Abstract
Objectives
To examine the influence of parental age at delivery and birth order on subsequent risk of childhood diabetes.
Design
Prospective population based family study.
Setting
Area formerly administered by the Oxford Regional Health Authority.
Participants
1375 families in which one child or more had diabetes. Of 3221 offspring, 1431 had diabetes (median age at diagnosis 10.5 years, range 0.4-28.5) and 1790 remained non-diabetic at a median age of 16.1 years.
Main outcome measures
Disease free survival and hazard ratios for the development of type 1 diabetes in all offspring, assessed by Cox proportional hazard regression.
Results
Maternal age at delivery was strongly related to risk of type 1 diabetes in the offspring; risk increased by 25% (95% confidence interval 17% to 34%) for each five year band of maternal age, so that maternal age at delivery of 45 years or more was associated with a relative risk of 3.11 (2.07 to 4.66) compared with a maternal age of less than 20 years. Paternal age was also associated with a 9% (3% to 16%) increase for each five year increase in paternal age. The relative risk of diabetes, adjusted for parental age at delivery and sex of offspring, decreased with increasing birth order; the overall effect was a 15% risk reduction (10% to 21%) per child born.
Conclusions
A strong association was found between increasing maternal age at delivery and risk of diabetes in the child. Risk was highest in firstborn children and decreased progressively with higher birth order. The fetal environment seems to have a strong influence on risk of type 1 diabetes in the child. The increase in maternal age at delivery in the United Kingdom over the past two decades could partly account for the increase in incidence of childhood diabetes over this period.
Introduction
Type 1 diabetes develops against a background of genetic susceptibility and is mediated by immune mechanisms activated many years before clinical onset of the disease. Studies of the appearance of autoantibodies in the offspring or siblings of individuals with type 1 diabetes suggest that autoimmune responses to islet antigens are generally well established by age 3-5 years, even though the disease may not develop until adult life.1 As in type 2 diabetes, prenatal and early postnatal factors are known to influence subsequent risk of type 1 diabetes.2 These include intrauterine exposure to viral infection with rubella or enterovirus and birth weight.3–7 Additionally, several studies have reported that high maternal age at delivery increases the risk of type 1 diabetes in the child.8–13 Studies of birth order have produced inconsistent results.12,13 We therefore examined the influence of parental age at delivery and birth order on subsequent risk of diabetes in the child in a large population based family cohort.
Participants and methods
The Bart's-Oxford family study
The Bart's-Oxford (BOX) family study of childhood diabetes is a prospective population based study, which since 1985 has recruited more than 90% of the families of children who have developed type 1 diabetes under the age of 21 in the former Oxford Regional Health Authority area. This area has a population of 2.6 million, including 730 000 people under the age of 21 years. Ascertainment of new cases is more than 95%, and incidence up to age 15 over the period 1985-95 averaged 18.6 (95% confidence interval 17.4 to 19.8) cases per 100 000/year.14 Data, including dates of birth and history of diabetes, are collected during home visits, and further contact with the families is maintained by visits and telephone calls from the field workers, supplemented by annual postal questionnaires. More than 90% of those recruited remain under regular follow up. Diabetes is defined according to World Health Organization criteria, and cases of secondary diabetes or maturity onset diabetes of the young are excluded by examination of the clinical records.
Our analysis included 3221 offspring from 1375 families. A further 91 offspring from 49 families were excluded because the date of birth of the father or mother, or both, was unavailable. The median age of mothers at delivery of the included offspring was 27 years (range 16-45) and the median age of fathers was 29 years (range 16-74). The analysis included 1294 firstborn children, 1177 second children, 516 third children, 171 fourth children, 41 fifth children, and 22 children who were sixth or more in the birth order. Of the 1790 offspring who remained non-diabetic at the time of the analysis, 21 had been randomised into a diabetes intervention trial at median age 15.7 years (range 7-25).
Data analysis
We used Cox proportional hazards regression to assess disease free survival and hazard ratios for development of diabetes. Survival time was from birth to the date of diagnosis of the disease. Offspring who did not develop diabetes were censored at the date of last contact or date of entry into a diabetes intervention trial. The sex of each offspring, maternal and paternal ages at delivery, and order of birth of the offspring were included in the regression model. Only offspring of both parents were included in the analysis, but birth order was defined by using all the mother's offspring, including both full and half siblings of the study proband. Maternal and paternal ages were modelled in five year bands. The model was built in stages according to the general strategy for model selection suggested by Collett.15 We used univariate analysis to identify variables individually predictive of the development of diabetes (stage 1). Those found to be significant at the 5% level were then fitted together in the multivariate model (stage 2). Each variable was then excluded in turn to examine whether, in the presence of other variables, they continued to be predictive of diabetes. This procedure was continued until the exclusion of any variable would have resulted in a significant change in model fit (P<0.05, as defined by a change in –2logL). Variables excluded after stage 1 were then added to this multivariate analysis to assess whether, in the presence of other significant variables, they became significant predictors of diabetes (stage 3). We examined interactions between variables retained at the end of stage 3. Linearity of effect was examined by comparison of model fit when parental age and birth order were fitted as ordered categorical variables and as separate categories. The assumption of proportionality was evaluated graphically by using log cumulative hazard against log time plots. Standard errors have been adjusted for clustering within families. Data were analysed with stata release 6.0. Results are expressed as relative risk (95% confidence interval).
The estimated effect of changes in distribution of maternal age on the incidence of diabetes was calculated by using demographic data for England and Wales (Office of Population Censuses and Surveys). An estimated cumulative incidence of diabetes up to age 15 of 2.5/1000 children was calculated on the basis of data on the incidence of childhood diabetes in the Oxford region in 1985.14 For each calendar year, the proportion of children born to mothers in each age group was multiplied by the percentage of children predicted to have developed diabetes by age 15, as estimated from the Cox regression model (assuming the child to be in the baseline category for all characteristics—that is, a firstborn female with a father aged less than 20 at delivery). This was summed to give a factor proportional to the cumulative incidence of diabetes in that annual birth cohort. This sum was then expressed relative to the estimate for the 1970 birth cohort.
Results
Survival analysis
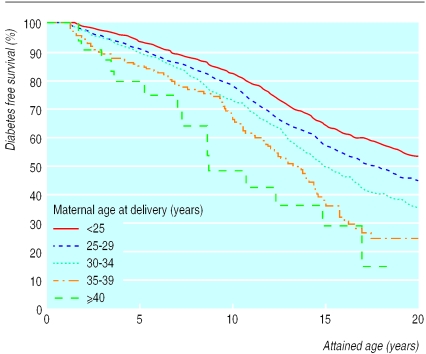
Of 3221 offspring included in this analysis, 1431 had developed diabetes at median age 10.5 years (range 0.4-28.5) and 1790 remained non-diabetic at median age 16.1 years (range 0-43). Overall, 57% (816 participants) of those with diabetes and 52% (931) of those who remained non-diabetic were male. Figure 1 shows the Kaplan-Meier survival curves for each five year band of maternal age at delivery. Cox regression analysis showed that maternal age, paternal age, birth order, and sex of offspring were independent determinants of risk. There was no evidence of significant non-linearity for parental age or birth order variables, and no significant first order interactions were found. The table shows the hazard ratios for these four variables in univariate and multivariate analyses. Risk increased with increasing maternal age at delivery and was less strongly associated with increasing paternal age. Overall risk of diabetes increased by 25% (95% confidence interval 17% to 34%) for each five year band of maternal age and by 9% (3% to 16%) for each five year increase in paternal age. Multivariate analysis showed that increasing birth order conferred some protection. The overall effect of increasing birth order was a 15% risk reduction (10% to 21%) per child. The risk in male offspring was 21% higher (8% to 35%) than in females. A small but significant inverse correlation was found between age at diagnosis and maternal age at delivery (r2=0.2, P<0.0001).
Figure 1.
Diabetes free survival in offspring in relation to maternal age at delivery
Estimated effect of temporal changes in maternal age distribution
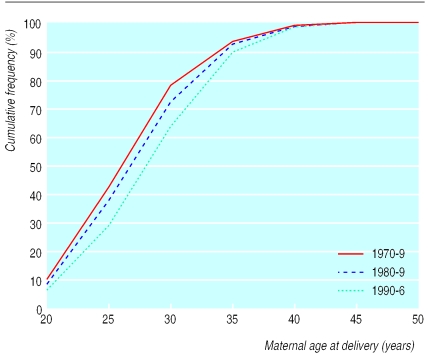
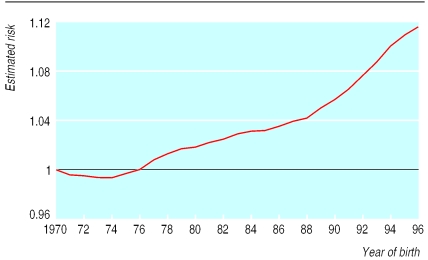
Figure 2 shows the distribution of maternal age at delivery in England and Wales since 1960. The most noticeable changes were that the proportion of children born to mothers aged 20-24 years decreased from 37% to 19% between 1970 and 1996, whereas the proportion born to mothers aged 30-34 years increased from 15% to 28% during this time. Figure 3 shows the estimated risk for development of diabetes in successive annual birth cohorts compared with the 1970 cohort on the basis of the maternal age distribution and estimated disease free survival for each age group derived above. This analysis suggests that changes in maternal age distribution account for an 11.4% increase in cumulative incidence of diabetes between the 1970 and 1996 birth cohorts.
Figure 2.
Maternal age at delivery in England and Wales 1970-9, 1980-9, and 1990-6
Figure 3.
Estimated risk of childhood diabetes in successive annual birth cohorts compared with 1970 cohort, calculated by using maternal age distribution and estimated disease free survival for each age group
Discussion
Several studies have shown that the risk of type 1 diabetes increases with high maternal age. These studies have generally compared cases with controls or census data. Flood et al studied 580 children or adolescents with diabetes and compared maternal age at delivery with data from a census bureau.10 The comparison showed fewer than expected births to mothers aged less than 25 and more than expected births to mothers aged more than 30. A previous study from the United Kingdom, again using census data for comparison, showed that mothers of diabetic children were significantly older at delivery than mothers in the general population.11 A population based Swedish study found no difference in mean maternal age at delivery between children with diabetes and referent children, but the proportion of mothers aged more than 35 years at delivery was higher for children with diabetes.9 Similar population based case-control studies found an increased risk of diabetes among children born to older mothers in Scotland but not in Northern Ireland, and a Danish study found a linear risk of diabetes with increasing maternal age for males but not for females.8,12 One case-control study found no association with maternal age.16
We studied the effect of parental age and risk of diabetes within a large population based cohort of children with diabetes and their siblings. This cohort is larger than the studies described above and has the additional advantage that probands are compared with siblings rather than unrelated controls. Survival analysis was therefore performed within a group in which genetic susceptibility to type 1 diabetes was increased and can be assumed to be independent of parental age at delivery. Our major finding was that increasing maternal age at delivery was associated with a log linear increase in risk of diabetes in the offspring. There was also a weaker association between risk and the father's age at delivery. The most comparable study to ours was that from Pittsburgh, which examined just over a thousand families and showed a significant association between a maternal age of more than 35 years and the cumulative frequency of diabetes in children. No trend was found in younger maternal age groups.13 The observation that children of older mothers have an increased risk of diabetes seems reasonably secure. We have extended this observation by showing a log linear relation throughout reproductive life sufficiently powerful to influence the overall rate of diabetes within a population.
Whereas most studies on maternal age at birth and risk of type 1 diabetes have had consistent results, the literature on birth order is conflicting.12,13 Our study, based on multivariate analysis, may help to explain this. We found that univariate analysis showed no association with birth order, but after adjustment for maternal age, an inverse association with birth order was apparent. In other words, later offspring have a higher risk of diabetes associated with maternal age but are also relatively protected compared with firstborn children. The combined effect of low birth order and high maternal age is such that risk of diabetes will be highest in firstborn children of mothers who start their families late.
The incidence of childhood diabetes has increased in many parts of the world since the 1950s. Studies from Finland, which has the highest incidence of childhood diabetes in the world, have shown that the incidence is now about four times as high as in 1953, when the first nationwide study was performed, with a linear trend over nearly 40 years.17 The increase in incidence has been most noticeable in developed countries, which have shown an increasing maternal age at first childbirth over a similar period.18 In the United Kingdom, for example, there are now fewer mothers in the 20-24 year age band and more in the 30-34 year age band (Figure 2). We estimate that changes in the distribution of maternal age at delivery could account for an 11% increase in the cumulative incidence of diabetes between 1970 and 1996.
The incidence of type 1 diabetes in childhood has increased rapidly in the population we are studying, with an annual increase of 4% from 1985 to 1996.14 Increasing maternal age at delivery can only partly explain an increase of this magnitude, and other as yet unknown factors must be involved. A trend to earlier onset of disease has also been observed in our area, as in Finland.14,19 The weak inverse correlation between maternal age at birth and age at diagnosis in the child shown in our present study and also in Pittsburgh13 would be expected to have a small influence on this trend.
What is already known on this topic
Several studies have shown that children born to older mothers have an increased risk of type 1 diabetes
Most studies have compared cases with census data or controls and therefore have not matched for genetic susceptibility
Most reports suggest that increased risk is limited to the offspring of mothers aged more than 35 years at delivery. Conflicting reports exist concerning birth order and subsequent risk of type 1 diabetes
What this study adds
A strong log linear inverse relation was found between maternal age at delivery and risk of diabetes, equivalent to a 25% increase in risk for each 5 year rise in maternal age
Multivariate analysis showed that risk, adjusted for the effects of parental age at delivery and sex of the offspring, is highest in firstborn children and decreased progressively with higher birth order
Increasing maternal age at delivery of the first child may have contributed to the rising incidence of childhood diabetes
Why should maternal age influence risk of type 1 diabetes? An acquired genetic abnormality seems unlikely because, although parental age is associated with several genetic disorders due to aneuploidy—most notably Down's syndrome20—no chromosomal abnormality is present in type 1 diabetes. Intrauterine viral infection can influence subsequent risk of diabetes in the child and might account for the higher risk in firstborn children, but it would not explain the effect of increasing maternal age.4,5 An alternative possibility, prompted by epidemiological observations in atopic conditions, including asthma, is that maturation of the immune system may be influenced by maternal age. Atopic disease is thought to be mediated via a predominant Th2 (T helper) lymphocyte response, whereas type 1 diabetes and other autoimmune diseases are mediated via Th1 responses. This is reflected in the clinical observation that children with type 1 diabetes have a lower prevalence of asthmatic symptoms than controls.21 Studies in asthma and other atopic disorders have shown that these Th2 mediated diseases are associated with low maternal age.22,23,24 We would therefore speculate that factors associated with higher maternal age influence maturation of the immune system in the offspring; possibly increasing predisposition to type 1 diabetes in later life by shifting the balance towards Th1 responses.
Conclusions
High maternal age—and possibly high paternal age—increases the risk of type 1 diabetes in offspring. Additionally, risk increases in a log linear fashion throughout the maternal age range. Higher maternal age at birth is associated with younger age of onset of type 1 diabetes in the child. Firstborn children are at greater risk than children of higher birth order. These observations, if confirmed, could account in part for the increasing incidence of diabetes and for the trend towards younger age at diagnosis. The fetal origins of type 2 diabetes, an aetiologically distinct disorder, are now well established.2 Fetal or neonatal influences could prove equally important in type 1 diabetes.
Supplementary Material
Table.
Relative risk (95% confidence interval) for development of diabetes assuming linear trend across categories
| Univariate analysis | P value* | Multivariate analysis† | P value* | |
|---|---|---|---|---|
| Maternal age (years) v <20 | ||||
| 20-24 | 1.25 (1.19 to 1.32) | <0.0001 | 1.25 (1.17 to 1.34) | <0.0001 |
| 25-29 | 1.57 (1.39 to 1.77) | 1.57 (1.33 to 1.85) | ||
| 30-34 | 1.97 (1.65 to 2.35) | 1.98 (1.55 to 2.52) | ||
| 35-39 | 2.47 (1.95 to 3.13) | 2.48 (1.79 to 3.43) | ||
| ⩾40 | 3.10 (2.31 to 4.16) | 3.11 (2.07 to 4.66) | ||
| Paternal age (years) v <20 | ||||
| 20-24 | 1.18 (1.12 to 1.24) | <0.0001 | 1.09 (1.03 to 1.16) | 0.003 |
| 25-29 | 1.39 (1.24 to 1.54) | 1.20 (1.05 to 1.37) | ||
| 30-34 | 1.63 (1.39 to 1.92) | 1.31 (1.08 to 1.59) | ||
| 35-39 | 1.92 (1.55 to 2.38) | 1.43 (1.10 to 1.86) | ||
| 40-45 | 2.27 (1.74 to 2.95) | 1.57 (1.13 to 2.17) | ||
| Birth order v 1st born | ||||
| 2nd | 0.97 (0.92 to 1.03) | 0.37 | 0.85 (0.79 to 0.90) | <0.0001 |
| 3rd | 0.95 (0.85 to 1.06) | 0.72 (0.64 to 0.80) | ||
| 4th | 0.93 (0.78 to 1.09) | 0.61 (0.52 to 0.71) | ||
| ⩾5th | 0.90 (0.72 to 1.13) | 0.52 (0.42 to 0.63) | ||
| Male v female | 1.20 (1.08 to 1.33) | 0.0007 | 1.21 (1.08 to 1.35) | <0.0001 |
Significance of overall effect of variable in model.
Relative risk adjusted for three other factors in model.
Acknowledgments
We thank the families taking part in the study; the physicians, paediatricians, and diabetes nurse specialists throughout the Oxford region for their continuing support; the project administrators, Hilary Gillmor and Suzanne Weeks; and the Bart's-Oxford family study fieldworkers, Kathryn Darvill, Louise Gorrod, Denise Morgans, Pam Sawtell, Rose Streeton, and Sallie Wall for all their help.
Footnotes
Funding: The Bart's-Oxford family study is supported by the British Diabetic Association. PJB was supported by a career development award from the Juvenile Diabetes Foundation. The Research and Development Support Unit at Southmead Hospital is supported by a grant from the South West NHS Research and Development Directorate.
Competing interests: None declared.
Members of the Bart's-Oxford Study Group are listed on the BMJ's website
References
- 1.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes. The 2-year analysis of the German BABYDIAB study. Diabetes. 1999;48:460–468. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJP. Type 2 (non-insulin dependent) diabetes mellitus; the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg-Fellner G, Witt ME, Fedun B, Doberson MJ, McEvoy RC, Cooper LZ, et al. Diabetes and autoimmunity in patients with the congenital rubella syndrome. Rev Infect Dis. 1985;7:170–16S. doi: 10.1093/clinids/7.supplement_1.s170. [DOI] [PubMed] [Google Scholar]
- 4.Dahlquist GG, Ivarsson S, Lindberg B, Forsgren M. Maternal enteroviral infection during pregnancy is a risk determinant for childhood IDDM. Diabetes. 1995;44:408–413. doi: 10.2337/diab.44.4.408. [DOI] [PubMed] [Google Scholar]
- 5.Hyoty H, Hiltunen M, Knip M, Laakonen M, Vahasalo P, Karjalainen J, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Diabetes. 1995;44:652–657. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 6.Khan N, Couper JJ. Low-birth-weight infants show earlier onset of IDDM. Diabetes Care. 1994;17:653–656. doi: 10.2337/diacare.17.7.653. [DOI] [PubMed] [Google Scholar]
- 7.Dahlquist G, Sandberg Bennich S, Kallen B. Intrauterine growth pattern and risk of childhood onset insulin dependent (type 1) diabetes: population based case-control study. BMJ. 1996;313:1174–1177. doi: 10.1136/bmj.313.7066.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bache I, Volund A, Bock T, Buschard K. Previous maternal abortion, longer gestation and younger maternal age decrease the risk of type 1 diabetes among male offspring. Diabetes Care. 1999;22:1063–1065. doi: 10.2337/diacare.22.7.1063. [DOI] [PubMed] [Google Scholar]
- 9.Blom L, Dahlquist G, Nystrom L, Sandstrom A, Wall S. The Swedish childhood diabetes study—social and perinatal determinants for diabetes in childhood. Diabetologia. 1989;32:7–13. doi: 10.1007/BF00265397. [DOI] [PubMed] [Google Scholar]
- 10.Flood TM, Brink SJ, Gleason RE. Increased incidence of type 1 diabetes in children of older mothers. Diabetes Care. 1982;5:571–573. doi: 10.2337/diacare.5.6.571. [DOI] [PubMed] [Google Scholar]
- 11.Metcalfe MA, Baum JD. Family characteristics and insulin-dependent diabetes. Arch Dis Child. 1992;67:731–736. doi: 10.1136/adc.67.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson CC, Carson DJ, Hadden DR, Waugh NR, Cole SK. A case-control investigation of perinatal risk factors for childhood IDDM in Northern Ireland and Scotland. Diabetes Care. 1994;17:376–381. doi: 10.2337/diacare.17.5.376. [DOI] [PubMed] [Google Scholar]
- 13.Wagener DK, LaPorte RE, Orchard TJ, Cavender D, Kuller LH, Drash AL. The Pittsburgh diabetes study 3: an increased prevalence with older maternal age. Diabetologia. 1983;25:82–85. doi: 10.1007/BF00250892. [DOI] [PubMed] [Google Scholar]
- 14.Gardner SG, Bingley PJ, Sawtell PA, Weeks S, Gale EAM the Bart's-Oxford Study Group. Rising incidence of insulin dependent diabetes in children aged under 5 years in the Oxford Region: time trend analysis. BMJ. 1997;315:713–717. doi: 10.1136/bmj.315.7110.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collett D. Modelling survival data in medical research. London: Chapman and Hall; 1994. pp. 54–106. [Google Scholar]
- 16.Mayer EJ, Hamman RF, Gay EC, Lezotte DC, Savitz DA, Klingensmith GJ. Reduced risk of IDDM among breast-fed children. Diabetes. 1988;37:1625–1632. doi: 10.2337/diab.37.12.1625. [DOI] [PubMed] [Google Scholar]
- 17.Tuomilehto J, Karvonen M, Pitkäniemi J, Virtala E, Kohtamaki K, Toivanen L, et al. Record high incidence of type I (insulin-dependent) diabetes mellitus in Finnish children. Diabetologia. 1999;42:655–660. doi: 10.1007/s001250051212. [DOI] [PubMed] [Google Scholar]
- 18.Organisation for Economic Cooperation and Development. Beyond 2000: the new social policy agenda. 1996. www.oecd.org//els/pdfs/isgd.pdf (accessed 25 Feb 2000).
- 19.Karvonen M, Pitkäniemi J, Tuomilehto J the Finnish Childhood Diabetes Registry Group. The onset age of type 1 diabetes in Finnish children has become younger. Diabetes Care. 1999;22:1066–1070. doi: 10.2337/diacare.22.7.1066. [DOI] [PubMed] [Google Scholar]
- 20.Wryobek AJ, Aardema M, Eichenlaub-Ritter U, Ferguson L, Marchetti F. Mechanisms and targets involved in maternal and paternal age effects on numerical aneuploidy. Exp Mol Mutagen. 1996;28:254–264. doi: 10.1002/(SICI)1098-2280(1996)28:3<254::AID-EM9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Douek IF, Leech NJ, Gillmor HA, Bingley PJ, Gale EAM. Children with type 1 diabetes and their unaffected siblings have fewer symptoms of asthma. Lancet. 1999;353:1850. doi: 10.1016/S0140-6736(99)00988-5. [DOI] [PubMed] [Google Scholar]
- 22.Lewis S, Butland B, Strachan D, Bynner J, Richards D, Butler N, et al. Study of the aetiology of wheezing illness at age 16 in two national British birth cohorts. Thorax. 1996;51:670–676. doi: 10.1136/thx.51.7.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events and race. Am Rev Resp Dis. 1990;142:555–562. doi: 10.1164/ajrccm/142.3.555. [DOI] [PubMed] [Google Scholar]
- 24.Svanes C, Omenaas E, Heuch JM, Irgens LM, Gulsvik A. Birth characteristics and asthma symptoms in young adults: results from a population-based cohort study in Norway. Eur Resp J. 1998;12:1366–1370. doi: 10.1183/09031936.98.12061366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.