Abstract
Experimental evidence indicates that leptin-deficient animals develop left ventricular (LV) hypertrophy, but data relating circulating leptin levels to cardiac structure and function in individuals older than 70 years are lacking. We related circulating leptin concentrations to echocardiographic measures of cardiac structure and function in 432 participants of the community-based Framingham Heart Study (mean age 75 years, 67% women) who underwent echocardiography at a routine examination (approximately 4 years before leptin concentrations were assayed). In multivariable linear regression, logarithmically-transformed sex-standardized leptin concentrations were related to the following echocardiographic measures: LV mass, left atrial (LA) size, and fractional shortening (primary analysis); LV wall thickness and LV end-diastolic dimensions (the 2 components of LV mass) and the transmitral E/A ratio (secondary analysis). Leptin concentrations were inversely associated with LV mass, LV wall thickness and LA size (p<0.04 for all). The top sex-specific tertile of leptin was associated with an adjusted-LV mass 16 gram lower compared to the lowest tertile (p=0.017 for trend across tertiles). Leptin levels were not associated with LV fractional shortening, the E/A ratio or LV end-diastolic diameter (p>0.16). In conclusion, our cross-sectional observations suggest a cardioprotective influence of leptin on LV remodeling consistent with experimental data, and may provide insight into the potential role of leptin-resistance as a mediator of obesity-associated cardiomyopathy.
Keywords: leptin, cardiac remodeling, left ventricular mass, left atrial size
Introduction
The adipokine Leptin regulates appetite and food consumption through central nervous system mechanisms1, but it is increasingly recognized that leptin also exerts a broad range of actions on peripheral organs, including the cardiovascular system. Increasing evidence indicates that leptin influences cardiac remodeling, although it remains unclear whether the net effect is cardioprotective versus adverse. Some studies indicated direct hypertrophic effects of leptin on isolated ventricular rat cardiomyocytes,2 whereas other animal models suggest that leptin is essential for maintaining normal cardiac structure.3, 4 Clinical studies have reported higher leptin levels in patients with heart failure.5 It is, however, unclear whether leptin contributes to the development of heart failure, or if the higher leptin levels are secondary responses to cardiac damage. We tested the hypothesis that leptin is cardioprotective by relating circulating leptin concentrations to echocardiographic measures of cardiac structure and function in an unselected community-based sample (mean age: 75 years, range 68 to 91 years).
Methods
Details about the design and the selection criteria of the Framingham Heart Study have been published elsewhere.6 A total of 1407 participants attended their 20th biennial examination cycle. Of these, 686 participants had available echocardiographic information from their 20th biennial examination. Participant without available echocardiographic data were older, heavier, more likely to be men and have diabetes mellitus, and more prevalent cardiovascular disease (CVD) as compared to those in whom echocardiograms could be obtained. No differences were observed between the two groups with respect to systolic and diastolic blood pressure, smoking, total/high-density lipoprotein cholesterol ratio and leptin levels. The worse cardiovascular risk profile in individuals with missing echocardiographic data is well known in epidemiological studies.7, 8 Serum leptin concentrations were assayed in attendees at their 22nd biennial examination, approximately 4 years later. We excluded an additional 254 participants because of non-available leptin levels. Participants with missing leptin levels were older, had a higher prevalence of CVD (including heart failure) and atrial fibrillation but did not differ with respect to traditional cardiovascular risk factors (except age) from participants with available leptin levels. After exclusions, 432 participants (mean age 75 years, 67% women) remained eligible for the present analyses. All participants provided written informed consent and the study was performed in accordance with the Helsinki Declaration of 1975 (as revised in 1983) and the study protocol was approved by the Institutional Review Board at the Boston University Medical Center.
Serum leptin concentrations were assayed at the examination cycle 22 with a commercially available radioimmunoassay (Linco Research, Inc., St. Louis, MO).9 The inter-assay coefficients of variation ranged from 3.0–6.2%. The lower sensitivity limit was 0.5 ng/ml.
At the 20th examination cycle, participants underwent routine transthoracic echocardiography with Doppler color flow imaging using a Sonos 1000 Hewlett-Packard machine. M-Mode measurements of the left ventricular (LV) septal and posterior wall thickness (both measured at end-diastole), left atrial (LA) size (end-systole) and LV internal dimensions at end-diastole and at end-systole were obtained using a leading edge technique as recommended by the American Society of Echocardiography.10 LV fractional shortening was defined as (LV end-diastolic diameter – LV end-systolic diameter)/LV end-diastolic diameter and served as a measure of LV systolic function. The ratio of the early/late (E/A) transmitral diastolic filling velocities was also measured at the index examination, as described previously.11 LV mass was calculated using the formula: LV mass= 0.8{1.04[(LV end-diastolic diameter+ LV posterior wall thickness + LV septal wall thickness)3−(LV end-diastolic diameter)3]}+0.6.12
Leptin levels were natural logarithmically-transformed, and standardized within each sex (to a mean of 0 and a standard deviation [SD] of 1), given the known sex-related differences in the distribution of leptin levels.13 Echocardiographic traits were likewise standardized within each sex (mean=0, SD=1), except for the E/A ratio. All analyses were for pooled sexes, given the lack of a statistically significant interaction of leptin with sex for any of the echocardiographic traits (p exceeded 0.20 for all traits).
In primary analyses, we related log-leptin (independent variable) to 3 echocardiographic traits (dependent variables), i.e., LV mass, LA size, and fractional shortening). Multivariable linear regression models adjusted for the following correlates of echocardiographic measurements: age, sex, height, and weight (model 1); age, sex, height, weight, systolic blood pressure, antihypertensive treatment, diabetes, current smoking (smoking within the year prior to the examination), and the ratio of total to high-density lipoprotein cholesterol (multivariable-adjusted model 2). We reran the analyses with LV mass and LA size indexed to body surface area as outcome variables, omitting height and weight from the multivariable-adjusted model.
We performed secondary analyses to evaluate if the association of LV mass with leptin was mediated by a relation to LV wall thickness versus LV end-diastolic dimensions, the 2 components used to calculate LV mass and we also related leptin to the E/A ratio. The multivariable model for the E/A ratio was additionally adjusted for heart rate.11 Since the primary analyses used log-transformed leptin levels and sex-standardized echocardiographic measures, we performed additional analyses to simplify the interpretation of changes in echocardiographic measurements in original units in relation to increments of leptin in original units. Therefore, we estimated least squares adjusted-means for LV mass (and it 2 components, LV wall thickness and LV end-diastolic dimensions) across sex-specific leptin tertiles using analyses of covariance adjusting for covariates in model 2. Furthermore, we repeated our analyses after excluding participants with prevalent myocardial infarction (n=29) or heart failure (n=12) at examination cycle 20.
Results
The clinical, biochemical and echocardiographic features of the study sample are shown in Table 1. The sample was predominantly female (67%) and characterized by a high prevalence of diabetes and cardiovascular disease. As previously reported in the literature,13 leptin levels were significantly higher in women. Mean age was 75 years (range 68 to 91 years).
Table 1.
Clinical, biochemical and echocardiographic characteristics of the study sample (n=432).a
| Variable | Women (n=290) | Men (n=142) |
|---|---|---|
| Age (years) | 74.8±4.5 | 74.2±4.1 |
| Systolic blood pressure (mm Hg) | 145±20 | 149±22 |
| Diastolic pressure (mm Hg) | 75±10 | 78±11 |
| Body mass index (kg/m2) | 26.0±4.6 | 27.0±3.8 |
| Antihypertensive treatment | 130 (45%) | 62 (44%) |
| Current smoker | 29 (10%) | 13 (9%) |
| Diabetes mellitus | 67 (23%) | 33 (23%) |
| Prevalent heart failure | 6 (2.0%) | 6 (4%) |
| Prevalent cardiovascular disease | 52 (18%) | 51 (36 %) |
| Total/high-density lipoprotein cholesterol (mg/dL) | 4.4±1.5 | 5.4±1.6 |
| Leptin (ng/ml) | 16.8 (9.9–25.5) | 6.5 (4.4–10.3) |
| Log-leptin | 2.8±0.7 | 1.9±0.7 |
| LV mass (g) | 161±36 | 216±54 |
| LV wall thickness (cm) | 1.99±0.24 | 2.15±0.33 |
| LV end-diastolic diameter (cm) | 4.62±0.41 | 5.18±0.43 |
| Left atrial size (cm) | 3.96±0.59 | 4.33±0.55 |
| LV fractional shortening (%) | 38.25 | 35.09 |
| Mean peak velocity E/A | 0.71±0.32 | 0.73±0.30 |
LV, left ventricular; E/A, early/late transmitral diastolic filling velocities.
Clinical and echocardiographic features were measured at the 20th biennial examination cycle; leptin was assayed at the 22nd examination cycle.
Data are percent for binary traits and mean±SD for continuous traits; except for leptin (median (Q1, Q3)).
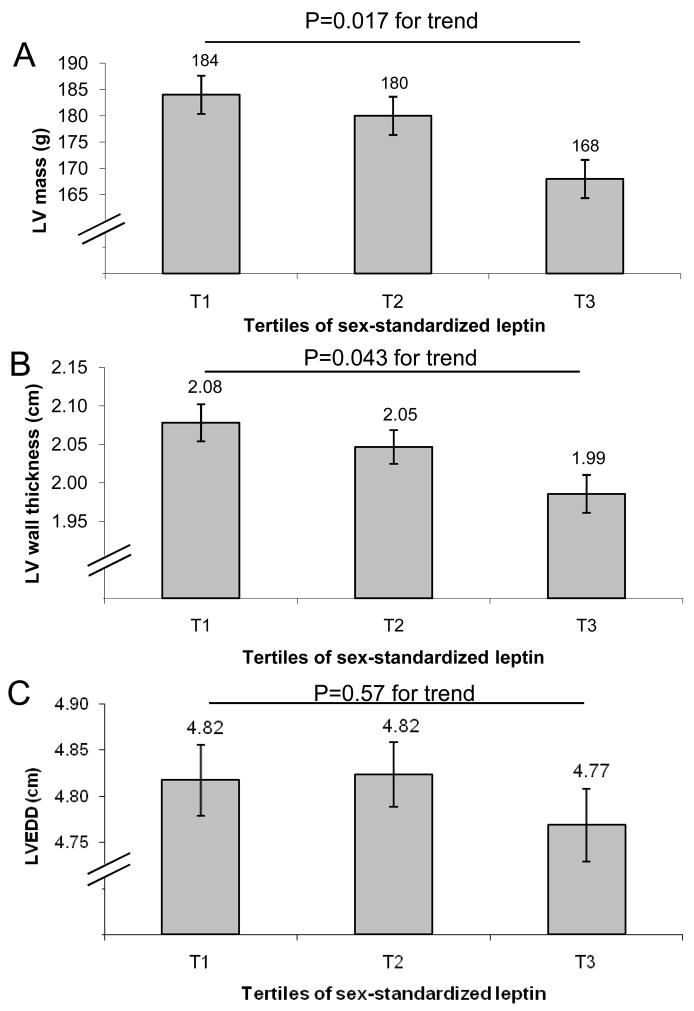
We observed a statistically significant inverse association of leptin levels with LV mass, LV wall thickness and LA size (Table 2). Consistent with these observations, LV mass decreased by 16 gm and LV wall thickness was about 1 mm lower in the top sex-specific leptin tertile compared to the lowest tertile (Figure 1, Panels A and B). Leptin levels were not associated with fractional shortening, the E/A ratio or with LV end-diastolic dimensions (Table 2 and Figure 1, Panel C). Indexing LV mass and LA size to body surface area (and omitting height and weight from the multivariable model) yielded comparable results (LV mass, β (SE): −0.110 (0.048) per 1-SD increment in sex-standardized log-leptin, p=0.02; LA size, β (SE): −240 (0.048) per 1-SD increment in sex-standardized log-leptin, p<0.0001). In secondary analyses after the exclusion of participants with prevalent myocardial infarction or heart failure, the association of leptin with LV mass and LA size remained robust, whereas the association with LV wall thickness was attenuated (Online Supplementary Table). Additional adjustment for weight change from exam 20 to 22 revealed similar results (data not shown).
Table 2.
Association of log-leptin with echocardiographic measures of left ventricular (LV) structure and function in the entire dataset (n=432).
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Beta (SE) | p-value | Beta (SE) | p-value | |
| Primary traits | ||||
| LV mass | −0.143 (0.056) | 0.01 | −0.134 (0.056) | 0.02 |
| Left atrial size | −0.115 (0.060) | 0.06 | −0.131 (0.062) | 0.04 |
| Fractional shortening | 0.005 (0.061) | 0.94 | −0.015 (0.062) | 0.81 |
| Secondary traits | ||||
| LV wall thickness | −0.148 (0.059) | 0.01 | −0.137 (0.059) | 0.02 |
| LV end-diastolic diameter | −0.032 (0.059) | 0.59 | −0.030 (0.061) | 0.62 |
| Mean peak velocity E/A* | −0.034 (0.019) | 0.075 | −0.028 (0.020) | 0.16 |
SE, standard error; E/A, early/late transmitral diastolic filling velocities.
The regression coefficient indicates the change in the echocardiographic traits (in standardized units) per one-SD increase in log-leptin. Thus, a change in leptin of eSD logleptin (which is an increase 2.0-fold in men and women) is associated with a decrement of LV mass of 9.1 gms (4.2%) in men and 6.1 gms (3.8%) in women.
Model 1 adjusted for age, sex, height and weight.
Model 2 adjusted for age, sex, height, weight, systolic blood pressure, antihypertensive treatment, diabetes, current smoking and total/high-density lipoprotein ratio.
Multivariable model for mean peak velocity E/A was additionally adjusted for heart rate.
Figure 1.
Least squares means of left ventricular (LV) mass (Panel A), wall thickness (Panel B) and LV end-diastolic diameters (LVEDD; Panel C) according to sex-specific tertiles of leptin. All models adjust for age, sex, height, weight, systolic blood pressure, antihypertensive treatment, diabetes, current smoking, and the ratio of total to high-density lipoprotein cholesterol.
Discussion
We observed a significant inverse association of circulating leptin concentrations with LV mass, LV wall thickness and with LA size in our community-based sample of individuals older than 68 years. In contrast, we did not observe any association of leptin with fractional shortening, the E/A ratio or LV end-diastolic diameter. These findings, although cross-sectional, are consistent with the notion that leptin favorably influences cardiac structure. This concept is in agreement with published epidemiological and experimental data. In the community, obesity (which is characterized by leptin resistance14) is associated with larger LA size15, higher LV mass16 and wall thickness16, diastolic dysfunction17, and confers an increased risk of heart failure.18
As noted above, recent experimental data also support a cardioprotective effect of leptin.3, 4 Other investigators also have reported worse cardiac function and prognosis after experimentally-induced myocardial infarction in leptin-deficient mice,4 consistent with this notion. However, the experimental and clinical data on leptin effects on the heart are not entirely consistent. A few experimental studies have reported adverse cardiac effects of leptin on isolated cardiomyocytes.2, 19 Thus, leptin inhibited contractility19 and promoted hypertrophy of rat ventricular myocytes in some2 but not in other reports.20 Likewise, some previous clinical studies found positive associations between leptin levels and LV mass, geometry and cardiac function in selected patient groups.21–23
We acknowledge several limitations. The sample size was moderate, which may have limited our statistical power to detect modest associations of leptin with other echocardiographic measurements. Furthermore, the generalizability of our results is limited: our sample included participants 68 to 91 years of age, predominantly white of European ancestry. The echocardiograms were obtained on average four years prior to the examination at which leptin was assayed. We would expect that this time lag between echocardiography and leptin measurements would result in random misclassification and would bias us towards the null hypothesis of no association of leptin with cardiac measurements. Also, the cross-sectional design precludes any causal inference. An investigation of serial leptin and echocardiographic measurements would be needed to more precisely quantify the relationship between leptin levels and echocardiographic traits. BMI does not distinguish between adipose and non-adipose tissue, and is thus a suboptimal measure of body composition. Additionally, no other adipokines or measures of insulin resistance were measured in the original cohort at the index examination; so we are unable to further elucidate the relations of other biomarkers of adipose tissue origin, insulin resistance and cardiac remodeling.
Supplementary Material
Online Supplementary Table. Association of leptin levels with echocardiographic measures of left ventricular structure and function after excluding participants with prevalent myocardial infarction or heart failure.
Acknowledgments
Source(s) of Funding: This work was supported through National Institutes of Health/National Heart, Lung, and Blood Institute Contract N01-HC-25195, 2 K24 HL04334, RO1HL080124, and 1R01DK080739 (all to RSV), and 6R01-NS 17950.
This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195); and 2K24 HL04334, RO1HL080124, 1R01DK080739 (all to RSV), and 6R01-NS 17950
Footnotes
Disclosure(s): Dr. Roubenoff is an employee of Biogen Idec, Inc, but reports no conflict of interest with the subject of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 2.Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 3.Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 4.McGaffin KR, Sun CK, Rager JJ, Romano LC, Zou B, Mathier MA, O’Doherty RM, McTiernan CF, O’Donnell CP. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc Res. 2008;77:54–63. doi: 10.1093/cvr/cvm023. [DOI] [PubMed] [Google Scholar]
- 5.Schulze PC, Kratzsch J, Linke A, Schoene N, Adams V, Gielen S, Erbs S, Moebius-Winkler S, Schuler G. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur J Heart Fail. 2003;5:33–40. doi: 10.1016/s1388-9842(02)00177-0. [DOI] [PubMed] [Google Scholar]
- 6.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage DD, Garrison RJ, Kannel WB, Anderson SJ, Feinleib M, Castelli WP. Considerations in the use of echocardiography in epidemiology. The Framingham Study. Hypertension. 1987;9:II40–II44. doi: 10.1161/01.hyp.9.2_pt_2.ii40. [DOI] [PubMed] [Google Scholar]
- 8.Devereux RB, Roman MJ, Liu JE, Lee ET, Wang W, Fabsitz RR, Welty TK, Howard BV. An appraisal of echocardiography as an epidemiological tool. The Strong Heart Study. Ann Epidemiol. 2003;13:238–244. doi: 10.1016/s1047-2797(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 9.Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M. Radioimmunoassay of leptin in human plasma. Clin Chem. 1996;42:942–946. [PubMed] [Google Scholar]
- 10.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 11.Galderisi M, Benjamin EJ, Evans JC, D’Agostino RB, Fuller DL, Lehman B, Levy D. Impact of heart rate and PR interval on Doppler indexes of left ventricular diastolic filling in an elderly cohort (the Framingham Heart Study) Am J Cardiol. 1993;72:1183–1187. doi: 10.1016/0002-9149(93)90991-k. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 13.Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82:579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Reed DR, Price RA. Leptin resistance is associated with extreme obesity and aggregates in families. Int J Obes Relat Metab Disord. 2001;25:1471–1473. doi: 10.1038/sj.ijo.0801736. [DOI] [PubMed] [Google Scholar]
- 15.Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension. 1995;25:1155–1160. doi: 10.1161/01.hyp.25.6.1155. [DOI] [PubMed] [Google Scholar]
- 16.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–236. [PubMed] [Google Scholar]
- 17.Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, Doring A, Broeckel U, Riegger G, Schunkert H. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320–328. doi: 10.1016/s0195-668x(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 18.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 19.Nickola MW, Wold LE, Colligan PB, Wang GJ, Samson WK, Ren J. Leptin attenuates cardiac contraction in rat ventricular myocytes. Role of NO. Hypertension. 2000;36:501–505. doi: 10.1161/01.hyp.36.4.501. [DOI] [PubMed] [Google Scholar]
- 20.Pinieiro R, Iglesias MJ, Eiras S, Vinuela J, Lago F, Gonzalez-Juanatey JR. Leptin does not induce hypertrophy, cell cycle alterations, or production of MCP-1 in cultured rat and mouse cardiomyocytes. Endocr Res. 2005;31:375–386. doi: 10.1080/07435800500456937. [DOI] [PubMed] [Google Scholar]
- 21.Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, Petrocelli A, Carella C, de Divitiis O, Varricchio M. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension. 1999;34:1047–1052. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- 22.Galderisi M, Tagliamonte MR, D’Errico A, Carella C, Varricchio G, Mondillo S, de Divitiis O, Paolisso G. Independent association of plasma leptin levels and left ventricular isovolumic relaxation in uncomplicated hypertension. Am J Hypertens. 2001;14:1019–1024. doi: 10.1016/s0895-7061(01)02137-9. [DOI] [PubMed] [Google Scholar]
- 23.Perego L, Pizzocri P, Corradi D, Maisano F, Paganelli M, Fiorina P, Barbieri M, Morabito A, Paolisso G, Folli F, Pontiroli AE. Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab. 2005;90:4087–4093. doi: 10.1210/jc.2004-1963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplementary Table. Association of leptin levels with echocardiographic measures of left ventricular structure and function after excluding participants with prevalent myocardial infarction or heart failure.