Abstract
Stereotypies are common in frontotemporal lobar degeneration (FTLD) however the anatomical correlates of stereotypies are unknown. We therefore set out to compare patterns of grey matter volume loss in FTLD subjects with and without stereotypies. Subjects with a diagnosis of FTLD that met international consensus criteria were prospectively recruited and separated into those with and without stereotypies. MRI and cognitive measures were obtained and voxel-based morphometry was used to assess the patterns of grey matter volume loss in those with and without stereotypies, compared to a group of age-and gender-matched controls. Demographic and clinical features were similar between subjects with and without stereotypies. FTLD subjects with stereotypies had greater volume loss in the striatum compared to those without stereotypies. Those without stereotypies showed a more widespread and typical pattern of cortical frontotemporal loss. Stereotypies in FTLD are therefore associated with a greater proportion of striatal to cortical volume loss than those without stereotypies.
Keywords: Frontotemporal dementia, voxel-based morphometry, stereotypy, striatum, MRI
1. INTRODUCTION
Frontotemporal lobar degeneration (FTLD) is a neurodegenerative disease characterized by changes in personality, executive dysfunction, and impairment of language and word meaning[15]. Three variants of FTLD are typically described: a behavioral variant frontotemporal dementia (bvFTD) with personality and behavioral changes, a language variant known as progressive non-fluent aphasia where speech is hesitant and agrammatic, and semantic dementia characterized by the loss of meaning or knowledge about objects. Head MRI in FTLD typically demonstrates varying degrees of frontal and temporal lobe atrophy depending on the clinical presentation[17].
Stereotypies are repetitive, predictable, coordinated movements that resemble a fragment of a normal action and are continually repeated without any purpose[4]. The movements can be simple such as rocking, tapping ones own leg, or protruding ones tongue. However, sometimes they are more complex such as spitting into ones hands and rubbing it into one’s hair. Stereotypies are common in FTLD[14], however the anatomical correlate is unknown.
In this study we set out to determine which anatomical structure or region would correlate with the presence of stereotypies in FTLD. We used voxel-based morphometry (VBM) to compare the patterns of grey matter volume loss in FTLD subjects with and without stereotypies.
2. METHODS
2.1 Subjects
Over the last 3 years (July 2003–July 2006) subjects who met research criteria for behavioral variant FTD[15] were prospectively evaluated by one of the authors with expertise in movement disorders and dementia (KAJ). Each subject’s family was administered a standardized questionnaire by the examining physician to determine whether or not any stereotypies had been observed over the past six months. The family members were given examples of stereotypies, (rubbing ones legs, making funny sounds over and over, rocking back and forth, etc.) when completing the questionnaire. If stereotypies were reported as being present, the family member was asked to describe the stereotypies in detail. The subject was coded as ‘positive’ if the movements met our criteria for stereotypies. Stereotypies were defined as involuntary, patterned, repetitive, purposeless but seemingly purposeful movements [7] and could be either simple or complex. If no stereotypies had been reported after detailed description and none noted during the examination, the subject was coded as ‘negative’. For each subject, at the time of evaluation, a measure of cognitive disease severity was conducted; the Short Test of Mental Status [10], and the subject also underwent volumetric head MRI scanning. The Short Test of Mental Status was chosen as a measure of cognitive disease because it includes a measure of learning, plus it tests seven different cognitive domains. Furthermore, it has been a standard test at our institution since the 1980’s, and has recently been shown to be superior to the Mini-Mental State Examination in detecting mild cognitive impairment [19]. Subjects were excluded if they had a prior or current exposure to neuroleptic drugs or another medical or psychiatric disease that could account for the presence of the stereotypies.
Each subject was matched by age and gender to a cognitively normal control subject. All controls were prospectively recruited and were cognitively normal individuals that were evaluated by a neurologist to verify the normal diagnosis. Controls were identified as individuals who a) were independently functioning community dwellers, b) did not have active neurologic or psychiatric conditions, c) had no cognitive complaints, d) had a normal neurological and neurocognitive examination, and e) were not taking any psychoactive medications in doses that would affect cognition. All controls had a normal Unified Parkinson’s Disease Rating Scale Motor Examination and was not noted to have any involuntary movements.[3]
2.2 Image analysis
T1-weighted volumetric MRI scans were acquired at 1.5T (22x16.5cm FOV, 25° flip angle, 124 contiguous 1.6mm thick coronal slices). An optimized method of VBM was used to assess group differences, implemented using SPM2 (http://www.fil.ion.ucl.ac.uk/spm)[2, 18]. A number of pre-processing steps were performed to ready the data for statistical analysis, including spatial normalization and segmentation. The spatial normalization step transforms all images into the same stereotactic space by registering each of the images to the same specific template, and the segmentation of the brain into grey matter (GM), white matter (WM) and CSF is performed using the voxel intensities combined with a priori knowledge of the spatial distribution of these tissues, derived from probability maps. In order to reduce any potential normalization or segmentation bias across the disease groups’ customized templates and prior probability maps were created from all subjects in the study, including both controls and FTLD subjects. To create the customized template and prior probability maps all images were registered to the Montreal Neurological Institute (MNI) template using a 12 degrees of freedom (dof) affine transformation and segmented into GM, WM and CSF using the MNI prior probability maps. GM images were normalized to the MNI GM prior probability map using a nonlinear discrete cosine transformation (DCT). The normalization parameters were applied to the original whole head and the images were segmented once again. Average images were created of whole head, GM, WM and CSF, and smoothed using 8mm full-width at half-maximum (FWHM) smoothing kernel. The average whole head image becomes the customized template, and the average GM, WM and CSF images are then used as the customized prior probability maps for subsequent segmentations. All images were then registered to the customized whole brain template using a 12dof affine transformation and segmented using the customized prior probability maps. The GM images were normalized to the custom GM prior probability map using a nonlinear DCT. The normalization parameters were then applied to the original whole head and the images were segmented once again. All the GM images were modulated and smoothed with 10mm FWHM smoothing kernel. In addition, a re-initialization routine was implemented. This uses the parameters from the initial normalization to the MNI template (performed to generate the customized template) to initialize the normalization to the custom template[18].
Two-sided t-tests were used to compare the smoothed modulated GM images between the FTLD subjects with stereotypies and FTLD subjects without stereotypies, and controls. Grey matter differences were assessed between each FTLD group and controls at a statistical threshold of p<0.05 corrected for multiple comparisons using the false discovery rate (FDR). The FDR is a well recognized and published statistical technique to adjust for multiple comparisons in VBM [5, 6, 13, 16, 20]. As a secondary analysis, we also performed a direct comparison between the FTLD subjects with and without stereotypies at an uncorrected threshold of p<0.05.
3. RESULTS
A total of seven FTLD subjects with stereotypies and 10 FTLD subjects without stereotypies were identified. The stereotypies were documented by the evaluating physician on average 2.1 years (range 1–3) into the disease course. The subject demographics are shown in table 1. There were no clinical or demographic differences between subjects with and without stereotypies. Two of the 17 subjects had died, one with stereotypies and one without. Both had a pathologic confirmed diagnosis of FTLD with ubiquitin-only immunoreactive changes[8].
Table 1.
Demographic and cognitive measures for all subjects
| FTLD with stereotypies N=7 | FTLD without stereotypies N=10 | Controls N=17 | |
|---|---|---|---|
| Age at onset, y | 61 (34–73) | 59 (47–71) | NA |
| Female: male ratio | 4:3 | 4:6 | 9:8 |
| Age at scan, y | 63 (36–75) | 64 (50–77) | 64 (50–77) |
| Years from onset to scan | 2.0 (0–5) | 1.9 (0–6) | NA |
| * STMS score at the time of Scan | 29 (4–35) | 24 (2–34) | 36 (34–38) |
| Parkinsonism | 0 (0%) | 1 (10%) | 0 (0%) |
| Psychosis | 2 (29%) | 1 (10%) | 0 (0%) |
| Mood disorder | 3 (43%) | 3 (30%) | 0 (0%) |
STMS = Short test of mental Status
Data shown in Median (range)
Comparisons made using the Mann Whitney U or Chi Square (for gender) tests with significance set at p< 0.05
Significant difference across all three groups
3.1 Stereotypies
For a description of the specific stereotypies see table 2. The stereotypies were simple, and in the majority of subjects involved repetitive movements of the hands and arms. Less common stereotypies that were observed included movements of the lower extremities, and the tongue. There were three subjects that were observed to also have vocalizations e.g. making humming sounds. In none of the subjects with stereotypies was there an urge to perform the movements, and when the subjects were asked to suppress the movements, which they could do, in no subject was there a feeling of internal uneasiness or increased tension arguing against these movements being tics[11].
Table 2.
Description of stereotypies in subjects with FTLD
| Subject | Description |
|---|---|
| 1 | Repetitive waving-like movements of his right hand |
| 2 | Repetitively moving her right hand back and forth |
| 3 | Repetitively making circles with her right hand |
| 4 | Repetitively hit the palm of his right hand against his right leg |
| 5 | Repetitively putting the right index finger into the mouth, tongue protrusions, and tapping the lips with her finger |
| 6 | Repetitively crossing the right leg over her left leg |
| 7 | Repetitively tapping his right hand on his right leg |
3.2 Image analysis
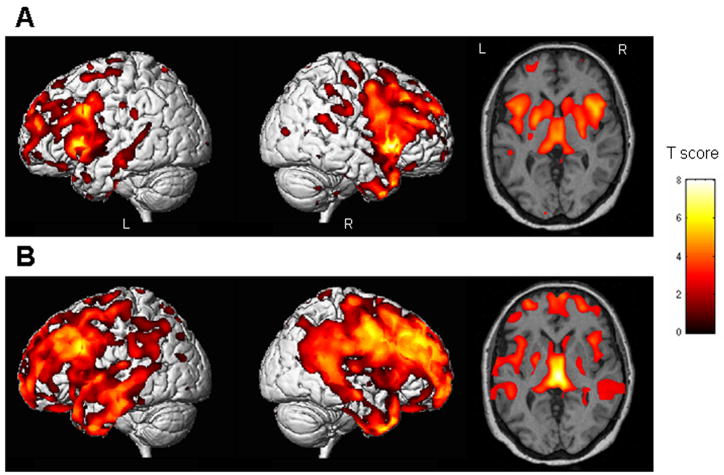
Compared to controls FTLD subjects with stereotypies showed a pattern of grey matter loss that was predominantly focused in the striatum, including the putamen and head of the caudate nucleus bilaterally (Figure 1A). There was significant involvement of the insula bilaterally. Grey matter losses were also observed in the frontal and anterior temporal lobes but to a lesser degree. The subjects without stereotypies showed less involvement of the striatum compared to controls, but widespread loss involving the frontal, temporal and parietal cortices, and the insula (Figure 1B).
Figure 1.
Regions of grey matter loss present in the group of FTLD subjects with stereotypic behaviors (A), and the group of FTLD subjects without stereotypic behaviors (B), when compared to the control group (Corrected for multiple comparisons, p<0.05). The patterns of cortical loss are shown on 3D surface renders, and patterns of loss in the basal ganglia regions are shown on a representative axial slice. The subjects with stereotypies showed loss in the striatum, with relatively less involvement of the frontal and temporal lobes. In comparison, the subjects without stereotypies showed widespread frontal and temporal losses, with less involvement of the striatum.
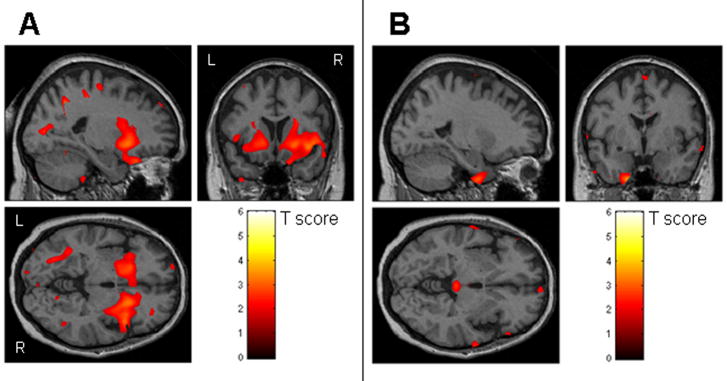
When the two FTLD groups were directly compared, the subjects with stereotypies showed significantly greater loss in the striatum than the subjects without stereotypies (Figure 2A). While both the left and right striatum was involved, the loss was more severe on the right where it also extended laterally to the right insula. Regions of greater loss were also seen in the orbitofrontal cortex. The subjects without stereotypies showed greater loss in regions scattered predominantly in the frontal and temporal cortices compared to those with stereotypies (Figure 2B).
Figure 2.
The results of a direct comparison performed between the FTLD subjects with and without stereotypies (uncorrected, p<0.05). Striatal loss was greater in the FTLD subjects with stereotypies compared to those without stereotypies (A), whereas the FTLD subjects without stereotypies showed greater loss in the frontal and temporal lobes compared to those with stereotypies (B).
4. DISCUSSION
In this study we demonstrate a different pattern of corticostriatal volume loss in FTLD subjects with and without stereotypies. In the subjects without stereotypies we see a typical pattern of frontotemporal volume loss with some involvement of the striatum. However, in the subjects with stereotypies the focus of significant atrophy was the striatum with relatively less cortical involvement.
The findings in this study suggest that although corticostriatal atrophy is a characteristic feature of FTLD, the degree of cortical and striatal loss determines whether stereotypies will manifest. There are two possible interpretations to our findings. First, stereotypies occur in FTLD when there is a certain amount of striatal atrophy independent of frontotemporal cortical atrophy. Second and more likely, stereotypies occur in FTLD when there is mixed striatal and frontotemporal atrophy, but the greater relative burden of volume loss occurs in the striatum. Specifically, it is unlikely that isolated striatal loss results in the presence of sterotypies in FTLD, but it is the imbalance between cortical and striatal loss that is important. A single patient with stereotypies and an isolated right putamen infarct has also been reported, highlighting the fact that striatal damage is associated with stereotypies [12]. Although there was varying degree of cortical and striatal atrophy in those with and without stereotypies, the overlapping regions of grey matter atrophy is evidence against FTLD with and without stereotypies being separate diseases, but more akin to the fact that FTLD is not a homogeneous clinical syndrome.
The group of subjects without stereotypies was found to have more frontotemporal neocortical atrophy compared to the group of subjects with stereotypies. This fits with the fact that the subjects without stereotypies had more advanced disease since the Short Test of Mental Status score[10] was 5-points lower, in that group. It is unlikely however that the more widespread striatal atrophy in the group of subjects with stereotypies was due to more advance disease since the Short Test of Mental Status score in those with stereotypies was 5-points higher than those without stereotypies.
The striatum receives many input fibers of which the corticostriatal and nigrostriatal are two of the most important. Since we did not observe an increased frequency of parkinsonism in our subjects with stereotypies we speculate that although the corticostriatal pathway is affected in FTLD with stereotypies, nigrostriatal pathways remain intact, at least earlier on in the disease course. The nigrostriatal pathway may later become affected, since parkinsonism is a common late occurrence in FTLD[9].
Repetitive behaviors are frequent in FTLD. One study reported that almost 80% of autopsy proven FTLD subjects had repetitive behaviors ranging from stereotypies to complex obsessive-compulsive behaviors [1]. The authors of that study concluded that repetitive behaviors in FTLD are secondary to combined damage to the frontal lobe, striatum, and globus pallidum. A more recent study demonstrated an increased frequency of sterotypies in FTLD compared to Alzheimer disease and suggested that repetitive movements in FTLD result from orbitofrontal and caudate injury. Indeed in our subjects with stereotypies, the striatum, including the caudate nuclei, and the orbitofrontal cortex were more affected than in those without stereotypies.
It is important to stress however that this is a group study and therefore the results may not be representative of each single subject in the analysis. The number of subjects in this study was also relatively small and hence results for the secondary analyses were considered significant at a p<0.05. There are also a number of limitations inherent to the technique of VBM. In particular, misclassification of tissue due to variable degrees of ventricular enlargement among subjects is likely to have contributed to the artifactual grey matter loss observed around the lateral ventricles. Regardless of these limitations, the results of our study help to shed light on the variability of the presence of stereotypies in FTLD.
In summary, we compare FTLD subjects with and without stereotypies and demonstrate a greater proportion of striatal to cortical volume loss in those with stereotypies compared to those without. The results suggest that stereotypies in FTLD are associated with greater striatal loss with an imbalance between cortical and striatal loss.
Acknowledgments
This study was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078, by grants P50 AG16574, U01 AG06786, R01 AG11378 and R01 AG15866 from the National Institute on Aging, Bethesda MD and the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer s Disease Research Program of the Mayo Foundation, U.S.A. The authors would also like to acknowledge Drs David Knopman, Bradley Boeve, and Ronald Petersen of the Mayo Alzheimer’s Disease Research Center who was involved with recruitment of some of the normal controls used in this study.
Footnotes
DISCLOSURE STATEMENT
None of the coauthors have any disclosures or conflicts of interest
References
- 1.Ames D, Cummings JL, Wirshing WC, Quinn B, Mahler M. Repetitive and compulsive behavior in frontal lobe degenerations. J Neuropsychiatry Clin Neurosci. 1994;6(2):100–13. doi: 10.1176/jnp.6.2.100. [DOI] [PubMed] [Google Scholar]
- 2.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S, Elton R. committee MotUd. United Parkinson’s Disease Rating Scale. In: Fahn S, Calne D, Golstein M, editors. Recent developments in Parkinson’s disease, M.D. Macmillina Health care Information; New Jersey: 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- 4.Friedman J. Stereotypy and Catatonia. In: Jankovic J, Tolosa E, editors. Parkinson’s disease and movement disorders. Williams and Wilkins; Baltimore: 1998. [Google Scholar]
- 5.Garraux G, Goldfine A, Bohlhalter S, Lerner A, Hanakawa T, Hallett M. Increased midbrain gray matter in Tourette’s syndrome. Ann Neurol. 2006;59(2):381–5. doi: 10.1002/ana.20765. [DOI] [PubMed] [Google Scholar]
- 6.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 7.Jankovic J. Stereotypies. In: Marsden CDF, editor. Movement Disorders. Butterworth Heinemann; London: 1994. pp. 503–517. [Google Scholar]
- 8.Josephs KA, Holton JL, Rossor MN, Godbolt AK, Ozawa T, Strand K, Khan N, Al-Sarraj S, Revesz T. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol. 2004;30(4):369–73. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 9.Knopman DS, Mastri AR, Frey WH, 2nd, Sung JH, Rustan T. Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology. 1990;40(2):251–6. doi: 10.1212/wnl.40.2.251. [DOI] [PubMed] [Google Scholar]
- 10.Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. 1987;62(4):281–8. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- 11.Lees AJ. Tics and related disorders. Edinburgh: Churchill Livingston; 1985. [Google Scholar]
- 12.Maraganore DM, Lees AJ, Marsden CD. Complex stereotypies after right putaminal infarction: a case report. Mov Disord. 1991;6(4):358–61. doi: 10.1002/mds.870060418. [DOI] [PubMed] [Google Scholar]
- 13.McMillan AB, Hermann BP, Johnson SC, Hansen RR, Seidenberg M, Meyerand ME. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage. 2004;23(1):167–74. doi: 10.1016/j.neuroimage.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Mendez MF, Shapira JS, Miller BL. Stereotypical movements and frontotemporal dementia. Mov Disord. 2005;20(6):742–5. doi: 10.1002/mds.20465. [DOI] [PubMed] [Google Scholar]
- 15.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 16.Padovani A, Borroni B, Brambati SM, Agosti C, Broli M, Alonso R, Scifo P, Bellelli G, Alberici A, Gasparotti R, Perani D. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2006;77(4):457–63. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 18.Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26(2):600–8. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang-Wai DF, Knopman DS, Geda YE, Edland SD, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Petersen RC. Comparison of the short test of mental status and the mini-mental state examination in mild cognitive impairment. Arch Neurol. 2003;60(12):1777–81. doi: 10.1001/archneur.60.12.1777. [DOI] [PubMed] [Google Scholar]
- 20.Teipel SJ, Alexander GE, Schapiro MB, Moller HJ, Rapoport SI, Hampel H. Age-related cortical grey matter reductions in non-demented Down’s syndrome adults determined by MRI with voxel-based morphometry. Brain. 2004;127(Pt 4):811–24. doi: 10.1093/brain/awh101. [DOI] [PubMed] [Google Scholar]