Abstract
Background
In most tuberculosis (TB) endemic countries, bacillus Calmette Guérin (BCG) is usually given around birth to prevent severe TB in infants. The neonatal immune system is immature. Our hypothesis was that delaying BCG vaccination from birth to 10 weeks of age would enhance the vaccine-induced immune response.
Methods
In a randomized clinical trial, BCG was administered intradermally either at birth (n=25) or at 10 weeks of age (n=21). Ten weeks after vaccination, and at 1 year of age, vaccine-specific CD4 and CD8 T cell responses were measured with a whole blood intracellular cytokine assay.
Results
Infants who received delayed BCG vaccination demonstrated higher frequencies of BCG-specific CD4 T cells, particularly polyfunctional T cells co-expressing IFN-γ, TNF-α and IL-2, and most strikingly at 1 year of age.
Conclusions
Delaying BCG vaccination from birth to 10 weeks of age enhances the quantitative and qualitative BCG-specific T cell response, when measured at one year of age.
Keywords: BCG, vaccination, birth, delayed, polyfunctional CD4 T cells
Introduction
Bacillus Calmette Guérin (BCG), the only current tuberculosis (TB) vaccine, is a live attenuated strain of Mycobacterium bovis. Worldwide, more than 100 million children receive BCG each year [1]. BCG vaccination confers protection against severe forms of childhood TB, i.e. TB meningitis and miliary TB [1, 2]. The protection afforded by BCG vaccination against pulmonary TB, the most common form of TB, is variable and mostly poor [3]. Factors that have been implicated in variable protection of BCG against TB include the BCG strain, BCG dose, prior exposure to environmental nontuberculous mycobacteria (NTM), host genetic variations, Mycobacterium tuberculosis (M.tb) strain, and vaccination route [3–7].
CD4 T cells that produce the T-helper type 1 (Th1) cytokines IFN-γ, TNF-α and IL-2 are thought to be critical for protection against TB [8, 9]. This kind of Th1 response is characteristic following BCG vaccination [10, 11]. In areas where TB is highly prevalent, BCG vaccination is widely administered at or soon after birth because of the risk of early exposure to M.tb [12]. Evidence exists that the Th1 response at birth is “immature”, for example, progressively increasing specific Th1 cell-mediated immunity has been shown when measles vaccine was given at 6, 9 and 12 months of age, respectively [13]. We proposed that allowing maturation of the neonatal immune system to 10 weeks of age prior to BCG vaccination would enhance vaccination-induced T-cell immunity. The effect of optimizing vaccination timing could have important implications for ultimately improving protection against TB disease through BCG vaccination.
Marchant, et al. reported that varying the age of BCG vaccination did not affect BCG immunogenicity when assessing interferon gamma (IFN-γ) production by peripheral blood mononuclear cells (PBMC) stimulated with mycobacterial antigens [10]. In a small study, Hussey, et al. also reported that a delay in BCG vaccination from birth to 10 weeks of age did not influence IFN-γ secretion, proliferative responses, or cytotoxic potential of BCG-specific T cells, following incubation of PBMC with mycobacterial antigens [14]. Since these results have emerged, we have shown that measurement of IFN-γ alone underestimates the complexity of the BCG-induced Th1 response. BCG vaccination in infants induces multiple Th1 subsets, defined by expression of different combinations of IFN-γ, TNF-α and IL-2 by specific cells [11]. We wished to comprehensively address the effect of age on the magnitude and quality of Th1 immunity induced by BCG in infants.
Materials and Methods
Participant enrolment and follow-up
Participants were enrolled between April 2006 and March 2008 in Khayelitsha, a suburb of Cape Town with an extraordinarily high TB incidence, reported to be 1,614 per 100,000 in the first quarter of 2008 (Case notification rate, City of Cape Town).
This formed part of a larger study investigating clinical and immunological characteristics of HIV-exposed and unexposed infants. In brief, pregnant women were approached at a public antenatal clinic for enrolment of their infants. For the study reported here, infants who were born to HIV-infected women or women with unknown HIV status, exposed to active TB in the household, born prematurely (≤36 weeks gestational age) or had low birth weight (≤2.5kg), or who had significant perinatal complications were excluded. In addition, all infants with a positive IFN-γ response to ESAT-6/CFP-10 at 10 weeks of age were excluded (see below for assay details). Infants were randomly assigned during antenatal recruitment to receive BCG (intradermal Danish strain 1331, Statens Serum Institute) on the first day of life (“birth vaccination”), or the identical BCG vaccination at 10 weeks of age (“delayed vaccination”). Infants were followed at 10 weeks, 20 weeks and at 50 weeks of age. A window period of −2 to + 4 weeks around the week 10 visit was allowed (e.g. 8 to 14 weeks). The time period for the 20-week visit ranged from 18 to 28 weeks, and 41 to 54 weeks for the 50-week visit.
Regulatory approval was obtained from the research ethics committees of Stellenbosch University and the University of Cape Town. Written informed consent was obtained from all mothers in their home language. All HIV testing was completed in conjunction with informed consent and pre-and post-test counselling.
Blood collection and intracellular cytokine assay
One mL of blood was collected at 10, 20, and 50 weeks of age in heparinized syringes. Whole blood was processed within 2 hours of collection, as previously described [15]. Briefly, 250µL whole blood was incubated for 12 hours at 37°C with viable BCG (reconstituted from the vaccine vial, Danish strain, Staten Serum Institute, 1.2 × 106 CFU/mL) and the co-stimulatory antibodies anti-CD28 and anti-CD49d (BD Biosciences, 0.5µg/mL each). 250µL blood incubated with Staphylococcal enterotoxin B (SEB, Sigma, 10µg/mL) and 250µL incubated with the co-stimulatory antibodies alone (unstimulated) served as positive and negative controls, respectively. Brefeldin A (Sigma, 10µg/mL) was added for the last 5 hours of incubation. Following incubation, red blood cells were lysed and white cells fixed with BD FACS Lysing Solution (BD Biosciences), and the cells cryopreserved.
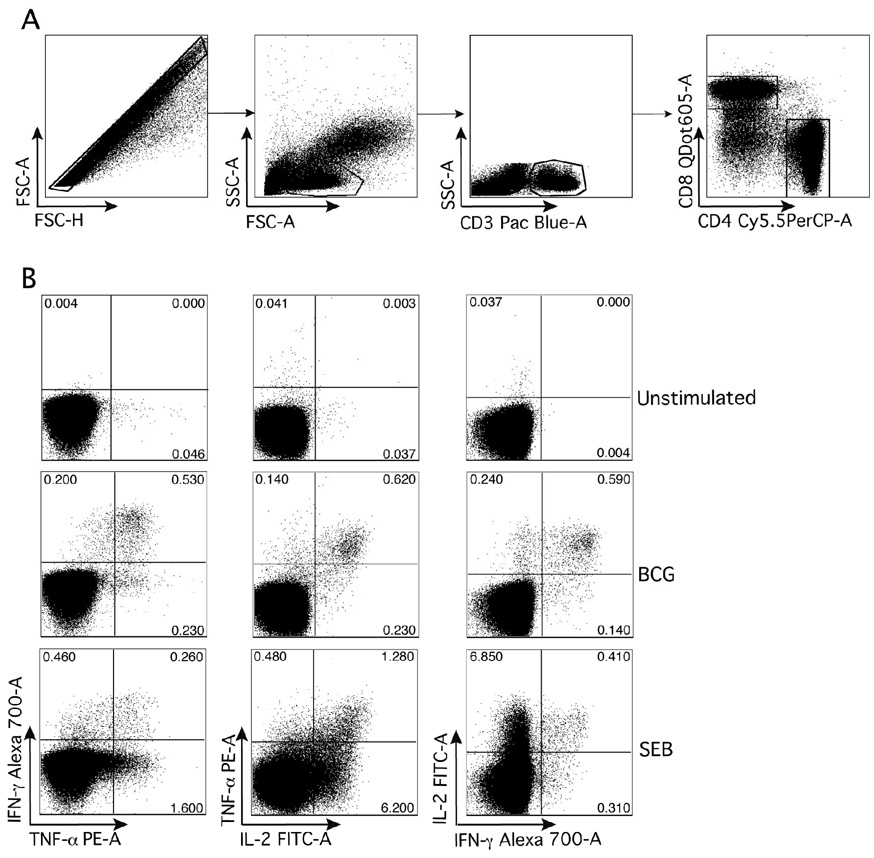
Cryopreserved cells were later thawed and washed in 1% bovine serum albumin (Sigma) in phosphate buffered saline (PBS, BioWhittaker), and permeabilised using Perm/Wash Solution (BD Biosciences). Cells were then stained with the following antibodies: anti-CD3 Pacific Blue (clone UCHT1), anti-CD4 PerCPCy5.5 (SK3), anti-CD45RA PECy7 (L48), anti-IFN-γ AlexaFluor700 (B27), anti-IL-2 FITC (5344.111), anti-TNF-α PE (MAb11; all from BD Biosciences), anti-CD8 Qdot605 (3B5, Invitrogen) and anti-CCR7 APC (150503, R&D Systems). After washes, cells were acquired on a LSR II flow cytometer (BD Biosciences), for this experiment configured with 3 lasers and 10 detectors, using FACS Diva 6.1 software. Compensation settings were set using anti-mouse kappa-beads (BD Biosciences), labelled with the respective fluorochrome-conjugated antibodies. Flowjo 8.7.1 (Treestar) was used to compensate and to analyze the flow cytometric data. Boolean gating was applied to generate combinations of cytokine expressing CD4 and CD8 T cell subsets (Figure 2B).
Figure 2. Gating strategy used to analyze CD4 and CD8 T cell cytokine responses.
Whole blood was incubated with BCG to complete a flow cytometric intracellular cytokine assay, as described in the Methods.
(A) Doublet cells were excluded by gating on Forward Scatter–Area (FSC-A) against Forward Scatter-Height (FSC-H), and lymphocytes were then selected in a FSC-A against Side Scatter-Area (SSC-A) dots plot. T cells were selected by gating on CD3+ events, which were further differentiated into CD4 and CD8 T cells. (B) Representative dot plots of cytokine co-expression patterns in CD4 T cells from unstimulated, BCG and SEB-stimulated conditions.
Assay of intercurrent M. tuberculosis infection
At each time point, whole blood was diluted 1:5 in RPMI-1640 tissue culture medium (Sigma), containing 1% L-glutamine (Sigma), and incubated with ESAT-6/CFP-10 fusion protein (provided by Tom Ottenhoff, Leiden University Medical Centre, 10µg/mL), phytohemagglutinin (PHA, Sigma, 5µg/mL) and SEB (1µg/mL; both positive controls), or no antigen (negative control), in triplicate, in 96-well plates (modified from Black et al) [16]. Plates were incubated at 37°C with 5% CO2 for 7 days, supernatants were harvested, triplicate wells pooled and stored at −80°C. Later, supernatants were thawed, and an IFN-γ sandwich ELISA was used to quantify IFN-γ in the supernatants derived from the 7-day whole blood assay, (BD Pharmingen). A positive IFN-γ response was defined as 62pg/mL, after background subtraction (twice the assay detection limit of 31pg/mL). Infants with evidence of intercurrent M. tuberculosis infection/exposure at 10, 20 or 50 weeks of age, were excluded from analysis.
Data analysis
For the intracellular cytokine assay, cytokine expression levels from unstimulated blood were subtracted from levels obtained after BCG stimulation. (The median level of expression of IFN-γ, IL-2 and TNF among CD4 T cells in unstimulated blood was 0.002%, 0.03% and 0.06%, respectively.) Differences in participant birth weights and gender were assessed using parametric tests. The Mann-Whitney U test was used to assess differences in frequencies of cytokine expressing CD4 and CD8 T cells between the 2 groups. P-values lower than 0.05 were considered to be significant.
Results
Participant characteristics
A total of 46 infants were enrolled into this study: 25 in the birth group and 21 in the delayed vaccination group. Infants not present for phlebotomy at a certain time point, bled beyond the defined window period at week 10 visit, or positive for M.tb infection were excluded from analysis (Figure 1). Results from infants with M.tb infection were excluded from the time point at which this was diagnosed, and from subsequent time points. At baseline, no differences were observed in birth weights or gender between the birth and delayed vaccination groups (mean birth weight, (SD): 3306g (435.6) birth vs. 3200g (371.9) delayed; p=0.390). Sixteen (64%) infants in the birth group were male compared with 9 (43%) in the delayed birth group (p=0.235).
Figure 1. Overview of eligibility, study flow and participants.
Detection of BCG-specific CD4 T cells using viable BCG as antigen
Before comparing BCG-induced immunity between infants who received birth and delayed vaccination, we evaluated whether incubation of whole blood with viable BCG might result in non-specific T cell activation, or whether a vaccination-specific response would be detectable. We compared CD4 T cell immunity, measured at 10 weeks of age with an intracellular cytokine detection assay (Figure 2: example of assay outcomes), between participants who had received BCG at birth and those who had not yet received BCG (infants in the delayed group). Cytokine production by CD4 T cells was readily detectable in infants from the birth-vaccinated arm, but was not detectable, or detectable at very low levels only, in infants who had not received the vaccine at birth (Figure 3). The birth-vaccinated infants had high frequencies of BCG-specific polyfunctional CD4 T cells, i.e., CD4 T cells that express IFN-γ, TNF-α and IL-2 together, and of CD4 T cells expressing other combinations of Th1 cytokines. Polyfunctional BCG-specific CD4 T cells were absent in infants in the delayed vaccination group. All infants had a positive response to the positive control, SEB (data not shown). We concluded that T cell responses measured by our assay system when BCG is used as antigen, are antigen specific and not due to non-specific T cell activation.
Figure 3. Comparison of CD4 T cell responses following incubation of whole blood from BCG-vaccinated and unvaccinated infants’ blood with BCG, at 10 weeks of age.
Frequencies of cytokine-expressing CD4 T cells detected by a whole blood intracellular cytokine assay (see Methods) are shown. The median is represented by the horizontal line, the interquartile range by the box, and the range by the whiskers. The Mann-Whitney U test was used to calculate p values of differences between the 2 groups.
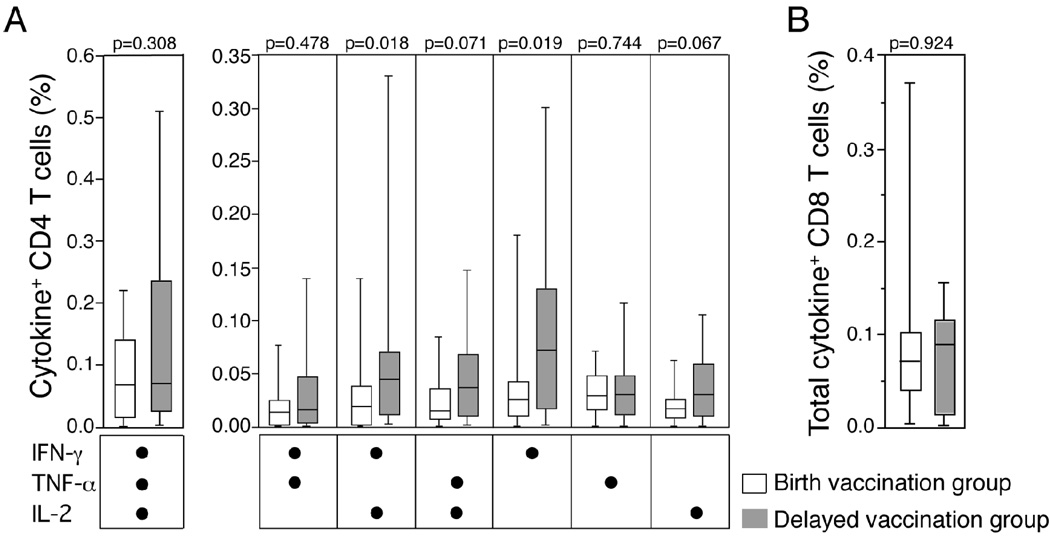
Delayed vaccination resulted in moderately higher frequencies of BCG-specific CD4 T cells 10 weeks post-vaccination
To assess differences in CD4 and CD8 T cell responses when the vaccine was given at birth or at 10 weeks at age, we measured the Th1-cytokine expression pattern induced by BCG 10 weeks after vaccination, i.e., at 10 weeks of age in the birth group and at 20 weeks of age in the delayed group. In both groups, high frequencies of polyfunctional CD4 T cells, or cells expressing other combinations of the Th1 cytokines, were observed (Figure 4A). The IFN-γ+ and IFN-γ+IL-2+ BCG-specific CD4 T cells subset frequencies were significantly higher in the delayed-vaccination group, compared with the birth-vaccination group (Figure 4A). As the frequencies of induced specific CD8 T cell subsets were very low, in both vaccination groups, total BCG-specific CD8 T cell responses only were evaluated: there was no difference between the two groups (Figure 4B). We concluded that delaying BCG vaccination by 10 weeks might result in an increased frequency of BCG-specific CD4 T cells, 10 weeks after vaccination.
Figure 4. BCG-specific T cell responses 10 weeks post-vaccination, in infants who received BCG at birth and those who received BCG at 10 weeks of age.
(A) Frequencies of BCG-specific cytokine expressing CD4 T cells are shown, as detected by the whole blood intracellular cytokine assay. (B) Frequencies of all expressing CD8 T cells, evaluated together, after incubation of whole blood with BCG. The median is represented by the horizontal line, the interquartile range by the box, and the range by the whiskers. The Mann-Whitney U test was used to calculate p values of differences between the 2 groups.
Delayed vaccination increased frequencies of polyfunctional BCG-specific CD4 T cells at one year of age
We also compared the BCG-specific memory response at 50 weeks of age. We observed significantly higher frequencies of BCG-specific polyfunctional IFN-γ+TNF-α+IL-2+ CD4 T cells in the delayed vaccination group, compared with the birth vaccination group (Figure 5). Frequencies of specific T cell subsets co-expressing TNF-α and IL-2, TNF-α and IFN-γ, or TNF-α alone, were also higher in the delayed vaccination group. At this age, total BCG-specific CD8 T cell responses were too low for reliable data analysis (data not shown). No difference between the 2 groups were observed when responses to the positive control, SEB, were compared (Supplementary Figure 1). We concluded that delaying BCG vaccination from birth to 10 weeks of age results in a quantitatively increased and qualitatively more optimal BCG-specific CD4 T cell memory response at one year of age.
Figure 5. BCG-specific CD4 T cell responses in the 2 groups of infants at 1 year of age.
Frequencies of cytokine-expressing CD4 T cells detected by a whole blood intracellular cytokine assay are shown. The median is represented by the horizontal line, the interquartile range by the box, and the range by the whiskers. The Mann-Whitney U test was used to calculate p values of differences between the 2 groups.
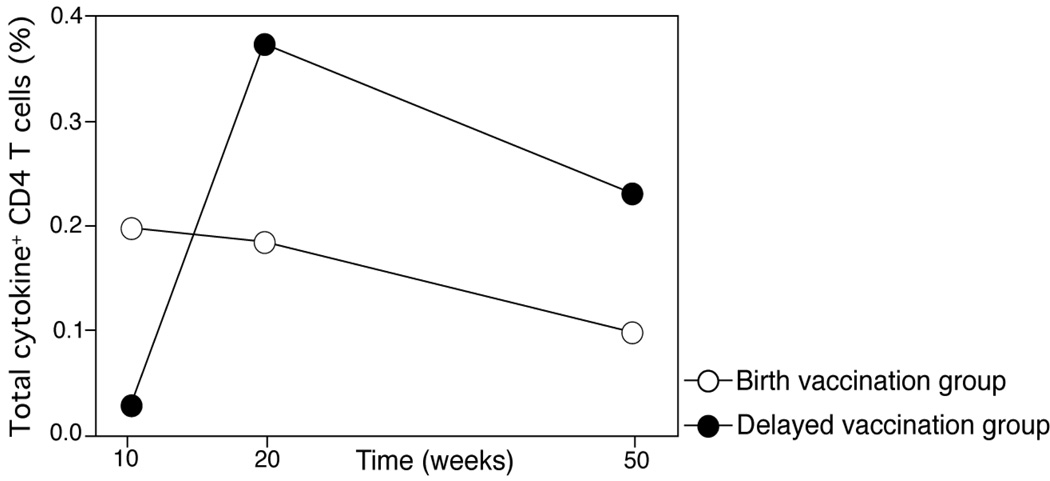
The total BCG-specific CD4 T cell response peaks at 10 weeks post-vaccination in the birth and delayed vaccination groups
To assess whether the BCG-specific CD4 T cell responses follow a similar kinetic pattern if vaccination is given at birth or at 10 weeks, we measured the total BCG-specific CD4 T cell memory response at 10, 20 and 50 weeks of age. Total responses are denoted by BCG-specific CD4 T cells that express IFN-γ, TNF-α, or IL-2, alone or in combination. In the birth and delayed vaccination groups, BCG-specific CD4 responses peaked 10 weeks post vaccination, and diminished gradually over the first year of life (Figure 6).
Figure 6. Kinetics of total BCG-specific CD4 T cells responses in the delayed and birth vaccination groups over one year.
Median frequencies of BCG-specific total cytokine-expressing CD4 T cells detected by a short term whole blood intracellular cytokine assay at 10, 20 and 50 weeks of age.
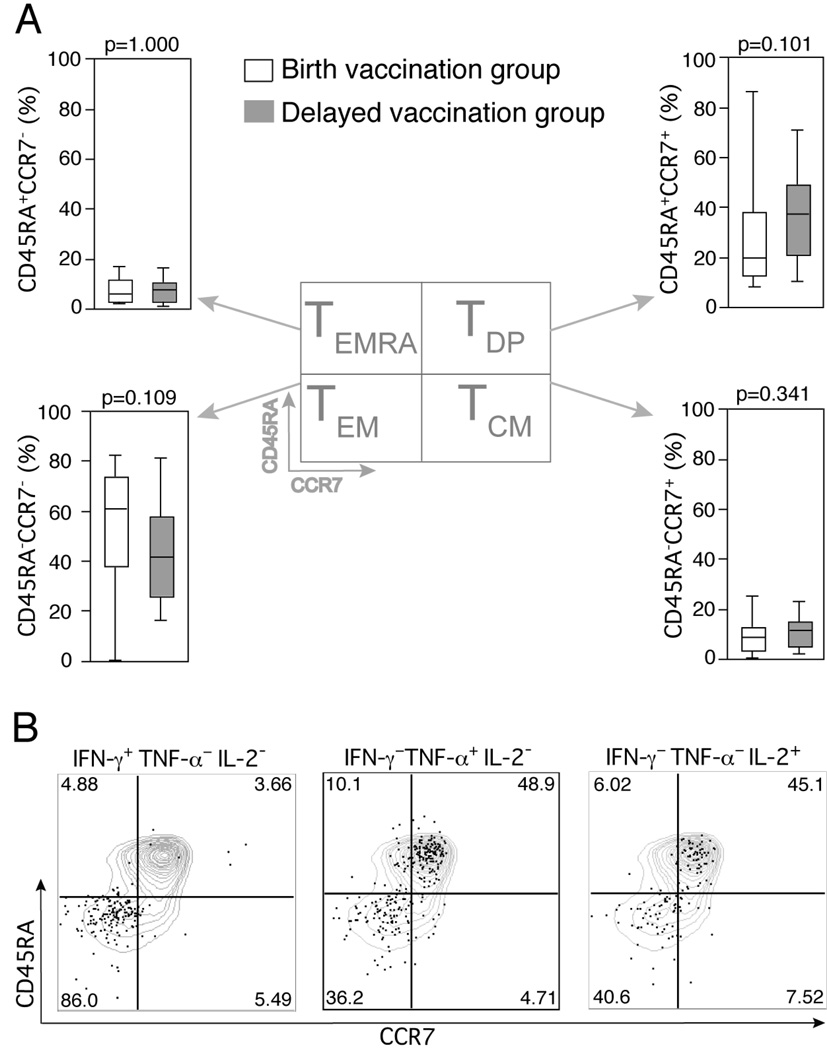
Comparable BCG-specific CD4 T cell memory phenotype between birth and delayed vaccination groups
To assess whether delaying BCG vaccination results in an altered vaccine specific CD4 T cell memory phenotype, we characterized cytokine expressing BCG-specific T cells. The memory phenotype of specific T cells, as defined by surface expression of CD45RA and CCR7, has in animal models been shown to determine vaccination outcome [17]. In both the birth-vaccination and delayed-vaccination groups effector memory (CD45RA-CCR7-, TEM) T cells predominated and at all time points evaluated (Figure 7 for data at 1 year of age). At one year of age, there was no significant difference for TEM or central memory (TCM, CD45RA−CCR7+) in the two infant groups (Figure 7).
Figure 7. Comparison of the surface memory phenotype of BCG-specific CD4 T cells between the 2 groups of infants, at 1 year of age.
Antigen specific CD4 T cells were identified by cytokine expression, by the whole blood intracellular cytokine assay. (A) Box and whisker plots depicting the memory phenotype of all BCG-specific CD4 T cells analysed together. (B) Representative dot plots of the surface phenotype of BCG-specific CD4 T cells (dots), superimposed on surface memory phenotype of all CD4 T cells (contour plots). The median is represented by the horizontal line, the interquartile range by the box, and the range by the whiskers. The Mann-Whitney U test was used to calculate p values of differences between the 2 groups.
Discussion
Our study showed that delaying BCG vaccination from birth to 10 weeks of age results in induction of higher frequencies of detectable specific CD4 T cells. In addition, the specific CD4 T cells were more likely to be polyfunctional in the delayed vaccine group, indicating that the “quality” of the BCG-induced response was enhanced.
Our results differ from those reported in 2 earlier studies, which did not show a difference in induced immunity when BCG vaccination was delayed beyond the immediate newborn period [10, 14]. Importantly, both those studies used IFN-γ measurement alone as outcome. Our results suggest that a detailed examination of the T cell response allows a more comprehensive, and contrasting, assessment of outcome. We showed that the most striking differences in induced immunity were at 1 year of age, highlighting the importance of a longitudinal design of such studies. Data on the kinetics of the BCG-induced immune response in infant cohorts is limited, especially in settings highly endemic for TB.
The immune correlates of vaccination-induced protection against TB are not known. Therefore, the question remains whether frequencies of specific cells, patterns of cytokine production and memory phenotype may be important for long-term protection against TB. Most vaccinologists would regard a quantitatively greater antigen-specific response as more optimal, following novel TB vaccination in clinical trials. This opinion has been substantiated by multiple animal studies, which have demonstrated that greater frequencies of specific T cells result in improved protection against TB [18–20]. In contrast, other studies have shown that the frequency of antigen-specific T cells induced by a TB vaccine may not necessarily correlate with outcome after virulent M.tb challenge [21–24]. Study design variables, such as the time point evaluated and the compartment analyzed, may be responsible for these discrepant results. In addition, many of these studies evaluated specific T cells responses by IFN-γ production only. Protection against TB without an optimal IFN-γ response is not possible. However, it is postulated that measuring other Th1 cytokines in addition to IFN-γ would allow a better assessment of the “quality” of the T cell response. For example, presence of polyfunctional T cells, co-expressing IFN-γ, TNF-α and IL-2, has emerged as a useful readout of “quality” immune responses [25, 26]. In animal models of vaccination against Leishmania major [27] and against TB [28], strategies that induce the highest frequency of polyfunctional antigen-specific CD4 T cells are associated with the best protection against subsequent challenge with the pathogen. Further, in HIV-1 infected individuals, slow disease progression is strongly associated with higher frequencies of polyfunctional HIV-specific T cells [29].
Classically, CD45RA and CCR7 have been used to delineate T cells into 4 memory subsets, namely naïve cells (CD45RA+CCR7+, TNaive), central memory cells (CD45RA−CCR7+, TCM), effector memory cells (CD45RA−CCR7−, TEM), and effector memory cells that have re-expressed CD45RA (CD45RA+CCR7−, TEMRA) [30, 31]. Long-lived TCM expand in lymph nodes, and differentiate into effector cells [30]. In contrast, TEM or the more terminally differentiated TEMRA populations can immediately home to a disease site for effector functions, but their proliferative capacity and longevity are limited [30]. From a hypothetical point of view, induction of longer-lived TCM should be a vaccination goal – this view has been substantiated in animal models of macaque infection with simian immunodeficiency virus (SIV) [17]. In contrast, induction of TEM or TEMRA may not be as optimal, as these short-lived populations cannot expand, and are more prone to exhaustion [32]. Interestingly, in most studies of human mycobacteria-specific T cells, TEM populations predominate and TCM are relatively infrequent [11, 33–35]. Our findings corroborate this; however there were no significant differences in these T cell subpopulations upon delaying the BCG vaccination.
Many variables are thought to affect BCG-induced immunity, as described in the introduction above. For example, Lalor et al [36] reported that seasonal changes affected the IFN-γ response in BCG vaccinated infants, when measured by PPD stimulation of whole blood for 6 days. In our study, at 50 weeks of age when the most significant differences in BCG-specific immunity were observed, there was no significant difference in the distribution of birth season between the delayed and birth vaccination groups. Further, it is possible that variable exposure to environmental mycobacteria could have confounded our results, although we regard this as unlikely, as all infants were enrolled from the same geographic area. We cannot exclude that other variables could have impacted on results.
Why did the immune response appear enhanced following delayed BCG vaccination? The newborn’s immune response is often regarded as “immature”, when compared with that of adults [37]. “Immature” may be a misnomer, as the response may be very appropriate for a baby emerging into a world of continuous antigenic challenge, and where excessive immunity to these stimuli may be detrimental. However, this may result in suboptimal responses to certain vaccines, such as BCG. We propose that the different nature of immunity induced by BCG at 10 weeks of age, compared with birth, could be ascribed to early “maturational” changes in infant immunity. Very limited data on “maturation” of the infant immune response over the first few months of life exists, but factors that may have contributed include inefficient antigen presenting cell function, including limited capacity to produce IL-12 [37], a critical cytokine for inducing the Th1 responses characteristic of successful mycobacterial immunity. In addition, animal and human evidence suggests that the newborn’s immune system is skewed towards a Th2 response [38–40], which may suppress induction of Th1 immunity.
Unlike many infant vaccines that confer increased protection upon boosting, a second BCG vaccination has been shown not to enhance protection [41]. New TB vaccination strategies focus on boosting immunity induced by BCG through use of heterologous vaccines. It is therefore critical that we use the prime vaccine most optimally. Our results suggest that the age at which BCG is administered may be a critical variable. The next step would be to confirm these findings in another cohort, prior to larger studies to assess whether delayed vaccination leads to increased protection against TB disease. Should this be the case, a comprehensive assessment of implications of delaying BCG vaccination to 6–14 weeks of age, when other childhood vaccines are given routinely, would be warranted.
Supplementary Material
Frequencies of cytokine-expressing CD4 T cells detected by a whole blood intracellular cytokine assay are shown for the positive control, SEB, used in this study. The median is represented by the horizontal line, the interquartile range by the box, and the range by the whiskers. The Mann-Whitney U test was used to calculate p values of differences between the 2 groups.
Acknowledgements
We thank the infants and mothers who took part in this study, their families, and the support of the excellent team at the field site, including Barbara Magwegwe, Nonkonzo Dziba, Sithembele Masimini, Sizwe Nqweniso and Stanzi Le Roux. We also thank Dr Tom Ottenhoff for providing antigens, and Dr Cheryl Day for critically reading the manuscript.
Funding
WAH is funded by the NIH (RO1-AI065653); ACH by the Thrasher Research Foundation and the South African Department of Health; WAH, GFB and GW by Bill and Melinda Gates Foundation through Grand Challenges in Global Health Grants; NN by the South African Medical Research Council; TJS by the Wellcome Trust; and BA by the South African National Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None declared
References
- 1.Trunz BB, Fine PEM, Dye C. Effect of BCG vaccination on childhood tuberculosis menengitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 2.Bonifachich E, Chort M, Astigarraga A, Diaz N, Brunet B, Pezzotto SM, et al. Protective effect of Bacillus Calmette-Guerin (BCG) vaccination in children with etra-pulmonary tuberculosis, but not the pulmonary disease, A case-control study in Rosario, Argentina. Vaccine. 2006;24:2894–2899. doi: 10.1016/j.vaccine.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 3.Fine PEM. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 4.Gorak-Stolinska P, Weir RE, Floyd S, Lalor MK, Stenson S, Branson K, et al. Immunogenicity of Danish-SSI 1331 BCG vaccine in the UK: Comparison with Glaxo-Evans 1077 BCG vaccine. Vaccine. 2006;24:5726–5733. doi: 10.1016/j.vaccine.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 5.Lagranderie MRR, Balazuc A-M, Deriaud E, Leclerc CD, Gheorghiu M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis vaccine strains. Infection and Immunity. 1996;64:1–9. doi: 10.1128/iai.64.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davids V, Hanekom W, Gelderbloem SJ, Hawkridge A, Hussey G, Sheperd R, et al. Dose-dependent immune response to Mycobacterium bovis BCG vaccination in neonates. Clinical and Vaccine Immunology. 2007;14:198–200. doi: 10.1128/CVI.00309-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt L, Cunha JF, Olsen AW, Chilima B, Hirsch P, Appelberg R, et al. Failure of the Mycobacterium bovis BCG Vaccine: Some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infection and Immunity. 2002;70:672–678. doi: 10.1128/iai.70.2.672-678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-g, yet succumb to tuberculosis. The Journal of Immunology. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 9.Wangoo A, Sparer T, Brown IN, Snewin VA, Janssen R, Thole J, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. The Journal of Immunology. 2001;166:3432–3439. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- 10.Marchant A, Goetghebuer T, Otta MO, Wolfe I, Ceesay SJ, Groote DD, et al. Newborns develop Th1-type Immune response to Mycobacterium bovis Bacillus Calmette-Gu'erin vaccination. The Journal of Immunology. 1999;163:2249–2255. [PubMed] [Google Scholar]
- 11.Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, et al. Bacillus Calmette-Guérin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. The Journal of Immunology. 2008;180:3569–3577. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine PEM, Carneiro IAM, Milstien JB, Clements CJ. Issues relating to the use of BCG in immunization programmes: a discussion document. [Accessed on 7th January 2009];World Health Organization WHO/V&B/99.23. 1999 Available at www.who.int/gpv-documents/
- 13.Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, Maldonado Y. Deficiency of the humoral immune responses to measles vaccine in infants immunized at age 6 months. Journal of American Medical Association. 1998;280:527–532. doi: 10.1001/jama.280.6.527. [DOI] [PubMed] [Google Scholar]
- 14.Hussey GD, Watkins MLV, Goddard EA, Gottschalk S, Hughes EJ, Iloni K, et al. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002;105:314–324. doi: 10.1046/j.1365-2567.2002.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, et al. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. Journal of Immunological Methods. 2004;291:185–195. doi: 10.1016/j.jim.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Black GF, Weir RE, Chaguluka SD, Warndorff D, Crampin AC, Mwaungulu L, et al. Gamma interferon responses induced by a panel of recombinant and purified mycobacterial antigens in healthy, non-mycobacterium bovis BCG-vaccinated Malawian young adults. Clinical and Diagnostic Laboratory Immunology. 2003;10(4):602–611. doi: 10.1128/CDLI.10.4.602-611.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaccari M, Trindade CJ, Trindade CJ, Zanetti M, Franchini G. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. The Journal of Immunology. 2005;175:3502–3507. doi: 10.4049/jimmunol.175.6.3502. [DOI] [PubMed] [Google Scholar]
- 18.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborneM. tuberculosis infection. Journal of Experimental Medicine. 2008;205(10):2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hervas-Stubbs S, Majlessi L, Simsova M, Morova J, Rojas M-J, Nouze' Cm, et al. High frequency of CD4+ T cells specific for the TB10.4 protein correlates with protection against Mycobacterium tuberculosis infection. Infection and Immunity. 2006;74(6):3396–3407. doi: 10.1128/IAI.02086-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AVS. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of Bacille Calmette-Gu'erin vaccine using mucosal administration and boosting with recombinant modified vaccinia virus ankara. The Journal of Immunology. 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 21.Mittrucker H-W, Steinhoff U, Kohler A, Krause M, Lazar D, Mex P, et al. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proccedings of the National Academy of Sciences. 2007;104(30):12434–12439. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchilian EZ, Desel C, Forbes EK, Bandermann S, Sander CR, Hill AVS, et al. Immunogenicity and protective efficacy of prime-boost regimens with recombinant ΔureC hly Mycobacterium bovis BCG and modified vaccinia virus ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infection and Immunity. 2009;77(2):622–631. doi: 10.1128/IAI.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas M-J, Bauche C, et al. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infection and Immunity. 2006;74(4):2128–2137. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennekov T, Dietrich J, Rosenkrands I, Stryhn A, Doherty TM, Andersen P. Alteration of epitope recognition pattern in Ag85B and ESAT-6 has a profound influence on vaccine-induced protection against Mycobacterium tuberculosis. European Journal of Immunology. 2006;36(12):3346–3355. doi: 10.1002/eji.200636128. [DOI] [PubMed] [Google Scholar]
- 25.Seder RA, Darrah PA, Roederer M. T-cell quality in memory protection: implications for vaccine design. Nature Reviews Immunology. 2008;8:247–259. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 26.Makedonas G, Betts MR. Polyfunctional analysis of human T cell responses: importance in vaccine immunogenicity and natural infection. Springer Seminars in Immunopathology. 2006;28:209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 27.Darrah PA, Patel DT, Luca PMD, Lindsay RWB, Davey DF, Flynn BJ, et al. Multifunctional Th1 cells define a correlate of vaccine mediated protection against Leishmania major. Nature Medicine. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 28.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. The Journal of Immunology. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Betts MR, Nason MC, West SM, Rosa SCD, Migueles SA, Abraham J, et al. HIV-1 non progressors preferentially maintain highly functional HIV-specific CD8+ T cells. Immunobiology. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Lenig D, rster RF, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocyteswith distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, Generation, and Maintenance. Annual Review of Immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 32.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4+ T cell responses in different conditions of antigen exposure and persistence. The Journal of Immunology. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 33.Beveridge NER, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE, et al. Immunization with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. European Journal of Immunology. 2007;37(11):3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller H, Detjen AK, Schuck SD, Gutschmidt A, Wahn U, magdorf K, et al. Mycobacterium tuberculosis-specific CD4+, IFN-g+ and TNF-a+ multifunctional memory T cells coexpress GM-CSF. Cytokine. 2008;43(2):143–148. doi: 10.1016/j.cyto.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Scriba TJ, Barbara K, Deborah-Ann A, Fatima I, Jessica H, Gillian B, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. The Journal of Immunology. 2008;180(3):1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalor MK, Ben-Smith A, Gorak-Stolinska P, Weir RE, Floyd S, Blitz R, et al. Population differences in immune responses to Bacille Calmette-Guérin vaccination in infancy. Journal of Infectious Diseases. 2009;199:795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clinical Immunology. 2006;121(3):251–259. doi: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byun H-J, Jung W-W, Lee J-B, Chung HY, Sul D, Kim SJ, et al. An evaluation of the neonatal immune system using a listeria infection model. Neonatology. 2006;92:83–90. doi: 10.1159/000100806. [DOI] [PubMed] [Google Scholar]
- 39.Rose S, Lichtenheld M, Foote MR, Adkins B. Murine neonatal CD4+ T cells are poised for rapid Th2 effector-like function. The Journal of Immunology. 2007;178(5):2667–2678. doi: 10.4049/jimmunol.178.5.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maro'di L. Down-regulation of Th1 responses in human neonates. Clinical and Experimental Immunology. 2002;128(1):1–2. doi: 10.1046/j.1365-2249.2002.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dantas OM, Ximenes RA, de Albuquerque Mde F da Silva NL, Montarroyos UR, de Souza WV, et al. A case-control study of protection against tuberculosis by BCG revaccination in Recife, Brazil. The International Journal of Tuberculosis and Lung Disease. 2006;10:536–541. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frequencies of cytokine-expressing CD4 T cells detected by a whole blood intracellular cytokine assay are shown for the positive control, SEB, used in this study. The median is represented by the horizontal line, the interquartile range by the box, and the range by the whiskers. The Mann-Whitney U test was used to calculate p values of differences between the 2 groups.