Abstract
Background/Aims
Acetaminophen overdose causes hepatotoxicity mediated by toxic metabolites generated through the cytochrome P450 enzyme. The objective of this study was to investigate whether acetaminophen (AAP) can enhance cisplatin (CDDP) cytotoxicity against human hepatocarcinoma and hepatoblastoma cells in vitro and whether this effect can be prevented by N-acetylcysteine (NAC).
Methods
In vitro studies (glutathione [GSH] level, cell viability, and immunoblot assays) were performed using human hepatocarcinoma and hepatoblastoma cells cultured in AAP, CDDP, and the combination of both with or without delayed NAC administration. The pharmacology and toxicology of high-dose AAP in rats were also examined.
Results
Acetaminophen decreased GSH levels in liver cancer cells in a dose- and time-dependent manner. Acetaminophen combined with CDDP had enhanced cytotoxicity over CDDP alone. The cytotoxicity caused by AAP plus CDDP was decreased by NAC, with the effectiveness being time-dependent. The GSH level was lowered in the liver but not in the blood or the brain in rats treated with a high dose of AAP (1000 mg/kg). The expression of CYP2E1 protein, a key cytochrome P450 enzyme, varies among species but is not correlated to AAP sensitivity in liver cancer cells.
Conclusions
Our results suggest that a chemotherapeutic regimen containing both AAP and CDDP with delayed NAC rescue has the potential to enhance chemotherapeutic efficacy while decreasing adverse effects. This would be a promising approach particularly for hepatoblastomas regardless of cellular CYP2E1 protein level but could also be beneficial in other malignancies.
Introduction
Hepatoblastoma (HB) is the most common tumor of the liver observed in pediatric patients and accounts for 0.9% of all pediatric solid tumors [1]. Currently, a wide range of chemotherapeutic agents is being used in the treatment of HB presenting patients, including irinotecan, vincristine, 5-fluorouracil, doxorubicin, and cisplatin (CDDP) [2]. The use of such treatment options, applied in combination with surgical resection, has resulted in overall survival rates in standard-risk cases of greater than 90% [3]. However, treatment options for high-risk and recurrent disease remain less than satisfactory, and chemotherapy regimens with improved efficacy are needed [4]. CDDP, a common platinum-based agent, is effective against a broad spectrum of cancer malignancies. CDDP forms both intrastrand and interstrand cross-links of DNA, thus shielding the DNA from repair mechanisms and leading the cell into autophagy and apoptosis [5]. CDDP causes covalent cross-links on both proteins and DNA [6]. The causes of tumor cell resistance to CDDP and its analogs (i.e., carboplatin and oxaloplatin) are incompletely understood, and the various analogs differ in their degree of cross-resistance with CDDP in experimental tumor systems. A major cause of CDDP resistance seems to be inactivation of the drug by endogenous thiols, including glutathione (GSH) and other sulfhydryls such as metallothionein [7]. One study found that low levels of messenger RNA for GSH S-transferase inversely correlate with high sensitivity to CDDP and carboplatin [8]. Another study developed a CDDP-resistant human small cell carcinoma cell line that was found to have significantly increased levels of GSH when compared with the CDDP-sensitive cell lines [9].
Acetaminophen (AAP, paracetamol) is a commonly used analgesic and antipyretic drug. When administered in high doses, AAP proved to be cytotoxic to hepatocytes and other cells containing mixed-function oxidase. The mixed-function oxidase system of enzymes (most relevantly cytochrome P450 2E1 [CYP2E1]) generates a reactive arylating intermediate that is normally detoxified by reduced GSH [10]. Large doses of AAP overwhelm GSH stores allowing the toxic metabolite, N-acetyl-p-benzoquinonimine (NAPQI), to bind macromolecules and induce cell death [11]. AAP has been shown to decrease intracellular GSH levels in different cell types [12–15]. There is evidence that AAP administration has a wide range of activity against many different tumor types that contain the mixed-function oxidase system [16].
N-acetylcysteine (NAC) is the US Food Drug Administration-approved antidote for AAP toxicity when given within 8 hours [17] but has also been shown to be protective against platinum-based chemotherapy-associated myelotoxicity, ototoxicity, hepatotoxicity, nephrotoxicity, and neuropathy. In animal models, NAC can prevent platinum-induced myelotoxicity, neuropathy, nephropathy, and ototoxicity when administered intravenously at doses significantly higher than the standard protocol [18,19]. The mechanism of action for this protection is unclear; however, it likely acts by inducing synthesis of GSH [20], by scavenging free radicals directly [21], or by serving as a substrate for direct chemical conjugation and detoxification [22]. However, whereas NAC has been shown to neutralize the cytotoxicity of various chemotherapeutic agents, it does not compromise chemotherapy efficacy in a rat brain tumor two-compartment model, where NAC was administered at time points both before and after a three-drug chemotherapy cocktail (carboplatin, melphalan, and etoposide phosphate) [23].
Kobrinsky et al. [24] reported a clinical case of an African American girl with unresectable stage III HB who had failed front-line CDDP-based therapies. This patient was disease-free 7 years after surgery after receiving a treatment regimen that combined CDDP after high dose of AAP and followed with NAC 8 hours later. The intent of this study was to explore whether AAP has a dual effect on HB cell lines by directly inducing apoptosis, and/or by decreasing intracellular GSH levels, thus enhancing CDDP-mediated cytotoxicity. This study also examined whether the delayed administration of NAC after combination therapy of AAP and CDDP would be capable of inhibiting therapy-related adverse effects. These results may provide clinically relevant information about a potential chemotherapeutic regimen in which AAP, combined with CDDP with delayed NAC rescue, can be used for the treatment of high-risk HB or other malignancies.
Materials and Methods
Pharmacological Agents and Antibodies
Sterile solutions of CDDP and NAC were obtained from the Medical University of Gdansk pharmacy. Acetaminophen and mouse anti-tubulin antibody were purchased from Sigma (St Louis, MO). Rabbit anti-poly ADP ribose polymerase (PARP) was purchased from Cell Signaling through New England BioLabs (Frankfurt, Germany). Rabbit anti-CYP2E1 polyclonal antibody was from Stressgen (Ann Arbor, MI).
Cell Culture
Human HepG2 hepatocarcinoma and HUH6 and HepT1 HB cell lines were obtained from Dr. Steven Warmann (Tuebingen, Germany) and were cultured in Dulbecco's modified Eagle medium in 10% FBS and 1% streptomycin/penicillin cocktail (Sigma-Aldrich, Poznan, Poland). Cells were maintained at 37°C and in an atmosphere containing 5% CO2. Normal human hepatocytes were obtained through Liver Tissue Cell Distribution System (Pittsburgh, PA), which was funded by the National Institutes of Health contract no. N01-DK-7-0004/HHSN26700700004C. Normal rat and mouse hepatocytes were obtained from the Department of Comparative Medicine of Oregon Health & Science University.
Cell Viability Assay
Cell viability was determined using a WST-1 Cell Proliferation Assay Kit obtained from Millipore (Warsaw, Poland) following a previously outlined protocol [25]. In preparation for experiments, cells were trypsinized, collected, and then plated in 96-well plates. One day later, the cells were treated with NAC, AAP, and/or CDDP. After an incubation period of 18 to 24 hours, 10 µl of WST-1 reagent was added to each of the wells, and 2 hours later, the plates were read at 450 nm. Relative cell viability was normalized with vehicle and presented as a fraction of the vehicle.
GSH Assay
A total of 4 x 105 cells were plated onto 60-mm tissue culture plates in 5 ml of medium and were allowed to incubate overnight. The medium was removed, and fresh medium was added containing the desired concentrations of AAP, CDDP, and/or NAC. At the appropriate time point, both the attached and detached cells were collected, washed with PBS, and resuspended in PBS containing 1 mM EDTA. The mixtures were then vortexed, freeze-thawed twice, and centrifuged at 10,000g for 15 minutes at 4°C. The supernatants were analyzed for GSH concentration using a Quanticrom Glutathione Assay Kit from BioAssay Systems (Hayward, CA) according to the manufacturer's protocol. The protein content was determined using a BCA assay kit (Pierce Biotechnology, Rockford, IL), and results were normalized for total protein concentration. Cellular GSH levels were also monitored and analyzed using the ThiolTracker Violet GSH detection reagent (Invitrogen/Molecular Probes, Eugene, OR) according to the manufacturer's protocol. Cells were seeded in a six-well plate, incubated overnight, and treated the following day. After designated treatment times, the cells were washed with PBS and incubated in Dulbecco's PBS containing 10 µM (final concentration) ThiolTracker Violet for 30 minutes at 37°C. Then the cells were viewed using a Zeiss Observer fluorescent microscope, and photographs of representative cells were taken by a Zeiss AxioCam camera (Maple Grove, MN).
Western Blot Analysis
The cellular protein level among the different cell types and/or different treatments was measured by Western blot analysis following a previously outlined protocol [25]. Briefly, cells were seeded, treated the following day, and allowed to incubate for the designated time before being collected, washed with PBS, and centrifuged (2000g for 5 minutes). The cells were then resuspended in a lysing buffer (0.138 M NaCl, 0.0027 M KCl, pH 7.4, 1% nonionic detergent [Igepal CA-630], 100 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail [Calbiochem, Germany]), and the protein content of the resulting solution was quantified using a BCA kit, as described previously. The samples were then diluted into a loading buffer (120 mM Tris-HCl pH 6.8, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.002% bromophenol blue), incubated at 95°C for 5 minutes, and separated on a 7% or 12% polyacrylamide gel (25 µg protein per well) under reducing conditions. The separated proteins were then transferred to a nitrocellulose membrane. The membrane was incubated with primary antibody for 1 hour at 25°C, washed with TBST (10 mM Tris-HCl, 150 mM NaCl, pH 7.5 and 0.1% Tween-20), and incubated for 1 hour with a peroxidase-labeled rabbit anti-mouse immunoglobulin G antibody (1:5000 dilution; Molecular Probes).
The following primary antibodies were used: Rabbit anti-PARP polyclonal antibody from Cell Signaling that cross-reacts with human, rat, and mouse was used at a 1:1000 dilution. Mouse anti-β-tubulin monoclonal antibody from Sigma-Aldrich that cross-reacts with human, rat, mouse, monkey, and chicken was used at a 1:5000 dilution. Rabbit anti-CYP2E1 polyclonal antibody from Stressgen that cross-reacts with human, rat, mouse, dog, monkey, guinea pig, and horse was used at a 1:1000 dilution.
Signals were detected using a Chemiluminescence Blotting Substrate Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's protocol. Quantification of the immunoblot signal of respective proteins (cleaved PARP, CYP2E1, and tubulin) was performed using UN-SCAN-IT Gel software (Silk Scientific, Inc, Orem, UT).
In Vivo Toxicology and Pharmacology Studies
Normal animal studies were performed in accordance with guidelines established by the Oregon Health & Science University's Institutional Animal Care Committee. A dose-response study of AAP on serum AAP concentration was performed. Female Long-Evans rats were administered 0, 300, 600, and 1000 mg/kg (0, 2.1, 4.2, and 7 g/m2, respectively) AAP intraperitoneally (IP); blood was collected at 0.5, 1, 2, and 4 hours after treatment. Serum samples were obtained by centrifugation, and AAP concentration was determined using an AAP direct ELISA (Immunalysis Corp, Pomona, CA). The in vivo effect of AAP on GSH level was also investigated. Female Long-Evans rats were placed under isoflurane anesthesia and then administered an IP dose of either a vehicle DMSO solution or AAP at 1000 mg/kg (approximately 7 g/m2). The rats were allowed to recover, and then 6 hours after treatment, the animals were killed and whole blood, liver, and brain were harvested to test for GSH levels. GSH levels were analyzed using a Quanticrom Glutathione Assay Kit (BioAssay Systems) as described in a previous paragraph.
Statistical Analysis
The results were expressed as mean ± SEM, and the significance of the difference between the mean values of treated cells/animals and controls was determined by the Student's t test. The level of significance was corrected by multiplying the P value by the number of comparisons performed (n) according to Tukey's correction. For data analysis, two-way analysis of variance test was performed by comparing the different arms of treatment of the two variables. Significance was determined at the 5% level, two-sided. Statistical significance between treatment and control (or vehicle) group or any two other groups was indicated by asterisks: *P < .05 or **P < .01.
Results
AAP Reduced Cellular GSH Levels in HB Cells, Which Was Reversed by NAC Administration In Vitro
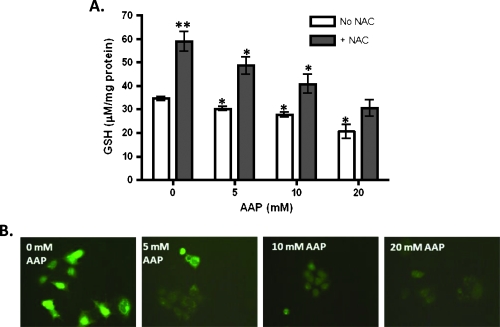
The effects of AAP and NAC on intracellular GSH levels were determined using a Quanticrom Glutathione Assay Kit and the ThiolTracker Violet GSH detection reagent. AAP reduced GSH levels in a dose-dependent manner in HUH6 HB cells. When controlling for total protein concentration, 5 mM AAP lowered intracellular GSH levels by 11% and 20 mM AAP lowered levels by 38%. NAC, a GSH precursor, reversed this effect when given in conjunction with AAP and raised GSH levels in HUH6 cells roughly two-fold when administered independently (Figure 1). Indeed, as in Figure 1, we found that NAC (1 mg/ml) blocked GSH reduction even at high concentrations of AAP (20 mM; last right gray bar) when compared with the DMSO alone vehicle control (first left open bar).
Figure 1.
Acetaminophen reduced cellular GSH levels in HUH6 cells in a dose-response manner. (A) HUH6 cells were treated with 5, 10, or 20 mM AAP, with or without 1 mg/ml of NAC, and collected the following day (n = 3, mean ± SEM). GSH levels were analyzed using a Quanticrom Glutathione Assay Kit and normalized for protein concentration. (B) HUH6 cells were treated with AAP and then were stained using a ThiolTracker Violet dye from Invitrogen. Shown are representative pictures of cells from each treatment group. Statistical significance between treatment and control group was indicated by asterisks: *P < .05 or **P < .01.
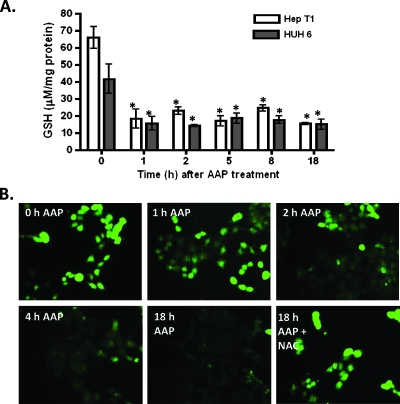
Furthermore, it was demonstrated that 20 mM AAP reduces intracellular GSH levels within 1 hour, with no significant difference in GSH concentration measured at 1, 2, 5, 8, or 18 hours post-20 mM AAP treatment (Figure 2A). However, 10 mM AAP lowered intracellular GSH levels more gradually, taking 4 hours to significantly reduce the levels in HUH6 cells (Figure 2B). Similar results were obtained for HepT1 cells, although in this line, untreated cells had relatively higher baseline levels of intracellular GSH. In addition, GSH levels did not increase as substantially in HepT1 cells treated with NAC (data not shown). Interestingly, CDDP administered independently had no significant effect on GSH levels, although cells treated with both CDDP and AAP had lower levels of GSH after treatment in comparison to those treated with AAP alone.
Figure 2.
Time course of GSH depletion in HB cells by AAP in vitro. (A) HepT1 and HUH6 HB cells were treated with 20 mM AAP at time 0, collected at various times thereafter, and analyzed for GSH content with a Quanticrom Glutathione Assay Kit (n = 3, mean ± SEM). (B) HUH6 cells were treated with 10 mM AAP and stained with ThiolTracker Violet dye at various time points. Also shown are cells treated with both 1 mg/ml NAC and 10 mM AAP and allowed to incubate overnight. Shown are representative pictures of each treatment group. Statistical significance between treatment and control group was indicated by an asterisk: *P < .05.
Acetaminophen Synergistically Increased CDDP-Induced Cytotoxicity in HB Cells
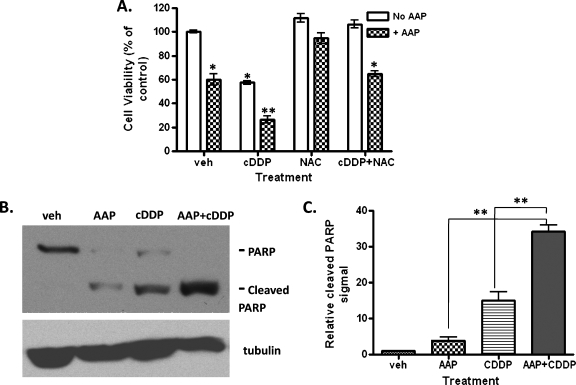
To determine the cytotoxic effects of a combination chemotherapy regimen, a WST-1 cell viability study was performed on cells treated with CDDP, NAC, and/or AAP. As shown in Figure 3A, HepT1 cells treated with 5 µg/ml CDDP in combination with 10 mM AAP had a relative viability of 26%, whereas cells receiving CDDP or AAP alone had relative viabilities of 58% and 60%, respectively. Similar results were obtained for HUH6 cells (data not shown).Western blot analysis for cleaved PARP was performed to determine the levels of apoptosis in cells receiving the various treatments. It was determined that 5 µg/ml CDDP in combination with 10 mM AAP induced significantly more apoptosis in both HepT1 (Figure 3B) and HUH6 cells (Figure 4A) than either AAP or CDDP alone. In Figure 3C, the signal intensity of cleaved PARP protein significantly (P < .01) increased in AAP + CDDP-treated group compared with either AAP- or CDDP-alone group. We found that the addition of AAP enhanced CDDP-induced apoptosis in both HB cell lines. Interestingly, similar results were found in a mouse in vivo animal study; however, HUH6 instead of HepT1 tumors had a better response to AAP and CDDP combination treatment (Warmann et al., unpublished in vivo data). This effect was independent of CYP2E1 expression, which suggests possible alternative mechanisms of AAP-induced oxidative stress and cytotoxicity in HB cancer cells.
Figure 3.
Acetaminophen synergistically enhanced CDDP-induced cytotoxicity of HB cells in vitro. (A) HepT1 cells seeded in a 96-well plate were treated with 5 µg/ml CDDP, 10 mMAAP, and/or 1 mg/ml NAC and allowed to incubate overnight. Ten microliters of WST reagent was then added to each well, and the plate was incubated in normal culturing conditions at 37°C for 2 hours. The plates were read using a UV spectrometer at 450 nm. Results shown are normalized relative to the vehicle (n = 3, mean ± SEM). Statistical significance between treatment and control group was indicated by asterisks: *P < .05 or **P < .01. (B) Western blot analysis for levels of PARP and cleaved PARP was performed to measure apoptosis in the treated cells. Tubulin protein detection was used to confirm and normalize equal protein loading. (C) Quantification of the immunoblot signal of cleaved PARP in panel B was performed using UN-SCAN-IT Gel software (Silk Scientific) from three independent experiments. Statistical significance between two treatment groups was indicated: **P < .01.
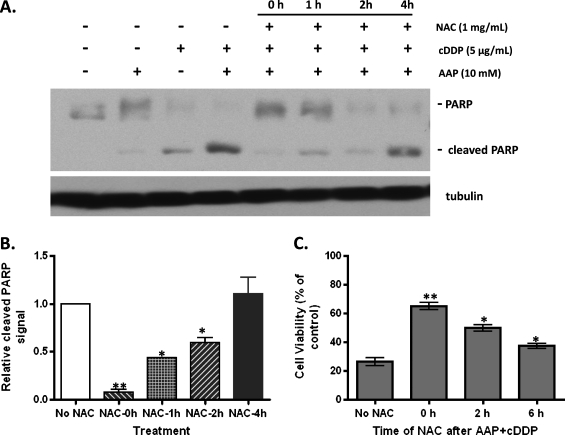
Figure 4.
Delayed administration of NAC exhibits decreased levels of protection against AAP and CDDP-induced cytotoxicity. (A) Western blot analysis of HUH6 cells detecting cleaved PARP as a marker for apoptosis and tubulin to confirm equal protein loading. Five micrograms of CDDP per milliliter and/or 10 mM AAP was added at time 0, and 1 mg/ml NAC was added at times 0, 1, 2, and 4 hours. Cells were incubated in treated medium overnight. (B) Quantification of the immunoblot signal of cleaved PARP in panel A was performed from three independent experiments. Statistical significance between two treatment groups was indicated by asterisks: *P < .05 or **P < .01. (C) WST cell viability assay of HepT1 cells. All cells were treated with 5 µg/ml CDDP and 10 mM AAP at time 0, and then either no NAC, or 1 mg/ml NAC at 0, 2, or 6 hours (n = 3, mean ± SEM). Cells were incubated in treated medium overnight before being developed for analysis. Statistical significance between treatment and “No NAC” control group was indicated by asterisks: *P < .05 or **P < .01.
NAC Reduces Apoptosis in HB Cell Lines Exposed to a Combination of AAP and CDDP
NAC was tested to determine whether it could prevent AAP and CDDP-induced cytotoxicity. When all drugs were administered simultaneously, the WST and Western blot analysis for cleaved PARP indicated that 1 mg/ml NAC is at least partially effective in reversing the cytotoxic effects of the various treatment regimens. In Figure 4, A and B, the presence and signal intensity of cleaved PARP show that NAC significantly (P < .05) lowered apoptosis induced by the combination of AAP and CDDP when added simultaneously 1 or 2 hours after but not 4 hours after the induction. Similar results were found in a WST cell viability assay, which showed the protective effect of NAC at 0 hour (65.0 ± 2.5% relative viability), 2 hours (49.9 ± 2.5%), and 6 hours (37.4 ± 1.6%) after the combination AAP and CDDP (26.4 ± 2.9%) treatment (Figure 4C). The in vitro protective effect of NAC on the AAP + CDDP-induced apoptosis of HB cells was dependent on the time of NAC administration in different assays used to quantify cell viability.
In Vivo Pharmacology and Toxicology Studies
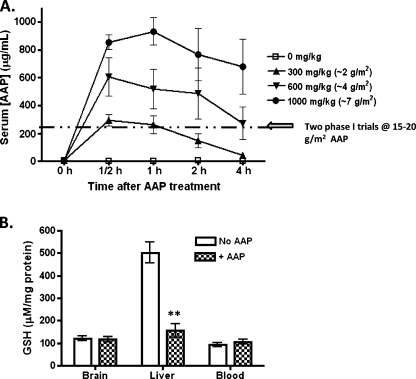
Rats were administered 0, 300, 600, or 1000 mg/kg (∼0, 2, 4 and 7 g/m2) AAP with serum levels of AAP checked at 0.5, 1, 2, and 4 hours after administration. Rats administered 1000 mg/kg (∼7 g/m2) AAP had peak serum AAP levels of 931.9 ± 98.9 µg/ml at 1 hour, whereas rats administered 300 and 600 mg/kg (∼2 and 4 g/m2) achieved peak plasma levels of 292.5 ± 40.2 and 605.6 ± 136 µg/ml at 0.5 hour after administration (Figure 5A). As already reported, brief exposure of animals to isoflurane did not significantly affect the activity of liver CYP2E1 [26], and thus, it was unlikely to have substantially biased the AAP pharmacokinetic study. Further, the levels of GSH were measured in the blood and in tissue samples from the brain and liver of treated and untreated rats. GSH levels were reduced in the liver of treated rats compared with vehicle. GSH levels in whole blood and the brain did not significantly differ between treated and untreated animals (Figure 5B).
Figure 5.
In vivo studies of AAP pharmacokinetics and AAP effect on cellular GSH levels. (A) Levels of AAP in the serum of rats (n = 5 per group) administered 0, 300, 600, or 1000 mg/kg (0, 2, 4, and 7 g/m2, respectively) AAP IP at times 0, 0.5, 1, 2, and 4 hours as measured using a direct ELISA test. Data were expressed as mean ± SEM. The dashed line indicates the peak levels of AAP measured in the serum of human patients during two phase 1 toxicity trials in which the patients were administered 15 to 20 g/m2 AAP [16,36]. (B) Rats were administered an IP dose of either vehicle or AAP at 1000 mg/kg. The rats were allowed to recover, and the liver, the brain, and whole blood were harvested for GSH levels at 6 hours after treatment (B). Statistical significance between treatment (+AAP) and control (No AAP) group was indicated by asterisks: **P < .01.
In Vitro Analysis of CYP2E1 Protein Levels in Different Species
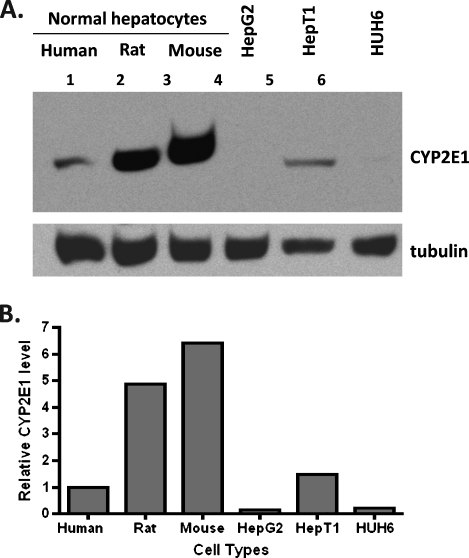
Testing the hypothesis that AAP overdose toxicity is highly dependent on the animal model, we found different CYP2E1 levels in normal hepatocytes among three different species (human, rat, and mouse). The CYP2E1 level in normal hepatocytes was measured in the following order: human < rat < mouse (Figure 6A). When the immunoblot signal was normalized to tubulin level, the CYP2E1 protein level found in rat and mouse hepatocytes was 4.9- and 6.4-fold higher, respectively, than normal human hepatocytes (Figure 6B). In addition, HB HepT1 cells expressed a high level of CYP2E1, relative to HepG2 and HUH6 cells, where CYP2E1 protein was not detected. We found that CYP2E1 protein level varied among different cell types and different species (Figure 6B). However, hepatocarcinoma and HB cells used in this study were responsive to AAP treatment, and drug sensitivity was not correlated to the CYP2E1 protein level.
Figure 6.
Immunoblot analysis of CYP2E1 protein level difference in normal and malignantly transformed hepatocytes. Normal hepatocytes, hepatocarcinoma HepG2, and HB cells (HepT1 and HUH6) were obtained and prepared for immunoblot analysis as described in Materials and Methods section. (A) CYP2E1 protein level was measured using rabbit anti-CYP2E1 polyclonal antibody from Stressgen that cross-reacts with human, rat, and mouse. Antitubulin monoclonal antibody from Sigma that cross-reacts with human, rat, and mouse was used to confirm and normalize equal protein loading. (B) Quantification of the immunoblot signal of CYP2E1 presented in panel A was measured by normalization with tubulin protein level.
Discussion
Acetaminophen and other nonsteroidal anti-inflammatory drugs have been extensively evaluated for their preventative role in cancer. However, their use as a chemotherapeutic agent has been less thoroughly explored. AAP has been evaluated multiple times in hepatocellular carcinoma cell lines that have shown AAP-induced toxicity but interestingly has not been rescued with NAC [12]. Glioma cell lines have also been evaluated, and AAP has consistently shown toxic effects in culture [27,28]. Breast cancer cell lines have been evaluated with mixed results [29,30]. More recently, the combination of doxorubicin and AAP was attempted in cell culture. This study showed that AAP was cytoprotective for HepG2 cell lines when combined with doxorubicin [31].
Treatment options for patients that present with unresectable or metastatic HB remain limited, with 25% to 30% of these patients resistant to standard chemotherapeutic regimens. Thus, alternative approaches to treatment are needed. Kobrinsky et al. [24] described a case report of the successful treatment of a patient with a doxorubicin- and CDDP-resistant HB with high-dose AAP and CDDP rescued with 8 hours of delayed NAC administration. In this study, we have demonstrated that a combined regimen of CDDP and AAP has enhanced cytotoxicity against HepT1 and HUH6 HB cells relative to CDDP alone in vitro. Posadas et al. [15] reported that AAP potentiated staurosporine-induced cell death in SH-SY5Y human neuroblastoma cell by reducing the GSH level. Further, the rescue agent and antioxidant NAC, when administered concurrently, acts to at least partially reverse the cytotoxicity of treatments consisting of CDDP and AAP.
CDDP is a commonly prescribed anticancer drug, and its cytotoxicity has recently been linked to the inhibition of thioredoxin reductase activity, an enzyme thought to help cells protect against oxidative stress caused by hydroperoxides [32]. That CDDP cytotoxicity proceeds through a mechanism involving oxidative stress is supported by studies that show an inverse relationship of CDDP toxicity and intracellular GSH levels [33]. AAP is also thought to damage both normal and cancer cells through mechanisms involving oxidative stress. Intracellularly, AAP is thought to be activated to the reactive metabolite NAPQI by CYP2E1, which covalently binds proteins and DNA within the cell, causing irreversible damage. NAPQI is detoxified by GSH, and thus, its buildup is associated with a decrease in intracellular GSH levels [34]. Indeed, our studies demonstrated that AAP reduces GSH levels in HUH6 and HepT1 cells within 1 hour of treatment. However, CYP2E1 was not detected in HUH6 cells, suggesting an alternative mechanism of AAP-induced oxidative stress and cytotoxicity in this cell line. Further, perhaps because both CDDP and AAP are thought to cause oxidative stress within treated cells, NAC, an antioxidant and free radical scavenger, protects against both AAP- and CDDP-induced toxicities.
Substantial interest has been demonstrated in combining drugs, such as buthionine sulfoxamine, 2-deoxy-d-glucose, and AAP, with CDDP that are thought to chemosensitize the tumor cells by depleting intracellular GSH levels [32]. However, before such drug combinations can be translated to clinical trials, studies need to be performed to determine their efficacy, pharmacology, and safety in preclinical settings. In the current study, we present data that indicate that AAP enhances CDDP cytotoxicity against human HB cell lines and that NAC acts to reverse this effect when all drugs are administered simultaneously. Other sulfur-containing antioxidants, such as sodium thiosulfate, demonstrate protection against CDDP-mediated cytotoxicity when both the medications are administered concurrently. However, when administration of CDDP and sodium thiosulfate is separated by 4 to 6 hours, cytotoxicity is not compromised [35]. Further, when chemoprotectants are administered at delayed time points, adverse effects of chemotherapy, such as high-frequency hearing loss, can be avoided [18]. Our studies demonstrated that whereas NAC administered simultaneously with CDDP and AAP protects HB cells in vitro, it does not reverse AAP and CDDP-induced cytotoxicity when administered 4 hours after AAP/CDDP combination.
There have been two phase 1 dose-escalation studies examining the potential of AAP as a chemotherapeutic agent. The first study was a phase 1 for multiple relapsed cancers. AAP was started at 6 g/m2 and escalated in a stepwise manner to 20 g/m2. Eleven rounds of 20 g/m2 of AAP were given to five patients, without achieving a maximum tolerated dose [36]. AAP concentrations peaked within 4 to 8 hours of administration and achieved a mean peak serum concentration of 245 µg/ml (range, 95–473 µg/ml). The second phase 1 trial used a combination of carmustine and AAP. AAP was escalated initially with a low dose of carmustine. The authors noted two significant toxicities at the 20-g/m2 dose level: one patient developed a grade 3 toxicity with significant aspartate amino transferase elevations, which resolved without specific treatment. A second patient developed a grade 4 pulmonary toxicity after AAP administration at 20 mg/m2. However, the patient's pulmonary toxicity was due to aspiration secondary to mental status change. Because of these toxicities, the maximum tolerated dose of AAP for this study was set at 15 g/m2 [16]. Serum AAP reached a peak concentration of 253 µg/ml between 1 and 4 hours at the 15-g/m2 dose.
In contrast, rats in this study treated with 300, 600, and 1000 mg/kg (∼2, 4, and 7 g/m2) AAP achieved peak serum AAP levels at 292.5 ± 40.2, 605.6 ± 136, and 931.9 ± 98.9 µg/ml within 1 to 2 hours after treatment, respectively (Figure 5A). It is also interesting to note that rats receiving 1000 mg/kg (∼7 g/m2) AAP became very weak, shivered, and exhibited hypothermia during treatment. This finding is consistent with studies indicating that rat hepatocytes are far more sensitive to AAP treatment than human hepatocytes [37]. In normal hepatocytes, we also found that rat (4.9-fold) and mouse (6.4-fold) contained a higher level of CYP2E1 protein than human tissue (Figure 6). The level of CYP2E1 protein expression by immunoblot analysis was found to be negatively correlated to in vitro hepatotoxicity induced by AAP (half maximal effective concentration: 3.8, 7.6, and 28.2 mM in mouse, rat, and human hepatocyte cultures, respectively) [37]. These data supported the hypothesis that the toxicity induced by AAP overdose greatly depends on the animal model applied. This may also be the major reason why 15 to 20 g/m2 is a tolerated dose in humans, whereas 500 mg/kg (approximately 3.5 g/m2) is toxic to mice. Our results exemplify the limitations of using a rat model to study the potential of incorporating AAP into a preclinical trial of chemotherapeutic regimens. In addition, the activity of rodent GSH S-transferase is approximately 10 to 20 times higher than that in humans [38]. Therefore, active metabolites produced in vivo in rat would be immediately detoxified by GSH conjugation, which would make the prediction of drug-induced hepatotoxicity in human more difficult by using either rat or mouse models.
Also notable from the phase 1 clinical study of Wolchok et al. [16] is that patients administered with up to 20 g/m2 of AAP were found to have no drop in serum GSH levels. Similarly, we found no drop in whole blood or brain GSH levels in rats in which substantially higher plasma AAP levels were achieved than is clinically feasible. However, we did observe substantial drops in GSH in the livers of the treated rats, the organ thought to be most active in AAP metabolism. This finding suggests that the chemoenhancement of CDDP-based chemotherapeutic regimens using AAP may be most effective against liver-based tumors despite the difference of CYP2E1 protein level. All of the human hepatocarcinoma and HB cells used in this study were responsive to AAP treatment in vitro.
In conclusion, we believe that the incorporation of AAP, an inexpensive, relatively safe, and widely available drug, into CDDP-containing chemotherapeutic regimens with 4 hours of delayed NAC administration is a promising approach to enhance the efficacy of CDDP chemotherapy while reducing the associated negative chemotherapy adverse effects. Thus, this is an approach that merits further examination because the potential treatment benefits could impact not only liver-based tumors but also various different malignancies.
Acknowledgments
The authors thank Sheila Taylor and Seth Lewin for their technical assistance; John Ozolek of Children's Hospital of Pittsburgh and Stephen Storm of University of Pittsburgh for providing the HUH6 HB cells and normal human hepatocytes.
Abbreviations
- AAP
acetaminophen
- CDDP
cisplatin
- CYP2E1
cytochrome P450 2E1
- HB
hepatoblastoma
- GSH
glutathione
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinonimine
- PARP
poly ADP ribose polymerase
Footnotes
This work was supported by the Biogen Idec Corporation and Fulbright Foundation to A.J.N. and by a grant from the National Institutes of Health (R37-NS044687) to E.A.N. E.A.N., Oregon Health & Science University, Portland Veterans Affairs Medical Center, and the Department of Veterans Affairs have a significant financial interest in Adherex, a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest was reviewed and managed by the Oregon Health & Science University Integrity Program Oversight Council and the Portland Veterans Affairs Medical Center's Conflict of Interest in Research Committee. E.A.N. has divested his financial interests in Adherex.
References
- 1.Roebuck DJ, Perilongo G. Hepatoblastoma: an oncological review. Pediatr Radiol. 2006;36:183–186. doi: 10.1007/s00247-005-0064-3. [DOI] [PubMed] [Google Scholar]
- 2.Malogolowkin MH, Katzenstein HM, Krailo M, Chen Z, Quinn JJ, Reynolds M, Ortega JA. Redefining the role of doxorubicin for the treatment of children with hepatoblastoma. J Clin Oncol. 2008;26:2379–2383. doi: 10.1200/JCO.2006.09.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perilongo G, Shafford E, Maibach R, Aronson D, Brugieres L, Brock P, Childs M, Czauderna P, MacKinlay G, Otte JB, et al. Risk-adapted treatment for childhood hepatoblastoma. Final report of the second study of the International Society of Paediatric Oncology—SIOPEL 2. Eur J Cancer. 2004;40:411–421. doi: 10.1016/j.ejca.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Roebuck DJ, Aronson D, Clapuyt P, Czauderna P, de Ville de Goyet J, Gauthier F, Mackinlay G, Maibach R, McHugh K, Olsen OE, et al. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37:123–132. doi: 10.1007/s00247-006-0361-5. quiz 249–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–F787. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- 6.Kartalou M, Essigmann JM. Recognition of cisplatin adducts by cellular proteins. Mutat Res. 2001;478:1–21. doi: 10.1016/s0027-5107(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 7.Meijer C, Mulder NH, Hospers GA, Uges DR, de Vries EG. The role of glutathione in resistance to cisplatin in a human small cell lung cancer cell line. Br J Cancer. 1990;62:72–77. doi: 10.1038/bjc.1990.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagawa K, Yokota J, Wada M, Sasaki Y, Fujiwara Y, Sakai M, Muramatsu M, Terasaki T, Tsunokawa Y, Terada M, et al. Levels of glutathione S transferase pi mRNA in human lung cancer cell lines correlate with the resistance to cisplatin and carboplatin. Jpn J Cancer Res. 1988;79:301–304. doi: 10.1111/j.1349-7006.1988.tb01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hospers GA, Mulder NH, de Jong B, de Ley L, Uges DR, Fichtinger-Schepman AM, Scheper RJ, de Vries EG. Characterization of a human small cell lung carcinoma cell line with acquired resistance to cis-diamminedichloroplatinum (II) in vitro. Cancer Res. 1988;48:6803–6807. [PubMed] [Google Scholar]
- 10.Estabrook RW. A passion for P450s (remembrances of the early history of research on cytochrome P450) Drug Metab Dispos. 2003;31:1461–1473. doi: 10.1124/dmd.31.12.1461. [DOI] [PubMed] [Google Scholar]
- 11.Kon K, Ikejima K, Okumura K, Aoyama T, Arai K, Takei Y, Lemasters JJ, Sato N. Role of apoptosis in acetaminophen hepatotoxicity. J Gastroenterol Hepatol. 2007;22(Suppl 1):S49–S52. doi: 10.1111/j.1440-1746.2007.04962.x. [DOI] [PubMed] [Google Scholar]
- 12.Manov I, Hirsh M, Iancu TC. N-acetylcysteine does not protect HepG2 cells against acetaminophen-induced apoptosis. Basic Clin Pharmacol Toxicol. 2004;94:213–225. doi: 10.1111/j.1742-7843.2004.pto940504.x. [DOI] [PubMed] [Google Scholar]
- 13.Wan J, Bae MA, Song BJ. Acetoaminophen-induced accumulation of 8-oxodeoxyguanosine through reduction of Ogg1 DNA repair enzyme in C6 glioma cells. Exp Mol Med. 2004;36:71–77. doi: 10.1038/emm.2004.10. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke H, Bajt ML. Intracellular signalingmechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 15.Posadas I, Vellecco V, Santos P, Prieto-Lloret J, Cena V. Acetaminophen potentiates staurosporine-induced death in a human neuroblastoma cell line. Br J Pharmacol. 2007;150:577–585. doi: 10.1038/sj.bjp.0706993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolchok JD, Williams L, Pinto JT, Fleisher M, Krown SE, Hwu WJ, Livingston PO, Chang C, Chapman PB. Phase I trial of high dose paracetamol and carmustine in patients with metastatic melanoma. Melanoma Res. 2003;13:189–196. doi: 10.1097/00008390-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–292. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther. 2005;314:1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 19.Dickey DT, Muldoon LL, Doolittle ND, Peterson DR, Kraemer DF, Neuwelt EA. Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin-induced toxicity in rat models. Cancer Chemother Pharmacol. 2008;62:235–241. doi: 10.1007/s00280-007-0597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safirstein R, Andrade L, Vieira JM. Acetylcysteine and nephrotoxic effects of radiographic contrast agents—a new use for an old drug. N Engl J Med. 2000;343:210–212. doi: 10.1056/NEJM200007203430311. [DOI] [PubMed] [Google Scholar]
- 22.Jarvinen K, Pietarinen-Runtti P, Linnainmaa K, Raivio KO, Krejsa CM, Kavanagh T, Kinnula VL. Antioxidant defense mechanisms of human mesothelioma and lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L696–L702. doi: 10.1152/ajplung.2000.278.4.L696. [DOI] [PubMed] [Google Scholar]
- 23.Neuwelt EA, Pagel MA, Kraemer DF, Peterson DR, Muldoon LL. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther. 2004;309:594–599. doi: 10.1124/jpet.103.063347. [DOI] [PubMed] [Google Scholar]
- 24.Kobrinsky NL, Sjolander DE, Goldenberg JA, Ortmeier TC. Successful treatment of doxorubicin and cisplatin resistant hepatoblastoma in a child with Beckwith-Wiedemann syndrome with high dose acetaminophen and N-acetylcysteine rescue. Pediatr Blood Cancer. 2005;45:222–225. doi: 10.1002/pbc.20330. [DOI] [PubMed] [Google Scholar]
- 25.Wu YJ, Muldoon LL, Neuwelt EA. The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J Pharmacol Exp Ther. 2005;312:424–431. doi: 10.1124/jpet.104.075119. [DOI] [PubMed] [Google Scholar]
- 26.Plate AY, Crankshaw DL, Gallaher DD. The effect of anesthesia by diethyl ether or isoflurane on activity of cytochrome P450 2E1 and P450 reductases in rat liver. Anesth Analg. 2005;101:1063–1064. doi: 10.1213/01.ane.0000166791.30963.ef. table of contents. [DOI] [PubMed] [Google Scholar]
- 27.Casper D, Lekhraj R, Yaparpalvi US, Pidel A, Jaggernauth WA, Werner P, Tribius S, Rowe JD, LaSala PA. Acetaminophen selectively reduces glioma cell growth and increases radiosensitivity in culture. J Neurooncol. 2000;46:215–229. doi: 10.1023/a:1006492423666. [DOI] [PubMed] [Google Scholar]
- 28.Bae MA, Pie JE, Song BJ. Acetaminophen induces apoptosis of C6 glioma cells by activating the c-Jun NH(2)-terminal protein kinase-related cell death pathway. Mol Pharmacol. 2001;60:847–856. [PubMed] [Google Scholar]
- 29.Harnagea-Theophilus E, Gadd SL, Knight-Trent AH, DeGeorge GL, Miller MR. Acetaminophen-induced proliferation of breast cancer cells involves estrogen receptors. Toxicol Appl Pharmacol. 1999;155:273–279. doi: 10.1006/taap.1998.8619. [DOI] [PubMed] [Google Scholar]
- 30.Gadd SL, Hobbs G, Miller MR. Acetaminophen-induced proliferation of estrogen-responsive breast cancer cells is associated with increases in c-myc RNA expression and NF-kappaB activity. Toxicol Sci. 2002;66:233–243. doi: 10.1093/toxsci/66.2.233. [DOI] [PubMed] [Google Scholar]
- 31.Manov I, Bashenko Y, Eliaz-Wolkowicz A, Mizrahi M, Liran O, Iancu TC. High-dose acetaminophen inhibits the lethal effect of doxorubicin in HepG2 cells: the role of P-glycoprotein and mitogen-activated protein kinase p44/42 pathway. J Pharmacol Exp Ther. 2007;322:1013–1022. doi: 10.1124/jpet.107.121772. [DOI] [PubMed] [Google Scholar]
- 32.Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-d-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67:3364–3370. doi: 10.1158/0008-5472.CAN-06-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA. 1992;89:3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- 35.Harned TM, Kalous O, Neuwelt A, Loera J, Ji L, Iovine P, Sposto R, Neuwelt EA, Reynolds CP. Sodium thiosulfate administered six hours after cisplatin does not compromise antineuroblastoma activity. Clin Cancer Res. 2008;14:533–540. doi: 10.1158/1078-0432.CCR-06-2289. [DOI] [PubMed] [Google Scholar]
- 36.Kobrinsky NL, Hartfield D, Horner H, Maksymiuk A, Minuk GY, White DF, Feldstein TJ. Treatment of advanced malignancies with high-dose acetaminophen and N-acetylcysteine rescue. Cancer Invest. 1996;14:202–210. doi: 10.3109/07357909609012140. [DOI] [PubMed] [Google Scholar]
- 37.Jemnitz K, Veres Z, Monostory K, Kobori L, Vereczkey L. Interspecies differences in acetaminophen sensitivity of human, rat, and mouse primary hepatocytes. Toxicol In Vitro. 2008;22:961–967. doi: 10.1016/j.tiv.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Akai S, Hosomi H, Minami K, Tsuneyama K, Katoh M, Nakajima M, Yokoi T. Knock down of gamma-glutamylcysteine synthetase in rat causes acetaminophen-induced hepatotoxicity. J Biol Chem. 2007;282:23996–24003. doi: 10.1074/jbc.M702819200. [DOI] [PubMed] [Google Scholar]