Abstract
Chemokine CXCL12 is proposed to promote multiple steps in growth of primary tumors and progression to metastatic disease in more than 20 different cancers. Functions of CXCL12 previously were believed to be controlled only by receptor CXCR4, but CXCR7 was recently identified as a second receptor for this chemokine. CXCR7 increases tumor formation and metastasis in mouse models, suggesting that this receptor may also be a key target for blocking effects of CXCL12 in cancer. To image activation of CXCR7 in intact cells and living mice, we tested the hypothesis that binding of chemokine ligands to CXCR7 recruits β-arrestins, a family of cytosolic adapter proteins that interact with many activated chemokine and related seven-transmembrane receptors. Using firefly luciferase protein fragment complementation, we established that chemokine ligands CXCL12 and CXCL11 significantly increase association of CXCR7 and β-arrestins with preferential interaction of the receptor with β-arrestin 2. The magnitude of interactions between CXCR7 and β-arrestin 2 increased over time after treatment with ligands, contrasting with transient association of β-arrestin 2 and CXCR4. β-Arrestin 2 increased uptake of CXCL12 in cells expressing CXCR7, emphasizing the functional relevance of the interaction between CXCR7 and β-arrestin 2. In an orthotopic xenograft model of human breast cancer, we used bioluminescence imaging to quantify changes in the association of CXCR7 and β-arrestin 2. These studies demonstrate ligand-dependent interactions of CXCR7 with β-arrestin 2 that promote accumulation of chemokines and establish an imaging assay for the dynamic regulation of CXCR7 by chemokines and candidate therapeutic agents in cell-based assays and living mice.
Introduction
Chemokine receptors are members of the large family of seven-transmembrane (7-TM) receptors, also referred to as G-protein-coupled receptors. Chemokine receptors were initially identified because of functions in trafficking of hematopoietic cells under physiologic conditions and in response to inflammatory stimuli. More recently, it has become evident that many different types of cancer cells co-opt chemokine receptors to promote growth of primary tumors, metastasis, and resistance to chemotherapy. The emerging roles of chemokines and their receptors in cancer are motivating ongoing efforts to target these pathways therapeutically [1].
In particular, chemokine CXCL12 has been linked to multiple key processes in cancer, including cell proliferation, survival, migration, invasion, and chemotaxis of cancer cells to characteristic sites of metastasis [2–7]. Effects of CXCL12 in normal physiology and cancer have been ascribed to signaling through receptor CXCR4 [8,9] based in large part on the comparable phenotypes of mice lacking either CXCL12 or CXCR4 [10–12]. Work by our laboratory and others has shown that CXCL12-CXCR4 signaling increases growth of orthotopic breast cancer xenografts and both spontaneous and experimentally induced metastases [13–15]. Indeed, studies have shown similar effects of CXCL12-CXCR4 in more than 20 types of cancer, providing compelling evidence for the importance of this chemokine signaling pathway in cancer.
Recently, CXCR7 was identified as a second chemokine receptor for CXCL12, suggesting that functions of CXCL12 in cancer may be regulated at least in part through this receptor. CXCR7 also binds to chemokine CXCL11, a molecule that has been implicated in cancer progression through binding to receptor CXCR3 [16]. We have shown that expression of CXCR7 promotes growth and metastasis of breast and lung cancer cells in animal models, and similar results have been obtained in a mouse model of prostate cancer [17,18]. Whereas these studies strongly link CXCR7 to cancer biology, functions of CXCR7 and its molecular interactions in cells after ligand binding remain poorly defined and controversial. Some studies suggest that CXCR7 functions as a signaling receptor, promoting cell adhesion, chemotaxis, and activation of downstream signaling molecules such as AKT [18–22]. However, these effects of CXCR7 have not been identified consistently in different model systems [23,24]. CXCR7 may heterodimerize with CXCR4 and modulate signaling pathways initiated through CXCL12-CXCR4 [25,26], but some effects of CXCR7 in growth and metastasis of cancer cells and cell migration seem to be independent of CXCR4 [17,27]. Recent data also suggest that CXCR7 may act as a decoy receptor to scavenge CXCL12 and establish appropriate gradients of this chemokine for germ cell migration in zebrafish [24]. Collectively, these data highlight complex functions of CXCR7 in both normal development and cancer, emphasizing the need to define fundamental mechanisms of receptor activation.
Ligand binding to most chemokine receptors, like other 7-TM receptors, activates the receptor and leads to phosphorylation of the receptor by a G protein receptor kinase (GRK; reviewed in Moore et al. [28]). Phosphorylation of the receptor causes recruitment of a cytosolic adapter protein, β-arrestin. The complex of ligand, receptor, and β-arrestin is then internalized, removing the receptor from the cell membrane. However, internalization of 7-TM receptors may be independent of β-arrestin [29], including some members of the class of decoy chemokine receptors [30,31]. Decoy chemokine receptors bind chemokine ligands and internalize without initiating signaling pathways characteristically associated with activated chemokine receptors. Such decoy receptors, including CCX-CKR and Darc, apparently function to sequester chemokines, limiting inflammation and/or maintaining well-defined gradients of chemokines for signaling through other receptors.
Because CXCR7 may have functions of both signaling and decoy chemokine receptors, we investigated the effects of chemokine ligands on binding of the receptor to β-arrestin molecules. To accomplish this goal, we used a protein fragment complementation assay (PCA) based on firefly luciferase [32]. The luciferase enzyme fragments have minimal background association when coexpressed as individual proteins in the same cell. When these enzyme fragments are fused to interacting proteins, association of the target proteins reconstitutes active firefly luciferase enzyme. Therefore, changes in luciferase activity can be used to quantify the magnitude and kinetics of protein interactions in intact cells and living animals. We recently used this PCA strategy to analyze chemokine-dependent interactions of CXCR4 with β-arrestin molecules, validating the system for our current investigation of CXCR7 and β-arrestin [33]. Using this PCA, we established that chemokines drive recruitment of β-arrestin molecules to CXCR7 and show that the receptor preferentially interacts with β-arrestin 2 relative to β-arrestin 1. Unlike the brief interaction between CXCR4 and β-arrestin 2, association of CXCR7 with β-arrestin 2 increased over time. Loss of functional β-arrestin 2 reduced CXCR7-dependent uptake of CXCL12 from the extracellular space, establishing that β-arrestin 2 is necessary for this function of the receptor. Using association of β-arrestin 2 and CXCR7 as a marker of receptor activation, we were able to image in vivo regulation of this receptor in human breast tumor xenografts after treatment with a CXCR7-targeted small molecule. By enabling CXCR7 activation to be quantified in intact cells and the in vivo tumor microenvironment, this imaging strategy will advance functional studies of CXCR7 and accelerate identification and validation of CXCR7-targeted therapeutic agents.
Experimental Procedures
DNA Constructs
Luciferase complementation plasmids in vector pEF for N- and C-terminal fragments of firefly luciferase (NLuc and CLuc) and c-fos fused to CLuc (fos-CLuc) were provided by Alnawaz Rehemtulla (University of Michigan). CLuc-FKBP12 was provided by David Piwnica-Worms (Washington University) [32]. A plasmid expressing constitutively active GRK2 (GRK2-C20) was provided by John Tesmer (University of Michigan). Plasmids for human CXCR4 or CXCR7 fused to NLuc or CLuc, β-arrestin 1 and β-arrestin 2 fused to CLuc, and CXCL12 fused to Gaussia luciferase (CXCL12-GL) have been described previously [33–35]. Plasmids for firefly and Gaussia luciferases (pGL3-control and pCMV-GL, respectively) were from Promega (Madison, WI).
CXCR7-green fluorescent protein (GFP) and CXCR7-mPlum were generated by amplifying CXCR7 with polymerase chain reaction (PCR) primers 5′-ATTACTCGAGACCGCCATGGATCTGCATCTCTTCGACTA-3′ and 5′-TAATGACCGGTCCACCGCCTGAAGAACCGCCTCCTTTGGTGCTCTGCTCCAAGGCAG-3′ and ligating the product into corresponding XhoI and AgeI sites of vector EGFP-N1 (BD Biosciences, San Jose, CA) or mPlum in the same N1 vector (a gift from Roger Tsien, University of California, San Diego, CA; restriction sites added for cloning are underlined) [36]. The C-terminus of β-arrestin 2 was fused to fluorescent protein mCherry (a gift from Roger Tsien) by amplifying mCherry with PCR primers 5′-ATCCACCGGTCGGCGGTGGCTCATCTGGCGGAGGTGTGAGCAAGGGCGAGGAGGATAAC-3′ and 5′-GCATGCGGCCGCCTACTTGTACAGCTCGTCCATG-3′. Restriction sites for AgeI and NotI, respectively, are underlined. The PCR product was ligated to the corresponding sites in plasmid β-arrestin 2-CLuc [33], removing the C-terminal fragment of firefly luciferase. All PCR products were verified by DNA sequencing.
Cells
Human embryonic kidney 293 cells stably expressing large T antigen from SV40 virus (293T; Open Biosystems, Huntsville, AL), human MDA-MB-231 and MCF-7 breast cancer cells (ATCC, Manassas, VA), and wild-type and β-arrestin 2-/- mouse embryonic fibroblasts (MEFs; gift from Robert Lefkowitz, Duke University) were cultured in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA), 10% fetal bovine serum, 1% glutamine, and 0.1% penicillin/streptomycin/gentamicin. Cells were maintained in a 37°C incubator with 5% CO2. 293T cells stably expressing CXCL12-GL and unfused GL have been described previously [35].
To generate stable cell lines coexpressing CXCR7-NLuc and β-arrestin 2-CLuc or fos-CLuc, we prepared recombinant lentiviruses expressing each of these proteins as described previously [14,37]. 293T or 231 cells were first transduced with supernatants containing CXCR7-NLuc in FUGW. Transduction efficiency was 100% as determined by fluorescence microscopy. These cells were then transduced with supernatants for β-arrestin 2-CLuc or fos-CLuc in FUPW. Again, all cells were transduced with CLuc constructs as determined by fluorescence microscopy for mPlum. Batch populations of 293T reporter cells were used for all experiments, whereas clonal cell lines of 231 cells transduced with CXCR7-NLuc and β-arrestin-2-CLuc were isolated for cell culture and animal studies. Wild-type or β-arrestin 2-/- MEFs were transduced with lentiviruses expressing CXCR7-GFP, and batch populations of transduced MEF lines were used for all experiments.
CXCL12-GL and Unfused GL Supernatants
Supernatants with CXCL12-GL or unfused GL were prepared from stably transduced 293T cells as described previously [35].
Live Cell Bioluminescence Imaging
293T cells were transfected with firefly luciferase complementation plasmids by calcium phosphate precipitation as described previously [37]. Cells were also cotransfected with constitutively expressed GL as a transfection control. One day after transfection, cells were split into black-walled 96-well plates (Corning, Lowell, MA) at 1 x 104 cells per well using a Multidrop 384 dispensing system (Labsystems, Waltham, MA), and experiments were performed 2 days after transfection. For competition experiments with CXCR4 or CXCR7, 293T cells stably expressing the CXCR7/β-arrestin 2 luciferase complementation pair were transiently transfected with 3.5 µg per 6-cm dish of CXCR4-mPlum or CXCR7-mPlum. These cells were split into 96-well plates the following day and used for assays 2 days after transfection. For other experiments with stable cell lines, cells were plated into 96-well plates as described previously 1 day before each assay. Quadruplicate samples were used for all experimental conditions. Cells were treated with various concentrations of CXCL11, CXCL12 (R&D Systems, Minneapolis, MN), AMD3100 (Sigma, St. Louis, MO), TF14013 (a gift from N. Fujii), or CCX733 or CCX754 (a gift from Chemocentryx, Mountain View, CA) as described in figure legends. Incubation times for each agent are also listed in figure legends.
To quantify uptake of CXCL12-GL or unfused GL, stably transduced MEFs were plated at 1.2 x 104 cells per well in black-walled 96-well plates 1 day before experiments. MEFs were wild-type or β-arrestin 2-/- cell lines stably expressing CXCR7-GFP. Cells were incubated with ≈ 10 ng/ml CXCL12-GL or a comparable amount of unfused GL based on bioluminescence for 1 hour. At the end of the incubation period, cells were washed twice with either phosphate-buffered saline (PBS) at room temperature or an acidic solution (0.2 M acetic acid, 0.5 M NaCl) at 4°C. Washing with acidic solution removes chemokine bound to the cell surface [38]. Cells were then washed once with PBS to restore neutral pH. Bioluminescence was quantified immediately after adding 1 µg/ml coelenterazine diluted in PBS (Fluka, St. Louis, MO).
Bioluminescence imaging of firefly luciferase activity in live cells was performed on a cryogenically cooled camera system (IVIS 100; Caliper, Hopkinton, MA) as described previously [32], using 1- to 5-minute acquisition times, high sensitivity, and field of view B. Imaging parameters for accumulation of CXCL12-GL were 2-minute acquisition, medium sensitivity, and field of view B. For firefly luciferase complementation experiments with transiently transfected cells, GL activity was measured in parallel wells of transfected cells incubated with 1 µg/ml coelenterazine. Bioluminescence was assayed on the IVIS using 1- to 2-minute acquisition, medium sensitivity, and field of view B immediately after adding coelenterazine. Previous research has shown that amounts of GL are directly related to the numbers of cells expressing this enzyme [39]. The same protocol was used to quantify amounts of CXCL12-GL or unfused GL in experiments with MEFs. For transient transfections with luciferase complementation, data for firefly luciferase activity were normalized to GL activity to account for differences in transfection efficiency and numbers of cells. Data for stable cell lines and cellular accumulation of CXCL12-GL were normalized to total protein per well measured by sulforhodamine B staining [40].
Fluorescence Microscopy
293T cells were transiently transfected with CXCR7-GFP and a wild-type or mutant β-arrestin 2-mCherry plasmid as described previously, split into 35-mmdishes with glass bottoms (MatTek, Ashland, MA) and then used for experiments 2 days after transfection. Cells were treated with 300 ng/mlCXCL12 for 2 hours and then imaged by epifluorescence microscopy using a 40x objective (Olympus, Center Valley, PA). Fluorescence images were obtained with Spot Advanced software (Sterling Heights, MI), and merged images were created with Adobe Photoshop (San Jose, CA).
Flow Cytometry
Cell surface expression of CXCR7 was quantified with monoclonal antibody 11G8 as we have described previously [17].
In Vivo Bioluminescence Imaging
All animal procedures were approved by the University of Michigan Committee for Use and Care of Animals. A total of 1 x 106 MDAMB-231 human breast cancer cells stably coexpressing CXCR7-NLuc and β-arrestin-2-CLuc were orthotopically implanted into bilateral inguinal mammary fat pads of 6-week-old female severe combined immunodeficient (SCID) mice (Taconic, Hudson, NY) [14]. After tumors reached approximately 8 to 10 mm in diameter, animals were injected subcutaneously with CCX754 or vehicle control (a gift from Chemocentryx) [20] at times and amounts listed in the figure legend.
Bioluminescence imaging was performed on an IVIS spectrum system (Caliper) as we have described previously [41]. Data for bioluminescence were quantified as photons using Living Image software (Caliper). Photon data for bioluminescence after injection with CCX754 or vehicle were normalized to pretreatment images to account for differences in volumes of various tumors.
Statistics
Data are plotted as mean values with SEM. Pairs of data were analyzed by t test to determine statistically significant differences (GraphPad Prism, La Jolla, CA).
Results
Recruitment of β-Arrestin to CXCR7
In response to ligand binding, chemokine receptors and other 7-TM receptors typically are phosphorylated by a GRK, resulting in the interaction of the phosphorylated receptor with a cytoplasmic adapter protein, β-arrestin. β-Arrestin then recruits the 7-TM receptor to clathrin and clathrin-coated pits, resulting in the internalization of the complex of β-arrestin and 7-TM receptor. However, some chemokine decoy receptors, such as D6, may cycle constitutively without ligand-induced binding to β-arrestin [42], and other chemokine decoy receptors, including Darc and CCX-CKR, do not interact with β-arrestin [30,31]. Because recent studies suggest that CXCR7 may function at least in part as a decoy chemokine receptor for CXCL12, we investigated the interactions of CXCR7 with β-arrestin and the effects of ligands on this process.
To analyze association of CXCR7 and β-arrestin molecules, we used a firefly luciferase PCA that we recently validated for analyzing interactions between CXCR4 and β-arrestin in intact cells and living mice [33]. The PCA uses N- and C-terminal fragments of firefly luciferase (NLuc and CLuc, respectively) that have essentially undetectable bioluminescence when expressed singly or together in living cells [32]. When NLuc and CLuc are fused to proteins of interest, association of the target proteins also reconstitutes luciferase activity, providing a quantitative measure of the magnitude and kinetics of protein interactions. We fused the intracellular C-terminus of CXCR7 to NLuc (CXCR7-NLuc) and β-arrestin 2 to CLuc (β-arrestin 2-CLuc), analogous to our PCA for CXCR4 and β-arrestin 2 (Figure 1A). As negative controls, we used either c-fos, a cytoplasmic protein not known to associate with 7-TM receptors, fused to CLuc (fos-CLuc) or CLuc-FKBP12, a fusion protein that we previously have shown to interact with FRB-NLuc in a rapamycin-dependent manner [32]. Expression of CXCR7-NLuc was verified by flow cytometry (Figure 1B), whereas cell membrane expression of the receptor was undetectable in control 293T cells (data not shown). Levels of CXCR7 on the surface of transiently transfected 293T cells were only slightly greater than the amounts expressed endogenously by MCF-7 human breast cancer cells. We previously have established that our constructs for β-arrestin 2-CLuc, fos-CLuc, and CLuc-FKBP12 fusion proteins express in 293T cells and note that these cells express higher levels of endogenous β-arrestin 2 than many other commonly used cell lines [32,33,43].
Figure 1.
Firefly luciferase PCA for recruitment of β-arrestin to CXCR7. (A) Diagram of PCA showing fusion of the NLuc and CLuc fragments of firefly luciferase to the 7-TM receptor CXCR7 and the cytoplasmic adapter molecule β-arrestin 2, respectively. Binding of CXCL11 or CXCL12 to CXCR7 recruits β-arrestin 2 to the receptor, bringing NLuc and CLuc enzyme fragments into proximity and reconstituting firefly luciferase activity. Bioluminescence provides a quantitative measure of the magnitude and kinetics of protein interactions between CXCR7 and β-arrestin. (B) 293T cells were transfected transiently with CXCR7-NLuc, and cell surface expression of the receptor was determined by flow cytometry 48 hours later. Endogenous expression of CXCR7 in MCF-7 human breast cancer cells is shown for comparison. Open symbol indicates isotype control; solid symbol, antibody to CXCR7. (C) 293T cells were transfected transiently with PCA reporters CXCR7-NLuc and β-arrestin 2-CLuc and a plasmid for constitutively active GRK2 (GRK2-C20) or empty vector control (n = 4 per condition). Cells were also cotransfected with an expression plasmid for GL as a control for transfection efficiency. Firefly luciferase bioluminescence in intact cells was quantified 48 hours after transfection and normalized to GL activity. Data are mean values ± SEM representative of three independent experiments. ***P < .005.
As an initial test of phosphorylation-dependent recruitment of β-arrestin 2 to CXCR7, we transiently transfected cells with CXCR7-NLuc and a plasmid for β-arrestin 2-CLuc, fos-CLuc, or CLuc-FKBP12, respectively. Separate cells were also transfected with only one of the four complementation vectors alone. To drive phosphorylation of CXCR7, we cotransfected cells with isoprenylated, constitutively active GRK2 or empty vector [44]. Forty-eight hours after transfection, bioluminescence from the interaction of CXCR7-NLuc and β-arrestin 2-CLuc was increased by ≈10-fold in cells expressing constitutively active GRK2 relative to vector control (Figure 1C; P < .005). By comparison, bioluminescence produced by the combination of CXCR7-NLuc and fos-CLuc or CXCR7-NLuc and CLuc-FKBP12 was not detectable above background, showing specificity of the interaction between CXCR7 and β-arrestin 2 (data not shown). Cells transfected with CXCR7-NLuc, β-arrestin 2-CLuc, fos-CLuc, and CLuc-FKBP12 alone also did not produce bioluminescence above background levels (data not shown). These data demonstrate that the association of CXCR7 and β-arrestin 2 is increased substantially in response to GRK activity.
Having established that CXCR7 interacts with β-arrestin 2, we compared the magnitude of basal and ligand-dependent recruitment of β-arrestin 2 to CXCR7 relative to CXCR4. CXCR4 is the only other chemokine receptor known to bind chemokine CXCL12. We used batch populations of 293T cells stably transduced with PCA reporters for CXCR7-NLuc/β-arrestin 2-CLuc or CXCR7-NLuc/fos-CLuc, in addition to our previously described stable cell lines for CXCR4-NLuc/β-arrestin 2-CLuc or CXCR4-NLuc/fos-CLuc [33]. We treated cells with either CXCL12, which binds to both CXCR4 and CXCR7, or CXCL11, a chemokine that is recognized by CXCR7 but not CXCR4 [19,20]. We measured bioluminescence in intact cells under basal conditions and ≈10 minutes after adding chemokines.
In the absence of chemokines, there was more luciferase activity in CXCR7-NLuc/β-arrestin 2-CLuc cells compared with cells expressing the CXCR4 reporter, suggesting a higher basal association of β-arrestin 2 with CXCR7 (Figure 2). Approximately 10 minutes after adding CXCL11 or CXCL12, bioluminescence increased by ≈1.5-fold above baseline for cells expressing CXCR7-NLuc/β-arrestin 2-CLuc (P < .05). By comparison, incubation with CXCL12 increased bioluminescence by approximately six-fold for recruitment of β-arrestin 2-CLuc to CXCR4-NLuc (P < .005). Treatment of CXCR4-NLuc/β-arrestin 2-CLuc cells with CXCL11 did not alter baseline association of these proteins, showing specificity of the assay for ligand-receptor binding. Bioluminescence from cells coexpressing fos-CLuc with either CXCR4- or CXCR7-NLuc did not differ from 293T cells without any firefly luciferase fragments. Overall, these data demonstrate that immediate recruitment of β-arrestin 2 to CXCR7 is substantially less than CXCR4 in response to chemokine ligands.
Figure 2.
Chemokine-dependent recruitment of β-arrestin 2 to CXCR7. 293T cells stably expressing firefly luciferase PCA reporters for CXCR7-NLuc/β-arrestin 2-CLuc, CXCR7-NLuc/fos-CLuc, CXCR4-NLuc/β-arrestin 2-CLuc, or CXCR4-NLuc/fos-CLuc were treated with 1 µg/ml CXCL11 or CXCL12. Bioluminescence produced by intact cells was quantified ≈10 minutes after adding chemokine or medium alone (n = 4 per condition). Parental 293T cells not expressing any firefly luciferase PCA reporters are shown for comparison. Data are mean values for firefly luciferase activity ± SEM and are representative of four independent experiments. *P < .05; ***P < .005.
Kinetics of Ligand-Dependent Recruitment of β-Arrestin 2 to CXCR7
In the initial experiments described in the previous paragraphs, we measured luciferase activity shortly after treating cells with a chemokine. This experimental protocol was based on our previous research with CXCR4 in which bioluminescence from recruitment of β-arrestin 2 to this receptor peaked ≈5 to 10 minutes after adding CXCL12 and then returned to basal levels within 40 minutes [33]. A relatively brief association of CXCR4 with β-arrestin 2 characterizes a subset of 7-TM receptors defined as class A [28,45]. However, other 7-TM receptors form more prolonged interactions with β-arrestin 2 in intracellular compartments, defining class B receptors. The existence of these two different classes suggested that kinetics of interaction between CXCR7 and β-arrestin 2 may be substantially longer for this receptor relative to CXCR4.
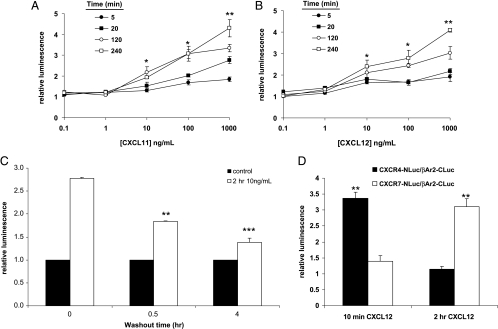
To analyze the time course of β-arrestin 2 recruitment to CXCR7, we treated stable 293T CXCR7-NLuc/β-arrestin 2-CLuc PCA cells with increasing concentrations of CXCL11 or CXCL12 for periods extending up to 4 hours before measuring luciferase activity in living cells. Incubation with CXCL11 or CXCL12 produced both time- and dose-dependent increases in bioluminescence from recruitment of β-arrestin 2 to CXCR7 (Figure 3, A and B). For example, treatment with 1 µg/ml CXCL12 increased bioluminescence by 1.3-fold above baseline levels 5 minutes after adding chemokine to cells, whereas the reporter signal from CXCR7 and β-arrestin 2 increased by four-fold after 4 hours of continuous incubation (P < .01). We observed similar kinetics and changes in the magnitude of bioluminescence after treatment with CXCL11.
Figure 3.
Time- and dose-dependent recruitment of β-arrestin 2 to CXCR7 in response to chemokine ligands. (A and B) Stably transduced CXCR7/β-arrestin 2 PCA reporter cells were incubated with increasing concentrations of CXCL11 (A) or CXCL12 (B) for various periods before quantifying bioluminescence in intact cells (n = 4, representative of three independent experiments). Data are reported as the fold increase in photons relative to control values in unstimulated cells. Error bars, SEM. (C) Reporter cells were incubated with 10 ng/ml CXCL12 for 2 hours, washed with PBS, and then incubated without chemokine for 0.5 or 4 hours of washout. Control cells were maintained in normal medium with no added chemokine. Data are expressed as mean values ± SEM relative to a value of 1 for control cells (n = 4 per condition, representative of two independent experiments). (D) 293T cells stably transduced with the CXCR7/β-arrestin 2 PCA or CXCR4/β-arrestin 2 PCA reporter were incubated with 100 ng/ml CXCL12 for either 10 minutes or 2 hours before quantifying firefly luciferase activity in living cells (n = 4 per condition). Data are presented as the increase in luminescence relative to baseline values in untreated cells with error bars denoting SEM. Results are representative of three independent experiments. *P < .05; **P < .01; ***P < .005.
We also investigated a reversal of association between CXCR7 and β-arrestin 2 after extended treatment with the chemokine ligand. We incubated stable reporter cells with 10 ng/ml CXCL12 or vehicle control for 2 hours to stimulate an interaction between CXCR4 and β-arrestin 2. Cells were washed with PBS and then incubated in a medium without chemokine for an additional 0.5 or 4 hours before quantifying luciferase activity in intact cells. Incubation with CXCL12 increased bioluminescence from the interaction of CXCR7 and β-arrestin 2 by more than 2.5-fold above control cells (Figure 3C). PCA signal decreased by ≈50% in the first 30 minutes after removing CXCL12 and decreased to ≈35%above control levels by 4 hours. These data demonstrate that most CXCR7-β-arrestin 2 complexes dissociate after removing the chemokine ligand, approaching the basal level of interaction between these two proteins in unstimulated cells.
To directly compare responses of β-arrestin 2 recruitment to CXCR4 versus CXCR7, we incubated stable reporter cell lines with 100 ng/ml CXCL12 and quantified luciferase activity within the first 10 minutes after adding chemokine or after 2 hours of continuous incubation (Figure 3D). Recruitment of β-arrestin 2 to CXCR4 occurred rapidly in response to CXCL12 and returned to baseline values by 2 hours. By comparison, the interaction of CXCR7 with β-arrestin 2 showed the opposite pattern with very low levels of bioluminescence produced within the first 10 minutes and more than a three-fold induction of luciferase activity above baseline after 2 hours. These experiments show that continuous exposure to chemokine ligands drives progressive increases in association of β-arrestin 2 with CXCR7, contrasting with the rapid onset and short duration of this process for CXCR4 and most other chemokine receptors.
We also used fluorescence microscopy to verify recruitment of β-arrestin 2 to CXCR7. 293T cells were transfected with CXCR7 and β-arrestin 2 fused to GFP and mCherry, respectively, and then imaged before and after treatment with 300 ng/ml CXCL12 or BSA control for 2 hours. Under basal conditions, CXCR7-GFP predominantly localized intracellularly as has been described previously [24], whereas β-arrestin 2-mCherry distributed diffusely throughout the cytoplasm with a limited number of more discrete intracellular foci (Figure 4). After treatment with CXCL12, β-arrestin 2 was present in more numerous intracellular structures that frequently had a ringlike appearance suggestive of vesicles. Many of these foci of β-arrestin 2 colocalized with CXCR7. By comparison, treatment with BSA did not alter distribution of β-arrestin 2 from the basal state (data not shown) and/or affect colocalization with CXCR7. In combination with data from luciferase complementation assays, results from fluorescence microscopy show that CXCL12 promotes association of CXCR7 with β-arrestin 2, predominantly on intracellular structures.
Figure 4.
Ligand-dependent colocalization of CXCR7 and β-arrestin 2. 293T cells were transfected with CXCR7-GFP and β-arrestin 2-mCherry and imaged before and after incubation with 300 ng/ml CXCL12 for 2 hours. Green, red, and merged fluorescence images are shown under basal conditions and after treatment with CXCL12. Blue arrows show colocalization of CXCR7-GFP and β-arrestin 2-mCherry in intracellular structures.
Effects of Nonbioluminescent CXCR4 or CXCR7 on Complementation Signal
To investigate luciferase complementation in the presence of CXCR4 or CXCR7 receptors without an enzyme fragment, we transiently expressed CXCR4-mPlum or CXCR7-mPlum in 293T cells stably expressing the CXCR7/β-arrestin 2 complementation pair. Cells transfected with unfused mPlum were used as a control. All fluorescent proteins were expressed in comparable numbers of cells based on fluorescence microscopy (data not shown). After treatment for 2 hours with 300 ng/ml CXCL12, luciferase activity was approximately 35% (P < .05) and 50% (P < .01) lower in cells coexpressing nonbioluminescent CXCR4 or CXCR7, respectively, relative to control cells (Figure 5). Differences between nonbioluminescent CXCR4 and CXCR7 to reduce luciferase activity were also significant (P < .05). Luciferase activity from the CXCR7-NLuc/β-arrestin 2-CLuc PCA also increased in response to CXCL12 when constructs were expressed stably in human MCF-7 breast cancer cells, a cell line that endogenously expresses CXCR4 and CXCR7 (data not shown) [20,46]. Collectively, these data show that the CXCR7/β-arrestin 2 complementation pair functions in the context of nonbioluminescent chemokine receptors, although the magnitude of induction is less than in cells lacking CXCR4 and CXCR7. In addition, CXCR7 is more effective than CXCR4 as a competitor for the CXCR7-NLuc/β-arrestin 2-CLuc PCA.
Figure 5.
Induction of complementation signal in cells coexpressing nonbioluminescent CXCR4 or CXCR7. 293T cells stably expressing the CXCR7/β-arrestin 2 PCA were transfected with CXCR4-mPlum, CXCR7-mPlum, or unfused mPlum control. Cells were treated with 300 ng/ml CXCL12 for 2 hours before quantifying firefly luciferase activity for association of CXCR7 and β-arrestin 2. Data are presented as percent induction of bioluminescence relative to mPlum control set at 100% and are representative of three independent experiments (n = 4 per condition). *P < .05; **P < .01.
CXCR7 Preferentially Interacts with β-Arrestin 2 Relative to β-Arrestin 1
Epithelial cells express two different β-arrestin molecules, β-arrestin 1 and β-arrestin 2. Class A 7-TM receptors preferentially interact with β-arrestin 2, whereas class B receptors have similar affinity for forming complexes with β-arrestin 1 or β-arrestin 2 [45]. To investigate the association of CXCR7 with β-arrestin 1 or β-arrestin 2, we transiently transfected cells with CXCR7-NLuc and β-arrestin 1-CLuc or β-arrestin 2-CLuc, respectively, and treated cells with increasing concentrations of CXCL12 for 1 hour before measuring luciferase activity. For CXCR7-NLuc and β-arrestin 2-CLuc, CXCL12 produced concentration-dependent increases in bioluminescence (Figure 6). Luciferase activity could be detected above basal levels in untreated cells at 10 ng/ml CXCL12 (the lowest tested concentration; P < .05) and bioluminescence increased by ≈2.5-fold at 1 µg/ml CXCL12 (P < .01). Incubation with CXCL12 had minimal effects on the CXCR7-β-arrestin 1 PCA. Relative to untreated cells, luciferase activity increased by ≈1.4-fold at 10 ng/ml CXCL12 and did not rise further through 1 µg/ml CXCL12 (P < .05). These data establish that CXCR7 preferentially interacts with β-arrestin 2 in response to chemokine binding.
Figure 6.
Enhanced reporter signal from association of CXCR7 with β-arrestin-2. 293T cells were transiently transfected with CXCR7-NLuc and β-arrestin 1-CLuc or β-arrestin 2-CLuc, respectively. Cells were cotransfected with GL to normalize for transfection efficiency. Forty-eight hours after transfection, cells were treated with increasing concentrations of CXCL12. Firefly luciferase bioluminescence produced by living cells was quantified 1 hour after adding chemokine (n = 4 per condition), and these values were normalized to GL activity. Bioluminescence data for chemokine-stimulated recruitment of β-arrestin 1 or β-arrestin 2 to CXCR7 are expressed as mean values relative to bioluminescence in unstimulated cells. Error bars, SEM. Data are representative of three independent experiments. *P < .05; **P < .01.
Small Molecules Regulate Recruitment of β-Arrestin 2 to CXCR7
Recent studies have identified small-molecule compounds (CCX733 and CCX754) that block binding of CXCL11 and CXCL12 to CXCR7 with a half maximal inhibitory concentration of ≈5 nMin ligand binding assays performed in vitro at 4°C [47]. CCX754 also inhibits tumor growth in mouse xenograft models of cancer [20]. We initially tested effects of these compounds to block CXCL11- and CXCL12-dependent recruitment of β-arrestin 2 to CXCR7. Stable 293 Treporter cells were incubated with increasing concentrations of either compound for 30 minutes before treating with 100 ng/ml CXCL11 or CXCL12 for 2 hours. Unexpectedly, neither compound inhibited chemokine dependent increases in bioluminescence from recruitment of β-arrestin 2 to CXCR7 (Figure 7, A and B). Treatment with 10 nM CCX733 (P < .01) or CCX754 (P < .05) activated the CXCR7/β-arrestin 2 PCA with dose-dependent increases through 1 µM. Incubation with 1 µM CCX733 and CCX754 increased bioluminescence by approximately four- and six-fold above untreated cells, respectively, after 2.5 hours. These data establish that both small molecules function similarly to chemokine ligands to drive recruitment of β-arrestin 2 to CXCR7 under physiologic conditions.
Figure 7.
Regulation of β-arrestin 2 recruitment to CXCR7 by small molecules. (A and B) 293T cells stably transduced with the CXCR7/β-arrestin 2 PCA were pretreated with increasing concentrations of CXCR7-targeted small molecules CCX733 (A) or CCX754 (B) for 30 minutes. Cells were then maintained in the same concentration of CCX733 or CCX754 and treated with 100 ng/ml CXCL11 or CXCL12 or vehicle control for an additional 2 hours before quantifying bioluminescence from firefly luciferase. Luminescence data were normalized to a value of 1 for cells not treated with either small molecule or chemokine. Data are mean values ± SEM (n = 4 per condition, representative of three independent experiments). *P < .05; **P < .01.
Having determined that two small-molecule inhibitors of CXCL11 and CXCL12 binding to CXCR7 functioned as agonists, we investigated the effects of two different inhibitors of CXCR4, namely, AMD3100 and TF14013, on the recruitment of β-arrestin 2 to CXCR7. We incubated 293T/β-arrestin 2 reporter cells with various concentrations of AMD3100 or TF14013 for 30 minutes before adding 100 ng/ml CXCL12 or BSA control for an additional 2 hours. At concentrations as low as 100 nM, treatment with TF14013 increased bioluminescence above untreated cells (P < .05), and luciferase activity increased by approximately two-fold in response to 1 µM of this compound (P < .01; Figure 8). Although AMD3100 did not alter the PCA signal at 100 nM, there was a 1.5-fold increase in bioluminescence in cells treated with 1 µM of this agent (P < .05). Neither TF14013 nor AMD3100 significantly altered the effects of CXCL12 to drive the recruitment of β-arrestin 2 to CXCR7, supporting results with other assay systems showing that these compounds do not alter binding of CXCL12 to CXCR7 [20,48].
Figure 8.
Effects of CXCR4-targeted agents on association of CXCR7 with β-arrestin 2. Stably transduced CXCR7/β-arrestin 2 PCA reporter cells were treated for 30 minutes with CXCR4-targeted inhibitors AMD3100 or TF14013 before addition of 100 ng/ml CXCL12 or control for an additional 2 hours before quantifying luciferase activity. Data are mean values ± SEM for luminescence relative to reporter activity in cells not treated with either CXCR4-targeted agent or CXCL12 (n = 4 per condition, representative of 2 independent experiments). *P < .05; **P < .01.
Interaction of CXCR7 and β-Arrestin 2 Regulates Accumulation of CXCL12
A recent study demonstrated that cells expressing CXCR7 accumulate CXCL12 [24], leading us to investigate effects of β-arrestin 2 on this process. We transduced CXCR7-GFP into MEF cell lines derived from mice lacking β-arrestin 2 or matched wild-type animals. These stable β-arrestin 2-/- and wild-type MEF cell lines had similar levels of CXCR7 on cell membranes as determined by flow cytometry (Figure 9A). We incubated cells for 1 hour with either ≈10 ng/ml CXCL12-GL or a comparable amount of unfused GL based on bioluminescence. Incubations also included 100 nM of CXCR7-specific compounds CCX733 or CCX754 or matched vehicle control. After incubating for 1 hour, cells were then washed with PBS or with an acidic solution, the latter of which removes chemokines bound to the cell surface.
Figure 9.
β-arrestin 2 regulates CXCR7-dependent accumulation of CXCL12. (A) Expression of CXCR7 on the cell membrane of wild-type and β-arrestin 2-/- MEFs was determined by flow cytometry. Histogram plots are shown for MEFs stably transduced with CXCR7-GFP and endogenous CXCR7 in untransduced cells. Open symbol denotes isotype control antibody and filled symbol is staining with antibody to CXCR7. (B and C) CXCR7-GFP-wild-type and β-arrestin 2-/- MEFs were incubated with ≈10 ng/ml CXCL12-GL for 1 hour and then washed twice with either PBS (B) or an acidic solution to remove chemokine from the cell surface (C) before quantifying bioluminescence. Data are mean values ± SEM for photons from CXCL12-GL normalized to bioluminescence measured in wild-type-CXCR7-GFPMEFs treated with vehicle control (n = 4 per condition, representative of three independent experiments). (D) Untransduced wild-type and β-arrestin 2-/- MEFs were incubated with ≈10 ng/ml CXCL12-GL for 1 hour and then washed with acidic solution as described in C (n = 4 per condition, representative of two independent experiments). *P < .05; **P < .01.
Wild-type- and β-arrestin 2-/-CXCR7-GFP MEFs washed only with PBS had essentially the same levels of CXCL12-GL (Figure 9B), showing that the absence of β-arrestin 2 did not alter total amounts of chemokine bound to the cell surface. This result is consistent with previous research showing that binding of CXCL12 to cell membranes is mediated predominantly through proteoglycans expressed on the surface of cells [49]. By comparison, washing cells with an acidic solution to remove chemokine on the cell surface revealed that MEFs lacking β-arrestin 2 had significantly lower levels of internalized CXCL12-GL compared with wild-type cells (P < .01; Figure 9C). However, levels of intracellular CXCL12-GL remained readily detectable above background in β-arrestin 2-/-CXCR7-GFP MEFs, implying that CXCR7-dependent uptake of chemokines has both β-arrestin 2-dependent and -independent components. Both cell types had comparable levels of unfused GL that were minimally above background under conditions of PBS or acid wash, demonstrating that greater accumulation of intracellular CXCL12-GL in wild-type MEFs was not due to nonspecific increases in fluid-phase endocytosis (data not shown).
Incubation with specific CXCR7 inhibitors CCX733 or CCX754 reduced cell-associated CXCL12-GL in cells washed with PBS (P < .05), but the extent of inhibition did not differ between wild-type- and β-arrestin 2-/-CXCR7-GFP MEFs (Figure 9B). Pharmacologic inhibition of CXCR7 had a more pronounced effect to diminish intracellular uptake of CXCL12 in wild-type CXCR7-GFP MEFs under acid wash conditions (Figure 9C; P < .01), showing that these compounds are more effective at blocking CXCR-dependent internalization of CXCL12-GL relative to total binding of chemokine to cells. Inhibitors of CXCR7 also reduced intracellular CXCL12-GL in β-arrestin 2-/-CXCR7-GFP MEFs, albeit to a lesser extent than in cells with wild-type β-arrestin 2 (P < .05). This result in β-arrestin 2-/-CXCR7-GFP MEFs further suggests that CXCR7 also internalizes CXCL12-GL through a β-arrestin 2-independent pathway.
To establish that these effects were not dependent on the overexpression of CXCR7, we also performed experiments in untransduced β-arrestin 2-/- and wild-type MEFs. These cells express CXCR7 endogneously with slightly higher amounts of receptor at the surface of wild-type MEFs (Figure 9A). Similar to results with cells overexpressing CXCR7, wild-type MEFs accumulated significantly more intracellular CXCL12-GL than β-arrestin 2-/- cells, and this uptake was inhibited with CCX733 and CCX754. Collectively, these data show that β-arrestin 2 regulates the predominant pathway for CXCR7-dependent uptake of chemokines, emphasizing a key biologic function for the CXCR7-β- arrestin 2 protein interaction that is the basis of our imaging assay.
In Vivo Recruitment of β-Arrestin 2 to CXCR7
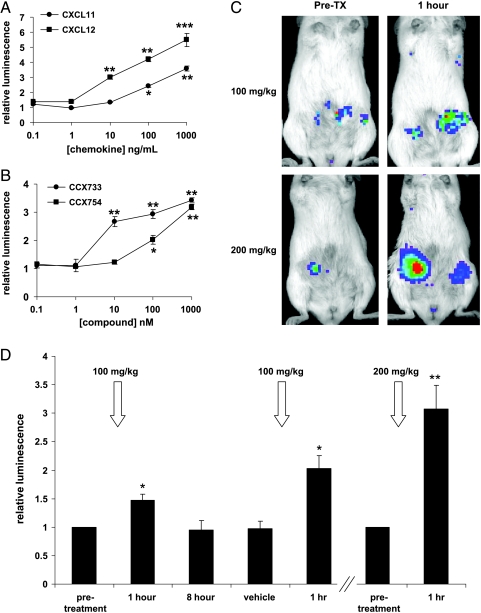
Recent studies show that CXCR7 is expressed by malignant cells in a variety of common cancers, including breast, prostate, and lung [17,18]. Having determined that small-molecule inhibitors of CXCR7-dependent tumor growth also cause association of the receptor with β-arrestin 2, we investigated to what extent this interaction could be detected in living animals. To accomplish this goal, we first established MDA-MB-231 human breast cancer cells stably transduced with the CXCR7/β-arrestin 2 PCA reporter. Similar to 293T cells, 231 reporter cells show dose-dependent increases in bioluminescence in response to CXCL11 or CXCL12. After 2 hours of incubation, CXCL11 and CXCL12 produced approximately three- and four-fold increases in bioluminescence above baseline levels, respectively (Figure 10A; P < .001). Treatment of these 231 cells with CCX733 and CCX754 also enhanced luciferase activity (P < .01; Figure 10B), confirming that these molecules drive interactions between CXCR7 and β-arrestin 2.
Figure 10.
In vivo recruitment of β-arrestin 2 to CXCR7 in breast tumor xenografts. (A and B) MDA-MB-231 human breast cancer cells stably transduced with the CXCR7-NLuc/β-arrestin 2-CLuc PCA were treated with increasing concentrations of chemokines CXCL11 or CXCL12 (A) or CXCR7-targeted small molecules CCX733 or CCX754 (B) for 2 hours. Reporter bioluminescence was normalized to luciferase activity in control cells (relative luminescence of 1), and these data are depicted as mean values ± SEM (n = 4 per condition, representative of two independent experiments). (C) Female SCIDmice were implanted with orthotopic tumor xenografts of MDA-MB-231 reporter cells. Bioluminescence imaging was performed under baseline conditions (Pre-TX) to measure association of CXCR7 with β-arrestin 2. The following day, mice were treated with CCX754 at the doses shown in the figure and then imaged 1 hour later. Paired images from pretreatment and 1 hour studies are shown for two different mice. Bioluminescence is presented as a pseudocolor scale, with red being the highest and blue being the lowest levels of emitted photons. The same pseudocolor scale was used for matched pairs of images. (D) Quantified bioluminescence imaging data formice under baseline conditions, 1 or 8 hours after treatment with CCX754, or vehicle control. Data are mean values ± SEM when error bars are visible (n = 8 tumors). *P < .05; **P < .01; ***P < .001.
We injected 231 reporter cells as orthotopic tumor xenografts into mammary fat pads of female SCID mice to reproduce the expression of CXCR7 in human breast cancer. We used bioluminescence imaging to detect and quantify basal interactions between CXCR7 and β-arrestin 2 in the tumor microenvironment. Images showed low levels of bioluminescence, consistent with the basal association of these proteins identified in cell culture studies. We then treated mice with 100 mg/kg of CCX754 injected subcutaneously, which produces serum levels that peak ≈1 hour later and subsequently decline to very low levels within 24 hours [20]. This route of administration has been used previously to block CXCR7-dependent tumor growth in mouse models. One hour after administering CCX754, in vivo imaging showed activation of the PCA for association of CXCR7 and β-arrestin 2 with luciferase activity ≈1.5-fold above baseline levels (Figure 10, C and D; P < .05). When these same mice were imaged 8 hours after administering this compound, reporter activity had returned to baseline levels. Subsequent treatment with vehicle control did not alter bioluminescence, whereas a second dose of 100 mg/kg of CCX754 again increased luciferase activity from the CXCR7/β-arrestin 2 PCA reporter. We also doubled the dose of CCX754 administered to mice to 200 mg/kg, resulting in a significantly greater increase in reporter activity (approximately three-fold above pretreatment levels) 1 hour after injecting the compound (P < .01). Collectively, these data establish a specific dose-dependent activation of CXCR7 and recruitment of β-arrestin 2 in a living animal model of human breast cancer.
Discussion
CXCR7 is the most recently identified chemokine receptor, binding with high affinity to both CXCL11 and CXCL12. Although chemokine receptor CXCR3 was known to bind chemokines CXCL9 and CXCL10 in addition to CXCL11, the discovery of CXCR7 as a second receptor for CXCL12 refuted previous conclusions that CXCL12 and CXCR4 comprised a unique ligand-receptor pair. As a result, there is intense interest in determining independent and integrated functions of CXCR7 versus CXCR4 in biologic effects of CXCL12 in cancer and other diseases. Several different cellular phenotypes have been attributed to CXCR7 either alone or in combination with CXCR4. However, many proposed functions of CXCR7 have not been consistent across different cell types and model systems. To understand general and cell type-specific functions of CXCR7 in normal physiology and cancer, it is essential to define molecular interactions of this receptor with potential downstream effector molecules in intact cells and living animals.
We used a firefly luciferase PCA to quantify association of CXCR7 with the cytosolic adapter protein β-arrestin 2, a molecule that is central to the biologic effects of many 7-TM receptors [50]. In the absence of chemokine ligand, the interaction between CXCR7 and β-arrestin 2 was significantly greater than that observed for control pairs of CXCR7 and c-fos or FKBP12, showing a specific association of CXCR7 and β-arrestin 2 under baseline conditions. Similar findings have been reported previously for ligand-independent interactions of CXCR4 and other 7-TM receptors with β-arrestin 2 [30,33,51]. Treatment with CXCL11 or CXCL12 significantly enhanced the interaction of CXCR7 with β-arrestin 2, and each chemokine produced comparable increases in bioluminescence from the PCA. Ligand-dependent association of CXCR7 and β-arrestin 2 was verified by fluorescence microscopy, which showed colocalization of both proteins in intracellular structures induced by treatment with CXCL12. In addition, we established that CXCR7 preferentially interacts with β-arrestin 2 compared with the related β-arrestin 1 adapter protein, as evidenced by the minimal change in bioluminescence produced by the CXCR7/β-arrestin 1 PCA pair in response to CXCL12. Taken together, these data establish that specific ligands drive recruitment of β-arrestin 2 to CXCR7.
7-TM receptors have been classified broadly into class A and class B families based on interactions with β-arrestin molecules [28,45]. In response to ligand, class A receptors have transient interactions with β-arrestin molecules, and these receptors recycle rapidly to the cell membrane through early endosomes. Class A receptors also have higher affinity binding to β-arrestin 2 versus β-arrestin 1. By comparison, class B 7-TM receptors remain associated with β-arrestin molecules for substantially longer periods because these protein complexes traffic to late endosomes and lysosomes. Class B 7-TM receptors also show comparable binding to both β-arrestin 1 and 2. Our data show that CXCR7 has properties of both class A and class B 7-TM receptors, differing notably from the class A kinetics of CXCR4 and β-arrestin 2 [33]. As described in previous paragraphs, CXCR7 has significantly greater association with β-arrestin 2, a characteristic of class A 7-TMreceptors. However, bioluminescence from the CXCR7-NLuc/β-arrestin 2-CLuc PCA continues to increase over time through at least 4 hours as would be expected for a class B receptor. These data suggest that stable complexes of CXCR7 and β-arrestin 2 persist over time, potentially on endosomes and other intracellular vesicular compartments. Increases in bioluminescence also indicate that new protein complexes continue to form during prolonged incubation with chemokine ligand, possibly because of the ongoing synthesis of new proteins and/or stabilization of existing proteins. Mixed patterns of class A and class B interactions with β-arrestin 2 have been reported previously for somatostatin receptors [52].
One recently identified biologic function of CXCR7 is sequestration of chemokine CXCL12, which is proposed to shape gradients of this chemokine for signaling through CXCR4 [24]. Using wild-type and β-arrestin 2-/- MEFs transduced with CXCR7, we established that β-arrestin 2 is necessary for the efficient uptake of chemokine ligands by CXCR7. We note that loss of β-arrestin 2 did not completely eliminate accumulation of CXCL12 in MEFs, indicating that CXCR7 may also use a β-arrestin 2-independent pathway for the uptake of chemokine ligands. We are currently investigating mechanisms CXCR7-dependent, β-arrestin 2-independent sequestration of chemokines. Nevertheless, these data show functional relevance for the protein interaction between CXCR7 and β-arrestin 2 and support the use of our imaging assay to monitor activation of CXCR7.
Previous studies have shown that CXCR7-targeted small molecules CCX733 and CCX754 inhibit binding of CXCL12 to CXCR7 at 4°C, a temperature at which endocytosis is blocked [20]. These molecules have also been reported to limit cell adhesion in vitro and growth of tumor xenografts in vivo [20,26]. Under physiologic conditions, we determined that both of these molecules potently drive association of CXCR7 with β-arrestin 2. In cell-based assays, we detected recruitment of β-arrestin 2 to CXCR7 in response to as little as 10 nM of compound, and effects of these compounds on the interaction of CXCR7 and β-arrestin 2 were comparable to those produced by chemokine ligands CXCL11 and CXCL12. In addition to CXCR7-targeted small molecules, we determined that agents directed against CXCR4 may also promote interactions between CXCR7 and β-arrestin 2. For AMD3100, this effect is modest and occurs only at a high concentration (1 µM) that is substantially greater than the half maximal effective concentration value (<100 nM) reported previously for this compound in CXCR4 bioassays [53]. Similar effects of high concentrations of AMD3100 to promote recruitment of β-arrestin 2 to CXCR7 have been described recently using bioluminescence resonance energy transfer to detect interactions between these two proteins [54]. In addition, we demonstrated a dose-dependent activation of the CXCR7/β-arrestin 2 PCA with the peptide analog TF14013 at concentrations as low as 100 nMin cell-based assays. These data imply that biologic effects of both CXCR4-targeted molecules potentially may be complicated by effects on CXCR7/β-arrestin 2 when the agents are used at saturating concentrations in cells that express both receptors.
One of the strengths of the firefly luciferase PCA is the ability to quantify the magnitude and dynamics of protein interactions in cell-based assays and then easily translate the system to mouse models. Using in vivo bioluminescence imaging, we demonstrated that one of the CXCR7-targeted compounds (CCX754) drives association of the receptor with β-arrestin 2 in cancer cells in an orthotopic mouse model of human breast cancer. Imaging data showed that the kinetics of association were consistent with previously reported data for pharmacokinetics of this compound in mice [20]. In addition, we established dose-dependent effects of this compound to activate the CXCR7/β-arrestin 2 PCA in breast tumors, providing an in vivo assay for pharmacodynamics of potential therapeutic agents targeted against CXCR7. Results from the cell-based and animal experiments imply that effects of these CXCR7-targeted agents on tumor growth are not dependent solely on blocking binding of CXCL11 or CXCL12 to the receptor. Potentially, these compounds drive association of CXCR7 and β-arrestin 2 without activating the same molecular pathways that are controlled by chemokine binding to CXCR7. Through such a mechanism, CXCR7 may internalize from the cell surface and no longer be available for binding CXCL12 and/or interacting with other intracellular molecules. Studies are ongoing to identify cell signaling pathways regulated by CXCR7. Defining these pathways and to what extent there is differential regulation by chemokines versus small molecules will advance efforts to target CXCR7 for cancer therapy.
CXCR7 seems to have critical functions in normal development, as evidenced by embryonic lethality of mice lacking this gene, and the receptor is also upregulated in disease processes including cancer and stroke [17,18,25,55]. These findings highlight important biologic functions of this newly discovered chemokine receptor and emphasize the need to identify molecular regulation of CXCR7. The current study establishes ligand-dependent interaction of CXCR7 with β-arrestin 2 with kinetics that are distinct from CXCR4, providing new insights into functional differences between these two receptors and mechanisms of action for CXCR7 as a signaling and/or decoy receptor. The firefly luciferase PCA provides a new quantitative assay for studies of CXCR7 and β-arrestin 2 in intact cells that would be compatible with high-throughput screening. In addition, we have demonstrated that the reporter can be used to image activation of CXCR7 in living animals, providing a noninvasive assay for studying CXCR7 in the tumor microenvironment and measuring pharmacodynamics of candidate therapeutic agents. Ongoing studies with this PCA will continue to define mechanisms of action for CXCR7 in tumor progression and metastasis and enable pharmacologic studies to develop CXCR7-targeted compounds as therapeutic agents in cancer and other diseases.
Acknowledgments
The authors thank AnnMarie Des Lauriers in the University of Michigan flow cytometry core for technical assistance.
Abbreviations
- CLuc
C-terminal fragment of firefly luciferase
- GRK
G protein receptor kinase
- GFP
green fluorescent protein
- GL
Gaussia luciferase
- MEFs
mouse embryonic fibroblasts
- NLuc
N-terminal fragment of firefly luciferase
- PCA
protein fragment complementation assay
- 7-TM
seven-transmembrane
Footnotes
This research was supported by National Institutes of Health grants P50CA093990, R01CA136553, R01CA136829, and R24CA083099 for the University of Michigan Small Animal Imaging Resource.
References
- 1.Mijatovic T, Mahieu T, Bruyere C, De Neve N, Dewelle J, Simon G, Dehoux M, van der Aar E, Haibe-Kains B, Bontempi G, et al. UNBS5162, a novel naphthalimide that decreases CXCL chemokine expression in experimental prostate cancers. Neoplasia. 2008;10:573–586. doi: 10.1593/neo.08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland J, Kochetkova M, Akekawatchai C, Dottore M, Lopez A, McColl S. Differential functional activation of chemokine receptor CXCR4 is mediated by G proteins in breast cancer cells. Cancer Res. 2006;66:4117–4124. doi: 10.1158/0008-5472.CAN-05-1631. [DOI] [PubMed] [Google Scholar]
- 3.Schabath H, Runz S, Journaa S, Altevogt P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci. 2006;119:314–325. doi: 10.1242/jcs.02741. [DOI] [PubMed] [Google Scholar]
- 4.Marlow R, Strickland P, Lee J, Wu X, Pebenito M, Binnewies M, Le E, Moran A, Macias H, Cardiff R, et al. SLITs suppress tumor growth in vivo by silencing Sdf1/CXCR4 within breast epithelium. Cancer Res. 2008;68:7819–7827. doi: 10.1158/0008-5472.CAN-08-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung B, Jhurani S, Ahn K, Mastuo Y, Yi T, Guha S, Liu M, Aggarwal B. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68:8938–8944. doi: 10.1158/0008-5472.CAN-08-2155. [DOI] [PubMed] [Google Scholar]
- 6.Engle T, Relja B, Marian D, Blumenberg C, Muller I, Beecken W, Jones J, Ringel E, Bereiter-Hahn J, Jonas D, et al. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through α5 and β3 integrins. Neoplasia. 2006;8:290–301. doi: 10.1593/neo.05694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kollmar O, Rupertus K, Scheuer C, Junker B, Tilton B, Schilling M, Menger M. Stromal cell-derived factor-1 promotes cell migration and tumor growth of colorectal metastasis. Neoplasia. 2007;9:862–870. doi: 10.1593/neo.07559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehtesham M, Yuan X, Kabos P, Chung N, Liu G, Akasaki Y, Black K, Yu S. Glioma tropic neural stem cells consist of astrocytic precursors and their migratory capacity is mediated by CXCR4. Neoplasia. 2004;6:287–293. doi: 10.1593/neo.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Yeger H, Das B, Irwin M, Baruchel S. Tissue microenvironment modulates CXCR4 expression and tumor metastasis in neuroblastoma. Neoplasia. 2007;9:36–46. doi: 10.1593/neo.06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 11.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 12.Zou Y, Kottman A, Kuroda M, Taniuchi I, Littman D. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 13.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanon M, McClanahan T, Murphy E, Yuan W, Wagner S, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 14.Smith M, Luker K, Garbow J, Prior J, Jackson E, Piwnica-Worms D, Luker G. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 15.Orimo A, Gupta P, Sgroi D, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey V, Richardson A, Weinberg R. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Ma X, Norsworthy K, Kundu N, Rodgers W, Gimotty P, Goloubeva O, Lipsky M, Li Y, Holt D, Fulton A. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther. 2009;8:490–498. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]
- 17.Miao Z, Luker K, Summers B, Berahovich R, Bhojani M, Rehemtulla A, Kleer C, Essner J, Nasevicius A, Luker G, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci USA. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta K, Mehra R, Loberg R, Taichman R. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 19.Balabanian K, Lagane B, Infantino S, Chow K, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 20.Burns J, Summers B, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold M, Sunshine M, Littman D, Kuo C, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J ExpMed. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentin G, Haas P, Gilmour D. The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr Biol. 2007;17:1026–1031. doi: 10.1016/j.cub.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Dambly-Chaudiere C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol. 2007;7:23. doi: 10.1186/1471-213X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infantino S, Moepps B, Thelen M. Expression and regulation of the orphan receptor RDC1 and its putative ligand in human dendritic and B cells. J Immunol. 2006;176:2197–2207. doi: 10.4049/jimmunol.176.4.2197. [DOI] [PubMed] [Google Scholar]
- 24.Boldajipour B, Mahabaleshwar S, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Rashohoff R, Li M, Woehl B, Leung H, Groom J, Batten M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmann T, Grabovsky V, Pasvolsky R, Shulman Z, Buss E, Spiegel A, Nagler A, Lapidot T, Thelen M, Alon R. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34+ cells. J Leukoc Biol. 2008;84:1130–1140. doi: 10.1189/jlb.0208088. [DOI] [PubMed] [Google Scholar]
- 27.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Bellerini L, Angelotti M, Parente E, Mancina R, Netti G, Becherucci F, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–790. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore C, Milano S, Benovic J. Regulation of receptor trafficking by GRKs and arrestins. Ann Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 29.Claing A, Laporte S, Caron M, Lefkowitz R. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and β-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 30.Chakera A, Seeber R, John A, Eidne K, Greaves D. The duffy antigen/receptor for chemokines exists in an oligomeric form in living cells and functionally antagonizes CCR5 signaling through hetero-oligomerization. Mol Pharmacol. 2008;73:1362–1370. doi: 10.1124/mol.107.040915. [DOI] [PubMed] [Google Scholar]
- 31.Comerford I, Milasta S, Morrow V, Milligan G, Nibbs R. The chemokine receptor CCX-CKR mediates effective scavenging of CCL19 in vitro. Eur J Immunol. 2006;36:1904–1916. doi: 10.1002/eji.200535716. [DOI] [PubMed] [Google Scholar]
- 32.Luker K, Smith M, Luker G, Gammon S, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci USA. 2004;101:12288–12293. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luker K, Gupta M, Luker G. Imaging CXCR4 signaling with firefly luciferase complementation. Anal Chem. 2008;80:5565–5573. doi: 10.1021/ac8005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luker K, Gupta M, Luker G. Imaging chemokine receptor dimerization with firefly luciferase complementation. FASEB J. 2008;23:823–834. doi: 10.1096/fj.08-116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luker K, Gupta M, Luker G. Bioluminescent CXCL12 fusion protein for cellular studies of CXCR4 and CXCR7. Biotechniques. 2009;47:625–632. doi: 10.2144/000113126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griesbeck O, Barid G, Campbell R, Zacharias D, Tsien R. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001;276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 37.Lois C, Hong E, Pease S, Brown E, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 38.Kelly K, Narhendorf M, Yu A, Reynolds F, Weissleder R. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Mol Imaging Biol. 2006;8:1536–1632. doi: 10.1007/s11307-006-0043-6. [DOI] [PubMed] [Google Scholar]
- 39.Tannous B, Kim D, Fernandez J, Weissleder R, Breakefield X. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11:435–443. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Sharma V, Crankshaw C, Piwnica-Worms D. Effects of multidrug resistance (MDR1) P-glycoprotein expression levels and coordination metal on the cytotoxic potency of multidentate (N4O2) (ethylenediamine)bis[propyl(R-benzylimino)] metal(III) cations. J Med Chem. 1996;39:3483–3490. doi: 10.1021/jm950823c. [DOI] [PubMed] [Google Scholar]
- 41.Luker G, Bardill J, Prior J, Pica C, Piwnica-Worms D, Leib D. Non-invasive bioluminescence imaging of herpes simplex virus type 1 infection and therapy in living mice. J Virol. 2002;76:12149–12161. doi: 10.1128/JVI.76.23.12149-12161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galliera E, Venkatakrishna R, Trent J, Bonecchi R, Signorelli P, Lefkowitz R, Mantovani A, Locati M, Haribabu B. β-Arrestin-dependent constitutive internalization of the human chemokine decoy receptor D6. J Biol Chem. 2004;279:25590–25597. doi: 10.1074/jbc.M400363200. [DOI] [PubMed] [Google Scholar]
- 43.Menard L, Ferguson S, Zhang J, Lin F, Lefkowitz R, Caron M, Barak L. Synergistic regulation of β2-adrenergic receptor sequestration: intracellular complement of β-adrenergic receptor kinase and β-arrestin determine kinetics of internalization. Mol Pharmacol. 1997;51:800–808. [PubMed] [Google Scholar]
- 44.Inglese J, Koch W, Caron M, Lefkowitz R. Isoprenylation in regulation of signal transduction by G-protein-coupled receptor kinases. Nature. 1992;359:147–150. doi: 10.1038/359147a0. [DOI] [PubMed] [Google Scholar]
- 45.Oakley R, Laporte S, Holt J, Caron M, Barak L. Differential affinities of visual arrestin, β arrestin1, and β arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 46.Hall J, Korach K. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17:792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- 47.Melikian A, Burns J, McMaster B, Schall T, Wright J. Inhibitors of human tumor-expressed CCX CKR2. 2004. Sep 2, Patent Cooperation Treaty Application WO04058705 and US patent publication US 20041070634.
- 48.Oishi S, Masuda R, Evans B, Ueda S, Goto Y, Ohno H, Hirasawa A, Tsujimoto G, Wang Z, Peiper S, et al. Synthesis and application of fluorescein- and biotin-labeled molecular probes for the chemokine receptor CXCR4. Chembiochem. 2008;9:1154–1158. doi: 10.1002/cbic.200700761. [DOI] [PubMed] [Google Scholar]
- 49.Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier J, Delepierre M, Baleux F, Lortat-Jacob H, et al. Stromal cell-derived factor-1α associates with heparan sulfates through the first β-strand of the chemokine. J Biol Chem. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 50.DeWire S, Ahn S, Lefkowitz R, Shenoy S. β-Arrestins and cell signaling. Ann Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 51.Cheng Z, Zhao J, Sun Y, Hu W, Wu Y, Cen B, Wu G, Pei G. β-Arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between β-arrestin and CXCR4. J Biol Chem. 2000;275:2479–2485. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- 52.Tulipano G, Stumm R, Pfeiffer M, Kreienkamp H, Hollt V, Schulz S. Differential β-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem. 2004;279:21374–21382. doi: 10.1074/jbc.M313522200. [DOI] [PubMed] [Google Scholar]
- 53.Percherancier Y, Berchiche Y, Slight I, Volkmer-Engert R, Tamamura H, Fujii N, Bouvier M, Heveker N. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280:9895–9903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- 54.Kalatskaya I, Berchiche Y, Gravel S, Limberg B, Rosenbaum J, Heveker N. AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol. 2009;75:1240–1247. doi: 10.1124/mol.108.053389. [DOI] [PubMed] [Google Scholar]
- 55.Schonemeier B, Schulz S, Hoellt V, Stumm R. Enhanced expression of the CXCL12/SDF-1 chemokine receptor CXCR7 after cerebral ischemia in the rat brain. J Neuroimmunol. 2008;198:39–45. doi: 10.1016/j.jneuroim.2008.04.010. [DOI] [PubMed] [Google Scholar]