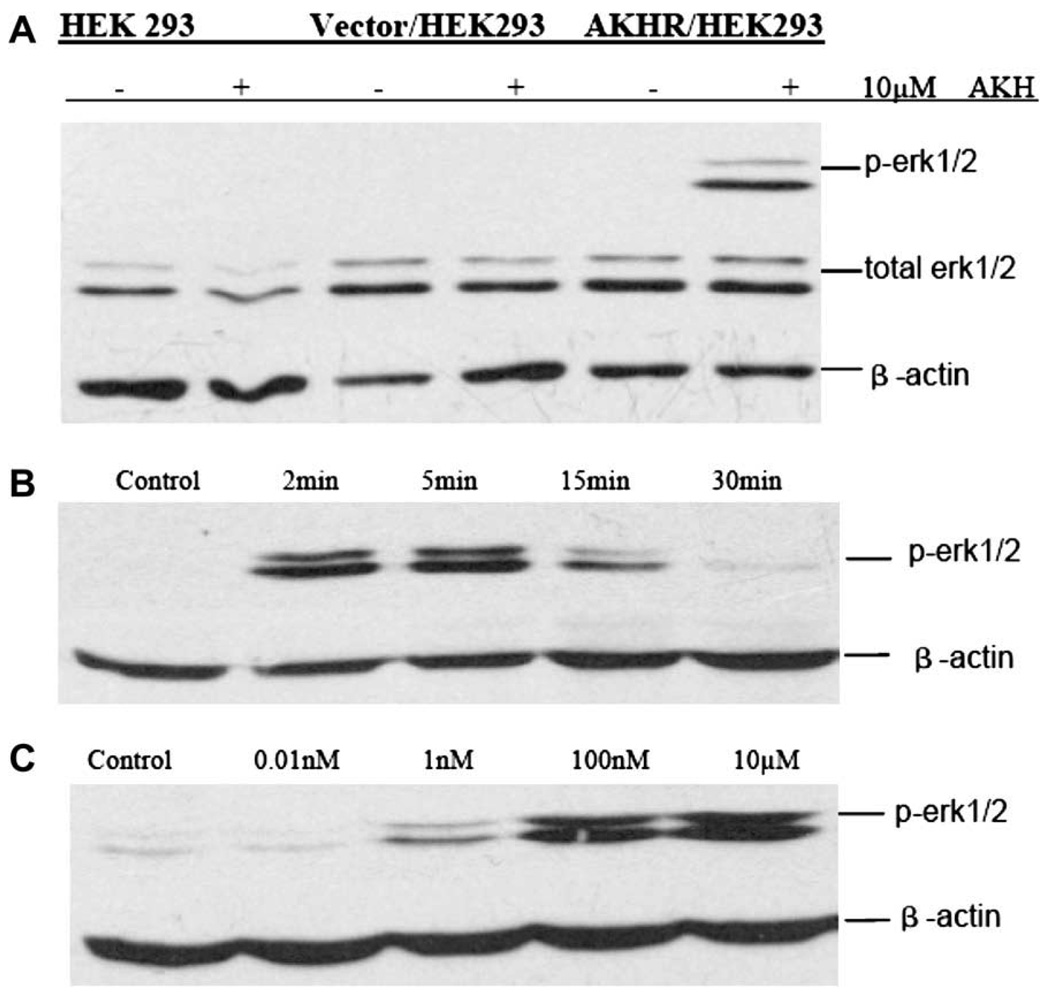
To investigate whether the activation of AKHR in stably transfected cells stimulates the phosphorylation of ERK1/2, the cells seeded in six-well plates were treated with AKH1 peptides, and assessed using a phospho-specific antibody known to bind only to the phosphorylated and activated forms of these kinases [20]. Fig. 3 shows that activation of AKHR elicited transient phosphorylation kinetics of ERK1/2 with maximal phosphorylation evident at 2–5 min and a return to almost basal levels by 15 min. By contrast, treatment with AKH1 did not provoke any appreciable effects on ERK1/2 in the parental 293 cells or transiently mock-transfected 293 cells. Fig. 3C illustrates the concentration dependence of AKH-mediated ERK1/2 phosphorylation and activation, with ERK1 and -2 phosphorylation increased significantly by nanomolar concentrations of AKH1 and a maximal ERK1/2 phosphorylation of at least three times the basal level.
Fig. 3.
Activation of ERK1/2 by AKH1. (A) AKH1 induce pERK1/2 only in transfected cells, not in controls of the experiment. (B) Time course of AKH-stimulated phosphorylation of ERK1/2 in stable AKHR-expressing HEK293 cells, cells were incubated with 10 µM AKH1 for the indicated times. (C) Concentration-dependent activation of ERK1/2 phosphorylation by AKH1 in HEK293 cells stably expressing Flag-AKHR. Cellular lysates were immunoblotted with phospho-specific (top lane) and non-specific (bottom lane) anti-ERK1/2 antibody, as described in Section 2. The results are representative of at least three independent experiments.
3.4. Rapid internalization of AKH receptors upon activation by AKH1
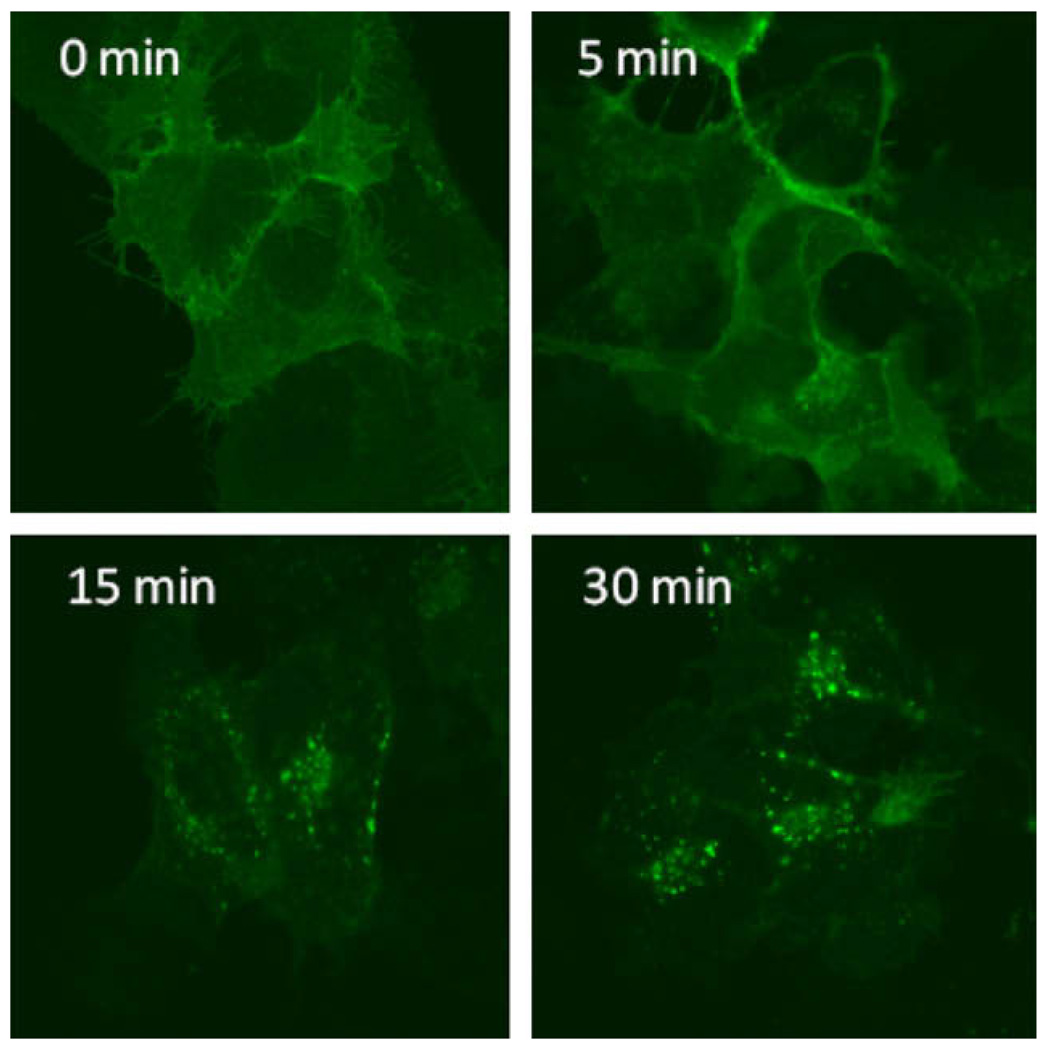
In order to visualize the internalization and trafficking of AKHR, we constructed a vector to express a chimeric protein in which enhanced green fluorescent protein (EGFP) is fused to the C-terminal end of AKHR (AKHR–EGFP) and established a stable HEK293 cell line expressing AKHR–EGFP. Observation of stable AKHR–EGFP-expressing HEK293 cells with fluorescence microscopy revealed that the fluorescence of AKHR–EGFP was mainly localized in the plasma membrane, and to a lesser extent in intracellular vesicles. Upon activation of AKHR–EGFP with ligand, the receptor was rapidly and dramatically redistributed in the cytoplasm with distinct perinuclear accumulation. The internalization of AKHR–EGFP was detectable 5 min after AKH1 stimulation, and reached a maximum within 30 min (Fig. 4).
Fig. 4.
Time course of AKHR–EGFP internalization induced by AKH1. Cells were incubated with 10 nM AKH at 37 °C for the indicated times, and after washing fixing, were examined by fluorescence microscopy as described in Section 2. The results are representative of three independent experiments.
3.5. Functional comparison of newly cloned AKH2 and AKH3 peptides with AKH1
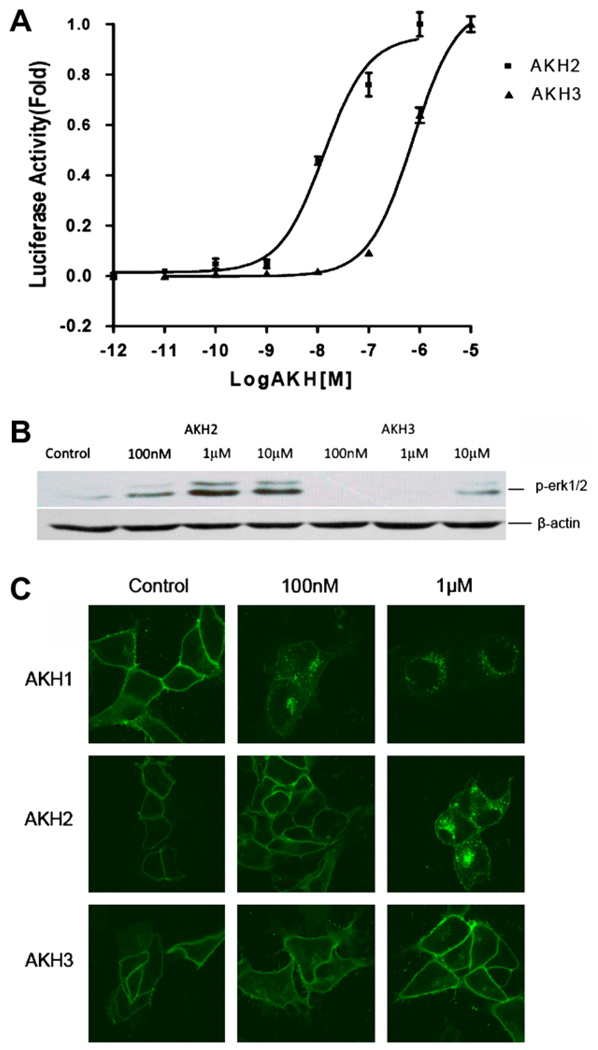
In Bombyx, recently another two distinct cDNAs encoding the prepro-AKH2, and 3 have been cloned [11], but never be characterized. We synthesized Bombyx AKH2 and AKH3 peptides (Table 1) and determine their functional activities on AKHR. As shown in Fig. 5, although AKH2 exhibited high activity in inducing cAMP accumulation (EC50 = 11.7 ± 1.6 nM) (Fig. 5A) comparable to AKH1 (EC50 = 6.4 ± 1.9 nM), showed lower activities in ERK1/2 phosphorylation (Fig. 5B) and receptor internalization (Fig. 5C) than that of AKH1. AKH3 did also activated AKHR in cAMP accumulation (EC50 = 1.07 ± 0.33 µM) with a much lower potency than that of AKH1 and AKH2 (Fig. 5A), but showed no activities on AKHR in ERK1/2 phosphorylation and receptor internalization (Fig. 5B and C).
Fig. 5.
Signaling activities and receptor internalization induced by synthesized AKH2 and AKH3 peptides. (A) cAMP accumulation in HEK293 cells stably expressing Flag-AKHR was assayed in response to different doses of AKH2 and AKH3. (B) Activation of ERK1/2 phosphorylation by different doses of AKH2 and AKH3 in HEK293 cells stably expressing AKHR was assayed as described in Section 2. (C) Induction of AKHR–EGFP internalization by AKH2 and AKH3. The stably Flag-AKHR-transfected 293 cells were treated with indicated concentrations of peptide for 45 min at 37 °C. The results are representative of at least three independent experiments.
4. Discussion
The signaling of AKH peptides has been studied in a few insects, but only in selected pathways such as cAMP and Ca2+ [19]. To date, most of our knowledge on signal transduction of the AKH peptide is derived from biological assays and use of purified fat cell membranes [21,22]. In this study, we molecularly and functionally characterized the signaling pathway of Bombyx AKHR, and demonstrated that activation of Bombyx AKHR not only led to cAMP accumulation and transient intracellular Ca2+ influxes in stable HEK293 cells, consistent with previous reports using biological assays and purified fat cell membranes, but also elicited transient activation of ERK1/2 pathway.
ERK1/2 has emerged as important effectors for GPCRs, and can be used to measure the functional outcome of receptor stimulation [23,24]. Therefore, characterization of the signaling pathways that stimulate ERK1/2 phosphorylation through a particular receptor is essential to understand its role in physiology and pathology. The ability of the Bom-AKHR to activate the phosphorylation of ERK1/2 in HEK293 cells and the determination of the G proteins’ responsibilities for coupling the AKHR to MAPK activation are examined in Fig. 3A. As shown in Fig. 3B and C, AKH induced a time- and concentration-dependent increase in MAPK activity. AKH-stimulated MAPK activity was maximal at 5 min of stimulation and the activity persisted for about 15 min. ERK1/2 phosphorylation have been reported to be involved in the regulation of lipid metabolism including regulation of contraction-induced activation of muscle hormone sensitive lipase (HSL) [25] and regulation of contraction-induced acetyl-CoA carboxylase phosphorylation and subsequent long-chain FA oxidation in mammalian cells [26,27]. Further investigations will be required to define the role of ERK1/2 in the transduction of the hyperlipaemic signal in insects.
The green fluorescent protein (GFP) has been widely used to study the localization, distribution, and function of other proteins by fusion expression in different systems. In this study, an expression vector of Bombyx AKHR fused with EGFP at its C-terminus was constructed and expressed stably in HEK293 cells for easy visualization of receptor localization, internalization and trafficking. Compared to the wild-type AKHR, AKHR–EGFP was found to be expressed and function normally. We showed for the first time that, upon binding and activation by AKH peptide, AKHR was rapidly internalized in a dose- and time-dependent manner. Further investigation of receptor trafficking and recycling is under way in our lab.
In Bombyx, a non-apeptide AKH1 has been first identified [10], and quite recently Roller et al. identified another two distinct cDNAs encoding the prepro-Bombyx AKH2, and 3. Bombyx AKH1 is identical to non-apeptides found only in moths, while Bombyx AKH2 is closely related to many other AKH/HrTH decapeptides. Although previous studies indicated that the Heliothis zea hypertrehalosaemic hormone (Hez-HrTH) activated Bombyx AKHR with a higher affinity than that of AKH1 [12], in our research, we demonstrated that AKH2 exhibit comparable activities in intracellular cAMP accumulation to AKH1, but much lower activities in phosphorylation of ERK1/2 and receptor internalization than that of AKH1. In structure, Bombyx AKH2 is closely related to Hez-HrTH, but with three amino acids different, these three amino acids may be responsible for the differences of activation on Bombyx AKHR between Bombyx AKH2 and Hez-HrTH. Bombyx AKH3 is more closely related to non-lepidopteran AKH peptides, and was much less effective in activating Bombyx AKHR we tested in this study, strongly implying that it is more likely that a second intrinsic AKHR exists as a high affinity receptor for AKH3 in Bombyx. The identification of AKHR-mediated signaling pathways is of importance to obtain a better understanding of the role of AKH/AKHR in the regulation of the molecular events responsible for sugar homeostasis and energy mobilization.
Acknowledgements
We thank Dr. Kerong He for providing silkworm larvae and pupae, thank Ms. Yanyan Gu for cloning of AKHR cDNA and the investigators mentioned in Section 2 for their generous gifts or reagents. We thank Dr. Ji-Ming Wang for critical reading of the manuscript. This work was supported in part by grants from the National Natural Science Foundation of China (30670425).
Abbreviations
- GPCR
G protein-coupled receptor
- AKH
adipokinetic hormone
- Hez-HrTH
Heliothis zea hypertrehalosaemic hormone
- MAPK
mitogen-activated protein kinase
- ERK1/2
extracellular signal-regulated kinase1/2
- PTX
pertussis toxin
- CTX
cholera toxin
References
- 1.Schooneveld H, Tesser GI, Veenstra JA, Romberg-Privee HM. Adipokinetic hormone and AKH-like peptide demonstrated in the corpora cardiaca and nervous system of Locusta migratoria by immunocytochemistry. Cell Tissue Res. 1983;230:67–76. doi: 10.1007/BF00216028. [DOI] [PubMed] [Google Scholar]
- 2.Schaffer MH, Noyes BE, Slaughter CA, Thorne GC, Gaskell SJ. The fruitfly Drosophila melanogaster contains a novel charged adipokinetic-hormone-family peptide. Biochem. J. 1990;269:315–320. doi: 10.1042/bj2690315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gade G. A unique charged tyrosine-containing member of the adipokinetic hormone/red-pigment-concentrating hormone peptide family isolated and sequenced from two beetle species. Biochem. J. 1991;275(Pt 3):671–677. doi: 10.1042/bj2750671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oudejans RC, Kooiman FP, Heerma W, Versluis C, Slotboom AJ, Beenakkers MT. Isolation and structure elucidation of a novel adipokinetic hormone (Lom-AKH-III) from the glandular lobes of the corpus cardiacum of the migratory locust, Locusta migratoria. Eur. J. Biochem. 1991;195:351–359. doi: 10.1111/j.1432-1033.1991.tb15713.x. [DOI] [PubMed] [Google Scholar]
- 5.Gade G, Janssens MP, Kellner R. A novel peptide in the AKH/RPCH family isolated from the corpora cardiaca of the Emperor dragonfly, Anax imperator. Peptides. 1994;15:1–6. doi: 10.1016/0196-9781(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 6.Gade G, Marco HG, Simek P, Marais E. The newly discovered insect order Mantophasmatodea contains a novel member of the adipokinetic hormone family of peptides. Biochem. Biophys. Res. Commun. 2005;330:598–603. doi: 10.1016/j.bbrc.2005.02.185. [DOI] [PubMed] [Google Scholar]
- 7.Gade G, Simek P, Clark KD, Auerswald L. Unique translational modification of an invertebrate neuropeptide: a phosphorylated member of the adipokinetic hormone peptide family. Biochem. J. 2006;393:705–713. doi: 10.1042/BJ20050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- 9.Oda Y, Uejuma M, Iwami M, Sakurai S. Involvement of adipokinetic hormone in the homeostatic control of hemolymph trehalose concentration in the larvae of Bombyx mori. Arch. Insect Biochem. Physiol. 2000;45:156–165. doi: 10.1002/1520-6327(200012)45:4<156::AID-ARCH3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi J, Kataoka H, Nagasawa H, Isogai A, Suzuki A. Isolation and identification of adipokinetic hormone of the silkworm, Bombyx mori. Biosci. Biotech. Biochem. 1992;56:66–70. [Google Scholar]
- 11.Roller L, Yamanaka N, Watanabe K, Daubnerova I, Zitnan D, Kataoka H, Tanaka Y. The unique evolution of neuropeptide genes in the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2008;38:1147–1157. doi: 10.1016/j.ibmb.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Staubli F, Jorgensen TJ, Cazzamali G, Williamson M, Lenz C, Sondergaard L, Roepstorff P, Grimmelikhuijzen CJ. Molecular identification of the insect adipokinetic hormone receptors. Proc. Natl. Acad. Sci. USA. 2002;99:3446–3451. doi: 10.1073/pnas.052556499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen KK, Hauser F, Cazzamali G, Williamson M, Grimmelikhuijzen CJ. Cloning and characterization of the adipokinetic hormone receptor from the cockroach Periplaneta americana. Biochem. Biophys. Res. Commun. 2006;343:638–643. doi: 10.1016/j.bbrc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Belmont M, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJ. Identification of four evolutionarily related G protein-coupled receptors from the malaria mosquito Anopheles gambiae. Biochem. Biophys. Res. Commun. 2006;344:160–165. doi: 10.1016/j.bbrc.2006.03.117. [DOI] [PubMed] [Google Scholar]
- 15.Arrese EL, Flowers MT, Gazard JL, Wells MA. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J. Lipid Res. 1999;40:556–564. [PubMed] [Google Scholar]
- 16.Vroemen SF, Van Marrewijk WJ, Van der Horst DJ. Stimulation of glycogenolysis by three locust adipokinetic hormones involves Gs and cAMP. Mol. Cell. Endocrinol. 1995;107:165–171. doi: 10.1016/0303-7207(94)03438-y. [DOI] [PubMed] [Google Scholar]
- 17.Vroemen SF, Van der Horst DJ, Van Marrewijk WJ. New insights into adipokinetic hormone signaling. Mol. Cell. Endocrinol. 1998;141:7–12. doi: 10.1016/s0303-7207(98)00079-3. [DOI] [PubMed] [Google Scholar]
- 18.Iwata K, Luo J, Penn RB, Benovic JL. Bimodal regulation of the human H1 histamine receptor by G protein-coupled receptor kinase 2. J. Biol. Chem. 2005;280:2197–2204. doi: 10.1074/jbc.M408834200. [DOI] [PubMed] [Google Scholar]
- 19.Gade G, Auerswald L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen. Comp. Endocrinol. 2003;132:10–20. doi: 10.1016/s0016-6480(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, et al. MAP kinases. Chem. Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 21.Gäde G. The Explosion of Structural Information on Insect Neuropeptides. Wien: Springer; 1997. [PubMed] [Google Scholar]
- 22.Ziegler R, Jasensky RD, Morimoto H. Characterization of the adipokinetic hormone receptor form the fat body of Manduca sexta. Regul. Pept. 1995;57:329–338. doi: 10.1016/0167-0115(95)00046-e. [DOI] [PubMed] [Google Scholar]
- 23.Pierce KL, Luttrell LM, Lefkowitz RJ. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene. 2001;20:1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- 24.Werry TD, Sexton PM, Christopoulos A. “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol. Metab. 2005;16:26–33. doi: 10.1016/j.tem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Donsmark M, Langfort J, Holm C, Ploug T, Galbo H. Contractions activate hormone-sensitive lipase in rat muscle by protein kinase C and mitogen-activated protein kinase. J. Physiol. 2003;550:845–854. doi: 10.1113/jphysiol.2003.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raney MA, Yee AJ, Todd MK, Turcotte LP. AMPK activation is not critical in the regulation of muscle FA uptake and oxidation during low-intensity muscle contraction. Am. J. Physiol. Endocrinol. Metab. 2005;288:E592–E598. doi: 10.1152/ajpendo.00301.2004. [DOI] [PubMed] [Google Scholar]
- 27.Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J. Appl. Physiol. 2001;91:1017–1028. doi: 10.1152/jappl.2001.91.3.1017. [DOI] [PubMed] [Google Scholar]