Abstract
Objective
This review highlights research on sex-based differences in pain perception and treatment. We sought to illuminate the complex factors contributing to differences in pain and analgesic responses between males and females, ranging from psychosocial to biological processes.
Design
We reviewed published studies of pain induction by chemical, electric, heat, surgical, or psychological means, and opioid and nonopioid analgesia comparing responses in men and women.
Results
A substantial body of research indicates that women experience greater clinical pain, suffer greater pain-related distress, and show heightened sensitivity to experimentally induced pain compared with men. Research on sex-based differences in the pain experience and treatment is beginning to uncover patterns that may enable tailoring of pain treatment to individual characteristics. The factors underpinning sex differences in the experience of pain are multifactorial and complex; for example, psychosocial factors such as pain-related catastrophizing may explain sex-based differences in reporting certain types of pain, as women tend to use catastrophizing to a greater degree. Gonadal hormone levels in cycling women also have a substantial impact on pain perception and analgesic response. Women perceive more pain during the luteal phase, and estrogen antagonists provide long-term pain relief in certain situations.
Conclusions
Collectively, greater understanding of the factors that commonly and differentially affect the disparity in pain perception, as well as analgesic response, are beginning to illuminate research targets and promising areas of therapeutic intervention for improved pain management.
Keywords: Sex, Sex Differences, Pain, Experimental Pain, Analgesia, Psychosocial
Introduction
Chronic pain leads to diminished quality of life, physical disability [1], and is a serious risk factor for suicide [2], rendering pain management an essential component of medical care. A broad range of variables, from genotype to psychosocial processes, contribute to individuals’ pain responses. Sex is a critical factor in modulating the experience of pain, with a growing body of research demonstrating that males and females experience pain differently and respond differentially to specific classes of analgesic medications.
In epidemiologic studies, women are more likely than men to report acute and chronic pain [3] and use pain-relieving medication significantly more often, even when equating the sexes on pain frequency and severity [4,5]. In fact, women are more likely than men to pursue a variety of treatments for many painful conditions [3]. Biological, cultural, psychological, and social factors are all hypothesized to contribute to these disparities in pain responses, reporting, and management [6]. Sex differences in pain are frequently substantial in magnitude, with moderate to large effect sizes ranging from 0.41 to 0.82 [7]. Consistency in the direction of sex differences across pain phenotypes is impressive, with women reporting greater pain than men in acute clinical pain models, such as postsurgical pain [3], and having an elevated prevalence (in many cases, 50–100% higher relative to men) of many chronic pain conditions, including headache, temporomandibular joint disorder (TMD), fibromyalgia, irritable bowel syndrome and arthritis [3].
Laboratory-based studies have also reported that women are more sensitive than men to experimentally induced pain, as measured by subjective pain threshold, tolerance, or ratings of pain intensity in response to standardized noxious stimuli [6,8], as well as by less subjective indices, such as electromyographically measured thresholds for muscle reflexes [9] or pupil dilation [10] in response to noxious stimulation. However, these studies, as described in the Greenspan consensus report [11], demonstrate the contrast between the often-striking epidemiological sex differences in chronic and acute pain and the generally subtle (though consistent) reported pain differences in experimental environments. While experimental pain studies provide different information than clinical reports, abundant research summarized in a meta-analysis by Riley and colleagues [6] has suggested the relevance of experimental pain induction procedures in predicting clinical pain outcomes [6]. Collectively, the findings in this experimental area complement clinical research and support the contention that sex differences in reports of pain are unlikely to simply be a function of reporting bias (or undue partiality), as involuntary physiological responses to painful stimulation are augmented in females. That is, the argument is sometimes advanced that the pain experience of men and women does not differ in a phenomenological sense, but rather that the two sexes describe their experiences in different terms (e.g., men may consciously or unconsciously underreport the pain that they “really” feel as a consequence of social forces and expectations). However, the documentation of sex differences in physiological responses to noxious stimulation that are not under voluntary control certainly appears to support the conclusion that sex and gender influence the processing of pain-related information in important ways. In the following review, we examine the complex spectrum of factors contributing to sex differences in pain responses, as data on individual differences may enhance clinical management. This article discusses several factors, including psychosocial, neurophysiologic, genetic, and gonadal hormonal. These variables and others are discussed in the context of the biopsychosocial model [12], which views the pain experience as a construct that is composed of fluid interactions among biologic, psychologic, and sociocultural factors, which are discussed in the context of the biopsychosocial model.
Factors that Contribute to Sex Differences in Pain Report
Experimental Methods
Models of noxious stimulation used to evoke pain in volunteers include pressure, thermal, mechanical, electrical, ischemic, chemical, and cold pressor stimulation [13]. The magnitude of sex differences observed in experimental pain studies may be affected by variations in the methodology used to induce pain, including stimulus type, anatomic location, stimulus duration, size of the stimulated area [14], number of repetitions [15], rate of rise of temperature in thermal stimulation [16], and even whether the stimulus is self-applied [17]. In evaluating the moderating influence of stimulus modality on sex differences in pain responses, a meta-analysis found a large to moderate effect size in sex differences when threshold or tolerance electrical stimulation was used, while effect sizes were smaller and more variable in thermal pain assessments [6].
Psychosocial Factors
Psychological factors, including anxiety, depression, attentional processes, use of specific pain-coping strategies, and catastrophizing, are associated with the experience and report of pain, and have all been shown to vary as a function of sex. A recent review nicely summarizes the broad network of contributing psychosocial factors including: age, race/ethnicity/culture, history, comorbidities and baseline health, disability, medications, physical variables, beliefs, coping, mood, the setting in which pain is studied, and the need for further pain-related studies on living arrangements, employment history, social support, marital status, and family composition on pain [11]. Here we would like to focus on a few less well-known factors in detail, including catastrophizing, abuse history, and health care provider characteristics.
Catastrophizing, a negative cognitive and affective response to pain, which includes feelings of helplessness, magnification, and ruminative thoughts, is more common in women. Sex difference in catastrophizing has been shown to mediate the sex difference in clinical pain in both patients with chronic pain [18] and in nonclinical samples of adults [19]. Catastrophizing is also prospectively associated with enhanced sensitivity to and reduced tolerance for thermal pain following acute dental procedures [19]. Catastrophizing appears to delay the resolution of sensitivity following an acutely painful condition, and has been associated with increased pain responses over time [19,20]. Similarly, depressive symptoms have been found to predict future musculoskeletal disorders, including lower back pain [21,22]. Positive emotions, such as positive mood (especially humor induced), and relaxation [23], are all associated with a reduction of reported pain; positive mood-inducing odors also reduce pain in women but not men [24]. Collectively, these findings suggest that emotional processes may influence pain differentially for men and women. Sex differences in the use of pain-coping strategies have been found in adults [25], and even in adolescents [26], with males reporting more use of behavioral distraction while females rely more on social support and positive self-statements.
Women’s higher reporting of pain could be linked to childhood abuse, as girls are more often victims of abuse, and a history of childhood abuse has been associated with chronic pain in adulthood [27]. A national study of childhood sexual abuse showed that 27% of women vs 16% of men reported abuse [28], and sexual maltreatment in childhood has been associated with a higher number of adult pain disorders (e.g., migraine, back pain, abdominal pain) [29]. Other studies have highlighted the complex association between abuse history and pain. Fillingim and Edwards [30], for example, reported an association between childhood abuse and decreased experimental pain sensitivity, but also with increased general pain complaints, poorer self-reported health, and greater negative affect in healthy adults. This link may be driven by hypothalamus–pituitary–adrenal axis abnormalities and/or neuroendocrine dysregulation caused by the initial trauma [31]. These findings highlight the complexities within this literature and the importance of incorporating psychosocial interventions when formulating treatment plans in clinical practice.
Interpersonal factors may also influence sex differences in the pain experience. For example, clinical research suggests that reports of symptoms such as pain may vary as a function of the health care provider’s sex, highlighting the importance of considering the interpersonal context of verbal reports of pain [32]. Sex differences have also been found in the diagnosis and treatment strategies physicians choose for patients reporting pain. A Swedish study found that both male and female physicians prescribed more drugs for women with extreme neck pain than for men, and ordered additional diagnostic work for the female patients [33]. In a U.S. study using hypothetical vignettes describing patients with persistent back pain, both male and female physicians prescribed higher doses of hydrocodone to same-sex “patients,” but male physicians prescribed more pain medication to whites, while female physicians prescribed more to blacks [34]. One suggested explanation for this difference may be that physicians differentially sympathize/identify with patients based on gender. However, findings of sex bias are not universal; no gender disparities were found in physician prescription of opioid analgesics in at least one emergency department study [35]. Manipulation of sex-role expectations can also significantly affect pain reporting, as boys and girls receive different reinforcement for their expression of pain-related experiences [36]. Consistent with this social learning perspective (i.e., men are conditioned to report less pain), women report greater pain with the same objective pathology [37,38], and both sexes expected men would report less pain for common events [39]. Robinson and colleagues found that significant sex differences in pain tolerance could be eliminated by manipulating gender role expectations [40]. Although studies such as these may suggest that psychosocial components are a principal cause of sex differences, autonomic indicators such as pupil dilation [10] and differential brain responses to noxious stimulation, measured by positron emission tomography (PET), have demonstrated that women do show enhanced physiologic responses to noxious stimuli [41], suggesting potential neurophysiological mechanisms as well.
Neurophysiological Mechanisms
Three opioid receptors, μ, κ, and δ, are significantly implicated in modulating pain [42]. Clinically, μ is the most relevant receptor, as morphine and most other opioid agonists act preferentially at that site; thus, studies have focused most attention on this opioid receptor. Human experiments consistently show that μ opioids have increased potency in females [43,44]. In animal studies, the intensity of noxious stimulation used during pain testing influences the observed sex effect (female predominance of μ-opioid analgesia is most notable at low to moderate intensities in thermal studies [45–47]). A 2001 review by Craft et al. summarizes several other animal studies reporting that κ opioids, butorphanol and nalbuphine, were far more potent and effective in male rodents, but only when the rodents were subjected to low or moderate intensity noxious stimuli [48].
Interestingly, pharmacokinetics does not appear to underlie the sex differences in analgesic effects of opioids, as most studies report no sex differences in plasma levels of morphine [49]. Male–female differences in opioid pharmacodynamics have been observed in opioid receptor density, in affinity of opioids for the receptor, and in opioid receptor-mediated signal transduction in rodents [50], and human postmortem analysis reveals greater μ-opioid receptor concentrations among females [51], which may partially account for the observation that μ-opioid agonists provide more analgesia among women. While analgesic efficacy may vary depending on sex, studies examining these differences remain inconclusive.
Genetics
While a growing body of pain research implicates several “pain genes” as a factor shaping variability in the experience of pain [52], available data on the genetic contribution to sex differences in pain is inconsistent. For example, a Swedish study of chronic pain in twins found that genetics and shared environmental influences accounted for nearly half the variance in chronic pain, but found no significant sex differences in the association of specific genes with pain phenotypes [53]. Similarly, a Danish study of twins of opposite sex found no sex difference in the genetic influence on neck pain [54]. Recent experimental pain research in healthy twins suggests that 60% of variance in cold pain responses and 26% in heat pain responses were attributable to genetic factors. While sex differences in pain report were evident, the heritability of these differences did not differ significantly by sex [55].
However, increasing evidence shows that certain genes mediate analgesic responses in a sex-specific manner. Women who have red hair and fair skin, caused by variants of the human melanocortin-1 receptor gene, demonstrated significantly greater analgesic effect from pentazocine, a κ-opioid, than did men or women without the variants [56]. Furthermore, the A118G single nucleotide polymorphism of the μ-opioid receptor gene (OPRM1) is directly associated with pressure pain responses in both sexes, and a rare allele is associated with higher heat pain ratings in women and lower heat pain ratings in men [57]. These profiles may eventually lead to patient genotype informing the pain management process in a sex-specific manner.
Sex Differences in Central Pain Processing
Both PET and functional magnetic resonance imaging (fMRI) techniques are being used to investigate the brain’s processing of acute and chronic pain, and can be used to study potential sex differences in central nervous system (CNS) pain processing. Research using fMRI has illuminated substantial sex differences in CNS processing of pain stimuli and even of anticipation of pain stimuli [58,59]. For example, mildly painful rectal distention in patients with irritable bowel syndrome (IBS) produced activation of the left thalamus and ventral striatum only in men. In women, areas of the amygdala and mid-cingulate were deactivated during uncomfortable and mild pain stimuli, respectively. During anticipation and uncomfortable (nonpainful) distension, men showed greater activation in areas of the insula [60]. Similarly, a study of pressure stimulation showed more frequent patterns of pain-related deactivation in parts of the pain neuromatrix in female subjects [59]. An earlier study using healthy patients found that in the anterior cingulate cortex/prefrontal region in women, but not men, progressive intensity of rectal distension corresponded with progressive increase in activation volume [61].
Using PET to assess sex differences in pain-related opioid receptor binding, researchers found sex differences in the magnitude and location of μ-opioid system activation. Reproductive-age women showed higher μ-opioid receptor binding values than men in several brain regions [62]. In another study, men demonstrated larger magnitudes of μ-opioid activation than woman in the anterior thalamus, ventral basal ganglia, and amygdala. Further, women showed reduced activation in the nucleus accumbens during the follicular phase (low progesterone, low estrogen state) of their menstrual cycle [41]. Naliboff used PET to study pain in IBS patients and found greater activation of the right dorsolateral prefrontal cortex, insula, and dorsal pons/periaqueductal gray in men, and greater activation in the ventromedial prefrontal cortex, right anterior cingulate cortex, and left amygdala in women [60]. Collectively, these imaging techniques suggest probable sex differences in brain activation within the pain neuromatrix following application of painful stimuli, though variability in the methods and findings is such that it seems premature to draw specific conclusion about which brain regions show differential pain-related activation as a function of sex.
Finally, sex differences in pain-relevant peripheral neuroanatomy have also been observed, suggesting structural and functional variation between sexes. Researchers harvested facial skin samples from male and female cadavers and found women to have twice the average nerve fiber density of men (34 +/− 19 vs 17 +/− 8 fibers per cm of skin) [63]. These findings suggest another potential mechanism, in this case involving structural aspects of the nervous system, contributing to sex differences in pain perception.
Gonadal Hormones
To date, studies report variable results when assessing potential changes in pain responses over the course of the menstrual cycle. In 2006, Sherman and LeResche reviewed experimental pain studies and concluded that there was little evidence that the menstrual cycle affected responses to noxious stimuli, with the possible exception of electrical pain stimulation; however, 9 of their 14 studies determined cycle status by self-report alone [64]. Their findings differed sharply from the meta-analysis of studies of experimental pain by Riley et al., which, after a more rigorous exclusion (i.e., they culled the number of included studies from 16 to 9) based on quality, and a standardization of cycle days, concluded that responses to painful stimuli are affected by the menstrual cycle [65]. Somewhat more consistency can be found in studies of clinical pain [66], as women reported higher rates of back pain, temporomandibular disorder (TMD), and migraine headache at the end of the luteal phase of the menstrual cycle, though again, the majority of these studies determined cycle status based on self-report [67]. In fibromyalgia patients, menstrual cycle onset and menopause resulted in higher pain scores, suggesting the importance of declining levels of estradiol [68]. As was recently addressed in the Consensus report by Greenspan et al., these variable results are complicated by vague gynecological nomenclature, the lack of standardization of determining hormone levels and the exact day of the menstrual cycles, and the difficulty of excluding irregularly cycling women solely by self-report [11].
In studies of exogenous hormone administration, estrogen administration is associated with increased reports of TMD in cycling women receiving oral contraceptives and an increase in reports of TMD in postmenopausal women receiving hormone supplements [69]. Relatedly, decreasing estrogen availability can provide analgesia; Kontostolis et al. found that administering the estrogen antagonist tamoxifen provided effective pain relief for 72% of subjects with cyclical mastalgia, and that 54% of patients were still symptom-free 12 months after treatment stopped [70]. Kuba et al. [66] noted that Kontostolis’ findings opened a promising line of research into the use of estrogen antagonists as analgesics in chronic pain conditions in which circulating hormones may be implicated. On the other hand, estrogen replacement treatment for women in menopause alleviated symptoms of oral discomfort and improved oral cytology in 56% of symptomatic women [71]. A summary of these findings is presented in Table 1.
Table 1.
A summary of the impact of gonadal hormones in pain and analgesia
| Hormonal milieu | Effects on pain state | |
|---|---|---|
| Endogenous hormonal states | ||
| Cycling women | End of luteal phase (premenstrual) vs other phases of the menstrual cycle | Higher rates of back pain, TMD, and migraine headache |
| Premenstrual period | TMD peaked | |
| Onset of menstrual cycle | Higher fibromyalgia pain scores | |
| Postmenopausal women | Postmenopausal women compared to cycling women | Greater orofacial pain |
| Exogenous hormone therapy | ||
| Cycling women | Cycling women administered exogenous estrogen | 20% increase in reported TMD |
| Postmenopausal women | Postmenopausal women administered exogenous estrogen | 30% increase in reported TMD |
| Estrogen replacement for postmenopausal women | Alleviated symptoms of oral discomfort |
TMD = temporomandibular joint disorder.
Estrogen may contribute to analgesia or hyperalgesia by modulating endogenous opioid neurotransmission. In ovarectomized mice, estradiol increases μ-opioid receptor (MOR) protein concentrations [72], the concentration of endogenous opioid peptides, and the release of endogenous opioid peptides in vitro [73]. Zubieta et al. used PET to show that women in a low estradiol state (early follicular phase) had lower in vivo μ-opioid receptor availability corresponding with higher endogenous μ-opioid activation, correlating with higher pain ratings and more negative affect when subjected to sustained pain [62]. Smith et al. found that women with high estrogen (i.e., those using a transdermal patch) showed increased regional μ-opioid receptor availability and increased pain-related activation of endogenous μ-opioid neurotransmission compared with low-estrogen-state women [74]. A recent fMRI study suggested that the affective component of pain may be enhanced during the low-estrogen phase of the menstrual cycle [75]. Interestingly, studies of pregnancy-induced analgesia report a steady increase in pain threshold throughout late pregnancy and then acutely prior to delivery [76], with evidence of estrogen involvement [77]. Finally, low androgen levels in women are associated with antianabolic features of fibromyalgia [78], high-lighting the multiple hormonal systems that likely interact to shape sex differences in the experience of pain.
Overall, fewer studies have focused on men, pain, and gonadal hormones. One such study found men receiving androgen deprivation therapy for prostate cancer reported significantly reduced quality of life, though no difference in pain was reported [79]. Moreover, low-dose testosterone administration did reduce pain in men with chronic, stable angina [80,81], and animal studies have noted analgesic effects of testosterone [82]. In a 2007 study of female-to-male transsexual chronic pain patients, half showed reduced pain with testosterone treatment [83]. However, there is substantial variability in findings; a study of exogenous estrogen administration in men showed no impact on exercise-induced muscle inflammation [84–87]. Collectively, substantial data suggests sex-related individual difference factors, such as hormone levels, that may influence person-to-person differences in pain processing both within and across sexes. Taken together, the literature suggests that either high or low estrogen in cycling women seems to enhance pain in most chronic pain conditions, while the data is mixed in postmenopausal women, depending on type of pain, and testosterone seems to improve quality of life in the context of pain. Table 1 summarizes research findings on the impact of gonadal hormones in pain and analgesia.
Sex Differences in Analgesic Responses
This section reviews research on sex differences in response to opioid and nonopioid pharmacological intervention, stress-induced analgesia, and other endogenous analgesic processes, and to behavioral and psychological interventions.
Sex Differences in Responses to Opioid Medications
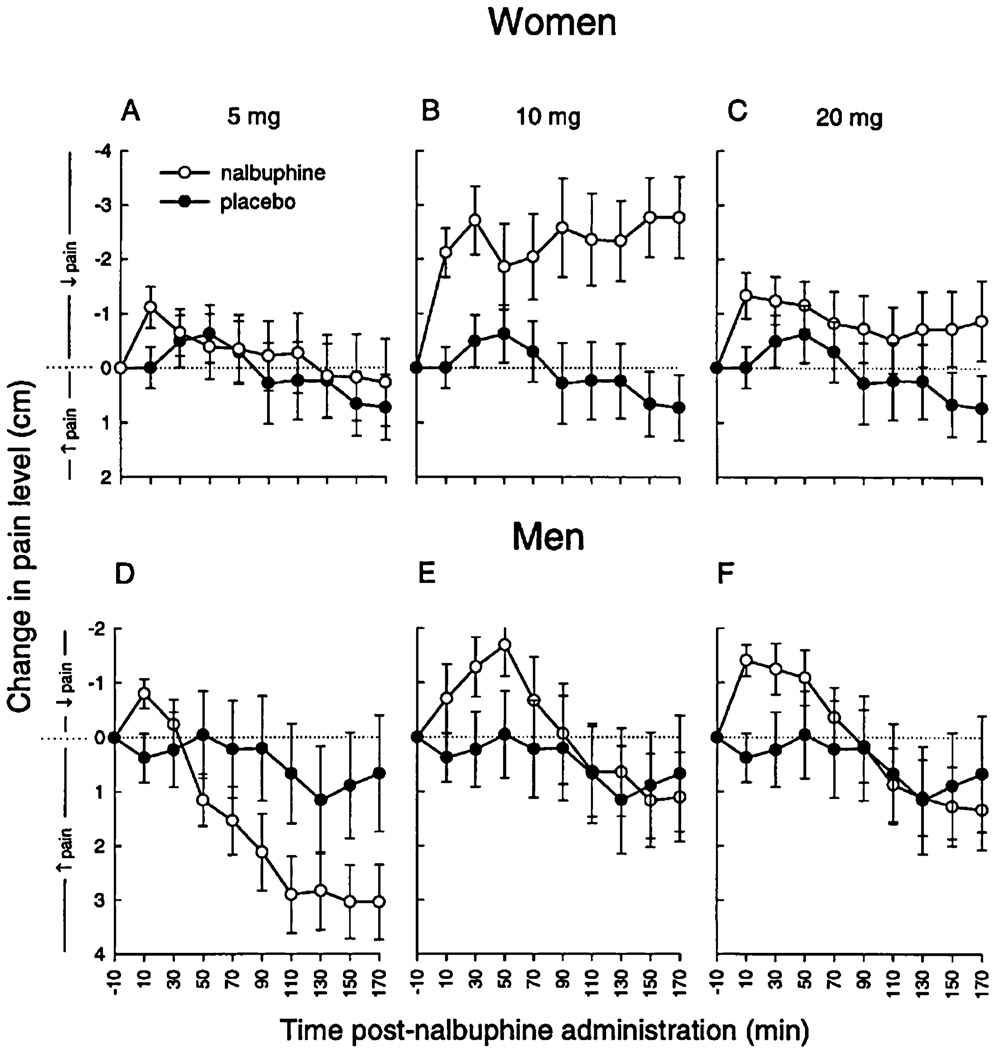
Several studies demonstrated greater opioid analgesia in females compared with males [88], mostly focusing on μ- and κ-opioid agonists. In a review of 18 studies of postsurgical opioid consumption, Miaskowski et al. found that in eight studies, men and women consumed equal amounts of opioids, while in 10 other studies, women consumed significantly less [89], suggesting potentially greater opioid analgesia in women per unit of administered opioid (though an alternative explanation may be that women experience greater respiratory depression from morphine) [49]. In a well-known series of studies, Gear et al. showed that women given κ-opioids have significantly greater analgesia than men after dental surgery [87]. Figure 1 summarizes these findings. Other studies of sex differences in opioid analgesia used experimental pain techniques, with some variation in results. Women showed greater analgesic response to morphine when experiencing electrical pain [90] and cold pressor pain [91]. However, Fillingim et al. tested sex differences in analgesic response to morphine (2005) and pentazocine (2004) using heat, pressure, and ischemic pain, and found no sex differences in analgesic response [92]. Women overall appear more sensitive to both dosage and type of analgesic medication.
Figure 1.
The effect of various doses of nalbuphine compared with placebo in women and men on postoperative pain [87] (used with permission).
Sex Differences in Response to Nonopioid Pharmacological Intervention
As there are relatively few studies specifically designed to test sex differences in nonopioid analgesic outcomes, findings in this area are somewhat discrepant. For example, no sex differences were found in analgesic response to ibuprofen in treating moderate to severe dental pain [93], while in a study of experimental electrically induced pain, only men showed a significant analgesic effect from ibuprofen [94]. Animal studies of the cyclooxygenase enzymes COX-1 and COX-2, and the ASIC (analgesics–acid-sensing ion channels) agents have suggested intriguing sex differences in nonopioid pain mechanisms. For example, administration of an inflammatory agent to COX-1 and COX-2 knockout mice had greater effects in females. Similarly, Chanda et al. found that a non-specific ASIC blocker (amiloride) had greater analgesic effect in female mice on formalin-induced nociception [95].
Sex Differences in Response to Endogenous Analgesic Processes
Many stress-related disorders, such as fibromyalgia [96] and chronic pain [97], are more prevalent in women, leading researchers to examine differences in endogenous analgesic processes. Although stress-induced analgesia (SIA) has been studied extensively in rodents, few studies have been conducted of SIA in humans. In 2005, Girdler et al. tested smokers’ and nonsmokers’ sensitivity to three types of pain following rest and following mental stress [98]. Only women had greater thresholds and tolerance for ischemic and thermal pain following a stressor compared with threshold and tolerance following rest. In contrast to these findings, counterirritation paradigms, thought to engage the endogenous opioid system, have been shown to be more effective in men when compared with women [99–101].
Remaining Research Questions
Despite inconsistencies, the rapidly increasing body of literature on sex differences in pain and analgesia offers glimpses into a future in which clinicians can expand diagnostic tools and tailor treatments to reflect individual differences. Although patterns in the data are still emerging, the current state of research allows us to suggest several research directions that could lead to changes in clinical approaches to pain treatment.
Given that catastrophizing may substantially account for sex differences in some pain conditions, could complementary psychological intervention (e.g., cognitive–behavioral therapy and training in effective pain-coping skills) be an important adjuvant in treating these conditions and reducing sex-related disparities in pain?
Given research showing κ-opioids to be more effective in women than in men after oral surgery, and that women have more respiratory depression from morphine than men, should κ-opioid treatment be considered as an alternative to morphine in females?
Given that chronic opioids worsen hypogonadal states in men and women, should supplemental hormones be administered? Relatedly, for pain conditions whose symptoms vary as a function of hormonal fluctuations, should treatment be tailored to particular menstrual phases in cycling women?
Given that fMRI technology provides a relatively clear topography of brain activity corresponding to pain conditions, could the use of this technology allow better targeting of therapeutic interventions? And could fMRI help us develop and test sex-specific analgesic interventions?
The experience of pain is affected by multiple variables, including psychosocial variables, genetics, sex, and type of analgesia. Exploring research questions as discussed above will permit us to better understand the multiple factors influencing a patient’s experience of pain and will allow us to develop more targeted treatment options.
Acknowledgment
Partial Funding from NIDA Grant R01 DA014098 and T32 MH75884.
References
- 1.Turner JA, Franklin G, Heagerty PJ, et al. The association between pain and disability. Pain. 2004;112(3):307–314. doi: 10.1016/j.pain.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Juurlink DN, Herrmann N, Szalai JP, Kopp A, Redelmeier DA. Medical illness and the risk of suicide in the elderly. Arch Intern Med. 2004;164(11):1179–1184. doi: 10.1001/archinte.164.11.1179. [DOI] [PubMed] [Google Scholar]
- 3.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65(2–3):123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 4.National Health Survey. Canberra, Australia: Australian Bureau of Statistics; Use of Medications. 1999
- 5.Isacson D, Bingefors K. Epidemiology of analgesic use: A gender perspective. Eur J Anaesthesiol Suppl. 2002;26:5–15. doi: 10.1097/00003643-200219261-00003. [DOI] [PubMed] [Google Scholar]
- 6.Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: A meta-analysis. Pain. 1998;74(2–3):181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 7.Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: Women report more pain than men after arthroscopic surgery. Pain. 2004;112(3):248–253. doi: 10.1016/j.pain.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Fillingim RB. Sex, gender and pain: Women and men really are different. Curr Rev Pain. 2000;4:24–30. doi: 10.1007/s11916-000-0006-6. [DOI] [PubMed] [Google Scholar]
- 9.France CR, Suchowiecki S. A comparison of diffuse noxious inhibitory controls in men and women. Pain. 1999;81(1–2):77–84. doi: 10.1016/s0304-3959(98)00272-3. [DOI] [PubMed] [Google Scholar]
- 10.Ellermeier W, Westphal W. Gender differences in pain ratings and pupil reactions to painful pressure stimuli. Pain. 1995;61(3):435–439. doi: 10.1016/0304-3959(94)00203-Q. [DOI] [PubMed] [Google Scholar]
- 11.Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: A consensus report. Pain. 2007;132 suppl 1:S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turk DC. Biopsychosocial perspective on chronic pain. In: Gatchel RJ, Turk DC, editors. Psychological Approaches to Pain Management: A Practitioner’s Handbook. New York: Guilford Press; 1996. pp. 3–32. [Google Scholar]
- 13.Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: Multiple domains of clinical relevance. Pain. 2005;114(3):315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Marchand S, Arsenault P. Spatial summation for pain perception: Interaction of inhibitory and excitatory mechanisms. Pain. 2002;95(3):201–206. doi: 10.1016/S0304-3959(01)00399-2. [DOI] [PubMed] [Google Scholar]
- 15.Carstens E, Wilson C. Rat tail flick reflex: Magnitude measurement of stimulus-response function, suppression by morphine and habituation. J Neurophysiol. 1993;70(2):630–639. doi: 10.1152/jn.1993.70.2.630. [DOI] [PubMed] [Google Scholar]
- 16.Fillingim RB, Maddux V, Shackelford JA. Sex differences in heat pain thresholds as a function of assessment method and rate of rise. Somatosens Mot Res. 1999;16(1):57–62. doi: 10.1080/08990229970654. [DOI] [PubMed] [Google Scholar]
- 17.Braid L, Cahusac PM. Decreased sensitivity to self-inflicted pain. Pain. 2006;124(1–2):134–139. doi: 10.1016/j.pain.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Keefe FJ, Lefebvre JC, et al. Understanding the adoption of arthritis self-management: Stages of change profiles among arthritis patients. Pain. 2000;87(3):303–313. doi: 10.1016/S0304-3959(00)00294-3. [DOI] [PubMed] [Google Scholar]
- 19.Edwards RR, Fillingim RB, Maixner W, Sigurdsson A, Haythornthwaite J. Catastrophizing predicts changes in thermal pain responses after resolution of acute dental pain. J Pain. 2004;5(3):164–170. doi: 10.1016/j.jpain.2004.02.226. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22(8):730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 21.Leino P, Magni G. Depressive and distress symptoms as predictors of low back pain, neck-shoulder pain, and other musculoskeletal morbidity: A 10-year follow-up of metal industry employees. Pain. 1993;53(1):89–94. doi: 10.1016/0304-3959(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 22.Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine. 2002;27(5):E109–E120. doi: 10.1097/00007632-200203010-00017. [DOI] [PubMed] [Google Scholar]
- 23.Cogan R, Cogan D, Waltz W, McCue M. Effects of laughter and relaxation on discomfort thresholds. J Behav Med. 1987;10(2):139–144. doi: 10.1007/BF00846422. [DOI] [PubMed] [Google Scholar]
- 24.Marchand S, Arsenault P. Odors modulate pain perception: A gender-specific effect. Physiol Behav. 2002;76(2):251–256. doi: 10.1016/s0031-9384(02)00703-5. [DOI] [PubMed] [Google Scholar]
- 25.Keogh E, Herdenfeldt M. Gender, coping and the perception of pain. Pain. 2002;97(3):195–201. doi: 10.1016/S0304-3959(01)00427-4. [DOI] [PubMed] [Google Scholar]
- 26.Keogh E, Eccleston C. Sex differences in adolescent chronic pain and pain-related coping. Pain. 2006;123(3):275–284. doi: 10.1016/j.pain.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg RT, Pachas WN, Keith D. Relationship between traumatic events in childhood and chronic pain. Disabil Rehabil. 1999;21(1):23–30. doi: 10.1080/096382899298061. [DOI] [PubMed] [Google Scholar]
- 28.Finkelhor D, Hotaling G, Lewis IA, Smith C. Sexual abuse in a national survey of adult men and women: Prevalence, characteristics, and risk factors. Child Abuse Negl. 1990;14(1):19–28. doi: 10.1016/0145-2134(90)90077-7. [DOI] [PubMed] [Google Scholar]
- 29.Walker EA, Gelfand A, Katon WJ, et al. Adult health status of women with histories of childhood abuse and neglect. Am J Med. 1999;107(4):332–339. doi: 10.1016/s0002-9343(99)00235-1. [DOI] [PubMed] [Google Scholar]
- 30.Fillingim RB, Edwards RR. Is self-reported childhood abuse history associated with pain perception among healthy young women and men? Clin J Pain. 2005;21(5):387–397. doi: 10.1097/01.ajp.0000149801.46864.39. [DOI] [PubMed] [Google Scholar]
- 31.Weissbecker I, Floyd A, Dedert E, Salmon P, Sephton S. Childhood trauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology. 2006;31(3):312–324. doi: 10.1016/j.psyneuen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Promoting pain relief and preventing abuse of pain medications: A critical balancing act. [accessed December 28, 2008];A joint statement from 21 health organizations and the Drug Enformcement Administration. Available at http://www.ampainsoc.org/advocacy/pdf/consensus_1.pdf. [PubMed]
- 33.Hamberg K, Risberg G, Johansson EE, Westman G. Gender bias in physicians’ management of neck pain: A study of the answers in a Swedish national examination. J Womens Health Gend Based Med. 2002;11(7):653–666. doi: 10.1089/152460902760360595. [DOI] [PubMed] [Google Scholar]
- 34.Weisse CS, Sorum PC, Sanders KN, Syat BL. Do gender and race affect decisions about pain management? J Gen Intern Med. 2001;16(4):211–217. doi: 10.1046/j.1525-1497.2001.016004211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heins JK, Heins A, Grammas M, et al. Disparities in analgesia and opioid prescribing practices for patients with musculoskeletal pain in the emergency department. J Emerg Nurs. 2006;32(3):219–224. doi: 10.1016/j.jen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Kupers R. Sex differences in pain—And now for something completely different. Behav Brain Sci. 1997;20:455–456. [Google Scholar]
- 37.Robinson ME, George SZ, Dannecker EA, et al. Sex differences in pain anchors revisited: Further investigation of “most intense” and common pain events. Eur J Pain. 2004;8(4):299–305. doi: 10.1016/j.ejpain.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Puntillo K, Weiss SJ. Pain: Its mediators and associated morbidity in critically ill cardiovascular surgical patients. Nurs Res. 1994;43(1):31–36. [PubMed] [Google Scholar]
- 39.Robinson ME, Gagnon CM, Dannecker EA, et al. Sex differences in common pain events: Expectations and anchors. J Pain. 2003;4(1):40–45. doi: 10.1054/jpai.2003.4. [DOI] [PubMed] [Google Scholar]
- 40.Robinson ME, Gagnon CM, Riley JL., 3rd Price DD. Altering gender role expectations: Effects on pain tolerance, pain threshold, and pain ratings. J Pain. 2003;4(5):284–288. doi: 10.1016/s1526-5900(03)00559-5. [DOI] [PubMed] [Google Scholar]
- 41.Zubieta JK, Smith YR, Bueller JA, et al. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22(12):5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przewlocki R, Przewlocka B. Opioids in chronic pain. Eur J Pharmacol. 2001;429(1–3):79–91. doi: 10.1016/s0014-2999(01)01308-5. [DOI] [PubMed] [Google Scholar]
- 43.Craft RM. Sex differences in opioid analgesia: “From mouse to man.”. Clin J Pain. 2003;19(3):175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: Importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology (Berl) 2000;150(4):430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- 45.Negus SS, Mello NK. Opioid antinociception in ovariectomized monkeys: Comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther. 1999;290(3):1132–1140. [PubMed] [Google Scholar]
- 46.Barrett AC, Cook CD, Terner JM, et al. Sex and rat strain determine sensitivity to kappa opioid-induced antinociception. Psychopharmacology (Berl) 2002;160(2):170–181. doi: 10.1007/s00213-001-0949-2. [DOI] [PubMed] [Google Scholar]
- 47.Negus SS, Zuzga DS, Mello NK. Sex differences in opioid antinociception in rhesus monkeys: Antagonism of fentanyl and U50,488 by quadazocine. J Pain. 2002;3(3):218–226. doi: 10.1054/jpai.2002.124734. [DOI] [PubMed] [Google Scholar]
- 48.Craft RM, Bernal SA. Sex differences in opioid antinociception: Kappa and “mixed action” agonists. Drug Alcohol Depend. 2001;63(3):215–228. doi: 10.1016/s0376-8716(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 49.Sarton E, Olofsen E, Romberg R, et al. Sex differences in morphine analgesia: An experimental study in healthy volunteers. Anesthesiology. 2000;93(5):1245–1254. doi: 10.1097/00000542-200011000-00018. discussion 6A. [DOI] [PubMed] [Google Scholar]
- 50.Baker L, Ratka A. Sex-specific differences in levels of morphine, morphine-3-glucuronide, and morphine antinociception in rats. Pain. 2002;95(1–2):65–74. doi: 10.1016/s0304-3959(01)00376-1. [DOI] [PubMed] [Google Scholar]
- 51.Gabilondo AM, Meana JJ, Garcia-Sevilla JA. Increased density of mu-opioid receptors in the postmortem brain of suicide victims. Brain Res. 1995;682(1–2):245–250. doi: 10.1016/0006-8993(95)00333-l. [DOI] [PubMed] [Google Scholar]
- 52.Mogil JS, Yu L, Basbaum AI. Pain genes? Natural variation and transgenic mutants. Annu Rev Neurosci. 2000;23:777–811. doi: 10.1146/annurev.neuro.23.1.777. [DOI] [PubMed] [Google Scholar]
- 53.Kato K, Sullivan PF, Evengard B, Pedersen NL. Importance of genetic influences on chronic widespread pain. Arthritis Rheum. 2006;54(5):1682–1686. doi: 10.1002/art.21798. [DOI] [PubMed] [Google Scholar]
- 54.Fejer R, Hartvigsen J, Kyvik KO. Sex differences in heritability of neck pain. Twin Res Hum Genet. 2006;9(2):198–204. doi: 10.1375/183242706776382482. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen CS, Stubhaug A, Price DD, et al. Individual differences in pain sensitivity: Genetic and environmental contributions. Pain. 2007 doi: 10.1016/j.pain.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Mogil JS, Wilson SG, Chesler EJ, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci USA. 2003;100(8):4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fillingim RB, Kaplan L, Staud R, et al. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6(3):159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- 59.Berman SM, Naliboff BD, Suyenobu B, et al. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R268–R276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- 60.Naliboff BD, Berman S, Chang L, et al. Sexrelated differences in IBS patients: Central processing of visceral stimuli. Gastroenterology. 2003;124(7):1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 61.Kern MK, Jaradeh S, Arndorfer RC, et al. Gender differences in cortical representation of rectal distension in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;281(6):G1512–G1523. doi: 10.1152/ajpgi.2001.281.6.G1512. [DOI] [PubMed] [Google Scholar]
- 62.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156(6):842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 63.Mowlavi A, Cooney D, Febus L, et al. Increased cutaneous nerve fibers in female specimens. Plast Reconstr Surg. 2005;116(5):1407–1410. doi: 10.1097/01.prs.0000182339.83156.06. [DOI] [PubMed] [Google Scholar]
- 64.Sherman JJ, Leresche L. Does experimental pain response vary across the menstrual cycle? A methodological review. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R245–R256. doi: 10.1152/ajpregu.00920.2005. [DOI] [PubMed] [Google Scholar]
- 65.Riley JL, 3rd, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81(3):225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 66.Kuba T, Quinones-Jenab V. The role of female gonadal hormones in behavioral sex differences in persistent and chronic pain: Clinical versus preclinical studies. Brain Res Bull. 2005;66(3):179–188. doi: 10.1016/j.brainresbull.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 67.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106(3):253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Pamuk ON, Cakir N. The variation in chronic widespread pain and other symptoms in fibromyalgia patients. The effects of menses and menopause. Clin Exp Rheumatol. 2005;23(6):778–782. [PubMed] [Google Scholar]
- 69.LeResche L, Saunders K, Von Korff MR, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69(1–2):153–160. doi: 10.1016/s0304-3959(96)03230-7. [DOI] [PubMed] [Google Scholar]
- 70.Kontostolis E, Stefanidis K, Navrozoglou I, Lolis D. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11(6):393–397. doi: 10.3109/09513599709152566. [DOI] [PubMed] [Google Scholar]
- 71.Forabosco A, Criscuolo M, Coukos G, et al. Efficacy of hormone replacement therapy in postmenopausal women with oral discomfort. Oral Surg Oral Med Oral Pathol. 1992;73(5):570–574. doi: 10.1016/0030-4220(92)90100-5. [DOI] [PubMed] [Google Scholar]
- 72.Quinones-Jenab V, Jenab S, Ogawa S, Inturrisi C, Pfaff DW. Estrogen regulation of muopioid receptor mRNA in the forebrain of female rats. Brain Res Mol Brain Res. 1997;47(1–2):134–138. doi: 10.1016/s0169-328x(97)00041-7. [DOI] [PubMed] [Google Scholar]
- 73.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18(10):3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith YR, Stohler CS, Nichols TE, et al. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26(21):5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Leeuw R, Albuquerque RJ, Andersen AH, Carlson CR. Influence of estrogen on brain activation during stimulation with painful heat. J Oral Maxillofac Surg. 2006;64(2):158–166. doi: 10.1016/j.joms.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Bajaj P, Bajaj P, Madsen H, Moller M, Arendt-Nielsen L. Antenatal women with or without pelvic pain can be characterized by generalized or segmental hypoalgesia in late pregnancy. J Pain. 2002;3(6):451–460. doi: 10.1054/jpai.2002.128065. [DOI] [PubMed] [Google Scholar]
- 77.Dawson-Basoa M, Gintzler AR. Gestational and ovarian sex steroid antinociception: Synergy between spinal kappa and delta opioid systems. Brain Res. 1998;794(1):61–67. doi: 10.1016/s0006-8993(98)00192-9. [DOI] [PubMed] [Google Scholar]
- 78.Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215–2222. doi: 10.1210/jcem.85.6.6615. [DOI] [PubMed] [Google Scholar]
- 79.Dacal K, Sereika SM, Greenspan SL. Quality of life in prostate cancer patients taking androgen deprivation therapy. J Am Geriatr Soc. 2006;54(1):85–90. doi: 10.1111/j.1532-5415.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 80.English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study. Circulation. 2000;102(16):1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 81.Hau M, Dominguez OA, Evrard HC. Testosterone reduces responsiveness to nociceptive stimuli in a wild bird. Horm Behav. 2004;46(2):165–170. doi: 10.1016/j.yhbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 82.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: The role of gonadal hormones. Eur J Pain. 2004;8(5):397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Aloisi AM, Bachiocco V, Costantino A, et al. Cross-sex hormone administration changes pain in transsexual women and men. Pain. 2007 doi: 10.1016/j.pain.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 84.Timmons BW, Hamadeh MJ, Tarnopolsky MA. No effect of short-term 17beta-estradiol supplementation in healthy men on systemic inflammatory responses to exercise. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R285–R290. doi: 10.1152/ajpregu.00605.2005. [DOI] [PubMed] [Google Scholar]
- 85.Gear RW, Gordon NC, Heller PH, et al. Gender difference in analgesic response to the kappaopioid pentazocine. Neurosci Lett. 1996;205(3):207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- 86.Gear RW, Miaskowski C, Gordon NC, et al. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2(11):1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 87.Gear RW, Miaskowski C, Gordon NC, et al. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83(2):339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 88.Gordon NC, Gear RW, Heller PH, et al. Enhancement of morphine analgesia by the GABAB agonist baclofen. Neuroscience. 1995;69(2):345–349. doi: 10.1016/0306-4522(95)00335-g. [DOI] [PubMed] [Google Scholar]
- 89.Miaskowski C, Gear RW, Levine JD. Sex-related differences in analgesic responses. In: Fillingim R, editor. Sex, Gender and Pain. Seattle, WA: IASP Press; 2000. pp. 209–230. [Google Scholar]
- 90.Sarton E, Teppema L, Dahan A. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology. 1999;90(5):1329–1338. doi: 10.1097/00000542-199905000-00017. [DOI] [PubMed] [Google Scholar]
- 91.Pud D, Yarnitsky D, Sprecher E, et al. Can personality traits and gender predict the response to morphine? An experimental cold pain study. Eur J Pain. 2006;10(2):103–112. doi: 10.1016/j.ejpain.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 92.Fillingim RB, Ness TJ, Glover TL, et al. Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6(2):116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Averbuch M, Katzper M. A search for sex differences in response to analgesia. Arch Intern Med. 2000;160(22):3424–3428. doi: 10.1001/archinte.160.22.3424. [DOI] [PubMed] [Google Scholar]
- 94.Walker JS, Carmody JJ. Experimental pain in healthy human subjects: Gender differences in nociception and in response to ibuprofen. Anesth Analg. 1998;86(6):1257–1262. doi: 10.1097/00000539-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 95.Chanda ML, Ritchie J, Austin JS, Seguela P, Mogil JS. Sex differences in the contribution of acid-sensing ion channels to nociceptive processing. Soc Neurosci Abstr. 2005;35:860–866. [Google Scholar]
- 96.Wolfe F, Ross K, Anderson J, Russell IJ. Aspects of fibromyalgia in the general population: Sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22(1):151–156. [PubMed] [Google Scholar]
- 97.Verhaak PF, Kerssens JJ, Dekker J, Sorbi MJ, Bensing JM. Prevalence of chronic benign pain disorder among adults: A review of the literature. Pain. 1998;77(3):231–239. doi: 10.1016/S0304-3959(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 98.Girdler SS, Maixner W, Naftel HA, et al. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114(3):372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 99.Serrao M, Rossi P, Sandrini G, et al. Effects of diffuse noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain. 2004;112(3):353–360. doi: 10.1016/j.pain.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 100.Ge HY, Madeleine P, Arendt-Nielsen L. Sex differences in temporal characteristics of descending inhibitory control: An evaluation using repeated bilateral experimental induction of muscle pain. Pain. 2004;110(1–2):72–78. doi: 10.1016/j.pain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 101.Staud R, Robinson ME, Vierck CJ, Jr, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101(1–2):167–174. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]