Summary
We investigated the phenomenon of cell–cell aggregation (flocculation) in a serotype D strain of Cryptococcus neoformans (ATCC 24067, isolate RC-2). Cell aggregation into clumps of 5–40 cells (clump+ cells) occurred during the early log phase and disappeared in the beginning of the stationary phase (clump− cells). The cell aggregation phenomenon was medium dependent. Clump+ cells could be dispersed by either vortexing or proteinase K digestion. Most importantly, the transient change in cellular phenotype changed several important host–pathogen interactions. Adherence of clump+ cells to murine macrophage-like cells J774.16 was significantly (P < 0.001) enhanced compared with adherence of clump− cells. Furthermore, complement-mediated phagocytosis efficacy of dispersed clump+ cells was significantly higher (P < 0.001) compared with clump− cells. Similar findings were documented with an in vivo phagocytosis assay. Infection of mice with a low inoculum (104) of clump+ cells resulted in lower fungal burden when compared with mice infected with clump− cells. Accordingly, mice infected with clump+ cells survived significantly longer than mice infected with clump− cells. These results indicate that the cellular phenotype undergoes significant changes that result in a transient flocculation-like phenotype. We hypothesize that this cell–cell aggregation is the result of changes in protein content in the polysaccharide capsule. We conclude from our data that the change in cellular phenotype has a dramatic effect on cell adherence, and on complement-mediated phagocytosis, both of which can affect the pathogenesis of the disease in the host. Our results underscore the complexity of studies that investigate host pathogen interactions and may explain differences and inconsistencies observed in in vitro and in vivo assays.
Introduction
Cryptococcus neoformans is an encapsulated yeast with a worldwide prevalence. The fungus is ubiquitous in the environment and infection is acquired via the respiratory tract. C. neoformans can cause asymptomatic pulmonary infection in normal individuals or disseminate to the central nervous system in immunocompromised individuals. Cryptococcal meningitis is one of the most common fungal infections in AIDS patients. Several important virulence factors including the ability to grow at 37°C (Jacobson and Emery, 1991), capsule formation (Chang and Kwon-Chung, 1994), laccase activity (Rhodes et al., 1982; Salas et al., 1996) and phospholipase activity (Chen et al., 1997; Cox et al., 2001) have been identified.
Flocculation has been described, and thoroughly investigated, in other yeasts, including Saccharomyces cerevisiae and various Candida species (Gaur et al., 1999; Verstrepen et al., 2003; Halme et al., 2004). In S. cerevisiae flocculation is important in the fermentation process of wine and beer (Verstrepen et al., 2003). Several proteins (FLO gene family) have been identified and the genes confer adherence to agar, solid surfaces and other yeasts (Guo et al., 2000; Reynolds and Fink, 2001). In pathogens such as C. albicans and C. glabrata FLO-like adherence proteins mediate adherence to mammalian tissues (Cormack et al., 1999; Hoyer, 2001; De Las Penas et al., 2003). Yeast flocculation is an asexual calcium-dependent and a reversible cell–cell aggregation phenomenon. It is caused by the interaction between specific flocculation proteins and the carbohydrate residues (receptors) of the fungal cell wall (Miki et al., 1982). In this adhesion process, calcium ions induce the correct conformation of the flocculation proteins (Miki et al., 1982; Stratford, 1989). Studies in different fungal species have shown that these gene families are epigenetically regulated (Halme et al., 2004; Iraqui et al., 2005). Thorough searches of the Cryptococcus genome database have not yielded homologues (Wormley et al., 2005) to genes associated with flocculation, including SFL1 (Fujita et al., 1989), FLO1 (Powderly, 1993) and FLO11 (Wiederrecht et al., 1988).
Although very little is known about cell aggregation in C. neoformans, this phenomenon has been observed in C. neoformans strains. Fries et al. (1999) and Goldman et al. (1998) described that cells from wrinkled colony type of both serotype A and D variants exhibited flocculence (clumps) in liquid broth and adhered to the agar surface. Wormley et al. (2005) reported flocculation in the skn7 mutant of H99 strain. The SKN7 gene encodes a transcription factor and has an important role in the cellular response to oxidative stress. Flocculation in this mutant appeared after overnight incubation and was not suppressed by mannose or glucose.
Notably, we also found flocculation like cell–cell aggregation in liquid culture of commonly used serotype A and D strains like ATCC 24067, B3501 and H99. Flocculation was transient, easily dispersed by vortexing, and thus distinguishable from clumps formed by acapsular mutants, which are resistant to vortexing. The serotype D strain ATCC 24067 was used to investigate the effects of cell aggregation on host–pathogen interactions. We conclude that cellular changes associated with flocculation have a profound effect on adherence and tendency of C. neoformans to be taken up by macrophages, which in turn significantly affects virulence.
Results
Characterization of cell aggregation of RC-2
Flocculation in C. neoformans was investigated because this cell phenotype is a virulence-associated trait in other yeasts (Cormack et al., 1999; Hoyer, 2001; De Las Penas et al., 2003). C. neoformans strain RC-2 transiently flocculated in Sabouraud dextrose broth (SAB) and exhibited clumps containing 5–40 cells during the early exponential phase of growth. The phenomenon was visible by naked eye examination of culture suspensions. Cell clumps disappeared in the beginning of the stationary phase (Fig. 1A). Similar timing was observed for cultures started with different inocula (ranging from 104 cells ml−1 to 5 × 107 cells ml−1). The clumping percentage varied between strains (Fig. 1B) and ranged from 0 to 70%. The clumping percentage in strain RC-2 was maximal at 13 h if the culture had been started from a density of 105 cells ml−1 whereas clump− cells of this strain contained less than 5% clumping cells if grown beyond 13 h. By elutriation small recently budded cells could be isolated. This is a simple method to synchronize a cell population without chemically arresting cells. There was no significant difference of timing of cell aggregation and clumping percentage observed between elutriated cells and non-elutriated cells (data not shown). Hence, cell aggregation in RC-2 was associated with growth phase and not with cell cycle. Maximum cell clumping was observed during early log phase. Clumping was observed in heat-killed cells. Clumping was not observed at room temperature.
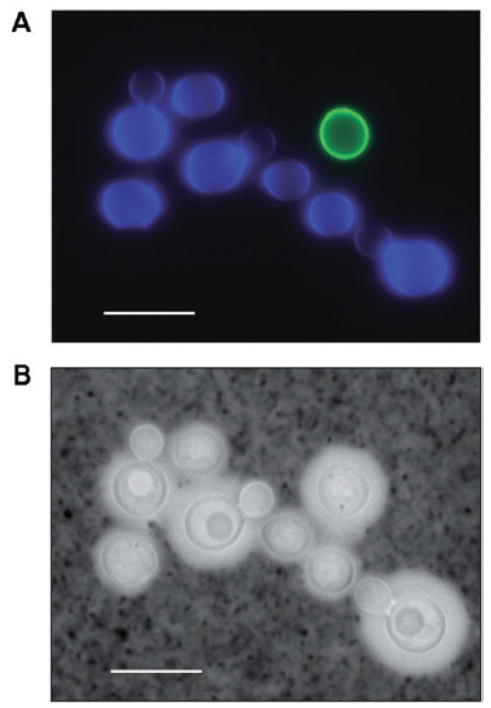
Fig. 1.
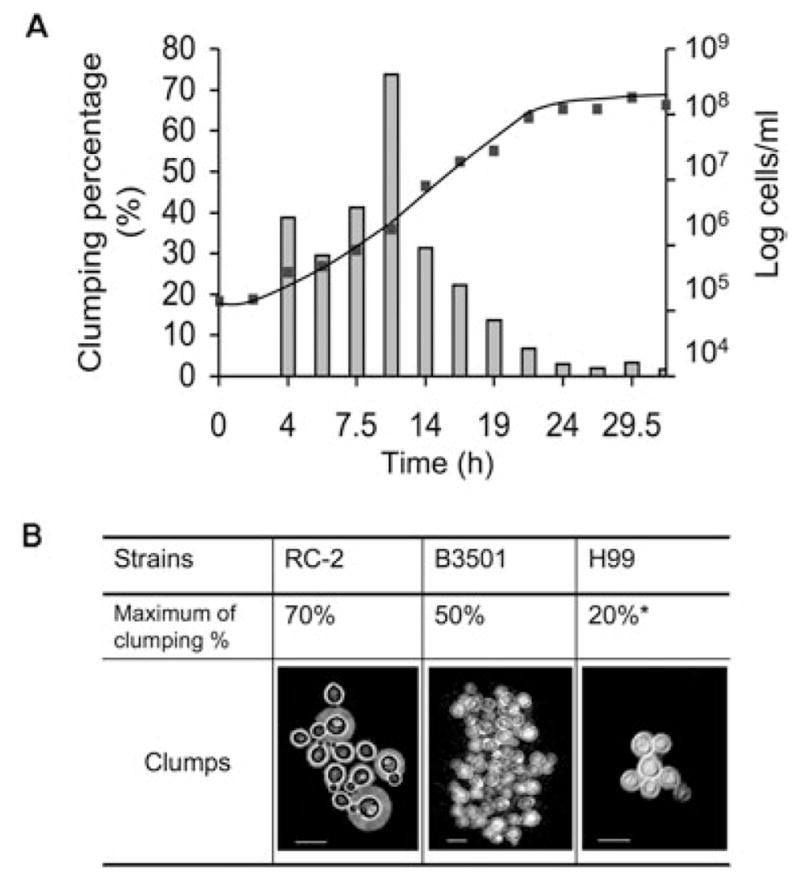
A. Growth curve (line) and cell aggregation (column) of RC-2. The culture was grown with a starting inoculum of 105 cells ml−1 in SAB broth. The number of cells at each specific time interval was counted on a haemocytometer.
B. Cell aggregation of C. neoformans strains grown in SAB broth. *Cells cultured in SD medium. Scale bar, 10 μm.
Cellular aggregation of the RC-2 strain differed in the various growth media (Table 1). In this regard, RC-2 exhibited no cell aggregation in SAB broth of lot number 4258140 whereas a 70% cell aggregation was observed in SAB of lot number 4148713. The molecules that facilitated clumping were small as they could be recovered from SAB broth after filtration through a membrane with a low molecule cut off (< 5 kDa microcon, Milipore, Bedford, MA). RC-2 grown in a capsule-inducing medium CO2-independent medium (CO2 IM) also displayed 50% clumps, whereas RC-2 cells grown in synthetic dextrose minimum medium (SD) displayed no clumps. Clumping was also observed in other C. neoformans strains but the optimal conditions differed for individual strains. H99 clumped more in SD (up to 20%) compared with SAB medium (less than 5%). In B3501 clumping was not dependent on media and was observed over longer growth times. We conclude that cell–cell aggregation occurs in various C. neoformans strains and that the degree of clumping can be medium dependent and variable.
Table 1.
Medium-dependent cell aggregation of RC-2.
| Media | Clumping percentagea |
|---|---|
| Sabouraud broth Lot 4148713 | 50%~70% |
| Sabouraud broth Lot 2134443 | ~30% |
| Sabouraud broth Lot 4258140 | ~2% |
| SD medium | ~3% |
| CO2 IM | ~50% |
Inoculum of 5 × 105 ml−1, cultured at 37°C, 150 rpm for 7 h.
Phenotypic characterization of clump+ and clump− cells
Staining of bud scars in aggregating cells determined that the areas of recent bud scars are not involved in cell aggregation (data not shown). Clump+ cells and clump− cells did not exhibit differences in capsular hydrophobicity. The percentage of hydrophobic cells was 70.7 ± 17.5 versus 71 ± 5.3 for clump+ and clump− cells respectively. The percentage of binding of mAb was also similar and documented as follows: percentage of binding of mAb 12A1 was 96.1 ± 0.85 versus 89.9 ± 8.9, percentage of binding of mAb 21D2 86.9 ± 4.4 versus 88.9 ± 3.7, and percentage of binding of mAb 2H1 98.7 ± 1.1 versus 99.5 ± 0.3 for clump+ and clump− cells respectively. There was no significant difference in mean fluorescent intensity (MFI) for the binding of 12A1, 21D2 and 2H1 (data not shown). In mixing experiments clump+ cells were labelled fluorescently and mixed with labelled clump− cells by a cell ratio of 5:1 (Fig. 2). These experiments demonstrated clumps contained predominately clump+ cells as only 1 in 150 ‘clumped’ cells was clump−. In addition we determined that clump− cells did not aggregate when diluted to 2 × 106 cells ml−1 and incubated in fresh clump inducing medium nor did clump+ cells aggregate in fresh non-inducing medium [or phosphate-buffered saline (PBS)]. These findings indicate that the cellular-aggregation phenomenon required changes in the cell surface and specific conditions in the medium and was not associated with changes in antibody epitope in the polysaccharide capsule or surface hydrophobicity.
Fig. 2.
A clump composed of clump+ cells and clump− cells by fluorescent microscopy. Clump+ cells were mixed with clump− cells by a cell ratio of 5:1 in the mixture experiment. The majority of clumps contained only clump+ cells.
A. Fluorescent merge. Clump+ cells were stained with UVITEX (blue) and clump− cells stained with Oregon green 448 (FITC) (green).
B. Phase contrast photomicrograph. Cells were stained with India ink. Scale bar, 10 μm.
Comparison of cell aggregation of RC-2 with flocculation in other yeasts
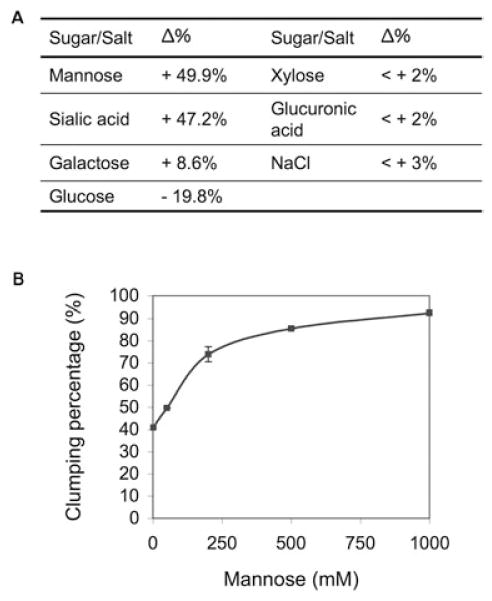
Saccharomyces cerevisiae flocculation is calcium dependent and mannose and EDTA can inhibit re-flocculation (Verstrepen et al., 2003). In contrast cell–cell aggregation in C. neoformans cannot be induced by calcium or dispersed by EDTA. Addition of mannose to C. neoformans cultures did not inhibit, but rather induced cell re-aggregation (Fig. 3A and B). Additionally, aggregation in RC-2 appeared in the early exponential phase of growth, while yeast flocculation happens constitutively or only in the stationary phase of growth. These results suggest that the cellular aggregation phenomenon observed for the RC-2 was different from cell flocculation in S. cerevisiae and we therefore refer to it as a flocculation-like cell aggregation. Interestingly, sugar and salt treatment also revealed that not only mannose but also sialic acid could induce clumping formation (Fig. 3). While glucose showed a weak inhibition, no significant effect of galactose, glucuronic acid, xylose and NaCl on cell aggregation was observed. The clump-forming ability of RC-2 was also irreversibly inhibited by proteinase K. A significant decrease (60%) in clumping was observed after exposure to 3 mg ml−1 proteinase K for 30 min (Fig. 4). Hence, our results suggest that cell aggregation in C. neoformans was due to sugar-induced protein interaction.
Fig. 3.
A. Effects of sugar and salt treatment on cell re-aggregation of RC-2. Δ%: percentage of increase (+) or decrease (−) of clumping percentage after treatment compared with control.
B. Induction of cell re-aggregation of RC-2 by mannose.
Fig. 4.
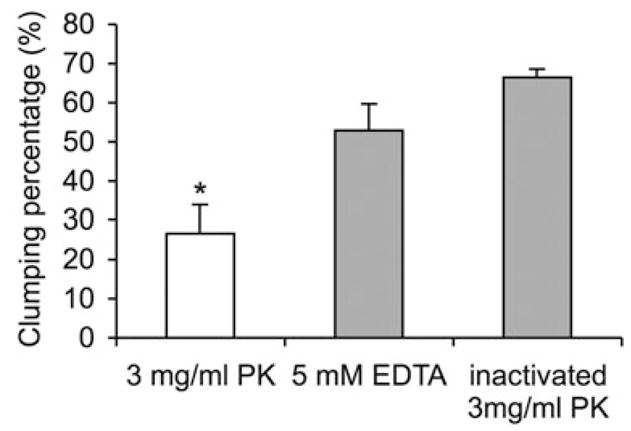
Proteinase K digestion of RC-2 clump+ cells. Digestion was performed in SAB medium by shaking at 37°C for 30 min. PK, proteinase K; *P < 0.001.
Effect of clumping on host pathogen interaction
Adherence and phagocytosis of clump+ cells
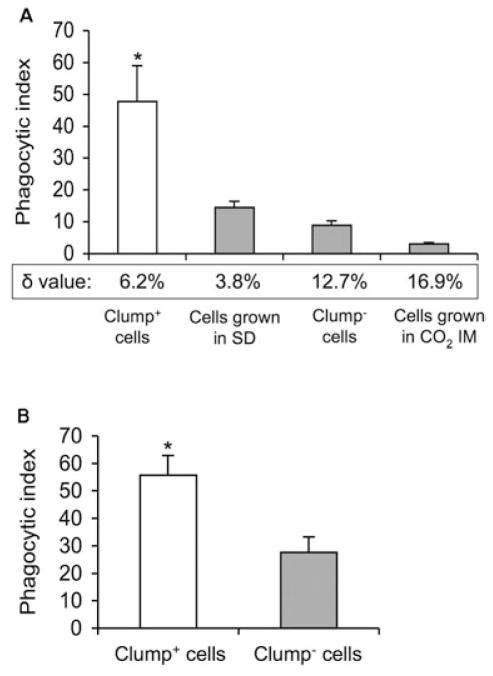
Both adherence to and phagocytosis by macrophages are key elements of an effective immune response. Clump+ cells exhibited significantly increased adherence to macrophage-like cells when compared with clump− cells (Fig. 5; P < 0.001). For cells grown in SAB medium, adherence correlated with presence of clumping cells. The adherence rate of clump+ cells was sevenfold higher than that of clump− cells. To further test the effect of adherence on phagocytosis, we compared the phagocytosis efficiency of clump+ cells and clump− cells (Fig. 6A). Without complement as an opsonin, no significant phagocytosis was observed in clump+ and clump− cells (data not shown). In complement-mediated phagocytosis assays, the phagocytic index (PI) of dispersed clump+ cells was significantly higher (P < 0.001) than the PI of clump− cells. Significantly greater (P < 0.05) complement-mediated phagocytosis was also found in two different experiments with H99. H99 cells grown in SD exhibited a higher PI when compared with the cells grown in SAB broth (19.6 ± 2.2 versus 12.9 ± 4.1, and 13.1 ± 1.5 versus 6.5 ± 2.6 respectively). Our findings were confirmed with an in vivo phagocytosis assay using a murine pulmonary infection model (Fig. 6B). Phagocytosis was significantly greater for clump+ cells when compared with clump− cells at 4 h post intratracheal inoculation (P < 0.001). Because differences in complement (C3) deposition (Zaragoza et al., 2003) on the cryptococcal polysaccharide can influence C3-mediated phagocytosis, we determined the relative location of C3 to the outer edge of the capsule in clump+ and clump− cells (Fig. 6A). A low δ-value (< 5%) is associated with efficient C3-mediated phagocytosis and indicates C3 localized to the outer edge of the capsule. A high δ-value (> 10%) indicates C3 is localized inside the capsule (Zaragoza et al., 2003) and is associated with poor C3-mediated phagocytosis. Although our data confirmed a lower δ-value (6.2%) in clump+ cells when compared with clump− cells (12.7%), we also found non-clumping cells that were grown in SD medium had a lower δ-value (3.8%). These cells neither exhibited good adherence nor high complement-mediated phagocytosis. The high δ-value (16.9%) in clumping cells grown in CO2 IM is consistent with their induced capsule state and their poor adherence and phagocytosis. We conclude that C3 deposition in the outer edge of polysaccharide capsule alone is not sufficient to enhance complement-mediated phagocytosis of RC-2. More importantly, our data suggest that enhanced adherence of clump+ cells increases the efficacy of complement-mediated phagocytosis. These host pathogen interactions are greatly affected by a presence of a flocculation-like phenotype in C. neoformans.
Fig. 5.
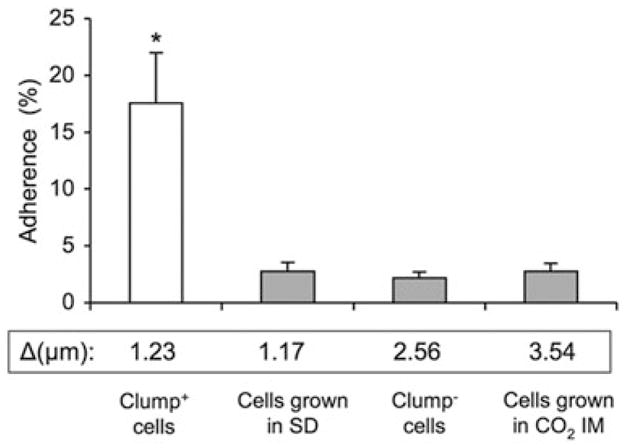
Per cent adherence of RC-2 to J774.16 cells on glass cover-slips. Δ: mean capsule thickness (μm) of RC-2 cells. SD, synthetic dextrose minimum medium; *P < 0.001.
Fig. 6.
Complement-mediated phagocytosis in vitro (A) and in vivo (B) of RC-2. δ-value indicates the distance of complement deposition to the outer edge of the capsule, measured by immunofluorescence (see Experimental procedures). SD, synthetic dextrose minimum medium; *P < 0.001.
Effects of clumping on virulence in a pulmonary infection model
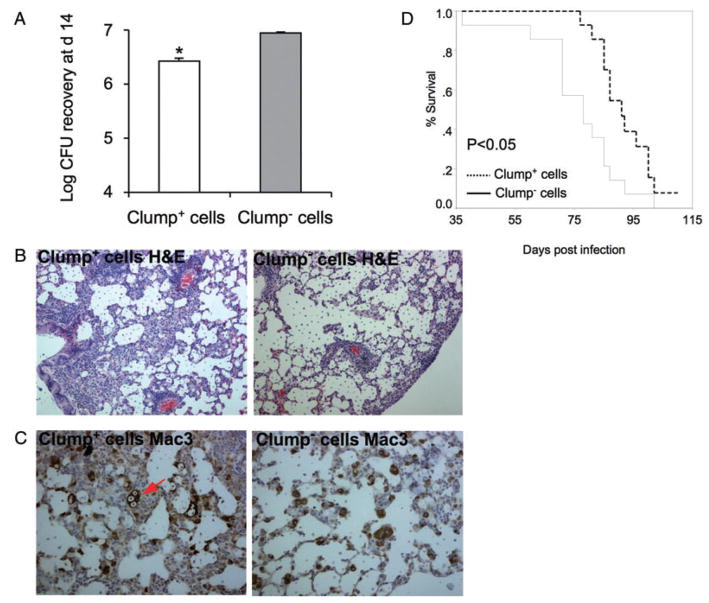
To determine the effect of cell aggregation on virulence, we inoculated BALB/c mice intratracheally with 104/106 cfu of clump+ cells or clump− cells and compared lung fungal burden at day 14 after infection and survival. With the high inoculum of 106 cfu per mouse, no significant differences were observed in colony recovery and mice survival after infection with clump+ cells and clump− cells (data not shown). However, infection with 104 clump+ cells resulted in significantly lower fungal burden at day 14 in the lung when compared with infection with clump− cells (Fig. 7A, P = 0.007). Consistent with a lower fungal burden, histological examination of lungs from mice infected with clump+ cells revealed increased inflammatory immune response (Fig. 7B) and more alveolar macrophages containing digested yeast cells (Fig. 7C) when compared with lungs from mice infected with clump− cells. Accordingly, with a low inoculum, the mean survival time of mice infected with clump+ cells was 15 days longer than that of mice infected with clump− cells (Fig. 7D). These data imply that phagocytosis efficiency and adherence property of clump+ cells promote effective clearance by the alveolar macrophage and thus explain the reduced virulence of clump+ cells. Most likely this effect is not seen in mice infected with a high-dose infectious inoculum because the initial fungal burden overwhelms the phagocytic capacity of the innate immune system.
Fig. 7.
Effects of clumping on the outcome of pulmonary infection. Mice were infected intratracheally with 104 C. neoformans.
A. Fungal burden (cfu) at day 14 post infection; *P = 0.007.
B. Inflammatory immune response in lungs at day 14 post infection (H&E).
C. Alveolar macrophages at day 14 post infection (Mac3). Arrow points to C. neoformans-loaded macrophage.
D. Survival curves for mice infected with clump+ cells and clump− cells. The average survival time of mice infected with clump+ cells and clump− cells was 91 and 76 days respectively (n = 14 per group).
Discussion
This study investigated transient flocculation-like cell–cell aggregation in C. neoformans and draws several important conclusions. Flocculation-like cell aggregation occurs in different C. neoformans strains and can be medium dependent. Transient changes of the yeast’s cellular phenotype greatly affect its ability to adhere and to be taken up by macrophages. The phenomenon is susceptible to protease digestion, but is not accompanied with changes in surface hydrophobicity or capsular epitope distribution. These changes in aggregation translate into differences in the way these cells interact with macrophages and into differences in virulence in a murine model of pulmonary infection.
Cryptococcus neoformans forms mostly yeast cells in vivo but even this cellular phenotype can vary among strains and switch variants (Franzot et al., 1998; Goldman et al., 1998; Fries et al., 1999). During growth in broth cell–cell aggregation was observed in several C. neoformans strains (Goldman et al., 1998; Fries et al., 1999; Wormley et al., 2005). This cell morphology has not been systematically investigated for this fungus. In contrast, cell–cell aggregation, also referred to as flocculation, has been thoroughly studied in S. cerevisiae and C. albicans and, for the latter fungus is believed to contribute to virulence (Cormack et al., 1999; Hoyer, 2001; De Las Penas et al., 2003). We found cell–cell aggregation in serotype A and D strains and specifically studied the implication of this cellular phenotype for host pathogen interactions. ATCC strain 24067 is a serotype D strain with known cellular heterogeneity that has been thoroughly studied by several investigators (Franzot et al., 1998; Fries et al., 1999; Lortholary et al., 2002; Maitta et al., 2004; Lindell et al., 2005). RC-2, a variant of this strain was used in this studies and exhibited reproducible prominent cell–cell aggregation in the exponential phase of growth, which was transient and disappeared in stationary phase. Other C. neoformans strains including H99 also exhibited cell–cell aggregation although the timing and presentation varied.
Cell aggregation in C. neoformans was compared with flocculation in S. cerevisiae and exhibited several similarities. Analogous to other yeasts, cell aggregation in C. neoformans can be abolished by dispersal and thorough vortexing. Proteins appear to be involved in cell aggregation as the process can be inhibited by proteinase K digestion. Flocculation-like cell aggregation in C. neoformans, however, also differed in several key points. First it was independent of calcium and thus could not be inhibited by EDTA. Second, it occurred in early log phase and appeared only transiently, whereas for S. cerevisiae flocculation is observed in the stationary growth phase.
One of the unique features of C. neoformans is its thick polysaccharide capsule that plays an important role in host pathogen interaction. The polysaccharide capsule inhibits phagocytosis by host inflammatory cells. Phagocytosis is a primary defence strategy by the innate immune response and in naïve hosts is primarily complement-mediated (Levitz and Tabuni, 1991; Kozel, 1996; Mansour and Levitz, 2002). Many studies have investigated this process in vitro and in vivo (Kozel, 1993; Taborda and Casadevall, 2002; Del Poeta, 2004). The efficiency of complement-mediated phagocytosis can vary greatly among strains (Zaragoza et al., 2003). High opsonization by complement was associated with a smaller capsule volume and complement disposition in the outer rim within the polysaccharide capsule. Induction of the capsule can affect the location of complement deposition and lower the efficacy of opsonization. C. neoformans strain 24067 was classified as ‘low-opsonized’ strain and showed low complement-mediated phagocytosis (Zaragoza et al., 2003). We now demonstrate that complement-mediated phagocytosis of this strain is also affected by the dynamic cellular phenotype and can rapidly change from low to high efficiency.
The mechanism by which C. neoformans forms clumps is not fully understood. Given that protease treatment results in cell dispersal we propose that clumping in C. neoformans is mediated by a protein adhesin. Consistent with this view no differences in cell hydrophobicity were measured for clump+ and clump− cells. Furthermore, we found no evidence for changes in capsule structure because the binding pattern of three mAbs that recognize different epitopes was indistinguishable in clump+ and clump− cells. In addition, our findings that cell aggregation was affected by co-incubation of cryptococcal cells with mannose and sialic acid suggested that these protein interactions involve sugar residues and, like FLO proteins are mediated by lectin like proteins (Miki et al., 1982; Suzzi et al., 1996). In contrast to other fungi C. neoformans is encapsulated and the presence of a thick polysaccharide affects all aspects of adherence. It is unlikely that a cell wall anchored protein mediates this cell aggregation. Conservative calculations (assuming a capsule thickness of 1.25 μm) would require this protein to be over 360 kDa to span the capsule and to be exposed beyond the capsule edge. It is conceivable, however, that the proteins involved in aggregation and adherence may be secreted and shed through the polysaccharide capsule in the early phase of logarithmic growth. Alternatively, it is possible that the biophysical properties such as density of the polysaccharide capsule change and permit better access to cell wall-anchored adhesions (Gates et al., 2004).
To investigate the effects of the cryptococcal capsule on pathogenesis, acapsular mutants have been studied (Ibrahim et al., 1995; Merkel and Scofield, 1997). Acapsular mutants constitutively form tight clumps, which cannot be dispersed by vortexing and thus are different from the clumps produced by the encapsulated wild-type strains. Acapsular mutants adhered more and are phagocytosed better than encapsulated strains (Ibrahim et al., 1995). Treatment with trypsin abolishes adherence, implying that a protein-containing adhesion was associated with adherence (Merkel and Scofield, 1997). Similar to our studies cryptococcal adherence in vitro was affected by yeast culture conditions and decreased in strains (acapsular and encapsulated) grown at 25°C when compared with those grown at 37°C. Similar finding was documented in our study where cell aggregation of RC-2 occurs at 30°C and 37°C, but not at room temperature (data not shown).
Our animal experiment data were consistent with the view that phagocytosis efficiency and adherence property of clump+ cells promoted effective clearance by alveolar macrophages and thus explains the reduced virulence of clump+ cells. This effect was only seen at low infecting inocula that can be effectively cleared by the innate immune system. Franzot et al. (1998) reported that strain ATCC 24067 could change in different laboratory environments over time. This microevolution was thought to explain inconsistencies in experimental results within and between laboratories. Our studies now highlight an even more pronounced complexity in that the cellular phenotype depends not only on the strains themselves but also on the growth conditions, such as media, culture age and temperature. This may not only explain inconsistencies that are observed during in vitro and in vivo experiments within and between laboratories but also highlight the need to further our understanding of dynamic phenotypic changes that occur in a growing pathogen population. Additional attention to growth conditions and individual colony morphology is needed.
We conclude that C. neoformans exhibits significant changes in cell aggregation during its growth in culture. These morphological transitions have been underemphasized, in comparison with other components of the large repertoire of fungal virulence factors. Nonetheless, transient morphological changes greatly affect the host pathogen interaction. The complexity of the variety of environmental signals that trigger morphogenesis and phenotype changes in any particular host niche complicate the analysis of individual genes involved and challenge the investigator.
Experimental procedures
Yeast strain and growth conditions
Strain 24067 is a serotype D strain from the American Type Culture Collection (ATCC, Rockville, MD, USA) and like other strains exhibits a heterogeneous cell morphology (Franzot et al., 1998; Garcia-Hermoso et al., 2004). Selection can occur during in vitro passaging and thus individual isolates from different laboratories exhibit phenotypic differences (Franzot et al., 1998). In this study, RC-2, a variant of the original ATCC 24067 strain was used. RC-2 can switch between a smooth and mucoid colony type (Fries et al., 2001). The parent smooth colony type was used in this study. The isolate was maintained by serial passages in Sabouraud dextrose agar (SDA). Media used for growing yeast were as follows: SAB (Difco, Sparks, MD, USA); CO2 IM (Gibco, Grand island, NY, USA); SD (0.67% Bacto yeast nitrogen base without amino acids and 2% dextrose). For strain RC-2 clump+ cells and clump− cells were generated as follows: a starter culture was grown overnight in SAB broth at 37°C with agitation (150 rpm). The cells were diluted to required concentrations. Clump+ cultures were prepared by 13 h cultivation in SAB medium with inocula of 105 cells ml−1. Clump− cells were inoculated at 107 cells ml−1 and grown in the same lot of SAB broth. Cells grown in SD and in CO2 IM were prepared by 13 h cultivation with inocula of 105 cells ml−1 and 106 cells ml−1 respectively. H99 was also grown at 105 cells ml−1 for 13 h in SD or SAB broth.
Characterization of cell clumping (flocculation)
Measurement of clumping percentage
Cell–cell aggregation was determined microscopically. A clump was defined as an aggregation of five or more cells. The degree of cell aggregation was measured by calculating the percentage of clumping cells before and after vortexing as follows:
For our studies comparing flocculation in different yeast species the standard protocol was modified (Soares and Vroman, 2003) in that yeast clumps were dispersed by vortexing in addition to washing in 30 mM EDTA, prior to induction of flocculation by incubation in a calcium-containing solution.
Measurement of hydrophobicity
The standard protocol to measure hydrophobicity is described in Hazen and Hazen (1988). Yeast cells were washed twice with cold (4°C) deionized water and suspended in phosphate-urea-MgSO4 buffer (PUM buffer: pH 7.1, 22.2 g K2HPO4·3H2O, 7.26 g KH2PO4, 1.8 g urea, 0.2 g MgSO4·7H2O and distilled water to 1000 ml) to a concentration of 4 × 106 cells ml−1. From a stock colloid suspension (2.6% solids) of dyed blue, sulphate polystyrene latex microspheres (0.928 ± 0.025 μm in diameter, Poly-sciences, Warrington, PA, USA), 24 μl was removed and mixed with 2.0 ml of PUM at 0–4°C. Equal volumes (100 μl each) of yeast cells and microsphere suspensions, were combined in Eppendorf tubes (1.5 ml), rapidly equilibrated (1–2 min) to room temperature, and vigorously mixed by vortexing for 30 s. Microsphere attachment to yeast cells was assessed immediately afterward by bright field microscopy (320× magnification). The percentage of cells with three or more attached microspheres was recorded as the per cent hydrophobicity of the cell population. Experiments were done in triplicates and at least 100 cells were counted.
Measurement of antibody binding to the polysaccharide capsule
Yeast cells were washed twice with PBS. Approximately 2 × 107 cells were suspended in 200 μl of PBS with or without addition of mAbs to the capsule (10 μg ml−1, protective IgG mAb 2H1, protective IgM mAb 12A1, and non-protective IgM mAb 21D2) and incubated at 4°C overnight. Cells were then washed twice with PBS, and incubated for 1 h at room temperature with 0.5 μg ml−1 of goat anti-mouse Kappa chain conjugated to FITC in PBS. After a final washing with PBS, the cells were suspended in 500 μl PBS for FACS analysis. The percentage of positive cells in the population was recorded.
Inhibition of clump formation by sugars and salts
For these assays clump+ cells were dispersed by vortexing and then concentrated 10× in SAB medium by centrifugation. Inhibition of clump formation was measured in medium containing 500 mM of mannose, galactose, glucose, glucuronic acid, sialic acid, xylose or NaCl by incubation at 37°C, 150 rpm for 2 h and compared with controls. Cell–cell re-aggregation was determined microscopically.
Enzyme treatment
Clump+ cells were incubated with 3 mg ml−1 proteinase K (fungal, Invitrogen, Carlsbad, CA, USA) in SAB medium. Control incubations were carried out with the same amount of heat-inactivated proteinase K (100°C for 10 min) and by adding 5 mM EDTA without proteinase K. The cell samples were shaken for 30 min at 37°C and the clumping percentage of the incubated samples was calculated.
Re-aggregation of mixture of clump+ cells and clump− cells
Clump+ cells were incubated for 5 min with the fluorescent probe UVITEX that binds to chitin in cell walls and dispersed by vortexing. Clump− cells were labelled with Oregon green 448 (FITC) at 37°C for 1 h. The labelled clump+ cells were concentrated 10× by centrifugation and mixed with labelled clump− cells by a cell ratio of 5:1 in SAB medium. The mixture of cells was shaken at 37°C for 1 h and cell re-aggregation was determined by fluorescence microscopy.
Capsule volume and complement localization in clump+ and clump− cells
The diameter of the cell body and total cell size (capsule included) was determined by India ink staining and light microscopy. The volume of the capsule was determined as the total cell volume minus the volume of the cell body. Complement localization was performed by immunofluorescence microscopy as described in Zaragoza et al. (2003) with the following modifications: Approximately 5 × 106 yeast cells were incubated for 1 h at 37°C in 100 μl of mouse serum and washed with 1% BSA/0.5% heat-inactivated FCS in PBS. The rest of the washings and incubations were done in this solution. Cells were incubated 5 μg ml−1 in FITC-conjugated goat antibody to mouse complement (Cappel, ICN, Aurora, OH, USA) for 30 min at 37°C and washed. This was followed by incubation with 18B7, an IgG1 mAb to the capsule (10 μg ml−1) for 30 min at 37°C, washing, and subsequent incubation for 30 min with 5 μg ml−1 of goat anti-mouse IgG conjugated to tetramethyl-rhodamine-isothiocyanate (Southern Biotechnology Associates, Birmingham, AL, USA). MAb 18B7 was used to determine the edge of the capsule. After a final washing, the cells were suspended in mounting medium (50% glycerol and 50 mM N-propyl gallate in PBS). The fluorescence intensity was determined with Scion Image software (http://rsb.info.nih.gov/ij/). To obtain a quantitative measure of complement localization, we measured the distance between the peaks of fluorescein intensity (fluorescence due to complement) and rhodamine intensity (outer edge of the capsule). To control for differences in cell size, the distance between peaks was calculated as a percentage (hereafter designated δ-value) of the total distance between the rhodamine intensity peaks. For each measurement at least eight cells were averaged.
Phagocytosis assay
The murine macrophage-like cell line J774.16 (Ralph and Nakoinz, 1975) was used in this study. J774.16 cells express Fc and CR3 and behave like primary murine peritoneal macrophages with regards to their ability to phagocytose C. neoformans (Dong and Murphy, 1997). In vitro phagocytosis assays were performed as described (Taborda and Casadevall, 2002) with the following modifications: J774.16 cells were grown on glass coverslips in 6 well tissue culture plates, and C. neoformans was added at a ratio of 5:1 (fungi:macrophages) after dispersing clumps by vortexing. As opsonins, 20% guinea pig sera (Sigma-aldrich, MO, USA) was used. After 2 h, monolayers were washed in PBS, fixed in ice-cold methanol and stained with Giemsa overnight at 4°C. The PI was determined as the number of ingested C. neoformans per 100 macrophages. For in vivo phagocytosis assays, groups of mice (n = 5) were infected by inoculation of 107C. neoformans cells in 50 μl sterile PBS into the trachea (i.t.) using a 26-gauge needle. After 4 h, the mice were killed and their tracheas were surgically exposed and cannulated with a 20-gauge plastic catheter (Angiocath, Sandy, UT, USA). Each mouse lung was lavaged 10 times with 1 ml of Hanks’ balanced salt solution (Gibco, Grand island, NY, USA) containing 1 mM EGTA. Cells were then pelleted and erythrocytes were lysed by incubation in NH4Cl solution for 10 min. The alveolar macrophages were allowed to adhere to tissue culture plates for 2 h at 37°C, then fixed and stained to determine PI.
Adherence assays
The murine macrophage-like J774.16 cells were grown on glass coverslips in each well of 6 well tissue culture plates, and washed with DMEM without FCS. C. neoformans was washed and resuspended in DMEM without FCS. Each well was inoculated with 107 vortexed yeast cells (cell amount was confirmed by cfu determinations) and incubated for 90 min at 37°C. After incubation cultures were rinsed five times with PBS to remove non-adherent C. neoformans cells. The glass coverslips were then removed and incubated in 1 ml of 0.2% Tween 20 for 30 min at 37°C to lyse macrophages. The amount of adherent C. neoformans cells was determined by cfu. All experiments were performed in triplicates and repeated. Adherence was expressed as a percentage of the inoculum added.
Animal experiments
BALB/c mice (female, 6–10 weeks old) were purchased from the National Cancer Institute (Bethesda, MD, USA). Mice were anaesthetized and infected by inoculation with 104 or 106C. neoformans cells (clumps were dispersed by vortexing) in 50 μl sterile PBS into the trachea using a 26-gauge needle as described (Feldmesser et al., 1996). To assure that comparable numbers of yeast cells were injected, dilutions of the inoculum (clump+ and clump− cells) were plated onto SAB agar. Cfu were determined by homogenizing lung tissue in PBS, and plating dilutions of the homogenate on SAB agar. For histological studies tissue sections were stained with haematoxylin and eosin (H&E). Immunohistochemistry staining was done with a macrophage-specific mAb (Mac 3; Pharmingen, San Diego, CA, USA) as the primary antibody. All protocols were done according to guidelines provided by the Albert-Einstein College of Medicine’s animal use and protection committee.
Statistical analysis
Standard deviation was used as an expression of variation. Comparisons of the means were made by using the Student’s t-test, and a P-value of 0.05 was considered significant. SPSS version 8.0 (SPSS, Chicago, IL) was used to generate Kaplan Meyer survival curves.
Acknowledgments
We thank Dr McFadden and Dr Goldman for critical reading and both Emily Cook and Dr McFadden for technical assistance. This work was supported by R0-1 AI 59681 to B.C.F. and by AECOM/MMC CFAR 1P30AI051519.
References
- Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Muller M, Zhou JZ, Wright LC, Sorrell TC. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J Infect Dis. 1997;175:414–420. doi: 10.1093/infdis/175.2.414. [DOI] [PubMed] [Google Scholar]
- Cormack BP, Ghori N, Falkow S. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science. 1999;285:578–582. doi: 10.1126/science.285.5427.578. [DOI] [PubMed] [Google Scholar]
- Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC, et al. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol. 2001;39:166–175. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- De Las Penas A, Pan SJ, Castano I, Alder J, Cregg R, Cormack BP. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003;17:2245–2258. doi: 10.1101/gad.1121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Poeta M. Role of phagocytosis in the virulence of Cryptococcus neoformans. Eukaryot Cell. 2004;3:1067–1075. doi: 10.1128/EC.3.5.1067-1075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZM, Murphy JW. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect Immun. 1997;65:557–563. doi: 10.1128/iai.65.2.557-563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, Mukherjee J, Casadevall A. Combination of 5-flucytosine and capsule-binding monoclonal antibody in the treatment of murine Cryptococcus neoformans infections and in vitro. J Antimicrob Chemother. 1996;37:617–622. doi: 10.1093/jac/37.3.617. [DOI] [PubMed] [Google Scholar]
- Franzot SP, Mukherjee J, Cherniak R, Chen LC, Hamdan JS, Casadevall A. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun. 1998;66:89–97. doi: 10.1128/iai.66.1.89-97.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries BC, Goldman DL, Cherniak R, Ju R, Casadevall A. Phenotypic switching in Cryptococcus neoformans results in changes in cellular morphology and glucuronoxylomannan structure. Infect Immun. 1999;67:6076–6083. doi: 10.1128/iai.67.11.6076-6083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries BC, Taborda CP, Serfass E, Casadevall A. Phenotypic switching of Cryptococcus neoformans occurs in vivo and influences the outcome of infection. J Clin Invest. 2001;108:1639–1648. doi: 10.1172/JCI13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Kikuchi Y, Kuhara S, Misumi Y, Matsumoto S, Kobayashi H. Domains of the SFL1 protein of yeasts are homologous to Myc oncoproteins or yeast heat-shock transcription factor. Gene. 1989;85:321–328. doi: 10.1016/0378-1119(89)90424-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Hermoso D, Dromer F, Janbon G. Cryptococcus neoformans capsule structure evolution in vitro and during murine infection. Infect Immun. 2004;72:3359–3365. doi: 10.1128/IAI.72.6.3359-3365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates MA, Thorkildson P, Kozel TR. Molecular architecture of the Cryptococcus neoformans capsule. Mol Microbiol. 2004;52:13–24. doi: 10.1111/j.1365-2958.2003.03957.x. [DOI] [PubMed] [Google Scholar]
- Gaur NK, Klotz SA, Henderson RL. Over-expression of the Candida albicans ALA1 gene in Saccharomyces cerevisiae results in aggregation following attachment of yeast cells to extracellular matrix proteins, adherence properties similar to those of Candida albicans. Infect Immun. 1999;67:6040–6047. doi: 10.1128/iai.67.11.6040-6047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DL, Fries BC, Franzot SP, Montella L, Casadevall A. Phenotypic switching in the human pathogenic fungus Cryptococcus neoformans is associated with changes in virulence and pulmonary inflammatory response in rodents. Proc Natl Acad Sci USA. 1998;95:14967–14972. doi: 10.1073/pnas.95.25.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Styles CA, Feng Q, Fink GR. A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc Natl Acad Sci USA. 2000;97:12158–12163. doi: 10.1073/pnas.220420397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme A, Bumgarner S, Styles C, Fink GR. Genetic and epigenetic regulation of the FLO gene family generates cell-surface variation in yeast. Cell. 2004;116:405–415. doi: 10.1016/s0092-8674(04)00118-7. [DOI] [PubMed] [Google Scholar]
- Hazen BW, Hazen KC. Modification and application of a simple, surface hydrophobicity detection method to immune cells. J Immunol Methods. 1988;107:157–163. doi: 10.1016/0022-1759(88)90214-1. [DOI] [PubMed] [Google Scholar]
- Hoyer LL. The ALS gene family of Candida albicans. Trends Microbiol. 2001;9:176–180. doi: 10.1016/s0966-842x(01)01984-9. [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, Filler SG, Alcouloumre MS, Kozel TR, Edwards JE, Jr, Ghannoum MA. Adherence to and damage of endothelial cells by Cryptococcus neoformans in vitro: role of the capsule. Infect Immun. 1995;63:4368–4374. doi: 10.1128/iai.63.11.4368-4374.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraqui I, Garcia-Sanchez S, Aubert S, Dromer F, Ghigo JM, d’Enfert C, Janbon G. The Yak1p kinase controls expression of adhesins and biofilm formation in Candida glabrata in a Sir4p-dependent pathway. Mol Microbiol. 2005;55:1259–1271. doi: 10.1111/j.1365-2958.2004.04475.x. [DOI] [PubMed] [Google Scholar]
- Jacobson ES, Emery HS. Temperature regulation of the cryptococcal phenoloxidase. J Med Vet Mycol. 1991;29:121–124. doi: 10.1080/02681219180000201. [DOI] [PubMed] [Google Scholar]
- Kozel TR. Opsonization and phagocytosis of Cryptococcus neoformans. Arch Med Res. 1993;24:211–218. [PubMed] [Google Scholar]
- Kozel TR. Activation of the complement system by pathogenic fungi. Clin Microbiol Rev. 1996;9:34–46. doi: 10.1128/cmr.9.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz SM, Tabuni A. Binding of Cryptococcus neoformans by human cultured macrophages. Requirements for multiple complement receptors and actin. J Clin Invest. 1991;87:528–535. doi: 10.1172/JCI115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell DM, Moore TA, McDonald RA, Toews GB, Huffnagle GB. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. J Immunol. 2005;174:7920–7928. doi: 10.4049/jimmunol.174.12.7920. [DOI] [PubMed] [Google Scholar]
- Lortholary O, Improvisi L, Fitting C, Cavaillon JM, Dromer F. Influence of gender and age on course of infection and cytokine responses in mice with disseminated Cryptococcus neoformans infection. Clin Microbiol Infect. 2002;8:31–37. doi: 10.1046/j.1469-0691.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- Maitta RW, Datta K, Chang Q, Luo RX, Witover B, Subramaniam K, Pirofski LA. Protective and nonprotective human immunoglobulin M monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan manifest different specificities and gene use profiles. Infect Immun. 2004;72:4810–4818. doi: 10.1128/IAI.72.8.4810-4818.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour MK, Levitz SM. Interactions of fungi with phagocytes. Curr Opin Microbiol. 2002;5:359–365. doi: 10.1016/s1369-5274(02)00342-9. [DOI] [PubMed] [Google Scholar]
- Merkel GJ, Scofield BA. The in vitro interaction of Cryptococcus neoformans with human lung epithelial cells. FEMS Immunol Med Microbiol. 1997;19:203–213. doi: 10.1111/j.1574-695X.1997.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Miki BL, Poon NH, James AP, Seligy VL. Possible mechanism for flocculation interactions governed by gene FLO1 in Saccharomyces cerevisiae. J Bacteriol. 1982;150:878–889. doi: 10.1128/jb.150.2.878-889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powderly WG. Cryptococcal meningitis and AIDS. Clin Infect Dis. 1993;17:837–842. doi: 10.1093/clinids/17.5.837. [DOI] [PubMed] [Google Scholar]
- Ralph P, Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. 1975;257:393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- Reynolds TB, Fink GR. Bakers’ yeast, a model for fungal biofilm formation. Science. 2001;291:878–881. doi: 10.1126/science.291.5505.878. [DOI] [PubMed] [Google Scholar]
- Rhodes JC, Polacheck I, Kwon-Chung KJ. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect Immun. 1982;36:1175–1184. doi: 10.1128/iai.36.3.1175-1184.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares EV, Vroman A. Effect of different starvation conditions on the flocculation of Saccharomyces cerevisiae. J Appl Microbiol. 2003;95:325–330. doi: 10.1046/j.1365-2672.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- Stratford M. Evidence for two mechanisms of flocculation in Saccharomyces cerevisiae. Yeast. 1989;5:S441–445. [PubMed] [Google Scholar]
- Suzzi G, Romano P, Westall F, Vannini L. The flocculation of wine yeasts: biochemical and morphological characteristics in Kloeckera apiculata. Antonie Van Leeuwenhoek. 1996;69:273–277. doi: 10.1007/BF00399616. [DOI] [PubMed] [Google Scholar]
- Taborda CP, Casadevall A. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity. 2002;16:791–802. doi: 10.1016/s1074-7613(02)00328-x. [DOI] [PubMed] [Google Scholar]
- Verstrepen KJ, Derdelinckx G, Verachtert H, Delvaux FR. Yeast flocculation: what brewers should know. Appl Microbiol Biotechnol. 2003;61:197–205. doi: 10.1007/s00253-002-1200-8. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G, Seto D, Parker CS. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell. 1988;54:841–853. doi: 10.1016/s0092-8674(88)91197-x. [DOI] [PubMed] [Google Scholar]
- Wormley FL, Jr, Heinrich G, Miller JL, Perfect JR, Cox GM. Identification and characterization of an SKN7 homologue in Cryptococcus neoformans. Infect Immun. 2005;73:5022–5030. doi: 10.1128/IAI.73.8.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Taborda CP, Casadevall A. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol. 2003;33:1957–1967. doi: 10.1002/eji.200323848. [DOI] [PubMed] [Google Scholar]