Abstract
Although presumed, damage in the remaining (intact) rotator cuff tendons in the presence of an isolated supraspinatus tendon tear or multiple tendon tear has not been well studied. This study utilized an animal model of multiple rotator cuff tendon tears to investigate alterations in the remaining (intact) tendon mechanical properties at 4 and 8 weeks post-injury. Twenty-four animals served as uninjured controls, while seventy-two were divided among the tendon detachment groups (supraspinatus tendon detachment, supraspinatus+infraspinatus tendon detachment, supraspinatus+subscapularis tendon detachment). We found the remaining (intact) rotator cuff tendons have decreased mechanical properties in the presence of rotator cuff tears. Remaining (intact) subscapularis and infraspinatus tendon cross-sectional area increased, while tendon modulus decreased after both one and two tendon tears. Additionally, the remaining (intact) tendon cross-sectional areas continued to increase with time post-injury. These alterations could potentially lead to further tendon damage and tear progression.
Introduction
The supraspinatus tendon is the most commonly torn rotator cuff tendon with isolated tears of it comprising about half of those presenting clinically [12, 13, 17]. Isolated supraspinatus tendon tears can progress to include more of the rotator cuff, which can greatly affect shoulder function. Of those that progress, 58 to 80% continue posteriorly from the supraspinatus into the infraspinatus tendon, while anterior tear extension, into the subscapularis tendon, occurs with moderate frequency [4, 6, 10, 11, 16].
Although presumed, damage in the remaining (intact) tendons in the presence of an isolated supraspinatus tendon tear or multiple tendon tear has not been well studied. It is believed that the rotator cuff functions as a force couple, with the forces in the cuff tendons balancing each other [2]. If one tendon was to be removed due to a tear, the loading in the remaining (intact) tendons would be altered in order to try to balance the force couple. This change in loading could lead to tendon damage and an increased risk of tear progression to the tendons.
An animal model would be useful in studying changes in the remaining (intact) rotator cuff tendons due to tears, as tear size, location, and length of time from the initial injury could be controlled. Unfortunately, shoulder animal models have not assessed damage in the remaining (intact) rotator cuff tendons as a result of a single tendon tear and have not included the study of multiple tendon tears [7, 15, 18, 20, 21]. Therefore, the objective of this study was first to determine the uninjured baseline mechanical data for three rotator cuff tendons in the rat and then to examine the effect of the number of tendons torn on the mechanical properties of the remaining (intact) tendons. Our hypotheses were: 1) the uninjured supraspinatus tendon will have decreased tensile mechanical properties compared to the other uninjured rotator cuff tendons, 2) an increase in the number of tendons torn will decrease the mechanical properties of the remaining (intact) tendons, 3) an increase in time post-tear will result in increased detrimental changes to the mechanical properties of the remaining (intact) tendons compared to normal changes, and 4) after an isolated supraspinatus tendon tear, changes in the intact infraspinatus tendon will be greater than changes in the intact subscapularis tendon.
Materials and Methods
Ninety-six Sprague-Dawley rats were used with IACUC approval. Twenty-four animals served as uninjured controls, while seventy-two were divided among the tendon detachment groups (supraspinatus tendon detachment, supraspinatus+infraspinatus tendon detachment, supraspinatus+subscapularis tendon detachment). In the tendon detachment groups, unilateral surgery was performed to detach the prescribed rotator cuff tendon(s) sharply from the bony insertion, similar to that previously described[8]. Briefly, with the arm in external rotation, a 2 cm skin incision was made followed by blunt dissection down to the rotator cuff musculature. The rotator cuff was exposed and the tendons visualized at their insertion on the humerus. The rotator cuff tendons were identified as the subscapularis, the most anterior and broadest rotator cuff tendon, the supraspinatus, the tendon that passes under the bony arch created by the acromion, coracoid, and clavicle, the infraspinatus, posterior to the other tendons with a similar insertion to the supraspinatus, and finally the teres minor, inferior to the infraspinatus with a similar insertion. Suture was passed under the acromion to apply upward traction for further exposure, and the supraspinatus was separated from the other cuff tendons before sharp detachment at its insertion on the greater tuberosity using a scalpel blade. A marking suture with long tails was placed at the end of the tendon to facilitate retrieval at the time of dissection. In the two-tendon detachment groups, the other tendon was detached in the same manner with sharp dissection from the insertion site and a marking suture. Any remaining fibrocartilage at the insertion was left intact, and detached tendons were allowed to retract freely without attempt at repair creating a gap of ~4 mm from their insertion sites. The overlying muscle and skin were closed, and the rats were allowed unrestricted cage activity. Animals in all groups were sacrificed at 4 and 8 weeks (n=12 in each group at each time point).
For biomechanical testing, the proximal humerus, rotator cuff tendons, and scapula complex were removed. The remaining (intact) cuff muscles were individually detached from the scapula, and the associated muscles were removed. The individual tendons were dissected under a microscope, while interdigitation fibers were carefully separated. Verhoeff stain lines were then placed along the length of each tendon. The first stain line was placed at the tendon insertion onto the humerus, and the remaining stain lines were placed at 4, 7, and 9mm from the insertion. These stain lines were used to define the gauge section and to determine the distribution of strain along the length of the tendon.
Tendon geometry was measured with a laser based system [5]. The tendon was placed bursal side down, and the data from three passes, 0.75 mm, 2 mm, and 4 mm from the humeral head, were recorded. A custom program was utilized to calculate the average cross-sectional area of the tendon. For biomechanical testing, the remaining portion of the humerus was embedded in a holding fixture using polymethylmethacrylate (PMMA). Using a 1 cc syringe, an additional amount of PMMA was used to cover the humeral head in order to prevent bone and/or growth plate fracture during the mechanical tests, as many specimens included more than one tendon. Care was taken to ensure that the tendon insertion sites did not touch the PMMA to prevent insertion site damage due to excessive tissue heating during PMMA curing. The holding fixture was inserted into a specially designed testing fixture. The proximal end of the tendon was then held at the third stain line (7mm) in a screw clamp lined with fine grit sandpaper. The specimen was immersed in a 39°C PBS bath, preloaded to 0.03N, preconditioned for 10 cycles from 0.03N to 0.07N at a rate of 1%/s, and held for 300sec. Immediately following, a stress relaxation experiment was performed by elongating the specimen to a strain of 6% at a rate of 50%/s (3.5 mm/sec), followed by a 600sec relaxation period. Ramp to failure was then applied at a rate of 0.3%/s.
Using the applied stain lines, local tissue strain in the tendon midsubstance was measured optically with a custom program. Elastic properties, such as stiffness and modulus, were calculated using linear regression from the near-linear region of the load-displacement and stress-strain curves, respectively. As measures of viscoelastic properties, peak and equilibrium load and percent relaxation were determined from the stress relaxation curve for each specimen. Uninjured differences between tendon types and differences between groups within the same tendon type were determined with one- way ANOVAs at each time point with Bonferroni corrections. Control tendons exhibited a difference between time points, therefore, a bootstrapping approach was used to pair 4 week and 8 week data from each group (control, supraspinatus tendon detachment, supraspinatus+infraspinatus tendon detachment, and supraspinatus+subscapularis tendon detachment), as published previously [19]. Change from 4 to 8 weeks was then compared between groups using a one-way ANOVA with a Bonferroni correction. Finally, to compare relative changes in the infraspinatus and subscapularis rigorously with a supraspinatus tendon tear, a paired bootstrapping approach was used [19]. Percent change from control tendons was evaluated with a one-way ANOVA and a Bonferroni correction. Significance was set at p<0.05 and trends at p<0.1.
Results
H1 – Uninjured tendons
The supraspinatus tendon had larger viscoelastic properties at 4 weeks (percent relaxation) and 8 weeks (peak and equilibrium load) compared to the other tendons (Table I). At both time points, the subscapularis had a significantly larger cross-sectional area than both the supraspinatus and the infraspinatus tendons (Table I). Additionally, the subscapularis had a significantly higher stiffness compared to the other rotator cuff tendons (Table I). The infraspinatus tendon was also larger than the supraspinatus at both time points (Table I).
Table I.
Uninjured tendon mechanical testing data.
| % Relaxation |
Peak Load (N) |
Equilibrium Load (N) |
Area (mm2) |
Stiffness (N/mm) | Modulus (MPa) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
|
| Subscapularis | 0.66 ±0.65 | 0.62 ±0.07 | 5.66 ±2.00 | 5.69 ±1.93 | 1.86 ±0.77 | 2.17 ±0.94 | 3.65 ±0.50b,c | 2.85 ±0.16b,c | 236.72 ±73.41b,c | 281.43± 12.94b,c | 274.58 ±101.55 | 381.42 ±33.38 |
| Supraspinatus | 0.75 ±0.08a,e | 0.67 ±0.03 | 6.34 ±1.10 | 7.92 ±1.82d,e | 1.70 ±0.67 | 2.55 ±0.81e | 2.10 ±0.30 | 1.61 ±0.15 | 122.08 ±31.04 | 144.59± 43.41 | 238.32 ±68.84 | 332.90 ±88.88 |
| Infraspinatus | 0.66 ±0.07 | 0.65 ±0.07 | 5.61 ±1.93 | 5.19 ±1.56 | 1.76 ±0.62 | 1.78 ±0.54 | 2.77 ±0.29b | 2.14 ±0.21b | 138.78 ±63.93 | 172.56± 46.73 | 203.04 ±100.50 | 320.50 ±89.06 |
= Significant from subscapularis tendon,
= Significant from supraspinatus tendon
= Significant from infraspinatus tendon,
= Trend from subscapularis tendon
= Trend from infraspinatus tendon
H2 – Increasing tear size
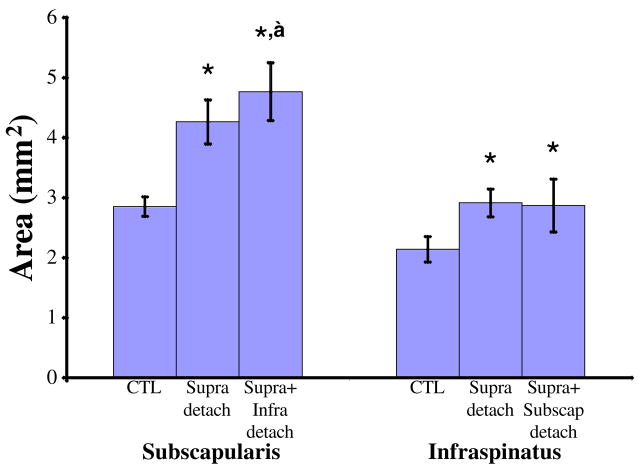
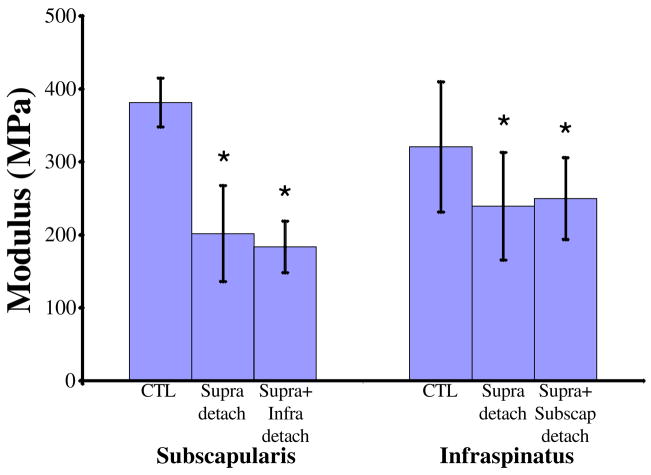
The infraspinatus tendon had a significantly larger cross-sectional area after both a one and two tendon tear compared to the uninjured control at 4 and 8 weeks (Table II, Figure 1). Modulus was significantly decreased in the infraspinatus tendon at 8 weeks for both tear types (Table II, Figure 2).
Table II.
Infraspinatus tendon mechanical testing data.
| % Relaxation |
Peak Load (N) |
Equilibrium Load (N) |
Area (mm2) |
Stiffness (N/mm) | Modulus (MPa) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
|
| Uninjured | 0.66 ±0.07 | 0.65 ±0.07 | 5.61 ±1.93 | 5.19 ±1.56 | 1.76 ±0.62 | 1.78 ±0.54 | 2.77 ±0.29 | 2.14 ±0.21 | 138.7 ±63.93 | 172.5 ±46.73 | 203.04 ±100.5 | 320.50 ±89.06 |
| Supra detach | 0.69 ±0.11 | 0.73 ±0.06f | 4.31 ±1.34 | 4.76 ±1.11 | 1.33 ±0.57 | 1.31 ±0.48h | 3.21 ±0.49f | 2.92 ±0.23f,m | 138.31 ±64.05 | 172.92 ±48.83 | 158.20 ±69.85 | 239.39 ±73.71f |
| Supra +Subscap detach | 0.73 ±0.07 | 0.74 ±0.04f | 5.72 ±2.60 | 6.26 ±2.06i | 1.50 ±0.67 | 1.65 ±0.52 | 3.07 ±0.53f | 2.87 ±0.44f,m | 130.62 ±45.48 | 174.37 ±51.42 | 178.35 ±74.81 | 249.78 ±56.10f |
= Significant from uninjured tendon,
= Significant from supraspinatus tendon detachment
= Trend from uninjured tendon,
= Trend from supraspinatus tendon detachment
= Significant from 4 weeks,
= Trend from 4 weeks
Figure 1.
At 8 weeks post-injury, the cross-sectional areas of the injured subscapularis and infraspinatus tendons are larger than their respective control tendons. Additionally, subscapularis tendons in the supraspinatus+infraspinatus detachment group are larger than those from the supraspinatus tendon detachment group. * = significant from control, ‡ = significant from supraspinatus tendon detachment.
Figure 2.
At 8 weeks post-injury, modulus of the injured subscapularis and infraspinatus tendons is decreased compared to their respective control tendons.
At 8 weeks, subscapularis tendon cross-sectional area significantly increased with tear size (Table III, Figure 1). Cross-sectional area was also significantly larger for both tear types at 4 weeks (Table III).
Table III.
Subscapularis tendon mechanical testing data.
| % Relaxation |
Peak Load (N) |
Equilibrium Load (N) |
Area (mm2) |
Stiffness (N/mm) | Modulus (MPa) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
4 week |
8 week |
|
| Uninjured | 0.66 ±0.05 | 0.62 ±0.07 | 5.66 ±2.00 | 5.69 ±1.93 | 1.86 ±0.77 | 2.17 ±0.94 | 3.65 ±0.50 | 2.85 ±0.16 | 236.72 ±73.41 | 281.43 ±12.94 | 274.58 ±101.5 | 381.42 ± 33.38 |
| Supra detach | 0.70 ±0.08 | 0.73 ±0.10f,m | 6.45 ±1.21 | 5.62 ±1.69 | 1.93 ±0.71 | 1.53 ±0.81 | 4.26 ±0.94f | 4.27 ±0.37f,k | 218.24 ±61.04 | 213.80 ±68.38f | 219.81 ±89.85 | 201.77 ± 65.83f,k |
| Supra +Infra detach | 0.65 ±0.06 | 0.66 ±0.08i | 4.56 ±1.67 | 5.52 ±1.68 | 1.53 ±0.64 | 1.84 ±0.61 | 4.78 ±0.71f | 4.77 ±0.48f,g,k | 191.89 ±71.59 | 217.05 ±36.09f | 167.05 ±71.4f | 183.69 ± 35.3f |
= Significant from uninjured tendon,
= Significant from supraspinatus tendon detachment
= Trend from uninjured tendon,
= Trend from supraspinatus tendon detachment
= Significant from 4 weeks,
= Trend from 4 weeks
Subscapularis tendon modulus was significantly decreased for a two-tendon tear at 4 weeks and for both tear sizes at 8 weeks (Table III, Figure 2). Stiffness of the subscapularis tendon was significantly decreased for all tears at 8 weeks (Table III). Subscapularis tendon percent relaxation was significantly increased at 8 weeks in the one-tendon tear group only (Table III).
H3 – Changes with time post-tear
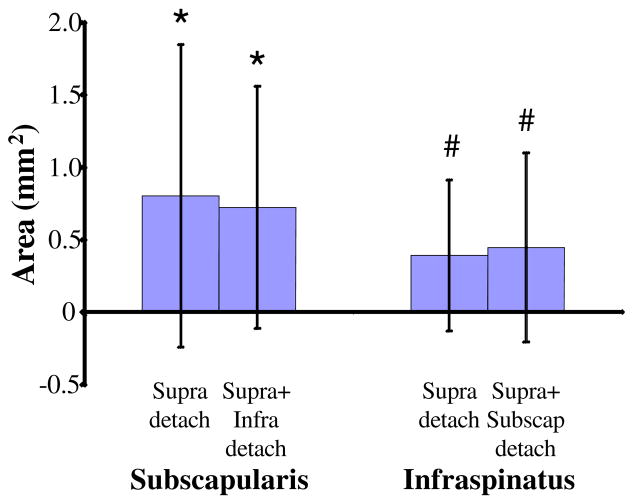
The infraspinatus (trend) and subscapularis tendons both had an increased cross-sectional area with time for one and two-tendon tears (Tables II and III, Figure 3). The subscapularis tendon had a significant decrease in modulus and an increase in percent relaxation with time in the one-tendon tear group (Table III).
Figure 3.
Cross-sectional area increases with time after tendon detachment. Values on graph represent the change from 4 weeks to 8 weeks and are relative to control. * = significant from control, # = trend from control.
H4 – Changes between tendon type with a supraspinatus tendon tear
At 4 weeks, the infraspinatus tendon decreased (trends) more in peak and equilibrium load from uninjured controls compared to the subscapularis tendon (Table IV). At 8 weeks, the subscapularis tendon significantly increased more in cross-sectional area and decreased (trends) more in modulus and stiffness from uninjured tendon than the infraspinatus tendon (Table IV).
Table IV.
Change of the remaining (untorn) tendons from uninjured after a supraspinatus tendon detachment.
| Area (mm2) | Stiffness (N/mm) | Modulus (MPa) | % Relaxation | Peak Load (N) | Equilbrium Load (N) | ||
|---|---|---|---|---|---|---|---|
| 4 week | Subscapularis change | 0.61 ±1.08 | −27.71 ± 111.16 | −87.17 ± 146.71 | 0.05 ±0.09 | 0.78 ±2.26# | −0.12 ±0.95# |
| Infraspinatus change | 0.42 ±0.63 | 4.30 ± 89.99 | −40.55 ± 107.47 | 0.04 ±0.13 | −1.24 ±2.21 | −0.42 ±0.91 | |
| 8 week | Subscapularis change | 1.37 ±0.41* | −66.42 ± 73.04# | −179.71 ± 74.50# | 0.12 ±0.14 | 0.19 ±2.62 | −0.58 ±1.37 |
| Infraspinatus change | 0.78 ±0.30 | −1.38 ± 75.63 | −87.75 ± 122.23 | 0.06 ±0.09 | −0.52 ±2.01 | −0.43 ±0.76 | |
= Significantly different from infraspinatus change
= Trend from infraspinatus change
Discussion
This is the first animal study to examine the normal rotator cuff tendons and the remaining (intact) tendons after tendon detachment or tears. We found that that the remaining (intact) tendons have altered mechanical properties with different tear sizes. These alterations could potentially lead to further tendon damage and tear progression.
We hypothesized that there would be differences in the uninjured rotator cuff tendons, specifically a decrease in the mechanical properties of the supraspinatus tendon due to its complex multi-axial loading environment caused by its anatomic location under the bony arch of the acromion. Surprisingly, most of the changes seen in the supraspinatus tendon were not in the elastic properties but in the viscoelastic properties. These alterations in the viscoelastic properties may be due to the differences in composition of the cuff tendons, including differences in glycosaminoglycans (GAGs) and water content [1]. The cross-sectional area of the supraspinatus was smaller than the other tendons. This may possibly be due to the space constraints caused by the acromion and enclosed arch through which the tendon must pass. Other differences in cross-sectional area and stiffness between uninjured tendons were seen in the subscapularis. The subscapularis is the only rotator cuff tendon located anteriorly and functions as a primary internal rotator and anterior stabilizer of the shoulder, supporting these results.
We also hypothesized that an increase in the number of tendons torn would result in decreases in the mechanical properties of the remaining (intact) tendons. Our results support this hypothesis, as many material, structural, and viscoelastic properties were altered with rotator cuff tendon tears. Increases in cross-sectional area in both the infraspinatus and subscapularis are presumably due to remodeling effects caused by mechanical overload on the remaining tendons. Combined with a decrease in tendon modulus, the remaining (intact) tendons appear to consist of a larger quantity of lesser quality material.
The subscapularis tendon had altered mechanical properties with differing tear sizes that continue to change with time post-injury. Early, properties were typically altered in subscapularis tendons associated with two tendon tears. But by 8 weeks, almost all properties were altered due to both one and two tendon tears. This suggests there is a slow progression in the accumulation of damage in the tendon after a one tendon tear. With time, the trauma to the tendon continues to accumulate, leading to decreases in the tendon properties and quality. With a two tendon tear, the damage to the tendon by 4 weeks is so great, that it has already reached a plateau.
Surprisingly, remaining (intact) infraspinatus tendon mechanical properties were not altered more with increasing tear size. In fact, occasionally there were more alterations after a one tendon tear than a two tendon tear. Therefore, adding a subscapularis tendon tear, does not always negatively affect the infraspinatus tendon. Based on anatomic location of the tendons, it is possible that tissues directly surrounding the subscapularis tendon, such as the long-head of the biceps tendon, are increasingly loaded and altered instead. Also, clinical data suggests a large reduction in function with supraspinatus-subscapularis tendon tears, even compared to a supraspinatus tear alone [2, 16]. This functional deficit may contribute to the animal using the limb less with the supraspinatus-subscapularis two tendon tear, thereby creating less force to be distributed throughout the intact tissues. With less loading on the joint compared to a one tendon tear, the infraspinatus tendon may remodel and adapt.
Finally, we hypothesized that after an isolated supraspinatus tendon tear, the infraspinatus tendon would be altered more than the subscapularis due to clinical data that rotator cuff tears are more likely to progress posteriorly and due to the common insertion site shared by the infraspinatus and supraspinatus [3, 4, 9–11]. Consistent with this hypothesis, at 4 weeks, the infraspinatus tendon had more differences than the subscapularis in viscoelastic properties. It is possible that the infraspinatus tendon may be performing some of the functions usually handled by the supraspinatus. This could lead to alterations in tendon water content or composition. Surprisingly, the subscapularis tendon was altered more than the infraspinatus in both structural and material properties at 8 weeks post-injury. Adaptations or compensation for the functional deficits associated with supraspinatus tendon tears could be occurring. Changes in the way a task is performed could further injure tendons that are not designed for that new motion or the altered loading associated with it. It is also possible that, in the rat model, the infraspinatus tendon is able to adapt to the alterations caused by the one tendon tear, but the subscapularis inherently cannot.
The rotator cuff is responsible for stabilization of the joint and many arm motions. It is thought to act as a force-couple, with the forces in all tendons balancing each other [2]. In order to balance the force-couple after removal of a tendon, as in the case of a tear, loading in the remaining (intact) tendons would be altered. Alterations in loading can lead to damage to and changes in the structure and composition of tendons. Increased loading can lead to the formation of microtears. Typically, if the increase in loading occurs once or in a short time frame, remodeling of the tendon matrix will occur, and the tissue will return to normal. But, if the increased loading is continued, the microtears will accumulate, eventually leading to a degenerative tendon. Further, it is thought that most chronic rotator cuff tears occur in degenerative tendons [14]. In this model, one or more cuff tendons have been torn acutely, leading to the assumption of an instantaneous increase in loading on the remaining (intact) tendons.
The results presented here support the concept that two-tendon tears result in worse mechanical properties than one-tendon tears. The remaining (intact) tendons were detrimentally altered with a supraspinatus tendon tear alone but were further damaged after a two-tendon tear, as seen with cross-sectional area and modulus of the subscapularis tendon. This is particularly interesting, as the increase in damage occurred after a supraspinatus+infraspinatus tendon tear, the most common tear progression direction.
There are some potential limitations associated with this study. First, an animal model was utilized to examine mechanical changes in the remaining (intact) tendons. While we agree that the rat is not an exact model for the human condition (as no model of any human disease or injury ever is), this model has been validated and used extensively in our lab and others Additionally, the tendon tears were performed by sharp surgical detachment. With an acute tear, changes in tendon loading occur immediately, rather than the gradual progression seen with more chronic tear scenarios. Although most tears of the rotator cuff are associated with tendon degeneration, surgical detachment of the tendons allowed for control over tear size and location. With the sharp dissection in the animal model, the injury process does not affect the initial quality of the tendons. Further, the results presented are only based on mechanical assays. Histologic, biologic, and imaging techniques could be used to correlate tissue quality and structure to the mechanical alterations. Also, a longer time may be necessary to examine the intact subscapularis tendon differences fully. After a one tendon detachment, the tendon properties continue to decrease with time, but after a two tendon detachment, they do not. Based on the time points in this study, it is unknown whether the tendon properties would continue to decrease or whether they have reached a plateau.
Results demonstrate not only differences in the uninjured rotator cuff tendons, but also that tears of one or two tendons affect the remaining (intact) tendons. Future studies could evaluate longer time points, the histologic and biologic alterations associated with the mechanical differences, and the role of tendon repair in the reversal of the degenerative process due to altered loading. Techniques, such as microMRI or microCT, could be used to examine the alterations in the muscle-tendon units further. Finally, the functional deficits associated with different numbers and combinations of rotator cuff tears could be examined.
Acknowledgments
This study was supported by NIH/NIAMS and the NIH/NIAMS sponsored Penn Center for Musculoskeletal Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blevins FT, Djurasovic M, Flatow EL, Vogel KG. Biology of the rotator cuff tendon. Orthopedic Clinics of North America. 1997;28(1):1–16. doi: 10.1016/s0030-5898(05)70260-1. [DOI] [PubMed] [Google Scholar]
- 2.Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clinical results and biomechanical rationale. Clinical Orthopaedics & Related Research. 1991;(267):45–56. [PubMed] [Google Scholar]
- 3.Clark JM, Harryman DT., 2nd Tendons, ligaments, and capsule of the rotator cuff. Gross and microscopic anatomy. Journal of Bone & Joint Surgery - American Volume. 1992;74(5):713–25. [PubMed] [Google Scholar]
- 4.Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM. Surgical repair of chronic rotator cuff tears. A prospective long-term study. Journal of Bone & Joint Surgery - American Volume. 2001;83-A(1):71–7. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Favata M. PhD thesis in Bioengineering. Vol. 216. University of Pennsylvania; Philadelphia: 2006. Scarless healing in the fetus: Implications and strategies for postnatal tendon repair. [Google Scholar]
- 6.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. Journal of Bone & Joint Surgery - American Volume. 2000;82(4):505–15. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. Journal of Bone & Joint Surgery - American Volume. 1999;81(9):1281–90. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Gimbel JA, Sarver JJ, Soslowsky LJ. The effect of overshooting the target strain on estimating viscoelastic properties from stress relaxation experiments. Journal of Biomechanical Engineering. 2004;126(6):844–8. doi: 10.1115/1.1824132. [DOI] [PubMed] [Google Scholar]
- 9.Gohlke F, Essigkrug B, Schmitz F. The pattern of the collagen fiber bundles of the capsule of the glenohumeral joint. Journal of Shoulder & Elbow Surgery. 1994;3:111–28. doi: 10.1016/S1058-2746(09)80090-6. [DOI] [PubMed] [Google Scholar]
- 10.Halder A, Zobitz ME, Schultz E, An KN. Structural properties of the subscapularis tendon. Journal of Orthopaedic Research. 2000;18(5):829–34. doi: 10.1002/jor.1100180522. [DOI] [PubMed] [Google Scholar]
- 11.Halder A, Zobitz ME, Schultz F, An KN. Mechanical properties of the posterior rotator cuff. Clinical Biomechanics. 2000;15(6):456–62. doi: 10.1016/s0268-0033(99)00095-9. [DOI] [PubMed] [Google Scholar]
- 12.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. Journal of Bone & Joint Surgery - American Volume. 1991;73(7):982–9. [PubMed] [Google Scholar]
- 13.Harryman DT, 2nd, Sidles JA, Harris SL, Matsen FA., 3rd The role of the rotator interval capsule in passive motion and stability of the shoulder. Journal of Bone & Joint Surgery - American Volume. 1992;74(1):53–66. [PubMed] [Google Scholar]
- 14.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clinical Orthopaedics & Related Research. 2003;(415):111–20. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 15.Hirose K, Kondo S, Choi HR, Mishima S, Iwata H, Ishiguro N. Spontaneous healing process of a supraspinatus tendon tear in rabbits. Archives of Orthopaedic & Trauma Surgery. 2004;124(6):374–7. doi: 10.1007/s00402-004-0663-8. [DOI] [PubMed] [Google Scholar]
- 16.Mansat P, Frankle MA, Cofield RH. Tears in the subscapularis tendon: descriptive analysis and results of surgical repair. Joint, Bone, Spine: Revue du Rhumatisme. 2003;70(5):342–7. doi: 10.1016/s1297-319x(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 17.Matsen FA, 3rd, Arntz CT, Lippitt SB. Rotator Cuff. In: Rockwood CA Jr, Matsen FA 3rd, editors. The Shoulder. Philadlephia: W.B. Saunders Co; 1998. pp. 755–839. [Google Scholar]
- 18.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. Journal of Shoulder & Elbow Surgery. 1996;5(5):383–92. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 19.Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD, 3rd, Carpenter JE. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Annals of Biomedical Engineering. 2002;30(8):1057–63. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 20.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, Soslowsky LJ. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. Journal of Orthopaedic Research. 2002;20(3):454–63. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 21.Uhthoff HK, Matsumoto F, Trudel G, Himori K. Early reattachment does not reverse atrophy and fat accumulation of the supraspinatus--an experimental study in rabbits. Journal of Orthopaedic Research. 2003;21(3):386–92. doi: 10.1016/S0736-0266(02)00208-5. [DOI] [PubMed] [Google Scholar]