Abstract
Tumor cells have evolved effective strategies to escape the host immune response. The objective of this study was to determine whether tumor cells can condition endothelial cells in a specific manner to prevent subsequent adhesion of polymorphonuclear neutrophils (PMNs) and/or peripheral blood lymphocytes (PBLs). Human umbilical vein endothelial cells (HUVECs) and UKF-NB-4 neuroblastoma tumor cells were established in coculture on opposite sides of porous transwell filters. After 24 hours with and without HUVEC conditioning, PMNs or PBLs were added to the HUVEC monolayer. Adhesion to conditioned HUVEC versus adhesion to nonconditioned HUVEC was compared. Effects on endothelial CD44v4, CD44v5, CD44v7, intercellular adhesion molecule 1 (ICAM-1), E-selectin, and vascular cell adhesion molecule 1 (VCAM-1) adhesion receptor expression were analyzed by flow cytometry, intracellular signaling proteins of the mitogen-activated protein kinase pathway and protein kinase C (PKC) subtypes quantified by Western blot analysis. Endothelial conditioning led to a distinct reduction in PMN but not in PBL adhesion to HUVEC. CD44 was significantly reduced, whereas ICAM-1, E-selectin, and VCAM-1 were not altered during HUVEC conditioning. Antibody blockade against CD44v4, CD44v5, and CD44v7 inhibited PMN but not PBL binding. The observed effects were caused by direct tumor cell-HUVEC contact because addition of isolated tumor cell membrane fragments but not of soluble cell culture supernatant to HUVEC induced the CD44 receptor loss. PKCα activity was strongly enhanced in conditioned HUVEC. Blocking PKC prevented the reduction in PMN binding, indicating that this protein is involved in PMN adhesion regulation. A novel tumor escape strategy is presented here. Cell contact-dependent adhesion of tumor cells to the vascular wall promotes down-regulation of endothelial CD44 receptor expression, impairing an effective neutrophil attack.
Introduction
Recent insights into the molecular and cellular mechanisms underlying cancer development have revealed that immune cells functionally regulate epithelial cancer development and progression. Although the immune response depends on phenotype and spatial distribution of infiltrating cells, leukocytes found in and around developing tumors are thought to be an attempt by the host to eradicate transformed neoplastic cells.
Lymphocyte populations are considered to be key cells in the immune system for tumor surveillance [1]. Polymorphonuclear neutrophils (PMN), the most abundant circulating blood leukocytes, have received little attention. Although it is still controversial how PMN may operate within the tumor tissue, recent studies have suggested that PMN can reject tumors by directly killing the tumor cells, by destroying tumor vessels and matrix, by inhibiting angiogenesis, or by inducing local inflammation [2–4].
On the basis of the observation that leukocyte infiltration into tumors may be associated with improved prognosis [5], immunodirected anti-tumor strategies, that is, adoptive or vaccination protocols, have been developed to treat cancer [6,7]. Unfortunately, immunotherapy has not been as effective as anticipated. The reasons for the disappointing results have not yet been fully elucidated, although several hypotheses exist.
Leukocytes must attach to and traverse the vascular endothelium to infiltrate from the bloodstream into the tumor tissue. Therefore, an attractive explanation might be that escape mechanisms have been evolved by the tumor cells to avoid leukocyte-endothelial cell interaction. Consequently, immune cells may become unable to migrate into tumor sites and may not be capable of destroying the tumor. Indeed, leukocyte delivery and adhesion were found to be reduced in tumor microvessels [8,9], and an in vivo microcirculation model revealed significantly diminished leukocyte-endothelium interaction in the tumor tissue compared with the healthy tissue [10].
It is not fully understood why immune cells are unable to traverse the vascular wall and eradicate the tumor. We speculated that tumor-endothelial cell cross talk leads to distinct alterations of the local vasculature, which prevents subsequent leukocyte transmigration. To address this issue, a three-culture assay was established in which human vascular endothelial cells were conditioned by tumor cells, and the adhesion capacity of isolated lymphocytes or PMN to endothelium was then assessed.
The results show, for the first time, that mechanical contact between tumor cells and endothelium induces distinct down-regulation of CD44 adhesion receptors (particularly CD44v4 and CD44v5 splice variants) on endothelial cells, leading to subsequent blockade of CD44-triggered neutrophil attachment. Lymphocytes attached to endothelial cells in a CD44-independent manner. This novel finding supplies an intriguing explanation as to how tumors may escape from PMN attack and PMN-dependent eradication.
Materials and Methods
Cell Cultures
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical veins and harvested by enzymatic treatment with chymotrypsin. HUVECs were grown in Medium 199 (M199; Biozol, Munich, Germany), supplemented with 10% fetal calf serum, 10% pooled human serum, 20 µg/ml endothelial cell growth factor (Boehringer, Mannheim, Germany), 0.1% heparin, 100 ng/ml gentamicin, and 20mM HEPES buffer (pH 7.4). Subcultures from passages 2 to 4 were selected for experimental use. For stimulation experiments, HUVECs were activated with 500 U/ml tumor necrosis factor α (TNFα; R&D Systems, Wiesbaden, Germany; 6 hours of incubation).
The neuroblastoma (NB) cell lines UKF-NB-3 and UKF-NB-4 were established from bone marrow metastasis of Evans stage IV NB [11]. UKF-NB-3 and UKF-NB-4 cells were grown and subcultured in Iscove's modified Dulbecco's medium (Seromed, Berlin, Germany) supplemented with 10% fetal calf serum, 100 IU/ml penicillin, and 100 µg/ml streptomycin at 37°C in a humidified 5% CO2 incubator. PMNs were isolated from the venous blood of healthy adult volunteers by centrifugation on granulocyte separation medium (Polymorphprep, Nycodenz; Axis-Shield, Oslo, Norway) and immediately used for experiments. The purity of the neutrophils was greater than 95%, and the viability was greater than 99% as determined by Trypan blue exclusion. Peripheral blood lymphocytes (PBLs) were isolated by Ficoll-Hypaque (Biochrome, Berlin, Germany) centrifugation and were resuspended at a density of 1 x 106 cells per milliliter of HUVEC medium.
PMN and PBL Coculture Adhesion Assay
Six-well plates (Sarstedt, Nürnbrecht, Germany) were equipped with porous cell culture membrane inserts with pore diameters of 3 µm, a pore density of 3 x 106/cm2, and an effective culture area of 4.2 cm2 (Nunc, Wiesbaden, Germany). The inserts were turned upside down, and 1 x 106 UKF-NB-4 or UKF-NB-3 cells per milliliter were poured onto the lower surface of the membrane units and allowed to settle for 12 hours at 37°C in a humidified 5% CO2 incubator. Inserts were then turned right side up, transferred to new six-well plates that were filled with a 1:1 mixture of NB/HUVEC medium, and 1 x 106 HUVECs per milliliter were added to the upper surface of the membrane units. HUVECs were conditioned by coculture for 24 hours before the PMN or PBL adhesion assay. In control studies, HUVECs were incubated on empty inserts. Either 0.5 x 106 PMNs or PBLs per milliliter were then added to HUVECs for 60 minutes. Subsequently, nonadherent cells were washed off using phosphate-buffered saline (Gibco, Karlsruhe, Germany). The remaining cells were detached from the upper surface of the membrane inserts by Accutase treatment (PAA Laboratories, Cölbe, Germany), stained with leukocyte-specific anti-CD45 PerCP monoclonal antibody (clone 2D1; BD Pharmingen, Heidelberg, Germany), and subjected to flow cytometry (FACscan; Becton Dickinson, Heidelberg, Germany). Sideward scatter (SSC) gating was set up to display percentage of PMNs or PBLs bound to HUVECs.
The purity of the HUVEC monolayer and contamination with migrated UKF-NB-3 or UKF-NB-4 were controlled in parallel experiments. Cells grown on the upper surface of the membrane inserts were detached by Accutase and stained with NB-specific phycoerythrin (PE)-conjugated monoclonal antibody anti-CD56, which detects the neural cell adhesion molecule (NCAM) 120-, 140-, and 180-kDa isoform (clone 16.2; immunoglobulin G2b [IgG2b]; BD Biosciences, Heidelberg, Germany). NCAM(CD56) expression was then measured using a FACscan (FL-2H [log] channel histogram analysis; 1 x 104 cells per scan). To evaluate background staining of PE-conjugated anti-CD56, goat-antimouse IgG2b-PE was used. Dot plot quadrant analyses were carried out to display percentage distribution of CD56+ (UKF-NB-3/UKF-NB-4) and CD56- (HUVEC) cells. UKF-NB-3 or UKF-NB-4 monocultures served as the positive controls.
Endothelial Adhesion Receptor Expression
Monoclonal antibodies were used, directed against CD44 splice variants (v) CD44v4 (clone VFF-11), CD44v5 (clone VFF-8), and CD44v7 (clone VFF-9; all from Bender MedSystems, Eching, Germany) or directed against CD54 (syn. intercellular adhesion molecule 1 [ICAM-1], clone 15.2), CD62E (syn. E-selectin, clone TEA2/1), and CD106 (syn. vascular cell adhesion molecule 1 [VCAM-1], clone 1G11.B1; all from Dianova, Hamburg, Germany). The primary antibodies were conjugated with fluorescein isothiocyanate (FITC). Two different strategies were applied to analyze adhesion receptor expression on HUVEC in a HUVEC-NB coculture system:
UKF-NB-4 cells (1 x 106 cells per ml; total volume, 5 ml) were added to subconfluent HUVEC monolayers grown in a 75-cm2 culture flask. After 16 hours of incubation, cells were detached by Accutase treatment, and receptor expression was analyzed by flow cytometry. To identify the endothelial cell population, the cell mixture was double stained using a monoclonal antibody directed against UKF-NB-4 (anti-CD56) and monoclonal antibodies directed against the receptor in question. Cells were washed twice in blocking solution (phosphate-buffered saline, 0.5% bovine serum albumin) and subsequently incubated for 60 minutes at 4°C with the monoclonal antibody anti-CD56-PE. Cells were then marked with FITC-conjugated monoclonal antibodies anti-CD44v4, anti-CD44v5, anti-CD44v7, anti-CD54 (ICAM-1), anti-CD62E (E-selectin), or anti CD106 (VCAM-1). To explore ICAM-1, VCAM-1, and E-selectin, HUVECs were prestimulated for 6 hours with 500 U/ml TNFα. Dot plot quadrant analyses (SSC gating) was carried out to obtain two distinct cell populations, population I (CD56+) as UKF-NB-4 cells and population II (CD56-) as HUVEC. Adhesion receptor expression of HUVEC was then detected by FACscan analysis (FL-1 [log] channel histogram analysis; 1 x 104 cells per scan). To evaluate the background staining of FITC-labeled antibodies, a mouse IgG1-FITC was used as an isotype control.
A membrane-separated coculture of UKF-NB-4 and HUVEC cells was established, in which UKF-NB-4 cells adhered to the lower surface of the membrane inserts and HUVECs were incubated on the upper surface of the membrane inserts (see previous paragraphs). HUVECs were detached from the upper surface of the membrane inserts by Accutase treatment, stained with monoclonal antibodies as indicated previously, and subjected to flow cytometry (FL-1 [log] channel histogram analysis).
Isolation of NB Plasma Membranes
UKF-NB-4 were pooled and washed in cold STM buffer (0.25 M sucrose, 5 mM Tris, pH 8.0, 0.5 mM MgCl2), minced, and homogenized using a Dounce homogenizer. Nuclei and cell debris were removed by centrifugation at 280g for 5 minutes. The supernatant was saved, and the pellet was resuspended in 0.25 M STM using a Dounce homogenizer. The suspension was again centrifuged as previously mentioned. First and second supernatants were combined and centrifuged at 1500g for 10 minutes. The resulting pellets, containing the plasma membrane fraction, were resuspended in 0.25 M STM and adjusted to 1.18 g/cm3 sucrose density using 2M sucrose in 5 mM Tris, pH 8.0, and 0.5 mM MgCl2. Sucrose density was determined at room temperature with an Abbe refractometer. Samples were transferred to ultracentrifuge tubes and overlaid with 0.25 M sucrose. After centrifugation for 60 minutes at 82,000g in a Beckman L5-65 centrifuge (SW 28 rotor), the pellicle at the interface was collected and resuspended in sufficient 0.25 M sucrose to obtain a density of 1.05 g/cm3. Protein concentration was determined by the Lowry method, using bovine serum albumin as a standard. One hundred micrograms of protein per milliliter was added to subconfluent HUVECs for 16 hours, and endothelial adhesion receptor expression was evaluated by flow cytometry as indicated previously.
CD44 Blocking Experiments
HUVECs were transferred to six-well plates and grown to subconfluency. They were then preincubated for 60 minutes with anti-CD44v4, anti-CD44v5, or anti-CD44v7 monoclonal antibodies at a concentration of 50 µg/ml each followed by repeated washing with fresh HUVEC medium. Controls remained untreated. Isolated PMNs or PBLs (1.5 x 105 cells per milliliter) were added to the HUVEC monolayer for 60 minutes. Subsequently, nonadherent tumor cells were washed off using warmed (37°C) culture medium. The remaining cells were fixed with 1% glutaraldehyde. In each experimental setting, adherent tumor cells were counted in five different fields of a defined size (5 x 0.25 mm2) using a phase-contrast microscope, and the mean cellular adhesion rate was calculated.
Analysis of Intracellular Signaling by Western Blot Analysis
Monoclonal antibodies were used and directed against proteins indicated subsequently: extracellular signal-regulated kinase 1 (ERK1, IgG1, clone MK12; 1:5000), ERK2 (IgG2b, clone 33; 1:5000), and phospho-ERK1/2 (IgG1, clone 20A; 1:1000) were obtained from BD Biosciences. PKCα (IgG2b, clone 3; 1:1000), PKCβ (IgG2b, clone 36; 1:250), PKCδ (IgG2b, clone 14; 1:500), PKCε (IgG2a, clone 21; 1:1000), and PKCι (IgG2b, clone 23; 1:250) were from BD Biosciences. Phospho-PKCα (rabbit, polyclonal; 1:1000) was from Millipore, Schwalbach, Germany. Phospho-PKCε (rabbit, polyclonal; 1:1000) and phospho-PKCι (rabbit, polyclonal; 1:1000) were from Abcam (Cambridge, United Kingdom). Anti-β-actin monoclonal antibody was obtained from Sigma (Taufenkirchen, Germany).
UKF-NB-4 cells (1 x 106 cells per milliliter; total volume, 5 ml) were added to subconfluent HUVEC monolayer grown in a 75-cm2 culture flask. After 16 hours of incubation, cells were detached by Accutase treatment. To isolate HUVECs, the cell suspension was incubated with magnetic beads (Dynabeads M450 IgG1; Dynal Biotech GmbH, Hamburg, Germany) conjugated to an anti-CD56 monoclonal antibody (negative selection). The selected population obtained by this procedure was routinely greater than 95% by flow cytometry.
HUVEC cell lysates were applied to a 7% polyacrylamide gel and electrophoresed for 90 minutes at 100 V. The protein was then transferred to nitrocellulose membranes. After blocking with nonfat dry milk for 1 hour, the membranes were incubated overnight with the antibodies listed previously. HRP-conjugated goat-antimouse IgG (Upstate Biotechnology, Lake Placid, NY; dilution 1:5000) served as the secondary antibody. The membranes were briefly incubated with enhanced chemiluminescence detection reagent (Amersham/GE Healthcare, München, Germany) to visualize the proteins and exposed to an x-ray film (Hyperfilm EC; Amersham).
Statistics
All experiments were performed three to six times. Statistical significance was investigated by the Wilcoxon-Mann-Whitney U test. Differences were considered statistically significant at P < .05.
Results
Preconditioning of HUVECs and Selection of Membrane Pore Size
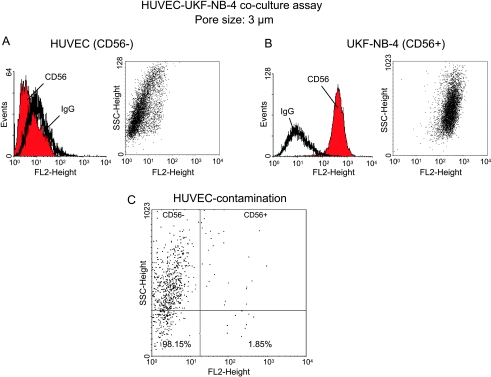
Tumor cell invasion through endothelium requires direct cell-to-cell contact. To allow direct contact and optimum conditioning of HUVECs by the tumor cells, a membrane-separated coculture was established, in which NB tumor cells adhered to the lower surface and HUVEC to the upper surface of membrane inserts. The objective was to promote a high degree of interaction between the two cell types but to leave the HUVEC monolayer intact (necessary for the subsequent PBL or PMN binding experiments). Therefore, a membrane pore size was chosen to guarantee the extension of tumor cell protrusions through the pores while restricting the tumor cell bodies to the seeded side. Three different pore-sized membranes were applied in initial studies, namely, 0.22, 3, or 8 µm. A 24-hour contamination rate of HUVEC by traversed NB cells was established by staining the cell population on the upper surface of the membrane inserts with an anti-CD56 monoclonal antibody. CD56 had previously been demonstrated to be exclusively expressed on NB cells but not on HUVEC (Figure 1, A and B, representative of UKF-NB-4). Anti-CD56 staining was therefore well qualified to discriminate between NB and endothelial cells and to quantify the percentage of NB that had been growing into the HUVEC monolayer. UKF-NB-4 cells easily traversed the 8-µm pores leading to a high contamination of the HUVEC monolayer (mean contamination rate, 24 ± 8%). No UKF-NB-3 or UKF-NB-4 cells passed through the 0.22-µm pores, but these pores were too small to allow sufficient tumor cell-endothelial cell contact. Optimum results were obtained with the 3-µm membrane inserts and a tumor cell contamination below 2% (Figure 1C, representative of UKF-NB-4). Therefore, adhesion of PBL or PMN measured after the 24-hour preconditioning phase could be related to HUVECs, and irregular interaction of leukocytes with NB cells could be excluded. Consequently, in all further experiments, the 3-µm membrane inserts were used.
Figure 1.
Fluorometric analysis of CD56 surface expression on HUVECs (A) versus UKF-NB-4 cells (B). Both cell types were stained separately with PE-conjugated monoclonal antibody anti-CD56, which detects the NCAM 120-, 140-, and 180-kDa isoforms. CD56 expression is depicted as dot blot (sideward scatter vs CD56) and FL-2H (log) channel histogram analysis. (C) A 24-hour contamination rate of CD56- HUVEC by traversed CD56+ UKF-NB-4 cells in the transwell coculture assay. Cell populations growing on the upper surface of 8-µm pore-sized membranes were stained with an anti-CD56 monoclonal antibody and dot blot analysis carried out by a FACscan. One representative of three tests is shown.
Tumor Cell-Endothelial Cell Interaction Alters PMN But Not PBL Adhesion
HUVECs were preconditioned for 24 hours with UKF-NB-3 or UKF-NB-4 on 3-µm membrane inserts, and lymphocytes or PMNs were then added to the HUVEC monolayer. PBLs or PMNs were subsequently gated by flow cytometry (population R2; Figure 2, A and B), and the percentage adhesion was calculated. Cross talk of HUVECs with UKF-NB-3 or UKF-NB-4 resulted in a selective and significant blockade of PMNs but not of PBLs binding to HUVECs compared with HUVEC controls that were cultivated on empty inserts (Figure 2C, representative of UKF-NB-4). Prestimulation of HUVECs with TNFα led to a further reduction of PMN binding. Separate cultivation of HUVECs and NBs, that is, HUVECs grown on the membrane insert and UKF-NB-3 or UKF-NB-4 on the bottom of the six-well plate, did not influence PBL or PMN binding behavior (data not shown).
Figure 2.
PMNs' versus PBLs' adhesion to HUVECs. Isolated PMNs or PBLs were added to preconditioned HUVECs as described in the Materials and Methods section. Anti-CD45 PerCP monoclonal antibody was then used to distinguish between HUVECs (R1) and PMNs (R2; A) or PBLs (R2; B) by flow cytometry (SSC gating) and to display the percentage of PMNs or PBLs, which bound to HUVECs (%R2). Controls, indicating PBL/PMN binding to unstimulated or TNFα-stimulated HUVECs, were each set to 100%. “HUVEC/NB” indicates percent PBL/PMN binding to preconditioned HUVEC; “HUVECact/NB” indicates percent PBL/PMN binding to preconditioned, TNFα-activated HUVECs (C). One representative of n = 6 experiments. *Significantly different from controls. #Significantly different from HUVEC-NB.
Analysis of Adhesion Receptor Expression
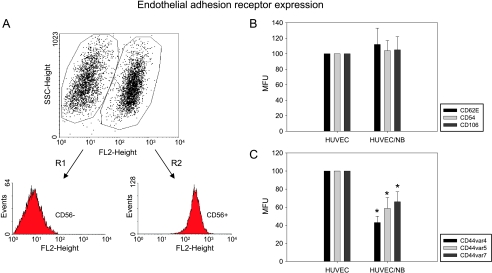
Endothelial adhesion receptors known to be involved in leukocyte recruitment were analyzed next. UKF-NB-4 cells were added to HUVEC for 16 hours, and HUVEC populations were separated thereafter by flow cytometry (Figure 3A). Expression levels of CD62E (E-selectin), CD54 (ICAM-1), and CD106 (VCAM-1) were not altered in TNFα-stimulated HUVECs cultivated in the presence of UKF-NB-4 cells (Figure 3B). However, CD44 splice variants CD44v4, CD44v5, and CD44v7 were all significantly downregulated on HUVECs in the coculture system compared with HUVECs that were seeded without UKF-NB-4 (Figure 3C). Effects on endothelial CD44v4, CD44v5, and CD44v7 adhesion receptor expression were similar between HUVECs pretreated and not pretreated with TNFα.
Figure 3.
Analysis of endothelial adhesion receptor expression. HUVECs were cocultivated with UKF-NB-4. CD56+ populations were gated out by flow cytometry (R1 vs R2) as described in the Materials and Methods section (A). E-selectin (CD62E), ICAM-1 (CD54), and P-selectin (CD106; B), and CD44v4, v5, and v7 (C) of CD56-negative HUVECs were then evaluated by FACS analysis (MFU indicates mean fluorescence units). “HUVEC” depicts isolated, nonconditioned endothelial cells (controls), MFU of which were set to 100%. “HUVEC/NB” indicates endothelial cells preconditioned by UKF-NB-4 tumor cells. One representative of n = 6 experiments. *Significantly different from control HUVECs.
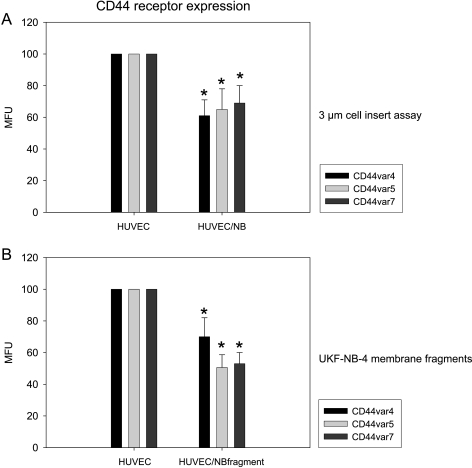
CD44 expression was further evaluated in the porous membrane coculture assay. HUVEC-UKF-NB-4 interaction led to distinct reductions of CD44v4, CD44v5, and CD44v7 levels (Figure 4A). To address the question of whether receptor loss was caused by direct contact of endothelial cells with tumor cells or indirectly by soluble mediators released into the cell culture supernatant, HUVECs were treated with isolated UKF-NB-4 membrane fragments, and CD44 expression was then analyzed. On the basis of this technique, CD44v4, CD44v5, and CD44v7 were all found to be diminished on HUVECs compared with untreated controls (Figure 4B). Separate cultivation of HUVECs (grown on the membrane insert) and UKF-NB-4 (grown on the bottom of the six-well plate) or stimulation of HUVECs with cell culture supernatant taken from a HUVEC-UKF-NB-4 coculture system did not change CD44 expression (data not shown).
Figure 4.
Evaluation of CD44 expression level on HUVECs. HUVECs and UKF-NB-4 cells were grown on opposite sides of 3-µm cell inserts and endothelial CD44 expression analyzed thereafter (A), or HUVECs were subjected to isolated UKF-NB-4 membrane fragments and CD44 expression was evaluated subsequently (B). CD44 analysis was carried out by a FACscan using the appropriate monoclonal antibodies as listed in the Materials and Methods section (MFU indicates mean fluorescence units). Nonconditioned HUVECs served as controls; MFU values were set to 100%. One representative of six experiments is shown. *Significant difference from control HUVECs.
CD44 Receptor Blockade Reduces PMN Binding
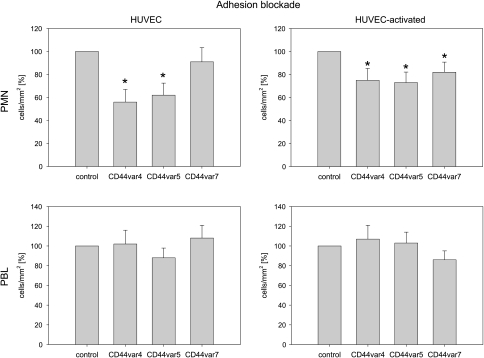
To evaluate the physiological relevance of CD44 adhesion receptors, HUVECs were incubated with monoclonal antibodies directed against CD44v4, CD44v5, or CD44v7, and leukocyte adhesion capacity was then measured. Blocking of CD44v4 and CD44v5, but not of CD44v7, strongly reduced the amount of PMN adhering to HUVEC (Figure 5, left). When HUVECs were preactivated with TNFα, blocking of each CD44 receptor subtype, namely, CD44v4, CD44v5, and CD44v7, resulted in a significant reduction in PMN's adhesion to HUVECs (Figure 5, right). Binding of PBLs to HUVECs was not influenced by CD44 blocking (Figure 5, down).
Figure 5.
CD44 blockade prevents PMNs' adhesion to HUVECs. PMNs or PBLs were added to HUVEC monolayers (nonactivated vs TNFα activated) and then added at a density of 1.5 x 105 cells per milliliter for 60 minutes. HUVECs have been preincubated for 60 minutes with anti-CD44v4, anti-CD44v5, or anti-CD44v7 monoclonal antibodies. Controls remained untreated. Nonadherent PMNs or PBLs were washed off in each sample; the remaining cells were fixed and counted in five different fields (5 x 0.25 mm2) using a phase-contrast microscope. Mean values were calculated from five counts. Mean adhesion capacity is depicted as counted cells per squared millimeter and related to control values that were set at 100%. One representative of six experiments is shown. *Significant difference from controls.
HUVEC Tumor Cell Cross Talk Modifies Endothelial Cell Signaling
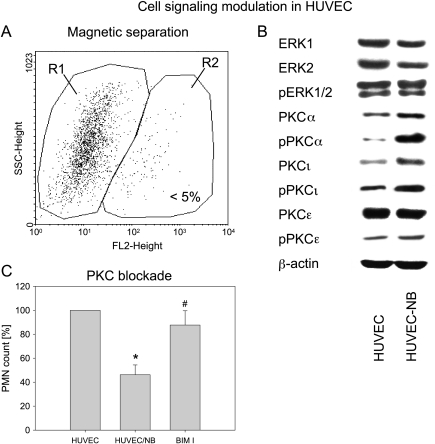
The intracellular signaling cascade in HUVEC after exposure to UKF-NB-4 cells was examined by purifying HUVEC from the coculture system by magnetic bead separation (Figure 6A) and subjecting them to Western blot analysis (Figure 6B). Conditioning of HUVECs by UKF-NB-4 cells caused a moderate reduction of ERK1 and ERK2 proteins. No changes were seen in ERK1/2 activation. PKCβ and PKCδ were not detected in HUVECs. PKCα and PKCι were slightly elevated in HUVECs exposed to UKF-NB-4, whereas PKCε remained unchanged. Notably, phosphorylated PKCα was drastically enhanced in HUVECs when cocultured with UKF-NB-4 cells. Phosphorylated PKCι was upregulated moderately compared with the controls (Figure 6B).
Figure 6.
Cell signaling in conditioned versus nonconditioned HUVECs. UKF-NB-4 cells were added to subconfluent HUVEC monolayer for 16 hours. Cells were then detached from the culture flask by Accutase treatment and HUVECs were isolated by magnetic separation (negative selection). The selected population (R1) obtained by this procedure was greater than 95% as controlled by flow cytometry (A). ERK and PKC signaling was explored in control HUVECs and HUVECs preconditioned with UKF-NB-4 cells (HUVEC-NB) by Western blot analysis (B). β-Actin served as the internal control. One representative of three experiments is shown. (C) Relevance of PKC blocking for PMNs' adhesion. A total of 0.5 x 106 PMNs per milliliter were added to preconditioned HUVECs (HUVEC-NB), to preconditioned HUVECs incubated with the PKC blocking antibody BIM I, or to control HUVECs for 60 minutes as described in the Materials and Methods section. Bound PMN were identified by flow cytometry using the leukocyte-specific anti-CD45 monoclonal antibody. SSC gating was set up to display percentage of PMNs that bound to HUVECs (controls = 100%). *Significant difference from controls. #Significant difference from HUVEC-NB.
Incubation of conditioned HUVECs with the PKC inhibitor bisin-dolylmaleimide I (BIM I, 2.5 µM) prevented the loss of PMN adhesion, which was induced by conditioned HUVECs not treated with BIM I (Figure 6C).
Discussion
Down-regulation of endothelial CAM and selectin adhesion molecules by angiogenic factors has recently been shown to be a potent escape mechanism used by disseminating tumor cells to protect themselves against T-lymphocyte attack [9]. A novel mechanism of endothelial cell anergy is presented here. On the basis of a three-cell culture assay, evidence is shown that tumor cell-endothelial cell communication triggers down-regulation of CD44 molecules on endothelial cells, resulting in diminished PMN-HUVEC interaction. CD44 down-regulation was caused by direct intercellular cross talk and not by soluble mediators because sensitization of endothelium by isolated tumor cell membrane fragments but not by soluble cell culture supernatant was responsible for the CD44 receptor loss. Therefore, the attenuation of vascular CD 44 expression may prevent immune surveillance of transmigrated tumor cells by PMN.
PMNs' impact on tumor destruction has not been completely elucidated, making final interpretation of the present results difficult. Nevertheless, several NB cell models document the importance of PMN attraction in tumor cell lysis and growth inhibition [12]. Accumulation of PMN has resulted in complete growth arrest of large, established tumors in NB-bearing animals [13], and Takamizawa et al. has reported targeting and destruction of NB clones by PMN in vivo, even in immune compromised hosts [14]. Antitumor characteristics of PMN have also been demonstrated in tumors other than NB. Neutrophil-depleted mice developed significantly larger tumors after subcutaneous injection of melanoma cells compared with control animals with intact PMN. Reactivation of neutrophils significantly delayed melanoma formation [15]. Also, PMNs have been shown to play a critical role in eradicating malignant mesothelioma or lung carcinoma cells in vivo, whereas complete abrogation of antitumor immunity has been seen in the absence of PMNs [16]. The mechanism of endothelial cell anergy presented in this report may therefore not be restricted to NB cells but rather may be valid for further tumor entities.
CD44 receptors are detectable on both PMNs and endothelial or epithelial cells. However, it has been demonstrated that CD44 receptors on PMNs do not play a fundamental role in facilitating PMN recruitment [17,18]. An elegant study on chimeric mice expressing CD44 either on their PMNs or on their endothelium presented evidence showing endothelial CD44 to be crucial for optimal PMN invasion [19]. In close analogy, rapid CD44 up-regulation on capillary endothelial cells rather than neutrophil CD44 expression mediated transendothelial neutrophil recruitment into the tissue. Endothelial CD44 deficiency led to decreased influx of neutrophils [20]. Accordingly, the blocking studies presented here point to the relevance of endothelial CD44 expression level in PMN binding control.
The CD44 splice variants v4, v5, and v7 were all downregulated in HUVECs conditioned with NB cells. However, the strongest alterations were seen in CD44v4 and CD44v5 expressions. This phenomenon may refer to a different significance of CD44 splice variants in initiating endothelial cell anergy. In fact, PMNs attached to unstimulated HUVECs through CD44v4 and CD44v5 but not through the CD44v7 receptor. CD44v7-dependent neutrophil adhesion was restricted to TNFα-stimulated HUVECs. Presumably, both CD44v4 and CD44v5 receptors are the dominant modifiers of transendothelial PMNs trafficking toward invaded tumor cells. CD44v7 may be considered an additional compensating mechanism to counteract TNFα-induced (and other proinflammatory cytokines?) leukocyte and endothelial cell activation. On the basis of this, it seems logical that PMN binding to TNFα-activated HUVECs was reduced to a higher extent than PMN binding to nonactivated HUVECs.
Binding of PBLs to HUVECs was not inhibited in our cell culture system, although lymphocytes have been described to be strongly involved in the escape of tumors from immune surveillance. Indeed, several in vitro and in vivo assays have revealed that tumors are able to block lymphocyte-vessel wall interactions as well. Nevertheless, PBL attachment seems to be regulated by mechanisms different from those regulating PMN adhesion. Dirkx et al. [9] and others [21–23] have discovered that lymphocyte invasion is hampered by diminishing the level of adhesion molecules ICAM-1, VCAM-1, and E-selectin expressed on endothelial cells. Receptor reduction is governed by exposing the endothelium to soluble angiogenic factors such as vascular endothelial cell growth factor or basic fibroblast growth factor.
On the basis of these data, suppression of ICAM-1,VCAM-1, and/or E-selectin by soluble mediators might be responsible for preventing lymphocyte extravasation toward engrafted tumor cells. We did not analyze the effect of soluble angiogenic factors on PMN recruitment. However, mechanical contact of NB cells with HUVECs was obligatory to modify CD44 expression and PMN adhesion capacity. In addition, neither cell culture supernatant taken from preconditioned HUVEC nor direct tumor cell-HUVEC interaction evoked significant alterations of ICAM-1, VCAM-1, and E-selectin, which had been attributed to control PBL trafficking. Taking into account that anti-CD44 monoclonal antibodies prevented PMNs' but not PBLs' attachment to HUVEC, it is possible that the binding capacity of PBL has not been modified in our in vitro model. Obviously, different tumor escape mechanisms exist, which affect the biologic interrelationship between vascular endothelium and PBL or PMN in two separate ways. Our experiments point to a direct (i.e., membrane triggered) CD44-dependent induction of PMN adhesion blockade, which opposes the indirect (i.e., not membrane triggered) CD44-independent modulation of PBL transmigration events.
HUVECs' contact with NB cells caused enhancement of phosphorylated PKCα and moderate up-regulation of activated PKCι in the endothelial cells. No data dealing with this issue are available from the literature. It is therefore difficult to make a final assessment about how this particular protein contributes to the regulation of CD44 expression and PMN binding. Blocking of PKC activity in HUVEC, which had previously been conditioned by UKF-NB-4, prevented the loss of PMN attachment seen in the control cocultures. This suggests at least a partial role for PKCα (and PKCι) in suppressing PMN adhesion. Recently, in good accordance with this view, activation of PKC in endothelial cells was demonstrated to abolish transendothelial PMN migration toward a chemoattractant [24]. Still, the impact of PKC expression level on endothelial cell function remains a matter of debate. On the basis of a hyperglycemia model, PKCα and PKCε isoforms positively correlated with PMN adhesion to HUVEC [25], whereas a further vascular inflammation model did not reveal any effect of PKC inhibition on PMN recruitment [26]. The putative role of PKCα on PMN recruitment may not be comparable in tumors and in inflammation [27]. Therefore, additional studies are required to clarify whether therapeutic blocking of PKCα could become an important tool to reactivate PMN migration.
Evidence that cell contact-dependent adhesion of tumor cells to the vascular wall promotes down-regulation of endothelial CD44 receptor expression and prevents subsequent CD44 triggered PMN binding to endothelial cells is presented here. This may serve as a tumor-protecting mechanism by impairing the development of an effective neutrophil attack. Silencing PKCα overcomes tumor escape processes, suggesting a new molecular mechanism toward which novel therapeutic approaches for cancer might be directed.
Acknowledgments
The authors thank Karen Nelson for critically reading the manuscript.
Footnotes
This work was supported by the “Hilfe fur krebskranke Kinder Frankfurt e.V.” and their foundation “Frankfurter Stiftung fur krebskranke Kinder”.
References
- 1.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71:907–920. [PubMed] [Google Scholar]
- 2.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- 3.Simons MP, O'Donnell MA, Griffith TS. Role of neutrophils in BCG immunotherapy for bladder cancer. Urol Oncol. 2008;26:341–345. doi: 10.1016/j.urolonc.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, Robinson BW, Nelson DJ. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol. 2008;20:1467–1479. doi: 10.1093/intimm/dxn104. [DOI] [PubMed] [Google Scholar]
- 5.Halapi E. Oligoclonal T cells in human cancer. Med Oncol. 1998;15:203–211. doi: 10.1007/BF02787202. [DOI] [PubMed] [Google Scholar]
- 6.Ribas A, Butterfield LH, Glaspy JA, Economou JS. Current developments in cancer vaccines and cellular immunotherapy. J Clin Oncol. 2003;21:2415–2432. doi: 10.1200/JCO.2003.06.041. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461–1463. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu NZ, Klitzman B, Dodge R, Dewhirst MW. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 1992;52:4265–4268. [PubMed] [Google Scholar]
- 9.Dirkx AE, Oude Egbrink MG, Kuijpers MJ, van der Niet ST, Heijnen VV, Boumater Steege JC, Wagstaff J, Griffioen AW. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003;63:2322–2329. [PubMed] [Google Scholar]
- 10.Maksan SM, Araib PM, Ryschich E, Gebhard MM, Schmidt J. Immune escape mechanism: defective resting and stimulated leukocyte-endothelium interaction in hepatocellular carcinoma of the rat. Dig Dis Sci. 2004;49:859–865. doi: 10.1023/b:ddas.0000030100.05979.b7. [DOI] [PubMed] [Google Scholar]
- 11.Cinatl J, Jr, Vogel JU, Cinatl J, Weber B, Rabenau H, Novak M, Kornhuber B, Doerr HW. Long-term productive human cytomegalovirus infection of a human neuroblastoma cell line. Int J Cancer. 1996;65:90–96. doi: 10.1002/(SICI)1097-0215(19960103)65:1<90::AID-IJC16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Chen RL, Reynolds CP, Seeger RC. Neutrophils are cytotoxic and growth-inhibiting for neuroblastoma cells with an anti-GD2 antibody but, without cytotoxicity, can be growth-stimulating. Cancer Immunol Immunother. 2000;48:603–612. doi: 10.1007/s002620050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David K, Ollert MW, Juhl H, Vollmert C, Erttmann R, Vogel CW, Bredehorst R. Growth arrest of solid human neuroblastoma xenografts in nude rats by natural IgM from healthy humans. Nat Med. 1996;2:686–689. doi: 10.1038/nm0696-686. [DOI] [PubMed] [Google Scholar]
- 14.Takamizawa S, Okamoto S, Wen J, Bishop W, Kimura K, Sandler A. Overexpression of Fas-ligand by neuroblastoma: a novel mechanism of tumor-cell killing. J Pediatr Surg. 2000;35:375–379. doi: 10.1016/s0022-3468(00)90044-7. [DOI] [PubMed] [Google Scholar]
- 15.Chen YL, Chen SH, Wang JY, Yang BC. Fas ligand on tumor cells mediates inactivation of neutrophils. J Immunol. 2003;171:1183–1191. doi: 10.4049/jimmunol.171.3.1183. [DOI] [PubMed] [Google Scholar]
- 16.Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, Robinson BW, Nelson DJ. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol. 2008;20:1467–1479. doi: 10.1093/intimm/dxn104. [DOI] [PubMed] [Google Scholar]
- 17.Zen K, Liu DQ, Li LM, Chen CX, Guo YL, Ha B, Chen X, Zhang CY, Liu Y. The heparan sulfate proteoglycan form of epithelial CD44v3 serves as a CD11b/CD18 counter-receptor during polymorphonuclear leukocyte transepithelial migration. J Biol Chem. 2009;284:3768–3776. doi: 10.1074/jbc.M807805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley BP, Sin A, McCormick BA. Adhesion molecules involved in hepoxilin A3-mediated neutrophil transepithelial migration. Clin Exp Immunol. 2008;151:297–305. doi: 10.1111/j.1365-2249.2007.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan AI, Kerfoot SM, Heit B, Liu L, Andonegui G, Ruffell B, Johnson P, Kubes P. Role of CD44 and hyaluronan in neutrophil recruitment. J Immunol. 2004;173:7594–7601. doi: 10.4049/jimmunol.173.12.7594. [DOI] [PubMed] [Google Scholar]
- 20.Rouschop KM, Roelofs JJ, Claessen N, da Costa Martins P, Zwaginga JJ, Pals ST, Weening JJ, Florquin S. Protection against renal ischemia reperfusion injury by CD44 disruption. J Am Soc Nephrol. 2005;16:2034–2043. doi: 10.1681/ASN.2005010054. [DOI] [PubMed] [Google Scholar]
- 21.Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J Exp Med. 1995;181:811–816. doi: 10.1084/jem.181.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellebrekers DM, Castermans K, Viré E, Dings RP, Hoebers NT, Mayo KH, Oude Egbrink MG, Molema G, Fuks F, van Engeland M, et al. Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule-1 by histone modifications. Cancer Res. 2006;66:10770–10777. doi: 10.1158/0008-5472.CAN-06-1609. [DOI] [PubMed] [Google Scholar]
- 23.Sawada T, Kimura K, Nishihara T, Onoda N, Teraoka H, Yamashita Y, Yamada N, Yashiro M, Ohira M, Hirakawa K. TGF-β1 down-regulates ICAM-1 expression and enhances liver metastasis of pancreatic cancer. Adv Med Sci. 2006;51:60–65. [PubMed] [Google Scholar]
- 24.Carpenter AC, Alexander JS. Endothelial PKCδ activation attenuates neutrophil transendothelial migration. Inflamm Res. 2008;57:216–229. doi: 10.1007/s00011-007-7031-4. [DOI] [PubMed] [Google Scholar]
- 25.Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-κB-dependent fashion. J Clin Invest. 1998;101:1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi Y, Okayama N, Imaeda K, Okouchi M, Omi H, Imai S, Akao M, Takeda Y, Hukutomi T, Itoh M. Effects of histamine 2 receptor antagonists on endothelial-neutrophil adhesion and surface expression of endothelial adhesion molecules induced by high glucose levels. J Diabetes Complications. 2007;21:50–55. doi: 10.1016/j.jdiacomp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Zittermann SI, Issekutz AC. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am J Pathol. 2006;168:835–846. doi: 10.2353/ajpath.2006.050479. [DOI] [PMC free article] [PubMed] [Google Scholar]