Abstract
Background
Older women with early-stage breast cancer experience higher rates of non—breast cancer-related death. We examined factors associated with cause-specific death in a large cohort of breast cancer patients treated with extended adjuvant endocrine therapy.
Methods
In the MA.17 trial, conducted by the National Cancer Institute of Canada Clinical Trials Group, 5170 breast cancer patients (median age = 62 years; range = 32–94 years) who were disease free after approximately 5 years of adjuvant tamoxifen treatment were randomly assigned to treatment with letrozole (2583 women) or placebo (2587 women). The median follow-up was 3.9 years (range = 0–7 years). We investigated the association of 11 baseline factors with the competing risks of death from breast cancer, other malignancies, and other causes. All statistical tests were two-sided likelihood ratio criterion tests.
Results
During follow-up, 256 deaths were reported (102 from breast cancer, 50 from other malignancies, 100 from other causes, and four from an unknown cause). Non—breast cancer deaths accounted for 60% of the 252 known deaths (72% for those ≥70 years and 48% for those <70 years). Two baseline factors were differentially associated with type of death: cardiovascular disease was associated with a statistically significant increased risk of death from other causes (P = .002), and osteoporosis was associated with a statistically significant increased risk of death from other malignancies (P = .05). An increased risk of breast cancer—specific death was associated with lymph node involvement (P < .001). Increased risk of death from all three causes was associated with older age (P < .001).
Conclusions
Non—breast cancer-related deaths were more common than breast cancer—specific deaths in this cohort of 5-year breast cancer survivors, especially among older women.
Overall survival is the most important therapeutically relevant outcome for cancer patients (1,2). Consideration of death from all causes among patients with breast cancer assumes that death is a sequela of breast cancer or its management. High treatment-related mortality or the presence of disease at death would justify combining non—breast cancer-related and breast cancer—specific deaths and a consideration of all-cause mortality. However, with increasing rates of early detection of breast cancer and improved therapies for breast cancer (3,4), more patients are expected to survive breast cancer and to be at risk of death from non—breast cancer-related causes. The risk of death from another cause that is unrelated to either breast cancer or its therapy is termed a competing risk of death. Different prognostic and predictive factors (5-7) may affect the type of competing death observed. For example, a woman with a HER2/neu-positive tumor would have a high risk of breast cancer death. However, a HER2/neu-positive tumor predicts response to targeted therapy with trastuzumab, and so she may survive breast cancer to die from another cause, especially if, for example, she was already at substantially higher risk of cardiovascular death. In general, a patient with breast cancer may develop non—breast cancer-related illnesses before, concurrent with, or after a diagnosis of breast cancer (8-19). The risk of death from other causes increases with age, as does the risk of having hormone receptor—positive breast cancer. Consequently, trials evaluating hormonal therapy that are open to patients of all ages with breast cancer may include more older women who are likely to die without a recurrence of breast cancer (16).
When a patient dies from another cause, it is, of course, unknown whether she might otherwise have died from breast cancer (8). Breast cancer—specific survival determined by a Kaplan—Meier analysis (13,14,16-19) may be substantively biased by the presence of an increased risk of death from other causes because, in that analysis, those who have died are assumed still to be able to die from breast cancer. An accurate proportion of deaths is obtained for a given time period with the cumulative incidence of each type of death (13,15-18). However, cumulative incidence may not accurately indicate treatment effect on risk of breast cancer death if the treatment also affects the risk of a competing cause of death (10,13,14,17-19).
In general, factors affecting death from all causes and from breast cancer—specific death are ascertained separately, with a comparison of whether the same factors statistically significantly affect both types of death. The number of deaths from all causes would usually contain a high proportion of breast cancer—specific deaths. Another approach to ascertaining differences by cause of death is to separately examine and compare the factors with statistically significant effects on different types of cause-specific death.
Lagakos (9) proposed a pragmatic framework to test whether various factors are differentially associated with cause-specific deaths. In this competing risk study, we considered death from breast cancer, other malignancies, and causes other than cancer for the 5170 breast cancer patients in the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) MA.17 trial. In this trial, patients who were disease free after about 5 years of tamoxifen treatment were randomly assigned to treatment with placebo or the aromatase inhibitor letrozole as an extended adjuvant hormonal therapy. There was no upper age restriction for enrollment to the trial; the median age at randomization was 62 years (20). Consequently, there was a substantial risk of death from a non—breast cancer cause (15,21). The large population of 5-year survivors in the MA.17 trial, which also included older women, was well suited to examination of the risk of non—breast cancer deaths and the factors associated with this risk; we examined the association of 11 baseline trial factors on the competing risks of death.
Patients and Methods
Patients
MA.17 was a randomized double-blind, placebo-controlled trial among women who had had breast cancer but were free of breast cancer after approximately 5 years of tamoxifen therapy. The goal of the trial was to evaluate maintenance endocrine therapy with letrozole (2.5 mg orally daily) versus placebo (orally daily), given for a period of 5 years (20). Criteria for eligibility in the trial included previous adjuvant tamoxifen therapy lasting 4.5–6 years; histologically confirmed primary breast cancer; a tumor that was estrogen receptor positive, progesterone receptor positive, or both (defined by a receptor level of 10 fmol/mg of protein or a positive result on immunohistochemical analysis of estrogen receptor or progesterone receptor); discontinuation of tamoxifen therapy less than 3 months before enrollment; an Eastern Cooperative Oncology Group performance status of 0, 1, or 2 (scored on a scale of 0–4, with lower scores indicating better function); a life expectancy of
CONTEXT AND CAVEATS.
Prior knowledge
Deaths of older women diagnosed with breast cancer are often due to causes other than breast cancer.
Study design
Prospective study of data from a large randomized trial of breast cancer patients treated with extended adjuvant endocrine therapy.
Contribution
Deaths from non—breast cancer causes were more common than breast cancer—related deaths, especially in older women. Cardiovascular disease at baseline was associated with a statistically significant increased risk of death from other causes. Osteoporosis at baseline was associated with increased risk of death from other malignancies.
Implications
Medical attention to the potential for death from other causes becomes increasingly important in older patients with breast cancer.
Limitations
The patient population was not representative of all breast cancer patients; it contained patients with hormone receptor—positive disease who were at low risk of recurrence and were disease free after 5 years of tamoxifen treatment. Younger women, who tend to have hormone-receptor negative disease and shorter survival, were excluded from this trial.
more than 5 years; and postmenopausal status. Women were defined as being postmenopausal if they were at least 50 years of age at the start of adjuvant tamoxifen therapy, were younger than 50 years but considered to be postmenopausal by their treating physician at the initiation of tamoxifen therapy, were younger than 50 years at the start of tamoxifen therapy but had undergone bilateral oophorectomy, were premenopausal and younger than 50 years at the start of tamoxifen therapy but became amenorrheic during chemotherapy or treatment with tamoxifen, or were any age but had postmenopausal levels of luteinizing hormone or follicle-stimulating hormone before enrollment in the study. Patients with unknown hormone receptor status were eligible if an effort had been made to determine the receptor status of the primary tumor. Patients were stratified according to tumor hormone receptor status (unknown or estrogen receptor positive and/or progesterone receptor positive), lymph node status (negative, unknown, or positive), and previous adjuvant chemotherapy (no or yes). The MA.17 trial was led by the NCIC CTG and included The Breast Cancer Intergroup of North America and the Breast International Group. Each participating institution’s ethics review board approved the study protocol. All centers followed good clinical practice guidelines. All patients gave written informed consent. Data were received, reviewed, and analyzed by the NCIC CTG, the study chairman (PEG), and the steering committee.
A total of 5187 patients free of breast cancer were randomly assigned to treatment with letrozole (2593 women) or placebo (2594 women). The study population for the current analyses was 5170 patients—2583 patients treated with letrozole and 2587 patients treated with placebo. Patients were unblinded at a median 2.4 years of follow-up because of statistically significantly better disease-free survival observed among patients in the letrozole arm (20). Of the 2587 women assigned to placebo, 2383 were alive at unblinding and 1579 (66%) were confirmed to have started letrozole. In this analysis, the use of letrozole and placebo refers to the assignment at randomization.
Events
The primary objective of MA.17 was to evaluate the relationship between letrozole treatment and disease-free survival. Disease-free survival was defined as the time between random assignment to treatment and breast cancer recurrence or the development of contralateral breast cancer; deaths from other causes were censored. A secondary endpoint was all-cause mortality, which was defined as the time between random assignment to treatment and death from any cause; this endpoint had been previously assessed with a stratified Cox proportional hazards analysis (20).
For these investigations, we considered time to death for the following cause-specific classifications: death from breast cancer, death from other malignancies, and death from other causes. The median follow-up was 3.9 years (range = 0–7 years). Patients were censored at time of death for a competing risk or at the date of their last evaluable follow-up. Data on all deaths were medically reviewed at NCIC CTG Central Office by the MA.17 Physician Coordinator (LS), who consulted with the study chairman and the steering committee (20). Autopsy was not mandatory.
Statistical Analyses
The potential for substantial competing risks was ascertained with plots of the times of death from breast cancer, other malignancies, and other causes. That is, substantial competing risks would exist for breast cancer if deaths from other malignancies and/or other causes occurred before or in the same period as deaths from breast cancer. The indication of substantial competing risks of death from other malignancies and death from other causes with death from breast cancer in preliminary analyses then prompted us to use the Lagakos method (9) to determine whether there were differential associations between various factors and types of death, under the assumption of independent cause-specific risks. The associations of each factor with each cause of death were examined in a model that included all of the factors (the “full-factor” model). For these investigations, we examined 11 baseline MA.17 factors: 1) treatment (placebo vs letrozole), 2) age (in years), 3) menopausal status [<50 years or missing vs ≥50 years (20)], 4) duration of previous tamoxifen treatment [≤5 years or missing vs >5 years (20)], 5) adjuvant radiotherapy (no or missing vs yes), 6) bone fracture (no or missing vs yes), 7) reported baseline diagnosis of osteoporosis (no or missing vs yes), 8) reported baseline cardiovascular disease (no or missing vs yes), and the stratification factors of 9) hormone receptor status (unknown vs yes), 10) lymph node status (negative vs unknown vs positive), and 11) adjuvant chemotherapy (no vs yes).
Three hypotheses (H1, H2, and H3) were tested in this analysis. The first hypothesis, H1, states that the factor under investigation does not affect the type or time to death, that is, βBC = βOM = βOC = 0, as tested with a likelihood ratio criterion that has an approximate chi-square distribution with , where β is the cause-specific effect of one of the 11 baseline factors listed above, BC is breast cancer, OM is other malignancies, and OC is other causes. If H1 was not rejected, then we stopped testing because there was no evidence that the factor was associated with mortality. If H1 was rejected, then we tested the second hypothesis, H2, which states that the factor has the same effect for all three types of death, that is, βBC = βOM = βOC, as tested with a likelihood ratio criterion that has an approximate chi-square distribution with . If H2 was rejected, then there was evidence that the factor was differentially associated with the three causes of death and we should assess each factor separately for cause-specific mortality; we reported values for β with its 95% confidence interval (CI), which are based on the assumption that β has an approximately normal distribution. If H2 was not rejected, then there was evidence of a common effect on all types of death, which was tested by the third hypothesis, H3 (9). H3 states that an association exists between the factor and all-cause mortality. Because no such factor was identified in this analysis (ie, H2 was rejected), H3 was not considered further.
Assessment of competing risks by this approach used a log-normal survival analysis. For more than 50 years, the log-normal analysis has been found to be an appropriate parametric model for breast cancer data (22). For the log-normal analysis (23), the natural logarithm of survival time, Y = ln(t), is a linear function, Y = α + Σβjzj + σW, where σ is a scale parameter; for the log-normal model, W is the standard normal distribution, zj is the jth baseline factor, j takes values 1–11 corresponding to the 11 baseline factors listed above, and βj is the effect of the jth baseline factor on mortality. Fourteen patients had no follow-up time in the study; these 14 patients were assigned 1 day of follow-up for analytic purposes so that logarithms of their survival time could be obtained.
The appropriateness of the log-normal model was examined graphically with density, hazard, and quantile plots (23) and with both the Akaike information criterion (24) and the Schwarz Bayesian criterion (25). Cox-Snell (26) and standardized residual (23) plots with the factors in the “best factor” model were used to examine the fit and data support for the model type. The choice of a log-normal model was consistent with the data from the extended adjuvant endocrine setting with time measurement beginning after about 5 years of primary therapy with tamoxifen.
We also examined the effects of the factors with separate cause-specific (breast cancer, other malignancies, or other causes) multivariable analyses by use of the log-normal model. The factor treatment (letrozole or placebo) and three stratification factors (hormone receptor status, lymph node status, and adjuvant chemotherapy) were always included in the log-normal cause-specific models to account for MA.17 design structure. All other factors (age, menopausal status, duration of previous tamoxifen treatment, adjuvant radiotherapy, bone fracture, reported baseline diagnosis of osteoporosis, and reported baseline cardiovascular disease) and interactions between treatment and each baseline factor were considered in stepwise forward regression analyses, with the inclusion of a factor if it had a P value of .05 or less as required by the test statistic—the two-sided likelihood ratio criterion has an approximate chi-square distribution with . The β value of the log-normal model permitted direct calculations of the percentage of survival for specific patient characteristics at a particular time (22); we report values of β with their 95% confidence intervals, which are based on the assumption that β has an approximately normal distribution. We used this technique to determine the 5-year survival from breast cancer for patients with specific characteristics: women who were hormone receptor positive; who were treated with letrozole and with adjuvant chemotherapy; and who were aged 50, 70, or 80 years and were either lymph node negative or lymph node positive. All statistical tests were two-sided. Similar results were obtained in reanalyses with both Cox and Weibull survival analyses (data not shown).
Results
Follow-up ended on October 9, 2005, with a median of 3.9 years (range = 0–7 years ). The rate of censoring was 95.0%, with 256 deaths (102 from breast cancer, 50 from other malignancies, 100 from other causes, and four from unknown causes) (Table 1). One (2%) of the 50 women classified as having died from other malignancies also had a recurrence of breast cancer. Of the 100 women who were classified as having died from other causes, two also had a local regional recurrence of breast cancer, two had contralateral breast cancer, and seven had distant metastases.
Table 1.
Cause-specific deaths by treatment arm and factor subgroups*
| Factor | No. of patients (%) |
Breast cancer deaths, No. |
Deaths from other malignancies, No. |
Deaths from other causes, No. |
||||
|---|---|---|---|---|---|---|---|---|
| Letrozole (n = 2583) |
Placebo (n = 2587) |
Letrozole | Placebo | Letrozole | Placebo | Letrozole | Placebo | |
| Age | ||||||||
| < 70 y | 1901 (73.6) | 1946 (75.2) | 31 | 37 | 12 | 14 | 19 | 17 |
| ≥70 y | 682 (26.4) | 641 (24.8) | 15 | 19 | 13 | 11 | 36 | 28 |
| Menopausal status† | ||||||||
| < 50 y + missing | 618 (23.9) | 626 (24.2) | 4 | 12 | 1 | 1 | 2 | 1 |
| ≥50 y | 1965 (76.1) | 1961 (75.8) | 42 | 44 | 24 | 24 | 53 | 44 |
| Tamoxifen treatment | ||||||||
| ≤5 y + missing | 1162 (45.0) | 1213 (46.9) | 25 | 25 | 15 | 7 | 30 | 19 |
| > 5 y | 1421 (55.0) | 1374 (53.1) | 21 | 31 | 10 | 18 | 25 | 26 |
| Radiation therapy | ||||||||
| No + missing | 1023 (39.6) | 1054 (40.7) | 13 | 25 | 11 | 9 | 25 | 27 |
| Yes | 1560 (60.4) | 1533 (59.3) | 33 | 31 | 14 | 16 | 30 | 18 |
| Bone fracture | ||||||||
| No + missing | 2295 (88.9) | 2281 (88.2) | 40 | 50 | 24 | 22 | 46 | 39 |
| Yes | 288 (11.1) | 306 (11.8) | 6 | 6 | 1 | 3 | 9 | 6 |
| Osteoporosis | ||||||||
| No + missing | 2273 (88.0) | 2280 (88.1) | 43 | 53 | 21 | 19 | 47 | 39 |
| Yes | 310 (12.0) | 307 (11.9) | 3 | 3 | 4 | 6 | 8 | 6 |
| Cardiovascular disease | ||||||||
| No + missing | 2268 (87.8) | 2294 (88.7) | 41 | 50 | 23 | 20 | 33 | 35 |
| Yes | 315 (12.2) | 293 (11.3) | 5 | 6 | 2 | 5 | 22 | 10 |
| Hormone receptor status | ||||||||
| Unknown | 55 (2.1) | 56 (2.2) | 2 | 2 | 2 | 1 | 2 | 0 |
| Positive | 2528 (97.9) | 2531 (97.8) | 44 | 54 | 23 | 24 | 53 | 45 |
| Lymph node status | ||||||||
| Negative | 1299 (50.3) | 1301 (50.3) | 9 | 9 | 14 | 11 | 24 | 17 |
| Unknown | 100 (3.9) | 98 (3.8) | 5 | 2 | 2 | 1 | 6 | 2 |
| Positive | 1184 (45.8) | 1188 (45.9) | 32 | 45 | 9 | 13 | 25 | 26 |
| Adjuvant chemotherapy | ||||||||
| No | 1385 (53.6) | 1388 (53.7) | 22 | 27 | 20 | 17 | 37 | 30 |
| Yes | 1198 (46.4) | 1199 (46.3) | 24 | 29 | 5 | 8 | 18 | 15 |
Four patients had a missing cause of death and are not included above. Deaths from other malignancies were as follows: 12 from lung cancer (11 from non—small cell lung cancer and one from small-cell lung cancer), eight from pancreatic cancer, five from colorectal cancer (four from colon cancer and one from rectal cancer), four from endometrial cancer, four from non-Hodgkin lymphoma, three from esophageal cancer, four from ovary or peritoneal cancer, two from head and neck cancer, two from sarcoma, one from leukemia, one from myelodysplastic disease, two from biliary tract cancer, one from melanoma, and one from an unknown type of cancer. Deaths from other causes were as follows: 39 from cardiovascular disease including stroke, 11 from organ failure other than cardiovascular disease, 12 from causes not otherwise specified (one possibly with unconfirmed pancreatic cancer), four from neurological disease, 14 from infection, six from hemorrhage, two from trauma, and 12 from multiple causes.
Menopausal status was defined in terms of a cut point at 50 years of age; those at least 50 years of age were considered to be postmenopausal.
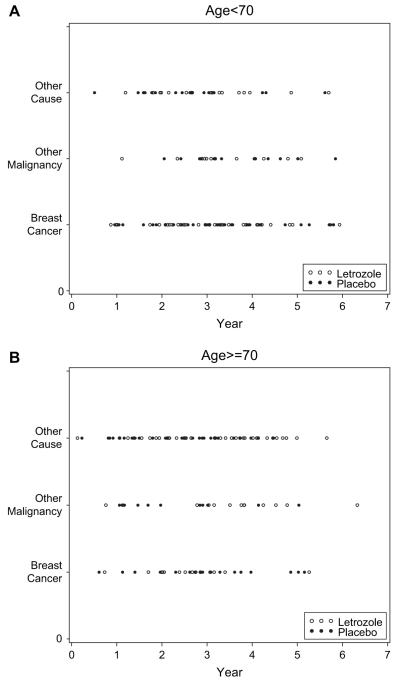
Non—breast cancer deaths accounted for 60% of known deaths (72% for those 70 years or older at study entry and 48% for those younger than 70 years). When the time of death was plotted against the type of death for women in both study arms who were younger than 70 years at study entry and for those who were 70 years or older, the time periods overlapped throughout follow-up for death from breast cancer, other malignancies, and other causes for both age groups and both trial arms (Fig. 1). These results thus provide evidence of substantial competing risks for both younger and older women, in that there would be a sizable risk of non—breast cancer death before breast cancer death.
Fig. 1.
Time of death by type of death, age group, and therapy group. A) Age group younger than 70 years. B) Age group 70 years or older. Type of event: 1 = death from breast cancer; 2 = death from other malignancy; 3 = death other than cancer. Open circles = letrozole treatment group; solid circles = control group.
Results of the competing risks analysis are presented in Table 2 for the 11 baseline trial factors. The factors age (from hypothesis H1, ; P < .001), cardiovascular disease (from hypothesis H1, ; P = .02), and lymph node status (from hypothesis H1, ; P < .001) were statistically significantly associated with death. There was weak evidence of an effect associated with a baseline report of osteoporosis (from hypothesis H1, ; P = .07). The remaining seven factors were not statistically significantly associated with breast cancer—specific death, death from other malignancies, or death from other causes: treatment (alone, without consideration of interactions with other factors), menopausal status, length of tamoxifen use, adjuvant radiotherapy, bone fracture, hormone receptor status, and adjuvant chemotherapy.
Table 2.
Factors associated with death by a Lagakos analysis
| Factor | Comparison | Association with death* |
Association by type of death† |
||
|---|---|---|---|---|---|
| Test statistic | P value | Test statistic | P value | ||
| Treatment | Placebo | 1.26 | .74 | ||
| Letrozole | |||||
| Age | In years | 53.07 | <.001 | 10.35 | .01 |
| Menopausal status‡ | <50 y + missing | 2.04 | .56 | ||
| ≥50 y | |||||
| Tamoxifen treatment | ≤5 y + missing | 2.30 | .51 | ||
| >5 y | |||||
| Adjuvant radiotherapy | No + missing | 2.11 | .55 | ||
| Yes | |||||
| Bone fracture | No + missing | 2.45 | .48 | ||
| Yes | |||||
| Diagnosis of osteoporosis | No + missing | 7.09 | .07 | 6.94 | .03 |
| Yes | |||||
| Cardiovascular disease | No + missing | 9.79 | .02 | 7.59 | .02 |
| Yes | |||||
| Hormone receptor status | Unknown | 3.45 | .33 | ||
| Positive | |||||
| Lymph node status | Negative | 31.05 | <.001 | 11.72 | <.001 |
| Unknown | |||||
| Positive | |||||
| Adjuvant chemotherapy | No | 0.79 | .85 | ||
| Yes | |||||
Hypothesis H1: Factor does not affect type or time to death. Test criterion was the likelihood ratio criterion that has an approximately chi-square distribution on . Only factors with an indication of statistical significance were considered in H2. All statistical tests were two-sided.
Hypothesis H2: Factor effect is same for all three causes of death. Test criterion was the likelihood ratio criterion that has an approximately chi-square distribution on .
Menopausal status was defined in terms of a cut point at 50 years of age; those at least 50 years of age were considered to be postmenopausal.
Age was strongly and statistically significantly associated with death (from hypothesis H1, ; P < .001) (Table 2). Older age was associated with statistically significantly increased mortality from all three causes of death (Table 3), with the strongest evidence of association being for death from other causes (from hypothesis H2, ; P = .01) (Table 2): breast cancer death (P = .02), death from other malignancies (P < .001), and death from other causes (P < .001) (Table 3). These results paralleled those in a cause-specific stepwise multivariable model, with older age (in years) at trial entry being associated with increased risk of death from breast cancer (effect β = -0.02, 95% CI = -0.04 to 0.0, with the confidence interval based on the assumption that values for β have an approximately normal distribution; P = .003, on the basis of the likelihood ratio criterion for factor effect in a stepwise model []), from other malignancies (effect β = -0.04, 95% CI = -0.06 to -0.02; P < .001), and from other causes (effect β = -0.06, 95% CI = -0.08 to -0.04, P < .001) (Table 4).
Table 3.
Factor effects by type of death*
| Covariate | Model† | Breast cancer—specific death |
Death from other malignancies |
Death from other causes |
|||
|---|---|---|---|---|---|---|---|
| β (SE) [95% CI]‡ |
P value§ | β (SE) [95% CI]‡ |
P value§ | β (SE) [95% CI]‡ |
P value§ | ||
| Age | In years | -0.02 (0.01) [-0.04 to 0.0] |
.02 | -0.03 (0.01) [-0.05 to -0.01] |
<.001 | -0.06 (0.01) [-0.08 to -0.04] |
<.001 |
| Lymph node status |
Negative, unknown, or positive |
-0.37 (0.07) [-0.51 to -0.23] |
<.001 | -0.04 (0.07) [-0.18 to 0.1] |
.53 | -0.12 (0.07) [-0.26 to 0.02] |
.10 |
| Diagnosis of osteoporosis |
No + missing or yes |
0.32 (0.19) [-0.06 to 0.7] |
.07 | -0.33 (0.16) [-0.65 to -0.01] |
.05 | 0.01 (0.18) [-0.35 to 0.37] |
.94 |
| Cardiovascular disease |
No + missing or yes |
0.09 (0.16) [-0.23 to 0.41] |
.55 | 0.03 (0.18) [-0.33 to 0.39] |
.86 | -0.46 (0.15) [-0.76 to -0.16] |
.002 |
β = factor effect; SE = standard error; CI = confidence interval based on assumption that β has an approximately normal distribution.
Factor effects are provided where there is rejection of hypothesis H2, that the factor effect is the same for all three causes of death. The associations of each factor with each cause of death were examined in the full-factor models, which included all of the factors.
All statistical tests were two-sided.
P value is based on inclusion of the factor in the full-factor model using the two-sided likelihood ratio criterion that has an approximate chi-square distribution with .
Table 4.
Multivariable analysis of factors by type of death*
| Covariate | Model† | Breast cancer—specific death‡ |
Death from other malignancies |
Death from other causes |
|||
|---|---|---|---|---|---|---|---|
| β (SE) [95% CI] |
P value§ | β (SE) [95% CI] |
P value§ | β (SE) [95% CI] |
P value§ | ||
| Treatment group | Placebo or letrozole |
-0.05 (0.12) [-0.29 to 0.19] |
.68 | 0.05 (0.13) [-0.21 to 0.31] |
.72 | 0.12 (0.13) [-0.14 to 0.38] |
.36 |
| Age | In years | -0.02 (0.01) [-0.04 to 0.0] |
.003 | -0.04 (0.01) [-0.06 to -0.02] |
<.001 | -0.06 (0.01) [-0.08 to -0.04] |
<.001 |
| Hormone receptor status |
Unknown or positive |
0.37 (0.28) [-0.19 to 0.93] |
.19 | 0.39 (0.31) [-0.23 to 1.01] |
.21 | -0.33 (0.47) [-1.27 to 0.61] |
.48 |
| Lymph node status | Negative, unknown, or positive |
-0.37 (0.07) [-0.51 to -0.23] |
<.001 | -0.05 (0.07) [-0.19 to 0.09] |
.50 | -0.11 (0.07) [-0.25 to 0.03] |
.13 |
| Adjuvant chemotherapy | No or yes | -0.03 (0.12) [-0.27 to 0.21] |
.84 | 0.08 (0.17) [-0.26 to 0.42] |
.63 | -0.12 (0.15) [-0.42 to 0.18] |
.43 |
| Interaction of treatment group and cardiovascular disease |
-0.68 (0.19) [-1.06 to -0.30] |
<.001 | |||||
| Interaction of treatment group and tamoxifen treatment |
0.29 (0.15) [-0.01 to 0.59] |
.05 | |||||
β = factor effect; SE = standard error; CI = confidence interval based on assumption that β has an approximately normal distribution.
Models were built with the stepwise forward addition of a factor with a likelihood ratio criterion that has an approximately chi-square distribution on having P ≤ .05. Treatment and the stratification factors hormone receptor status, lymph node status, and adjuvant chemotherapy had a forced entry in the models. All other factors, as well as all interactions between treatment and factors, were considered in stepwise selection. The log-normal model has the factors of a Cox model. Cells are empty if the factor was not included in the cause-specific model.
Cause-specific survival of breast cancer (Table 5) used the logarithm of survival time as a linear function of β × (factor values).
All statistical tests were two-sided.
Lymph node status was also strongly and statistically significantly associated with death (from hypothesis H1, ; P < .001) (Table 2). Lymph node involvement was associated with an increased risk of death from breast cancer, multivariable effect (effect β = -0.37, 95% CI = -0.51 to -0.23; P < .001) (Table 3), and was not associated with death from other malignancies or other causes (in stepwise modeling, effect β = -0.05, 95% CI = -0.19 to 0.09, P = .50; and effect β = -0.11, 95% CI = -0.25 to 0.03, P = .13) (Table 4).
A baseline report of osteoporosis was differentially associated with death (for hypothesis H2, ; P = .03) (Table 2), with a reduction in breast cancer deaths (effect β = 0.32, 95% CI = -0.06 to 0.7, P = .07), and increase in other malignancies (effect β = -0.33, 95% CI = -0.65 to -0.01, P = .05) (Table 3). However, a baseline report of osteoporosis was not included in any multivariable cause-specific stepwise model (Table 4) because it was not statistically significantly associated with survival.
A baseline report of cardiovascular disease was differently associated with mortality (for hypothesis H2, ; P = .02) (Table 2), leading to greatly increased risk of death from other causes (effect β = -0.46, 95% CI = -0.76 to -0.16, P = .002) (Table 3). The association between treatment and cardiovascular disease was included in the stepwise multivariable model for death from other causes (interaction effect β = -0.68, 95% CI = -1.06 to -0.30, P < .001) (Table 4). Treatment with letrozole was associated with a lower risk of death from other causes if cardiovascular disease was absent at baseline; with cardiovascular disease, treatment with placebo was associated with a lower risk of death from other causes (P < .001). The interaction effect between treatment and baseline report of cardiovascular disease was substantiated with two alternate survival analyses (P < .001 with both Cox and Weibull survival analysis models).
There was also a statistically significant association between trial therapy and length of previous tamoxifen use for breast cancer (interaction effect β = 0.29, 95% CI = -0.01 to 0.59, with the confidence interval based on the assumption that values for β have an approximately normal distribution; P = .05, from the likelihood ratio criterion for the interaction effect in a stepwise model []) (Table 4). Specifically, letrozole showed greater benefit in terms of lower risk of breast cancer mortality for those who had received more than 5 years of tamoxifen, and placebo was of greater benefit with lower risk of breast cancer mortality for those receiving 5 years or less of tamoxifen treatment.
The breast cancer model (Table 4) was used to provide 5-year survival of breast cancer for a group of patients with selected characteristics that were indicated by the results and an adjuvant chemotherapy question of interest (Table 5): patients who received letrozole, were estrogen and/or progesterone receptor positive, and had received adjuvant chemotherapy. A 50-year-old woman would be expected to have a breast cancer mortality rate of 1% (95% CI = 0% to 34%) if her breast cancer was lymph node negative or 4% (95% CI = 0.01% to 5.9%) if it was lymph node positive. A 70-year-old woman would have breast cancer mortality rates of 2% (95% CI = 0% to 46%) and 8% (95% CI = 0.03% to 70%), respectively, and an 80-year-old woman would have mortality rates of 3% (95% CI = 0.01% to 52%) and 10% (95% CI = 0.06% to 75%), respectively.
Table 5.
Five-year breast cancer survival of patients with early breast cancer treated with letrozole by age: a log-normal model*
| Age | Lymph node status | Survival†, % (95% CI) |
|---|---|---|
| 50 y | Negative | 0.99 (0.66 to 1.00) |
| Positive | 0.96 (0.41 to 0.9999) | |
| 70 y | Negative | 0.98 (0.54 to 1.00) |
| Positive | 0.92 (0.30 to 0.9997) | |
| 80 y | Negative | 0.97 (0.48 to 0.9999) |
| Positive | 0.90 (0.25 to 0.9994) |
Patients were estrogen and/or progesterone receptor positive and had received adjuvant chemotherapy. CI = confidence interval.
Survival was based on the log-normal breast cancer model in Table 3.
Discussion
We found that non—breast cancer-related deaths were more common than breast cancer—related deaths in this cohort of 5-year breast cancer survivors, accounting for 60% of known deaths (72% for those 70 years or older and 48% for those younger than 70 years). Two baseline factors had different associations by type of death: cardiovascular disease was associated with a statistically significant increased risk of death from other causes (P = .002), and osteoporosis was associated with a statistically significant increased risk of death from other malignancies (P = .05). An increased risk of breast cancer—specific death was associated with lymph node involvement (P < .001). Increased risk of death from all three causes was associated with older age (P < .001). A 50-year-old woman on letrozole who had an estrogen receptor— and/or progesterone receptor—positive tumor and had received adjuvant chemotherapy would be expected to have a breast cancer mortality rate of 1% if her breast cancer was lymph node negative or 4% if it was lymph node positive. A 70-year old woman would have breast cancer mortality rates of 2% and 8%, respectively, and an 80-year-old woman would have breast cancer mortality rates of 3% and 10%, respectively. Optimal management and prevention of non—breast cancer-related illness are therefore important for patients with early-stage breast cancer, particularly for older women.
Routine use of screening mammography and improved therapeutic management of breast cancer, especially with generally well-tolerated hormonal therapy or biologic agents, will mean that more women will survive breast cancer to older ages, at which they might have a higher risk of death from causes other than breast cancer. Consequently, one has to think beyond clinical trial assessments of overall survival because assessment of a patient’s likelihood of dying from breast cancer or treatment-related sequela will be confounded by the existence of competing risks of death (8-19). It becomes important at a general societal level to consider relative survival from breast cancer, which takes into account other risks of mortality in the population at large. In this study, we analyzed the available information from the MA.17 trial about dying from breast cancer, another malignancy, or causes other than cancer. Better ascertainment and understanding the risk of death from breast cancer versus the risk of death from other causes should be useful in selecting the most appropriate therapeutic management for individual patients.
We reported the results of competing risks analyses for the NCIC CTG MA.17 trial, a double-blinded, placebo-controlled multicenter trial that evaluated the use of maintenance hormonal therapy with 5 years of letrozole therapy among patients with breast cancer who have had no recurrence during approximately 5 years of adjuvant tamoxifen therapy (20). This trial was a particularly good setting in which to investigate competing risks of death because of the trial entry requirement that the breast cancer be hormone receptor positive (a characteristic more common with increasing age), the shift in follow-up to a period 5 years later than primary adjuvant therapy, and no upper age restriction for the trial. Women who were accrued to MA.17 thus would be at higher risk for non—breast cancer death (15,21), and 26% of them were at least 70 years of age.
This study had several limitations. All patients with hormone receptor—negative breast cancer, and thus poorer prognosis, were excluded from this trial. The excluded women were generally younger than those included in the trial. Women accrued to MA.17 were also at lower risk of recurrence than women with newly diagnosed breast cancer because they had to be disease free after 5 years of treatment with tamoxifen; 95% of patients had survived a median of 3.9 years on trial. Determination of cause of death was predominantly with clinical reports because no autopsy was mandated; however, there was an indication of competing risks from the overlapping occurrence of breast cancer, other malignancies, and other causes of death for women of all ages. Only 2% of women who were deemed by medical review to have died with other malignancies and 9% of those who died from other causes were reported to have had distant metastases or contralateral breast cancer. The potential for confounding with multiple concurrent causes of death was low, and the number of patients was too small to consider deaths from multiple causes separately. The assignment of deaths to other malignancies or to other causes when there has been breast cancer progression is conservative to the question addressed in this work of whether the baseline MA.17 trial factors differentially influenced whether a woman would die of breast cancer, other malignancies, or other causes. The focus of this report was the pragmatic assessment of factors associated with the three types of death. An indication of similar associations would have supported the standard approach of including all types of death and determining the factors affecting overall survival. We cannot extend our results to breast cancer patients of all ages, patients with receptor-negative disease, or even similar patients with other lengths of follow-up.
Another limitation is that the observation that letrozole treatment did not statistically significantly improve overall survival in the MA.17 trial (20) may have resulted from unblinding the MA.17 trial at a median follow-up of 2.4 years because of statistically significantly better disease-free survival among women treated with letrozole than among those treated with placebo. All patients were offered letrozole treatment, and most patients in the placebo arm accepted that offer. Of the 2587 women assigned to placebo, 2383 were alive at unblinding, and 1579 (66%) of the 2383 women were confirmed to have started letrozole treatment.
Another possible reason for letrozole not impacting overall survival might be the operation of competing risks of death, with factors differentially affecting different causes of death. Associations between each factor and the outcomes were examined in the presence of all other factors assessed, so that the indication that older age was associated with statistically significantly increased risk of death from all causes (P < .001) was obtained from an analysis that also included, for example, lymph node status, treatment modalities, and indicators of a woman’s baseline health status. We examined the effect of age continuously in years because both underlying risk of death and anticipated hormone responsiveness to letrozole were expected to increase with age. This study’s results reflected the full population of the MA.17 trial, with a median age of 62 years, who at baseline were disease free after 5 years of tamoxifen treatment. Cause-specific multivariable results support the competing risk assessments in that the older women had higher mortality from breast cancer (P = .003), from other malignancies (P < .001), and from other causes (P < .001) than younger women.
Lymph node involvement was also associated with statistically significantly increased mortality (P < .001), especially from breast cancer—specific death (P < .001), with a possible trend to shorter survival from other causes of death (P = .13). This result is consistent with a previous report (20) that letrozole treatment was statistically significantly associated with improved overall survival only among lymph node—positive patients. However, neither letrozole treatment by itself nor the combination of treatment and lymph node status was statistically significantly associated with mortality. Consequently, the association between lymph node status and other causes of death should be reexamined after longer follow-up.
A report of baseline osteoporosis was differentially associated with the type of death (P = .03)—that is, with fewer breast cancer deaths and more deaths from other malignancies. It is possible that women with osteoporosis at baseline were treated with bisphosphonates, which reduced breast cancer mortality. However, additional studies are required to examine this possibility.
The baseline presence of cardiovascular disease was also differentially associated with mortality (P = .02), with a greatly increased risk of death from other causes. In multivariable analyses, a treatment interaction was found with cardiovascular disease and a detrimental affect was observed with letrozole administration (P < .001) among patients who had cardiovascular disease at baseline. Baseline reporting, however, was not verified, and so this association should be examined more thoroughly, perhaps with adjudication, in other trials evaluating aromatase inhibitors.
Finally, we found a treatment interaction with length of previous tamoxifen use (P = .05)—patients who had used tamoxifen for more than 5 years benefited more from letrozole treatment than those who had used it for less than 5 years. It is not clear whether this association is an artifact related to confounding because patients with longer adjuvant tamoxifen therapy had events during the pretrial period and were not included in this study or whether letrozole treatment was associated with improved survival among patients who were beginning to experience an estrogenic effect (ie, have increased levels of estrogen) from longer tamoxifen usage. Elimination of the apparent differences between letrozole and placebo groups after 2.5 years of letrozole treatment may be attributed to the substantive crossover of original placebo patients who began to use letrozole.
In summary, in the maintenance adjuvant endocrine phase of follow-up in the MA.17 trial, older women had increased mortality, with a particularly strong (P < .001) association with death from other causes. The association between increased risk of death from other causes among women with baseline cardiovascular disease (P < .001) requires further study. Letrozole treatment was associated with decreased mortality among women who were treated for more than 5 years with tamoxifen (P = .05). Further, the increased mortality related to lymph node involvement appeared to be strongly associated with breast cancer—specific death. Although older women had a higher risk of dying from non—breast cancer causes, the reduced level of relapse with letrozole therapy was similar in younger and older patients, further supporting consideration of extended adjuvant endocrine therapy with letrozole for older women. Results of this study point out that medical attention to the potential for death from other causes becomes increasingly important in older populations of patients with breast cancer.
Acknowledgments
Funding: AstraZeneca Postdoctoral Fellow to D.M.; Canadian Cancer Society through the National Cancer Institute of Canada grant 10362; grants from the National Cancer Institute in the United States (CA31946, CA21115, CA25224, CA38926 and CA32102); Novartis Pharmaceuticals to National Cancer Institute of Canada Clinical Trials Group.
Notes: The reviewers’ and Senior Editor’s input was appreciated and greatly helped to improve the exposition of this work. The authors acknowledge the dedicated work of those who contributed to the NCIC CTG MA.17 trial; the international trialists; clinicians who enrolled patients; clinical research associates who acquired the data; NCIC CTG computer systems group, especially Liting Zhu; and most of all the patients who participated in the trial.
The authors had full responsibility for the design of the study, collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
Footnotes
Editor’s note: Dr J. N. Ingle received honoria from Novartis for consulting and for speaking. Dr P. E. Goss received consulting honoria from Novartis, Pfizer, and AstraZeneca.
References
- 1.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services . Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research Center for Biologics Evaluation and Research; 2007. http://www.fda.gov/cber/gdlns/clintrialend.pdf [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 5.Henderson IC, Patek AJ. The relationship between prognostic and predictive factors in the management of breast cancer. Breast Cancer Res Treat. 1998;52:261–288. doi: 10.1023/a:1006141703224. [DOI] [PubMed] [Google Scholar]
- 6.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–168. [PubMed] [Google Scholar]
- 7.Isaacs C, Stearns V, Hayes DF. New prognostic factors for breast cancer recurrence. Semin Oncol. 2001;28(1):53–67. doi: 10.1016/s0093-7754(01)90045-4. [DOI] [PubMed] [Google Scholar]
- 8.Gail M. A review and critique of some models use in competing risk analysis. Biometrics. 1975;31(1):209–222. [PubMed] [Google Scholar]
- 9.Lagakos SW. A covariate model for partially censored data subject to competing causes of failure. Appl Stat. 1978;27(3):235–241. [Google Scholar]
- 10.Prentice RL. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 11.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 12.Gelman R, Gelber R, Henderson IC, Coleman CN, Harris JR. Improved methodology for analyzing local and distant recurrence. J Clin Oncol. 1990;8(3):548–555. doi: 10.1200/JCO.1990.8.3.548. [DOI] [PubMed] [Google Scholar]
- 13.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12:737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 14.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat Assoc. 1993;88(422):400–409. [Google Scholar]
- 15.Fish EB, Chapman JW, Link MA. Competing causes of death for primary breast cancer. Ann Surg Oncol. 1998;5(4):368–375. doi: 10.1007/BF02303502. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Tai B-C, Machin D, White I, Gebski V. Competing risks analysis of patients with osteosarcoma: a comparison of four different approaches. Stat Med. 2001;20:661–684. doi: 10.1002/sim.711. [DOI] [PubMed] [Google Scholar]
- 19.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97(17):1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 21.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19(4):980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 22.Chapman JW, Lickley HLA, Trudeau ME, et al. Ascertaining prognosis for breast cancer in node-negative patients with innovative survival analysis. Breast J. 2006;12(1):37–47. doi: 10.1111/j.1075-122X.2006.00183.x. [DOI] [PubMed] [Google Scholar]
- 23.Lawless JF. Statistical models and methods for lifetime data. In: Balding DJ, Bloomfield P, Cressie NAC, et al., editors. Wiley Series in Probability and Statistics. 2nd ed John Wiley & Sons, Inc.; Hoboken, NJ: 2003. [Google Scholar]
- 24.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–723. [Google Scholar]
- 25.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 26.Cox DR, Snell EJ. Applied Statistics: Principles and Examples. Chapman and Hall; London, UK: 1981. [Google Scholar]