Abstract
Background
We recently reported marked hyporeactivity of the hypothalamo-pituitary-adrenal (HPA) axis in depressed females on job-stress related long-term sick-leave (LTSL). This unexpected finding prompted the question whether HPA-axis hypofunction in this group results from stress exposure, or reflects pre-existing vulnerability. Here, as a first step toward addressing this question, we assessed temporal stability of HPA-axis reactivity in these subjects.
Methods
We used the combined dexamethasone/corticotropin-releasing hormone (DEX-CRH) test to retest HPA-axis reactivity in 29 patients and 27 controls after 12 months follow-up. Clinical status and cognitive performance was also retested.
Results
Despite marked clinical improvement, and normalization of initially observed impairments in attention and working memory, marked HPA-axis hyporeactivity persisted in patients. A high test – retest correlation was found both at the level of ACTH (R=0.85, p<0.001) and cortisol (R=0.76, p<0.001) responses.
Conclusions
Hyporeactivity of the HPA was stable over 12 months in LTSL subjects, independently of clinical improvement and normalized cognitive function. The stability of this response over time suggests that decreased DEX-CRH responses in this group may be a trait rather than a state marker. This finding is compatible with a hypothesis that HPA-axis hyporeactivity may reflect a pre-existing vulnerability in these subjects.
Keywords: stress, depression, burnout, Dex-CRH-test, ACTH, cortisol, vulnerability
Introduction
Psychiatric diagnoses have surpassed other conditions as a cause of long-term sick-leave (LTSL) in Sweden and other industrialized countries. Diagnostic categories that account for the majority of this increase are mood and anxiety disorders, conditions thought to involve dysregulation of stress systems. This morbidity has been hypothesized to represent prolonged psychological responses to chronic job-related emotional and interpersonal stressors (28; 15). Commonly reported symptoms among subjects on job-stress related LTSL are emotional and physical exhaustion, manifested in depletion of energy and drive, and cognitive problems. These symptoms overlap with core symptoms of major depression, and in fact many of these subjects fulfill established diagnostic criteria for mood disorders. This prompts the question whether these two categories of conditions reflect a shared or distinct pathophysiology.
The glucocorticoid receptor hypothesis postulates that a key pathophysiological mechanism in major depression is an impaired negative feedback control of the hypothalamic-pituitary-adrenal (HPA)-axis, resulting in progressively unrestrained cortisol release (10; 19). Among a wide range of pathological consequences, this is thought to potentially result in hippocampal “endangerment” and volume reduction, in turn leading not only to further impairment of negative HPA-axis inhibition, but also to impaired cognitive function commonly reported in stress-related conditions (16). Original data on HPA-axis hyperactivity in depression were obtained using the dexamethasone suppression test (DST), subsequently found to possess limited sensitivity and specificity for depression (25). An improved test has therefore been developed to probe the dynamic status of the HPA-axis. In this combined DEX-CRH test, CRH is administered to stimulate ACTH release after dexamethasone pre-treatment. Using this test, a 80–90% sensitivity for detecting depression has been reported (9).
We recently applied the DEX-CRH text to probe whether depressed subjects on LTSL due to self-reported job stress share HPA-axis pathophysiology with major depression (23). Our hypothesis was that chronic stress exposure in LTSL subjects could have resulted in up-regulated reactivity of the HPA-axis in a manner commonly seen in major depression. We further hypothesized that this might be accompanied by hippocampal volume reduction with concomitant cognitive impairment. Unexpectedly, we found just the opposite, i.e. a marked decrease of HPA-axis reactivity in depressed LTSL subjects. HPA-axis responses to the DEX-CRH challenge in this group were decreased compared to healthy controls, in the absence of baseline differences. Rather than providing support for an impaired feedback inhibition of the HPA-axis, these data suggested a failure to mount an adequate response to the CRH stimulus, caused at a level upstream of the adrenal cortex. No hippocampal volume reduction was found, and cognitive impairment was limited to frontocortically localized functions, such as attention and working memory. Our study added to a growing literature on stress-related disorders in which hypo- rather than hypersecretion of cortisol is found (8; 6; 4)
A key question prompted by these findings is whether the hyporeactive HPA-axis observed in our LTSL subjects is likely to be the result of prolonged stress exposure, warranting the “burnout” label commonly used in occupational psychology (14), or whether it might instead reflect a pre-existing vulnerability. The latter possibility would be in line with the observation that, while an unrestrained HPA-axis activity leads to well recognized pathology, an inability to mount an adequate stress response is also detrimental for successful coping with stress (16). In fact, it has previously been proposed that a persistent lack of cortisol availability in traumatized or chronically stressed individuals may promote an increased vulnerability for the development of stress-related disorders with primarily bodily manifestations (8).
An interesting hypothesis is thus that a hyporeactivity of the HPA-axis, as measured by the DEX-CRH test, reflects pre-existing vulnerability. Several testable predictions arise from this hypothesis. One of these is that the hyporeactivity is expected to persist as a stable trait, rather than vary with state. The objective of the present study was to obtain initial data needed to address this question, by following up the LTSL subjects reported in (23) after 12 months, an interval during which a significant clinical improvement occurred in most of them. On follow-up, we re-evaluated DEX-CRH responses and cognitive function. Structural imaging was not repeated, since no difference between groups had been found on the initial assessment.
Methods
Subjects
The study was approved by the Human Subject Ethics Committee North of the Karolinska Institute (Dnr. 01/373). All subjects gave their written informed consent. The original population was 29 female patients and 28 matched healthy controls (see below). For details regarding the initial recruitment process, see (23). In brief, subjects were females, 40–55 years old, and employed ≥ 30 hrs/week for ≥ 3 yrs in the health care, social services or education sector in Stockholm County. Patients on LTSL were selected from a database over public service employees on long-term (i.e. ≥ 3 month) sick-leave during November 2002–November 2003. Additional criteria for patients were full-time long-term sick-leave, presence of major depression (26 subjects) or adjustment disorder with depressed mood (3 subjects) according to DSM IV, as determined using SCID interviews; job-related stressors reported as the main problem on axis IV; and presence of these stressors for > 6 months. Subects with any illicit drug use, hazardous alcohol consumption as determined by the Alcohol Use Disorder Identification Test (AUDIT), or a SCID diagnosis of a substance use disorder were excluded. On the initial assessment, 12 of the 29 patients were receiving antidepressant medication. By the time of the present study, this number was 15. Additional criteria for the control group were absence of past or present psychiatric or medical diagnosis, and absence of any medical or psychiatric treatment. Controls were screened for these criteria during an initial telephone interview, and then in writing. Those who negated any history of disease or treatment, with the exception of contraceptive use, and who scored within ±2 standard deviations from their population norm on all subscales as well as the Global Severity Index of the Symptom Check List-90 (2) were invited to participate. From 200 subjects eligible according to these criteria, 28 subject were selected to provide maximal matching for age, hormonal status, education, height, weight, family situation (including number of children living in the household), and nicotine use.
Following the initial study (23), all but 3 patients participated in cognitive group therapy, a therapy model that addresses job-related issues in groups of 6–8 participants. Weekly sessions were held by an experienced therapist for ten weeks. Every session was followed by homework assignment. Controls received no treatment. For the 12 month follow-up, subjects were contacted by telephone and admitted to the Clinical Research Centre (CRC) at the Karolinska University Hospital to undergo a retest with the combined DEX-CRH test and a cognitive re-evaluation. CRH challenge and cognitive testing were done on two consecutive afternoons and all subjects slept at home on the intervening night.
DEX-CRH test
The DEX-CRH test was carried out as previously described (9). The setting (Karolinska University Hospital CRC), procedure and biochemical analyses were identical to those used in the initial study (23). Briefly, subjects received 1.5 mg dexamethasone (Dexacortal, Organon) to take at 11 pm the day before the CRH challenge. On the following day, intravenous catheters were inserted at 2.00 pm, subjects rested for 1 h, and blood samples were drawn for basal ACTH and cortisol at 3.00 pm. Within 2 min, 100 μg of human CRH (Ferring, Kiel, Germany) was injected, and blood was drawn every 15 min 3.30 – 5.15 pm. This represents a 1 h extension of sampling time compared to established procedures (9), and was done to more fully capture the response. Total serum cortisol was determined by a commercial fluoroimmunoassay (AutoDELFIA cortisol-kit, Wallac, Oy). The lower detection limit was 5 nmol/L and the intra – and inter-assay coefficients of variation were below 8.5%. Plasma ACTH was measured using a chemiluminescence immunometric assay (Nichols, California, USA). The lower detection limit was 1 ng/L. Intra-and inter-assay coefficient of variation (CV) were below 8.5%, respectively, for both kits used.
To evaluate group-wise as well as individual stability of ACTH and cortisol responses, the area under the curve (AUC) was calculated for each subject on the respective test session. In order to make data from the two test sessions comparable, this was restricted to the the time-points assessed on both tests (3.00 – 4.15pm). Although these sampling intervals are established (9), they do not capture the full course of the response until it returns to baseline, and could potentially fail to capture differences that primarily affect the late phase of the response. We therefore repeated all analyses using individual peak responses as an alternative index of response magnitude. AUC and peak responses were highly correlated, and results on all analyses were virtually identical using either measure. The AUC-based analyses yielded lower residual variance, and are therefore the ones presented. AUC data were also used to evaluate a potential correlation between neuroendocrine responses on the two assessments, and a potential correlation between the neuroendocrine responses and ratings on the self-report version of the Montgomery-Åsberg Depression Rating Scale (MADRS) (27).
Cognitive evaluation
Cognitive testing was carried out as described for the initial study (23). Briefly, testing lasted 60 minutes, starting 2:30 or 3:30 pm. Attention was assessed using both a simple and a complex reaction task. Working memory was examined using a backward digit-span test. Declarative memory was examined using a test of associative memory for complex visual cues, delayed word recognition and picture recognition. None of the pictures or words used in the initial testing was used on the follow-up to avoid learning effects.
Statistics
Data for cortisol and ACTH on the 12 month follow-up session were analysed separately using two way ANOVA, with subject category as a between subjects factor and repeated measures over time within the session as a within subjects factor. AUC responses on the two test rounds were analyzed using a two way ANOVA with subject category as a between subjects, and test session (baseline or follow-up) as a within subjects factor. Correlations of AUC responses on the respective test round with each other, and correlations of AUC responses on follow-up with MADRS scores were evaluated using Pearson’s product-moment correlation. Data from the cognitive tests were analyzed using one-way ANOVA. Patient characterstics were compared between groups using two-tailed t-tests (continuous variables) or Fishers Exact test (frequency variables). All statistical analyses were carried out using Statistica 6.0 (Statsoft, Tulsa, OK).
Results
Subject characteristics and clinical state
All 29 patients, and all but one of the 28 controls from the original study participated in the follow-up. Descriptive subject data at the time of follow-up are given in Table 1. The groups continued to be comparable on key variables, although there was a trend for lower body weight in the control group. Among the LTSL patients who had all fulfilled criteria for major depression or adjustment disorder with depressed mood at the time of the initial study, 21 had now fully remitted and no longer fulfilled criteria for these disorders, while 8 patients were still in partial remission. There was a marked decrease in depressive symptomatology over the 1 year follow-up interval, as measured by the MADRS ratings (16.5±1.0 vs. 9.2±1.2, baseline study vs. follow-up, mean±SEM; F[1.28]=39.4, p≪0.0001). The reliability of these ratings was supported by the observation that, despite the overall decrease, there was a highly significant correlation between baseline and follow-up MADRS scores (r=0.51, p=0.007). Eighteen of the 29 LTSL patients had returned to part-time or full-time work at the time of follow-up, while 11 remained on LTSL.
Table 1.
Descriptive characteristics of subjects at follow-up. The groups continued to be well matched on key variables, although there was a trend for lower body weight in controls. Continuous variables are given as mean ± SD, with corresponding p-values generated using two-tailed t-test. Count variables are given as absolute frequencies, and compared using Fishers Exact test
| Patients (n=29) | Controls (n=27) | p | |
|---|---|---|---|
| Age, (years) | 48.9 ± 5.0 | 48.5 ± 4.2 | .78 |
| Weight (kg) | 71.2 ± 12.2 | 66.0 ± 9.4 | .09 |
| Current nicotine use | 9 | 7 | .77 |
| Hormonal phase: | |||
| Premenopause | 15 | 11 | .59 |
| estrogen-medication | 3 | 2 | 1.00 |
| Family situation: | |||
| Living with partner | 21 | 19 | 1.00 |
| Living with children ≤ 16 yrs | 9 | 8 | 1.00 |
Cognitive function
Results of the cognitive tests are given in Table 2. The impairments in attention (complex reaction time) and working memory found in the baseline study were no longer present.
Table 2.
No group differences were found on the neurocognitive tests carried out to assess reaction times, working memory, and long term memory. Mean ± SD values are given for the nr of correct responses on each test, as described in Methods, except for the reaction times, which are given in milliseconds. Some test data were lost due to computer failures; actual degrees of freedom are given for each analysis.
| Patients | Controls | ||
|---|---|---|---|
| Reaction time | |||
| Simple reaction task | 375.2±100.2 | 371.5±93.2 | F[1,51]=0.02; p=0.89 |
| Complex reaction task | 407.5±64.6 | 396.3±62.8 | F[1,52]=0.42; p=0.52 |
| Working memory | |||
| Backward digit span, no corr. seq. | 2.9±1.7 | 3.6±1.4 | F[1,54]=3.15; p=0.08 |
| Backward digit span, no tot. seq. | 6.5±2.0 | 7.0±1.7 | F[1,54]=1.09; p=0.30 |
| Long term memory | |||
| Picture recognition | 27.5±8.5 | 28.0±7.2 | F[1,52]=0.06; p=0.80 |
| Delayed word recognition | 18.1±2.4 | 18.4±1.3 | F[1,53]=0.25; p=0.62 |
| Visual Cues | 36.0±4.2 | 36.7±3.8 | F[1,53]=0.43; p=0.52 |
DEX-CRH test
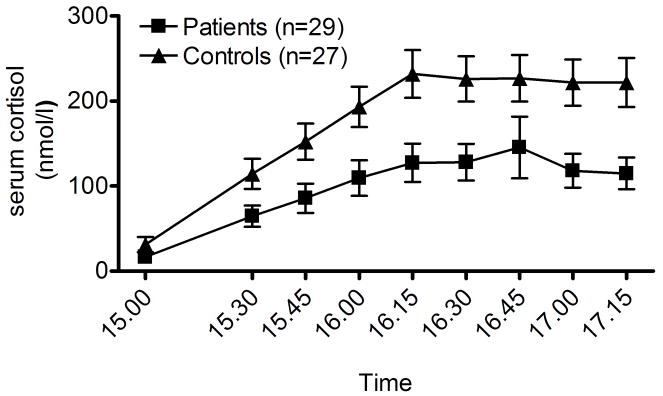
Cortisol data from the DEX-CRH test are shown in fig. 1A. There was a robust cortisol response to the CRH challenge (main time effect: F[8,434]=39.4, p≪0.0001). There was also a robust group difference, with a significantly lower response in the patient group (main group effect: F[1,54]=8.3, p=0.006), as well as group × time interaction (F[8,434]=3.2, p=0.002) indicative of a differential time course of the response between the groups.
Figure 1.
A. Persistent attenuated cortisol responses (p=0.006) to an 100 μg i.v. CRH challenge in depressed patients on long-term sick-leave related to job-stress compared to controls on 12 month follow-up. Datapoints are means ± SEM. Potential confounds from smoking or antidepressant treatment were excluded by replicating the results with these subjects excluded. For details and statistics, see Results.
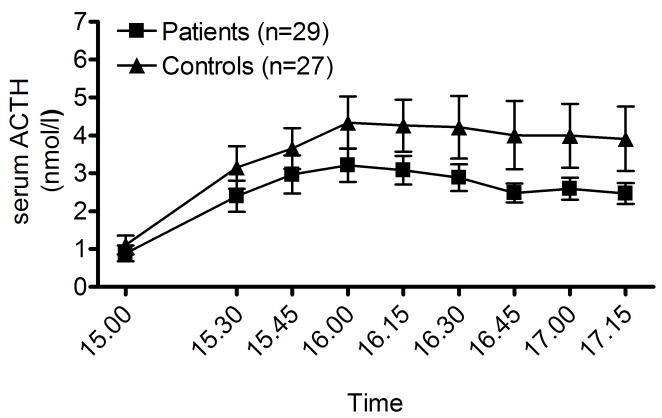
B) Despite greater inherent variability, a persistent attenuated ACTH response (p=0.03) to the CRH challenge was also found in the patient group on 12 month follow-up; it had only been observed as a trend on the initial assessment. Datapoints are means ± SEM. For details and statistics, see Results.
Despite the reduced power, this pattern remained after excluding all smokers (10 patients and 7 controls; (main time effect: F[8,304]=38.1, p≪0.0001; main group effect: F[1,37]=8.11, p=0.007; group × time interaction: F[8,304]=4.1, p=0.0001). The same was also true when the 15 antidepressant treated patients were excluded from analysis (main time effect: F[8,312]=21.3 p≪0.0001; main group effect: F[1,39]=5.4, p=0.02; group × time interaction: F[8,312]=2.2, p=0.027).
ACTH data from the DEX-CRH test are shown in fig 1B. There was a robust ACTH response to the CRH challenge (F[8,424]=14.1, p≪0.0001). Despite the inherently higher variance of ACTH data, the ACTH response in the patient group was significantly lower (main group effect: F[1,53]=4.1, p<0.05). The ACTH responses, measured as AUC, were highly correlated with the corresponding measure for cortisol (R=0.76, p≪0.0001). Because of this correlation, and the fact that ACTH data have a considerably higher variance, subgroup analyses aimed at eliminating potential confounds were limited to the cortisol responses, as indicated above.
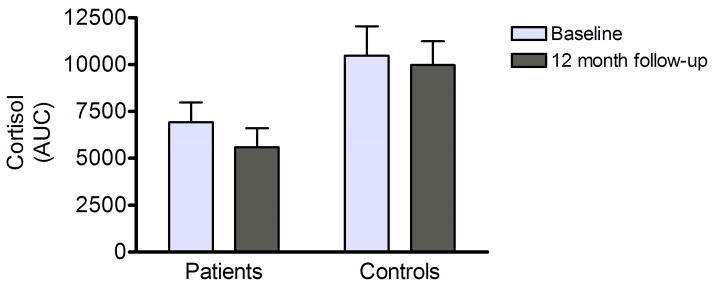
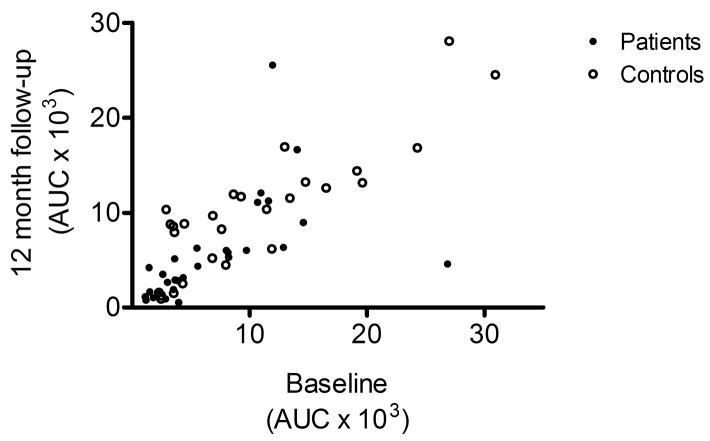
Expressed as AUC, cortisol responses within each group were very similar between the baseline assessment, and the 12-month follow-up (Fig. 2A). A mixed model ANOVA of AUC responses, with timepoint (baselineas vs. follow-up) as within-subjects factor and diagnostic group (patient vs. control) as between subject factor demonstrated a significant main effect of diagnostic group (F[1,54]=6.0, p=0.02), but no effect of, or trend for an effect of time, or of the group × time interaction. Furthermore, individual cortisol responses were highly correlated between the baseline study and this follow-up (Fig. 2B; R=0.76, p≪0.0001). Similar findings were obtained for ACTH responses, which were also highly correlated between baseline and follow-up (R=0.85, p≪0.0001). In contrast, no correlation or trend was found between MADRS-scores and ACTH or cortisol responses expressed as AUC. Consistent with this observation, MADRS-scores were not a significant co-variate neither in the cortisol- nor the ACTH-analysis.
Figure 2.
A) Comparison of cortisol responses measured as AUC indicated high stability between the baseline assessment and the 12-month follow-up reported here. Responses for patients and controls, respectively, are given (mean ± SEM). On a repeated measures analysis across the two rounds of testing, there was a significant main main effect of group (p=0.02), but no time effect, or group × time interaction. For details and statistics, see Results.
B) Cortisol responses also showed high individual stability, as shown by a highly significant correlation between the baseline and the present follow-up study (R=0.76, p≪0.0001).
Discussion
We recently reported the unexpected finding that depressed females on long-term sick-leave related to job stress had markedly suppressed reactivity of the HPA-axis in the DEX-CRH test, a pathology opposite to that typically reported in major depression (9). Here, we find that at 12 month follow-up, this pathology persists despite a marked clinical improvement shown by full remission in close to 75% of subjects, a highly significant reduction in depression symptom ratings, and normalized cognitive function. In our original paper, the attenuation of cortisol responses found at baseline had been statistically robust, while ACTH response attenuation was only at a trend level, following a typical pattern in which ACTH responses have a higher degree of variability. On the present follow-up, attenuation was significant both for cortisol and ACTH, and the effect sizes for both variables were slightly larger than those observed initially. Together, this demonstrates that HPA-axis hyporeactivity on follow-up was no less pronounced than that found on initial assessment. In both studies, we were able to rule out confounding influence of factors such as smoking or antidepressant treatment. Attenuated responses both at the level of the adrenals and the pituitary, and the observation that responses at these two levels were highly correlated, strongly suggest that the hyporeactivity is of central origin.
The stability of HPA-axis hyporeactivity in this group, and its lack of relation to clinical improvement is consistent with a hypothesis that the observed pathology may be a trait rather than a state marker. Trait markers are ideally independent of disease state, are expected to be found even before the onset of illness as well as during remission, and might be found in persons vulnerable to the illness who do not manifest it, e.g. unaffected first-degree relatives (29). Our findings of stability during symptomatic remission provides initial evidence that is thus suggestive, although not conclusive of, HPA-axis hyporeactivity in this population being a trait marker. An alternative possibility that cannot be excluded at this stage is that the stable deficit observed during remission could be a residual marker of the disease, as previously suggested for elevated DEX-CRH responses in major depression (3). It has long been debated whether long-lasting changes in hormonal responses following stress are pathological (33) or in fact adaptive (8). According to the latter view, adaptive down-regulation of pituitary CRH receptors in response to increased central CRH drive might ultimately result in hypocortisolism. These two views are not necessarily contradictory, since what starts out as an adaptive response may ultimately result in a persistent dysregulation and allostatic set-point shift (24). Prospective studies as well as studies of unaffected relatives will ultimately be helpful in determining whether the HPA axis hyporeactivity observed here in fact reflects a pre-existing vulnerability factor, and if so, whether this vulnerability interacts with stress exposure to produce disease.
Our sample was selected based on criteria of a depressive disorder as well as job stress related sick-leave. It is difficult to obtain reliable measures of cumulative stress burden that combine exposures both within and outside the workplace using retroactive report. A limitation of our study may therefore be that we were not able to directly assess the quantitative relation between degree of stress exposure and HPA axis pathology. However, using as inclusion criteria the constellation of job-stress related LTSL and a diagnosis of a depressive disorder appears to have identified a population distinct from subjects with depression alone, since up-rather than down-regulated reactivity of the HPA-axis has generally been observed in major depression (10; 15). Thus, increased DEX-CRH responses, indicative of impaired negative HPA-axis feedback control, have been reported during depressive episodes (9; 18), and these changes partially remitted with an improvement of depressive symptoms (12). Less pronounced abnormal responses were also found in non-depressed relatives of depressed subjects (11), but the relation of these abnormalities to depression susceptibility is complex. In the healthy high-risk probands, HPA-findings were remarkably stable over a period of four years (17). When these individuals were followed up for more than 10 years, those among them that ultimately developed an affective disorder did not have premorbid HPA-hyperactivity. Based on these findings, it was concluded that in major depression, exaggerated DEX-CRH responses are not likely to reflect pre-existing vulnerability, but rather be a state marker, reflect changes acquired as a result of illness, or both (13). In contrast to major depression, consistent abnormalities in DEX-CRH responses have not been found in chronically depressed patients (30) or in dysthymia (20).
Less research interest has been devoted to a potential pathophysiological role of attenuated HPA-axis reactivity. The best documented case where this is found is post-traumatic stress-disorder (PTSD), where peripheral cortisol levels are decreased, presumably due to up-regulated feedback inhibition (32). In agreement with these findings, it has recently been shown that the DEX-CRH response in this disorder is also attenuated (26). PTSD may, however, be a special case, where peripheral hypocortisolism is found despite increased central CRH drive (1), and where hippocampal volume reduction may be related to a specific pre-existing vulnerability (5). Hypocortisolism postulated to exist in chronic fatigue syndrome, fibromyalgia and other chronic stress-related conditions with primarily physical manifestations (8; 4; 22) may be of more direct relevance for our present findings, but has not until now been extensively studied mechanistically.
Our initial observation of attenuated DEX-CRH response in depressed subjects on LTSL related to job-stress were consistent with, and expanded on a previous report, which showed decreased morning saliva cortisol and increased dexamethasone suppression in subjects scoring high on measures of “burnout” (21). As indicated above, a key question prompted by these converging findings is whether HPA-axis hypoactivity in these subjects reflects consequences of prolonged stress exposure, or might be a stable endophenotype related to pre-existing vulnerability factors. If HPA-axis hyporeactivity is indeed a vulnerability trait, future research will have to take into account genetic or epigenetic factors as candidate mechanisms for its biological underpinnings. Both genetic variation and epigenetic regulation leading to hyperactive stress responses have been described in animal models (7; 31), but no data are yet available on mechanisms in either category with an ability to produce chronically and pathologically dampened responses.
It has been argued that workforce structure in industrialised economies has undergone changes over the last appr. 25 years, facing employees with powerful social stressors resulting from greater demands and less job security, and contributing to the incidence of stress-related disorders such as depression (28). Unrestrained HPA-axis reactivity to stressors has been linked to psychiatric morbidity, but it is equally clear that the ability to mount an adequate stress response is critical for coping with stressful challenges, and ultimately for survival and health (16). At low doses, corticosteroids preferentially activate the high-affinity mineralocorticoid rather than the low-affinity glucocorticoid receptor, and are neuroprotective, increase hippocampal neurogenesis and plasticity, and have positive effects on memory and affect. The adaptive value of stress-responses has typically been framed in metabolic terms, because cortisol-mediated mobilization of energy for short term use at the expense of long-term processes obviously subserves physical coping responses. However, emotional, behavioral, autonomic and endocrine stress responses act in concert to support coping, and are coordinated by central CRH-systems. It has previously been pointed out that hypoactivity of these systems may render subjects vulnerable to a particular category of depressive disorders, commonly labelled “atypical” (6). It is presently unknown what psychological coping deficits that might result from a persistent hypoactivity of stress systems. In the context of prolonged emotional and social stress in a changing workplace, it is, however, easy to speculate that an inability to mount adequate biological stress responses may impair active strategies to cope with chronic excessive job stress, such as refusing to accept unrealistic demands or leaving a dysfunctional workplace.
In conclusion, this one year follow-up showed a persistent decrease of HPA-reactivity in females on long-term sick-leave initially diagnosed with depression, despite remission of clinical symtoms and normalized cognitive function. A provocative hypothesis emerging from these data is that an inability to mount an adequate stress response to emotional and social stressors in the workplace may constitute a pre-existing vulnerability factor that results in impairment of coping ability, and increases the risk of LTSL.
Acknowledgments
Supported by an unrestricted grant from the Swedish insurance company AFA. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. American Journal of Psychiatry. 1997;154(5):624–9. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derogatis L. Symptom Checklist-90-Revised: Administration, Scoring, and Procedures Manual. 2. Towson, MD: Clinical Psychometrics Research; 1992. [Google Scholar]
- 3.Dettling M, Heinz A, Dufeu P, Rommelspacher H, Graf KJ, Schmidt LG. Dopaminergic Responsivity in Alcoholism - Trait, State, Or Residual Marker. Am J Psychiatry. 1995;152:1317–1321. doi: 10.1176/ajp.152.9.1317. [DOI] [PubMed] [Google Scholar]
- 4.Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. [Review] [100 refs] Biological Psychology. 2001 Aug;57(1–3):141–52. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- 5.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 7.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 9.Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 10.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 11.Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- 12.Holsboer-Trachsler E, Stohler R, Hatzinger M. Repeated administration of the combined dexamethasone-human corticotropin releasing hormone stimulation test during treatment of depression. Psychiatry Res. 1991;38:163–171. doi: 10.1016/0165-1781(91)90041-m. [DOI] [PubMed] [Google Scholar]
- 13.Ising M, Lauer CJ, Holsboer F, Modell S. The Munich vulnerability study on affective disorders: premorbid neuroendocrine profile of affected high-risk probands. J Psychiatr Res. 2005;39:21–28. doi: 10.1016/j.jpsychires.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Maslach C, Schaufeli WB, Leiter MP. Job burnout. Annu Rev Psychol. 2001;52:397–422. doi: 10.1146/annurev.psych.52.1.397. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 17.Modell S, Lauer CJ, Schreiber W, Huber J, Krieg JC, Holsboer F. Hormonal response pattern in the combined DEX-CRH test is stable over time in subjects at high familial risk for affective disorders. Neuropsychopharmacology. 1998;18:253–262. doi: 10.1016/S0893-133X(97)00144-9. [DOI] [PubMed] [Google Scholar]
- 18.Modell S, Yassouridis A, Huber J, Holsboer F. Corticosteroid receptor function is decreased in depressed patients. Neuroendocrinology. 1997;65:216–222. doi: 10.1159/000127275. [DOI] [PubMed] [Google Scholar]
- 19.Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci. 2002;5(Suppl):1068–1070. doi: 10.1038/nn943. [DOI] [PubMed] [Google Scholar]
- 20.Oshima A, Yamashita S, Owashi T, Murata T, Tadokoro C, Miyaoka H, et al. The differential ACTH responses to combined dexamethasone/CRH administration in major depressive and dysthymic disorders. Journal of Psychiatric Research. 2000 Oct;34(4–5):325–8. doi: 10.1016/s0022-3956(00)00021-2. [DOI] [PubMed] [Google Scholar]
- 21.Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 23.Rydmark I, Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, et al. Neuroendocrine, Cognitive and Structural Imaging Characteristics of Women on Longterm Sickleave with Job Stress-Induced Depression. Biol Psychiatry. 2006;60:867–873. doi: 10.1016/j.biopsych.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher R, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. pp. 629–649. [Google Scholar]
- 25.Strohle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl 3):S207–S214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- 26.Strohle A, Scheel M, Modell S, Holsboer F. Blunted ACTH response to dexamethasone suppression-CRH stimulation in posttraumatic stress disorder. J Psychiatr Res. doi: 10.1016/j.jpsychires.2008.01.015. (in press) [DOI] [PubMed] [Google Scholar]
- 27.Svanborg P, Asberg M. A new self-rating scale for depression and anxiety states based on the Comprehensive Psychopathological Rating Scale. Acta Psychiatr Scand. 1994;89:21–28. doi: 10.1111/j.1600-0447.1994.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 28.Tennant C. Work-related stress and depressive disorders. J Psychosom Res. 2001;51:697–704. doi: 10.1016/s0022-3999(01)00255-0. [DOI] [PubMed] [Google Scholar]
- 29.Usdin E, Hanin I. Biological markers in psychiatry and neurology. New York: Pergamon Press; 1982. [Google Scholar]
- 30.Watson S, Gallagher P, Del-Estal D, Hearn A, Ferrier IN, Young AH. Hypothalamic-pituitary-adrenal axis function in patients with chronic depression. Psychological Medicine. 2002;32(6):1021–8. doi: 10.1017/s0033291702005998. [DOI] [PubMed] [Google Scholar]
- 31.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 32.Yehuda R, Golier JA, Halligan SL, Meaney M, Bierer LM. The ACTH response to dexamethasone in PTSD. American Journal of Psychiatry. 2004;161(8):1397–403. doi: 10.1176/appi.ajp.161.8.1397. [DOI] [PubMed] [Google Scholar]
- 33.Yehuda R, Resnick H, Kahana B, Giller EL. Long-lasting hormonal alterations to extreme stress in humans: normative or maladaptive? Psychosomatic Medicine. 1993 Jun;55(3):287–97. doi: 10.1097/00006842-199305000-00006. [DOI] [PubMed] [Google Scholar]