Abstract
Introduction
The link between lung cancer and chronic obstructive lung diseases (COPD) has not been well studied in women even though lung cancer and COPD account for significant and growing morbidity and mortality among women.
Methods
We evaluated the relationship between COPD and non-small cell lung cancer (NSCLC) in a population-based case-control study of women and constructed a time course of chronic lung diseases in relation to onset of lung cancer. Five hundred sixty-two women aged 18–74, diagnosed with NSCLC and 564 population-based controls matched on race and age participated. Multivariable unconditional logistic regression models were used to estimate risk associated with a history of COPD, chronic bronchitis or emphysema.
Results
Lung cancer risk increased significantly for white women with a history of COPD (OR=1.85; 95% CI 1.21–2.81), but this was not seen in African American women. Risk associated with a history of chronic bronchitis was strongest when diagnosed at age 25 or earlier (OR=2.35, 95% CI 1.17–4.72); emphysema diagnosed within nine years of lung cancer was also associated with substantial risk (OR=6.36, 95% CI 2.36–17.13). Race, pack-years of smoking, exposure to environmental tobacco smoke as an adult, childhood asthma and exposure to asbestos were associated with a history of COPD among lung cancer cases.
Conclusions
In women, COPD is associated with risk of lung cancer differentially by race. Untangling whether COPD is in the causal pathway or simply shares risk factors will require future studies to focus on specific COPD features while exploring underlying genetic susceptibility to these diseases.
Introduction
Chronic obstructive lung diseases have been linked to lung cancer risk in multiple studies. These lung diseases share multiple risk factors, most importantly cigarette smoking. Approximately 15% of smokers will develop lung cancer and/or chronic obstructive pulmonary disease (COPD) and 10–15% of individuals with either of these diseases are never smokers (1, 2). Once COPD is diagnosed, risk of developing lung cancer increases about 2-fold (3–7), even among never smokers (8). Similarly, risk of lung cancer increases with decreasing forced expiratory volume in 1 second (FEV1); this was reported even in smokers with only minimal decreases in FEV1 of approximately 10% (9). A family history of COPD also increases risk of lung cancer development (10, 11) suggesting a common underlying genetic contribution to these diseases. An area of current research focuses on whether COPD pathogenesis directly affects the development of lung cancer or is a variation in manifestation of disease from the same exposures.
The definition of and diagnosis of COPD have evolved over time and includes a combination of two diseases that are frequently co-diagnosed, chronic bronchitis and emphysema, making COPD heterogeneous in observed clinical phenotype. Emphysema is characterized by parenchymal destruction, while chronic bronchitis is a small airways disease. Overall, COPD is characterized by airflow limitation that is usually progressive and associated with an abnormal inflammatory response.
COPD is the fourth leading cause of mortality in the United States. The death rates from COPD among women have surpassed those among men, with mortality rates in women increasing 5-fold between 1971 and 2000 (12). Studies have shown that female smokers demonstrate a sharper decline in FEV1 than their smoking male counterparts over time (13) and a recent meta-analysis found that at each quintile of reduced FEV1, women were approximately twice as likely as men to develop lung cancer (9). Lung cancer is the leading cause of cancer death in women and mortality rates have only recently begun to decline (14). The combination of increasing incidence of COPD and the high mortality from lung cancer in women, make this a population of growing importance for continued and renewed study.
In this case-control study, we evaluated the relationship between chronic obstructive lung diseases collectively, and emphysema and chronic bronchitis separately, and risk of lung cancer in a large population-based group of women. We also report on the time course of chronic lung diseases in relation to onset of lung cancer.
Materials and Methods
Study Subjects
Female residents of metropolitan Detroit, aged 18–74, diagnosed with primary NSCLC November, 2001 to October, 2005 were identified through the population-based Metropolitan Detroit Cancer Surveillance System (MDCSS), a participant in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. In-person interviews were completed with 577 women (54.5%); 129 women were too ill to participate, 89 women could not be located and 263 women refused. Women self-reporting race other than African American or white (n=15) were excluded, leaving 562 cases included in the analysis.
Population-based controls were identified through random digit dialing, frequency matched to cases on race and five-year age group. Of the households willing to complete the eligibility screening, 575 (69.7%) participated. Eleven controls reporting a race other than African American or white were excluded, leaving 564 controls included in the analysis.
Data Collection
All local institutional and review boards approved this study. Informed consent was obtained from each subject prior to study participation. In-person interviews were conducted to collect demographic information, smoking history, health history, family history of cancer and environmental tobacco smoke (ETS) exposure (15). Medical history included self-report of physician diagnoses of asthma, emphysema, allergies, pneumonia, chronic bronchitis, chronic obstructive pulmonary disease (COPD), tuberculosis and cancer. Reports of emphysema, COPD and/or chronic bronchitis were collapsed in the inclusive ‘chronic obstructive lung disease’ variable. Reference to COPD in the Results section refers only to those subjects who reported a diagnosis of COPD and not to the inclusive category. Only individuals with lung diseases diagnosed more than one year prior to lung cancer diagnosis (for cases) or interview (for the controls) were considered affected to limit inclusion of COPD diagnoses made in conjunction with the diagnosis of lung cancer. It is likely that COPD diagnoses made within one year of lung cancer diagnosis are less likely to be associated with lung cancer risk.
Follow-up for vital status and cause of death was obtained from the MDCSS for case-only survival analysis. Yearly active follow-up is conducted for all cases in the registry, with follow-up rates exceeding 98%.
Statistical Analysis
Comparisons of dichotomous risk factors between cases and controls were conducted using Chi-square tests; comparisons of means were conducted using Student’s T-tests. Multivariable unconditional logistic regression models were used to estimate odds ratios (OR) and 95% confidence intervals (CI) associated with a history of any chronic obstructive lung disease, chronic bronchitis or emphysema. Models included age, race, years of education, pack-years of smoking, current body mass index (BMI), family history of lung cancer, and regular use of adult strength aspirin (at least one pill three times per week for at least one month). Analyses were stratified on race, smoking status, smoking amount, age, and regular aspirin use. Cox proportional hazards models were used to evaluate the impact of chronic obstructive lung diseases on survival of women with lung cancer, constructed in the same manner as the logistic regression models. Survival analyses were also stratified on race, smoking status, smoking amount and age at diagnosis of lung cancer/age at interview. Differences between the median times between lung diagnoses by age at lung cancer diagnosis were evaluated using a median two-sample test. All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
Results
Cases and controls did not differ significantly by age, race, or history of pneumonia or asthma (Table 1). These groups did differ significantly by history of chronic obstructive lung disease diagnoses, including the combined variable, emphysema, COPD and chronic bronchitis, and childhood diagnosis of bronchitis. As expected, cases started smoking at an earlier age, had significantly higher levels of pack-years of smoking and quit smoking at a significantly later age than controls.
Table 1.
Subject Characteristics
| Category | Cases (N=562) | Controls (N=564) | p-value |
|---|---|---|---|
| Race | |||
| White | 427 (76.0) | 432 (76.6) | 0.81 |
| African American | 135 (24.0) | 132 (23.4) | |
| Age (mean, SD) | 60.1 (9.2) | 59.4 (9.4) | 0.20 |
| Smoking status | |||
| Never | 49 (8.7) | 275 (48.8) | <0.0001 |
| Exsmoker* | 183 (32.6) | 180 (31.9) | |
| Current smoker | 330 (58.7) | 105 (18.6) | |
| Unknown/not answered | 0 (0) | 4 (0.7) | |
| Smoking history | |||
| Packyears (mean, SD) | 46.6 (29.4) | 25.1 (22.4) | <0.0001 |
| Age began smoking | 16.8 (4.3) | 17.4 (4.4) | 0.07 |
| Age last smoked (exsmokers only, mean, SD) | 48.7 (12.1) | 39.6 (12.8) | <0.0001 |
| Ever diagnosed with pneumonia? | |||
| No | 356 (63.4) | 378 (67.0) | 0.19 |
| Yes | 202 (35.9) | 182 (32.3) | |
| Unknown/Not answered | 4 (0.7) | 4 (0.7) | |
| Ever diagnosed with childhood pneumonia? | |||
| No | 510 (90.7) | 505 (89.5) | 0.48 |
| Yes | 48 (8.6) | 55 (9.8) | |
| Unknown/Not answered | 4 (0.7) | 4 (0.7) | |
| Ever diagnosed with asthma? | |||
| No | 457 (81.3) | 465 (82.4) | 0.62 |
| Yes | 102 (18.2) | 96 (17.0) | |
| Unknown/Not answered | 3 (0.5) | 3 (0.5) | |
| Ever diagnosed with childhood asthma? | |||
| No | 528 (94.0) | 543 (96.3) | 0.06 |
| Yes | 31 (5.5) | 18 (3.2) | |
| Unknown/Not answered | 3 (0.5) | 3 (0.5) | |
| Ever diagnosed with chronic obstructive lung disease†? | |||
| No | 387 (68.9) | 483 (85.6) | <0.0001 |
| Yes | 175 (31.1) | 81 (14.4) | |
| Ever diagnosed with COPD? | |||
| No | 513 (91.3) | 541 (95.9) | 0.0005 |
| Yes | 46 (8.2) | 19 (3.4) | |
| Unknown/Not answered | 3 (0.5) | 4 (0.7) | |
| Ever diagnosed with emphysema? | |||
| No | 476 (84.7) | 549 (97.4) | <0.0001 |
| Yes | 85 (15.1) | 12 (2.1) | |
| Unknown/Not answered | 1 (0.2) | 3 (0.5) | |
| Ever diagnosed with chronic bronchitis? | |||
| No | 439 (78.1) | 498 (88.3) | <0.0001 |
| Yes | 120 (21.4) | 63 (11.2) | |
| Unknown/Not answered | 3 (0.5) | 3 (0.5) | |
| Ever diagnosed with bronchitis as a child? | |||
| No | 537 (95.6) | 550 (97.5) | 0.05 |
| Yes | 22 (3.9) | 11 (2.0) | |
| Unknown/Not answered | 3 (0.5) | 3 (0.5) | |
Quit at least 2 years prior to diagnosis (cases) or interview (controls)
Includes chronic bronchitis, emphysema or chronic obstructive pulmonary disease (COPD)
Table 2 presents stratified results for the risk of lung cancer associated with the inclusive chronic obstructive lung disease category, as well as for individually reported diagnoses. For the combined obstructive lung disease classification, a significantly increased risk was seen for the entire study population (OR=1.67, 95% CI 1.15–2.41) and in white women (OR=1.85; 95% CI 1.21–2.81). No increase in risk was seen in African American women. An increased risk was not identified in nonsmokers or light smokers, however, a significantly increased risk was found among heavy smoking women (OR=1.93; 95% CI 1.18–3.15). This risk was seen in both former and current smokers. For this combined lung disease category, lung cancer risk was highest in women diagnosed before age 55.
Table 2.
Risk of NSCLC in women associated with a history of chronic obstructive lung disease, emphysema and chronic bronchitis
| Category | Percent of cases affected/percent of controls affected | Chronic obstructive lung disease* OR† (95% CI) | Percent of cases affected/percent of controls affected | Emphysema OR† (95% CI) | Percent of cases affected/percent of controls affected | Chronic Bronchitis OR† (95% CI) |
|---|---|---|---|---|---|---|
| All subjects | 31.1/14.4 | 1.67 (1.15–2.41) | 15.1/2.1 | 3.21 (1.60–6.45) | 21.5/11.2 | 1.71 (1.13–2.59) |
| Race | ||||||
| White | 35.1/14.6 | 1.85 (1.21–2.81) | 17.4/2.1 | 3.75 (1.69–8.32) | 23.4/11.4 | 1.82 (1.13–2.92) |
| African American | 18.5/13.6 | 1.11 (0.49–2.49) | 8.1/2.3 | 1.86 (0.42–8.23) | 15.6/10.6 | 1.38 (0.56–3.37) |
| Smoking History | ||||||
| Never smokers | 4.1/12.7 | 0.34 (0.08–1.51) | 0.0/0.7 | NA | 4.2/11.8 | 0.37 (0.08–1.65) |
| Light smokers (<= 18 pack-years) | 19.4/12.1 | 2.29 (0.91–5.75) | 6.9/0.7 | 12.92 (1.30–128.8) | 13.9/9.3 | 2.29 (0.79–6.64) |
| Heavy smokers (>18 pack-years) | 34.0/20.4 | 1.93 (1.18–3.15) | 15.9/6.3 | 2.65 (1.24–5.68) | 23.4/12.7 | 2.13 (1.19–3.80) |
| Smoking Status | ||||||
| Former smokers‡ | 32.8/13.9 | 2.03 (1.11–3.71) | 18.6/2.2 | 5.33 (1.65–17.20) | 19.1/11.1 | 1.51 (0.76–3.02) |
| Current smokers | 34.2/20.0 | 1.93 (1.06–3.49) | 15.5/5.7 | 2.33 (0.91–5.97) | 25.3/10.5 | 3.08 (1.47–6.43) |
| Age at Lung Cancer Diagnosis/Interview | ||||||
| < 55 years | 26.3/11.5 | 2.05 (1.01–4.19) | 10.0/0.6 | 13.59 (1.49–124.3) | 20.8/9.9 | 1.96 (0.91–4.18) |
| 55+ years | 33.1/15.8 | 1.54 (1.00–2.40) | 17.2/2.9 | 2.53 (1.20–5.33) | 21.8/11.8 | 1.63 (0.96–2.69) |
| Adult Strength Aspirin Use | ||||||
| Never took aspirin regularly | 27.8/12.1 | 1.64 (1.05–2.57) | 13.0/1.0 | 6.34 (2.13–18.87) | 19.5/9.9 | 1.63 (0.99–2.68) |
| Took aspirin regularly for at least one month | 40.4/21.0 | 1.74 (0.89–3.41) | 20.6/5.4 | 1.69 (0.62–4.62) | 27.2/15.0 | 1.86 (0.86–3.99) |
Includes chronic bronchitis, emphysema or chronic obstructive pulmonary disease (COPD) diagnosis
Adjusted for age at diagnosis/interview, race (white/African American), pack-years, family history of lung cancer, education (less than high school, high school or GED, more than high school), current BMI and adult aspirin use (ever/never)
Quit at least 2 years before diagnosis
Controls were significantly more likely than cases to report only one chronic obstructive lung disease related illness (p=0.01). In particular, controls were more likely to report only a simple COPD or chronic bronchitis diagnosis. The number of chronic lung conditions was significantly higher in cases than controls (p=0.004); 10% of cases reported 2 or more illnesses while only 2% of controls did.
A history of emphysema was associated with a 3-fold increased risk of lung cancer (OR=3.21; 95% CI 1.60–6.45) after adjusting for age, race, pack-years, family history of lung cancer, education, current BMI, and regular strength aspirin use (Table 2). Risk was high in white women, but was not statistically significantly elevated in African American women. Very high ORs were reported for light smokers (OR=12.92, 95% CI 1.30–129) and earlier onset lung cancer (OR=13.59, 95% CI 1.49–124) most likely because emphysema was very rare in the controls in these categories. Significantly elevated lung cancer risk on the order of 5–6 fold was associated with a history of emphysema in former smokers and women never using aspirin regularly. Overall, a history of chronic bronchitis was associated with a 1.7-fold increased risk of lung cancer (95% CI 1.13–2.59), and an approximately 1.5 to 3.1-fold increased risk of lung cancer across strata, with highest risk in heavier smokers.
Table 3 presents lung cancer risk by age at diagnosis and time since diagnosis for selected lung diseases. Lung cancer risk did not vary by age at diagnosis of asthma or time since diagnosis of asthma. Similar findings were seen for pneumonia. Cases were significantly more likely to report an early diagnosis of chronic bronchitis (on or before age 25, reported in 6.9% of cases; OR=2.35, 95% CI 1.17–4.72) and a longer time between chronic bronchitis and lung cancer was associated with the greatest risk. For emphysema, a recent diagnosis (within nine years of lung cancer; OR=6.36, 95% CI 2.36–17.13) and an older age of diagnosis were associated with the greatest increase in risk of lung cancer.
Table 3.
Risk of NSCLC in women associated with age and years since diagnosis of lung diseases
| Age at first diagnosis of lung disease | Number of cases/Number of controls | Adjusted OR* 95% CI | Years since first diagnosed with lung disease | Number of cases/Number of controls | Adjusted OR* 95% CI |
|---|---|---|---|---|---|
| Asthma | |||||
| No | 457/465 | 1.00 (ref) | No | 457/465 | 1.00 (ref) |
| 0–25 | 35/29 | 1.07 (0.56–2.07) | 2–9† | 34/32 | 0.97 (0.50–1.91) |
| 26–49 | 32/37 | 0.93 (0.68–1.27) | 10–19 | 18/23 | 0.77 (0.52–1.14) |
| 50+ | 34/30 | 0.91 (0.72–1.15) | 20+ | 49/41 | 1.01 (0.84–1.22) |
| Pneumonia | |||||
| No | 356/378 | 1.00 (ref) | No | 356/378 | 1.00 (ref) |
| 0–25 | 74/72 | 0.90 (0.57–1.40) | 2–9† | 53/36 | 1.34 (0.76–2.35) |
| 26–49 | 82/74 | 1.04 (0.84–1.30) | 10–19 | 33/27 | 1.04 (0.75–1.45) |
| 50+ | 46/36 | 0.96 (0.79–1.18) | 20+ | 116/119 | 0.95 (0.84–1.07) |
| Chronic Bronchitis | |||||
| No | 439/498 | 1.00 (ref) | No | 439/498 | 1.00 (ref) |
| 0–25 | 39/19 | 2.35 (1.17–4.72) | 2–9† | 31/15 | 1.71 (0.78–3.71) |
| 26–49 | 45/26 | 1.21 (0.89–1.66) | 10–19 | 27/18 | 1.03 (0.71–1.50) |
| 50+ | 36/18 | 1.12 (0.88–1.43) | 20+ | 62/30 | 1.30 (1.07–1.57) |
| Emphysema | |||||
| No | 476/549 | 1.00 (ref) | No | 476/549 | 1.00 (ref) |
| 0–25 | 1/1 | 0.20 (0.01–3.34) | 2–9† | 61/5 | 6.36 (2.36–17.13) |
| 26–49 | 21/2 | 2.61 (1.14–5.95) | 10–19 | 20/4 | 1.11 (0.60–2.02) |
| 50+ | 63/9 | 1.44 (1.10–1.87) | 20+ | 4/3 | 0.78 (0.40–1.51) |
| Chronic obstructive lung disease‡ | |||||
| No | 387/483 | 1.00 (ref) | No | 387/483 | 1.00 (ref) |
| 0–25 | 40/21 | 2.08 (1.06–4.08) | 2–9† | 67/27 | 1.75 (1.00–3.06) |
| 26–49 | 58/30 | 1.25 (0.93–1.67) | 10–19 | 41/20 | 1.07 (0.76–1.51) |
| 50+ | 77/30 | 1.15 (0.95–1.38) | 20+ | 67/34 | 1.26 (1.05–1.51) |
Adjusted for age at diagnosis/interview, race (white/African American), pack-years, family history of lung cancer, education (less than high school, high school or GED, more than high school), current BMI and ever/never adult aspirin use
Only individuals with lung diseases diagnosed more than one year prior to lung cancer diagnosis (for cases) or interview (for the controls) were considered affected.
Includes chronic bronchitis, emphysema or chronic obstructive pulmonary disease (COPD)
To further characterize the occurrence of chronic obstructive lung diseases and lung cancer, cases with and without a chronic obstructive lung disease diagnosis were compared for a variety of risk factors (Table 4). In univariable analyses, cases with a chronic obstructive lung disease diagnosis were significantly older at diagnosis of lung cancer (p=0.01), were significantly more likely to be white (0.0003) and had significantly higher levels of all smoking and most ETS exposure measures than cases without such a history. In addition, cases with a diagnosis of chronic obstructive lung disease were significantly more likely to have been exposed to asbestos or report a childhood diagnosis of pneumonia or asthma (p=0.04, 0.05, 0.03, respectively). These two groups of cases were not significantly different, however, in their current BMI, years exposed to ETS as a child, family history of lung cancer or stage at diagnosis. While cases with a history of chronic obstructive lung disease (38.9%) were somewhat more likely than cases without such a history (31.5%) to be diagnosed at a localized stage, this difference was not statistically significant. In a multivariable model constructed using a step-wise approach, race, pack-years of smoking, exposure to ETS as an adult, childhood asthma and exposure to asbestos were associated with history of a chronic obstructive lung disease among lung cancer cases (Table 5).
Table 4.
Subject characteristics of women with NSCLC, with and without chronic obstructive lung disease
| Category | Cases without a chronic obstructive lung disease diagnosis (N=387) | Cases with a chronic obstructive lung disease diagnosis (N=175) | p-value |
|---|---|---|---|
| Race | |||
| White | 277 (71.6) | 150 (85.7) | 0.0003 |
| African American | 110 (28.4) | 25 (14.3) | |
| Age at lung cancer diagnosis (mean, SD) | 59.4 (9.5) | 61.4 (8.6) | 0.01 |
| Current BMI (mean, SD) | 26.2 (5.8) | 26.6 (6.3) | 0.46 |
| Smoking status | |||
| Never | 47 (12.1) | 2 (1.1) | <0.0001 |
| Exsmoker* | 123 (31.8) | 60 (34.3) | |
| Current smoker | 217 (56.1) | 113 (64.5) | |
| Smoking history for smokers only | |||
| Age began smoking | 17.1 (4.4) | 16.3 (4.1) | 0.05 |
| Packyears (exsmokers) (mean, SD) | 35.2 (24.7) | 52.5 (34.8) | 0.0009 |
| Packyears (current smokers) (mean, SD) | 46.6 (27.5) | 56.0 (30.9) | 0.005 |
| Age last smoked (exsmokers) (mean, SD) | 47.1 (12.5) | 52.0 (10.6) | 0.01 |
| Education | |||
| Less than high school | 68 (17.6) | 36 (20.6) | 0.02 |
| High school graduate or GED | 145 (37.4) | 82 (46.9) | |
| At least some college | 173 (44.7) | 57 (32.5) | |
| Unknown/not answered | 1 (0.3) | 0 (0) | |
| Exposure to ETS as a child | |||
| No | 94 (24.3) | 29 (16.6) | 0.04 |
| Yes | 293 (75.7) | 146 (83.4) | |
| Years (mean, SD among exposed) | 16.6 (3.7) | 16.0 (4.3) | 0.16 |
| Household exposure to ETS as an adult | |||
| No | 88 (22.7) | 20 (11.4) | 0.002 |
| Yes | 298 (77.0) | 155 (88.6) | |
| Unknown/Not answered | 1 (0.3) | 0 (0) | |
| Years (mean, SD among exposed) | 25.7 (16.1) | 28.9 (13.8) | 0.03 |
| Work expose to ETS | |||
| No | 93 (24.0) | 28 (16.0) | 0.03 |
| Yes | 293 (75.7) | 147 (84.0) | |
| Unknown/Not answered | 1 (0.3) | 0 (0) | |
| Years (mean, SD among exposed) | 16.0 (10.4) | 17.6 (11.1) | 0.13 |
| Ever took adult strength aspirin regularly? | |||
| No | 302 (78.0) | 116 (66.3) | 0.005 |
| Yes | 81 (21.0) | 55 (31.4) | |
| Unknown/Not answered | 4 (1.0) | 4 (2.3) | |
| Ever exposed to asbestos? | |||
| No | 317 (81.9) | 133 (76.0) | 0.04 |
| Yes | 63 (16.3) | 42 (24.0) | |
| Unknown/Not answered | 7 (1.8) | 0 (0) | |
| First-degree family history of lung cancer | |||
| No | 297 (76.7) | 127 (72.6) | 0.29 |
| Yes | 90 (23.3) | 48 (27.4) | |
| Childhood pneumonia | |||
| No | 357 (92.2) | 153 (87.4) | 0.05 |
| Yes | 27 (7.0) | 21 (12.0) | |
| Unknown/Not answered | 3 (0.8) | 1 (0.6) | |
| Childhood asthma | |||
| No | 370 (95.6) | 158 (90.4) | 0.03 |
| Yes | 16 (4.1) | 15 (9.5) | |
| Unknown/Not answered | 1 (0.3) | 2 (0.1) | |
| Stage at diagnosis | |||
| Local | 122 (31.5) | 68 (38.9) | 0.19 |
| Regional | 127 (32.8) | 57 (32.6) | |
| Distant | 132 (34.1) | 49 (28.0) | |
| Unknown | 6 (1.6) | 1 (0.5) | |
Quit at least 2 years prior to diagnosis (cases) or interview (controls)
Table 5.
Risk factors associated with a history of any chronic obstructive lung disease in women with NSCLC
| Category | Univariable OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
| Race (African American/white) | 0.42 (0.26–0.68) | 0.56 (0.34–0.93) |
| Age | 1.02 (1.00–1.04) | 1.02 (0.99–1.04) |
| Current BMI | 1.01 (0.98–1.04) | |
| Pack-years | 1.02 (1.01–1.02) | 1.01 (1.01–1.02) |
| Age began smoking | 0.95 (0.91–1.00) | |
| Education (less than high school, high school or GED, more than high school) | 0.76 (0.60–0.97) | |
| Exposure to childhood ETS | 1.62 (1.02–2.56) | |
| Years of childhood ETS exposure | 1.01 (0.99–1.04) | |
| Exposure to adulthood ETS | 2.29 (1.36–3.86) | 2.02 (1.17–3.49) |
| Years of adulthood ETS exposure | 1.02 (1.01–1.03) | |
| Exposure to ETS at work | 1.67 (1.05–2.66) | |
| Years of work ETS exposure | 1.02 (1.00–1.04) | |
| Family history of lung cancer | 1.25 (0.83–1.87) | |
| Childhood pneumonia | 1.82 (1.00–3.31) | |
| Childhood asthma | 2.20 (1.06–4.55) | 2.42 (1.11–5.24) |
| Ever took aspirin regularly | 1.77 (1.18–2.65) | |
| Ever exposed to asbestos | 1.59 (1.02–2.47) | 1.69 (1.05–2.72) |
Figure 1 represents time lines for chronic obstructive lung diseases and lung cancer diagnoses in cases less than 55 (Figure 1a) and 55 years of age and older (Figure 1b). The lung disease histories are fairly similar in these two age groups, but the time line is accelerated for all lung diseases in women diagnosed with lung cancer before age 55. A history of emphysema was more commonly reported among cases diagnosed at or after age 55 (17%) as compared with cases diagnosed at younger ages (10%) (p=0.03). Median time between emphysema diagnosis and lung cancer diagnosis was 8.5 and 5 years in the two lung cancer diagnosis age groups, respectively (p=0.30). Median time between any chronic obstructive lung disease diagnosis and lung cancer diagnosis (13.5 and 13 years) was also similar by age (p=0.92), but the initial age at onset of the chronic obstructive lung disease differed significantly (35.5 and 51 years, respectively, p<0.001) suggesting a group of women with early onset of lung diseases who are more susceptible to early onset lung cancer.
Figure 1.
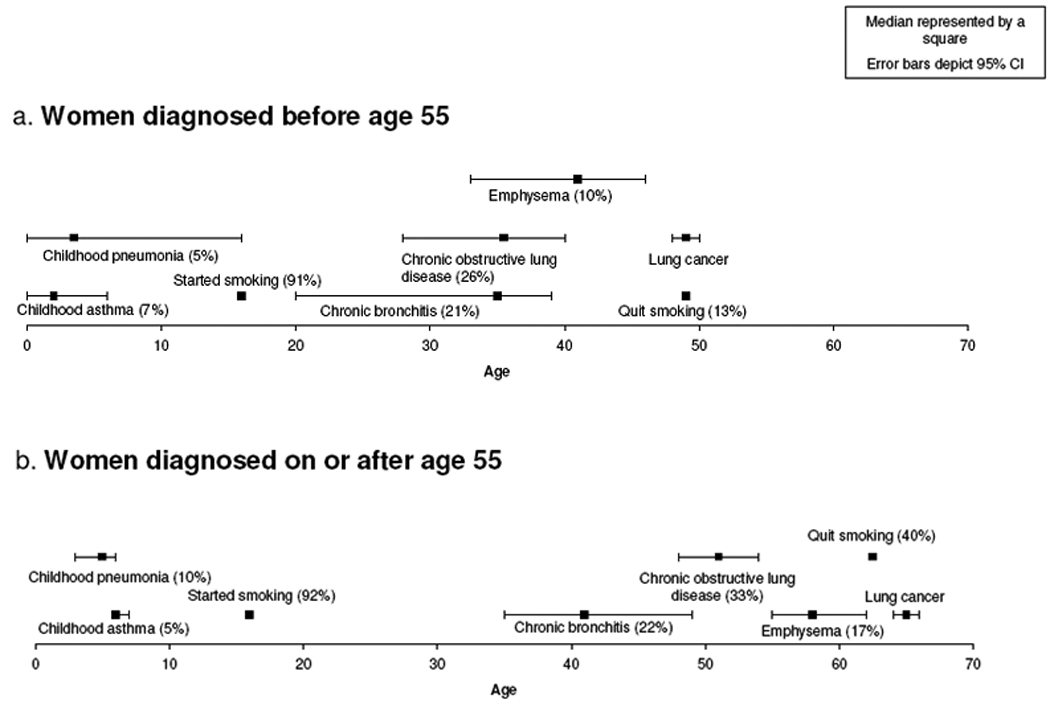
Time lines of lung disease history in women with NSCLC
African American women with NSCLC were less likely to be diagnosed with a chronic obstructive lung disease (19%) than white women (35%) (p=0.0003). When diagnosed, both chronic bronchitis and emphysema were diagnosed at ages approximately 8 years older than white women with these conditions (data not shown). This suggests the potential for delay in diagnosis, under-reporting and/or under-diagnosis in African American women.
Also evaluated was whether a history of chronic obstructive lung disease, emphysema, or chronic bronchitis impacted survival among cases. We found no significant differences in survival overall or when our data were stratified on race, smoking status, smoking amount, or age at diagnosis (data not shown).
Discussion
This study supports previous findings of an association between a history of chronic obstructive lung disease and NSCLC after adjustment for smoking history, extending previous work by including a large number of women and African Americans. We also present a time line for onset of lung cancer after chronic obstructive lung disease diagnoses. Overall, lung cancer risk was increased in women reporting a diagnosis of any chronic obstructive lung diseases, emphysema and/or chronic bronchitis. Two prospective studies reported similar findings with risk of lung cancer increased almost 3-fold with a history of COPD (HR: 2.8, 95% CI 1.8–4.4) (3), and with an emphysema diagnosis on radiography after adjustment for extend of airflow obstruction (OR: 3.1; 95% CI 1.9–5.2) (7). Numerous case-control studies are also in agreement (5, 16, 17).
Our cases were significantly more likely to report multiple chronic obstructive lung disease illnesses than controls and the timing of these illnesses appears to be distinct. We report that an early age of diagnosis of chronic bronchitis was related to a higher risk of lung cancer. In a previous analysis, Wu et al. also found chronic bronchitis was associated with a significant increase in lung cancer risk overall (18). Among their population of nonsmoking women, however, a later age of chronic bronchitis appeared to be associated with the most significant risk. Schabath et al. also did a similar analysis and found a borderline protective effect of chronic bronchitis diagnosed 10 years prior to lung cancer diagnosis/interview (5). Although we did not find a protective effect associated with a ten year lag time, we report null results for a 10–19 year lag time. It was only with lag times of over 20 years that we see increased lung cancer risk. These findings are not unexpected given that chronic bronchitis tends to be diagnosed earlier than emphysema. Individuals with emphysema tend present later, however once diagnosed they have a more rapid deterioration of lung function.
We report that lung cancer is typically diagnosed closer to an emphysema diagnosis than to a chronic bronchitis diagnosis. Schabath et al. found emphysema to be significantly related to an increased risk at all time periods before a lung cancer diagnosis (5). Both men and women were included in that study making it likely that smoking exposures were higher overall than seen in our study of women only. Two prospective studies show that smokers with an emphysema diagnosis based on low dose CT of the chest were at a 3-fold increased risk of developing lung cancer than smokers without emphysema (7, 19), at a rate of 25 lung cancers per 1,000 person-years (19). This relationship held even in individuals without airway obstruction based on spirometry readings. Smokers with both emphysema and airway obstruction were at even greater risk. These findings suggest that lung cancer occurrence is linked differentially to specific features of COPD.
African American women in our study were less likely than white women to report a physician diagnosis of COPD. This racial difference in prevalence has been reported previously (20). In addition, while lung cancer risk associated with a previous chronic obstructive lung disease was elevated in African American women, these findings were not statistically significant. Racial differences reported may be due to under-reporting, under-diagnosis, or lower risk of COPD among African American women associated with lower smoking exposures or genetic variations in susceptibility.
In previous published work based on this case-control study, we reported that lung cancer risk was inversely associated with use of adult strength aspirin (15). The association between COPD or emphysema and lung cancer risk was strongest in women reporting no regular aspirin use. The association between COPD and lung cancer risk was still elevated in women who did take aspirin regularly, although these findings were not statistically significant. It has been reported that use of inhaled corticosteroids among patients with COPD might reduce risk of subsequent lung cancer (21). We did not collect information about inhaled corticosteroids. The contribution of anti-inflammatory drug use among women with COPD to lung cancer risk should be further explored.
In characterizing the lung disease histories of women with lung cancer, we report that lung cancer cases with a chronic obstructive lung disease diagnosis were more likely to be white, heavy smokers, be exposed to ETS as an adult, have childhood asthma, and have a history of asbestos exposure. Each of these exposures are risk factors for both COPD and lung cancer. Therefore, the unanswered question is whether COPD is in the causal pathway in the development of lung cancer or a variation in manifestation from the same exposures. Shared risk factors include not only cigarette smoking, but occupational exposures and asbestos exposure (22). These exposures are associated with airway inflammation (23). Brody and Spira (24) review the pathways leading from inflammation to COPD and lung cancer and suggest that early on in the pathogenesis of these diseases divergent pathways emerge.
In addition to shared exposures, a number of candidate single gene polymorphisms (SNPs) have been associated with susceptibility to lung cancer and COPD (25–28). Common polymorphisms associated with both COPD and lung cancer are found in genes coding for epoxide hydrolase 1 (EPHX1), the matrix metalloprotienases, and interleukin 1β (IL1B). Gene expression studies in COPD and lung cancer also point to up- and down-regulation of genes functioning in inflammation and oxidative stress in both these diseases (29–31). Uncoupling shared risk factors from causal pathways will be difficult.
This study has a number of strengths including its large size, use of population-based cases and controls and a focus on women, including a large minority population. There are, however, some limitations. The biggest limitation to this study, and all studies that rely on self-report, is the possibility of misclassification of history of lung disease. Given the clinical complexities and changes in diagnostic classifications over the years, it is probably most reasonable to use the classification of any chronic obstructive lung disease that combines women who report a history of COPD, chronic bronchitis and/or emphysema to limit potential misclassification. Future studies need to include better defined COPD phenotypes that include measures of severity.
This study adds to the substantial literature on COPD and lung cancer by demonstrating an association between a history of chronic obstructive lung diseases and the development of NSCLC in women, a less frequently studied group. It further shows that African American women with lung cancer are less likely than white women with lung cancer to report a previous lung disease suggesting that they are either under-diagnosed or at lower risk of having such a diagnosis even with similar smoking histories. Women diagnosed with lung cancer before age 55 report a previous lung disease beginning at an earlier age than women with later onset lung cancer. These women represent a group of susceptible women who might benefit from more intensive follow-up. Future studies should focus on better defining chronic obstructive lung diseases using spirometry and CT, while exploring underlying genetic susceptibility to these diseases.
Acknowledgements
The authors thank Lynda Forbes, Yvonne Bush, Kelly Montgomery, Pat Campagna, Gina Claeys, Geoff Prysak and the staff of the Metropolitan Detroit Cancer Surveillance System for data collection and management.
This research was funded by NIH grants R01-CA87895 and contracts N01-PC35145 and P30CA22453.
References
- 1.Mattson ME, Pollack ES, Cullen JW. What are the odds that smoking will kill you? Am J Public Health. 1987;77:425–431. doi: 10.2105/ajph.77.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menezes AM, Hallal PC. Role of passive smoking on copd risk in non-smokers. Lancet. 2007;370:716–717. doi: 10.1016/S0140-6736(07)61353-1. [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: Data from the first national health and nutrition examination survey follow-up. Archives of internal medicine. 2003;163:1475–1480. doi: 10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- 4.Purdue MP, Gold L, Jarvholm B, Alavanja MC, Ward MH, Vermeulen R. Impaired lung function and lung cancer incidence in a cohort of swedish construction workers. Thorax. 2007;62:51–56. doi: 10.1136/thx.2006.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schabath MB, Delclos GL, Martynowicz MM, Greisinger AJ, Lu C, Wu X, Spitz MR. Opposing effects of emphysema, hay fever, and select genetic variants on lung cancer risk. Am J Epidemiol. 2005;161:412–422. doi: 10.1093/aje/kwi063. [DOI] [PubMed] [Google Scholar]
- 6.Littman AJ, Thornquist MD, White E, Jackson LA, Goodman GE, Vaughan TL. Prior lung disease and risk of lung cancer in a large prospective study. Cancer Causes Control. 2004;15:819–827. doi: 10.1023/B:CACO.0000043432.71626.45. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried J, Shapiro SD, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. American journal of respiratory and critical care medicine. 2008 doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. American journal of respiratory and critical care medicine. 2007;176:285–290. doi: 10.1164/rccm.200612-1792OC. [DOI] [PubMed] [Google Scholar]
- 9.Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: A systematic review and meta-analysis. Thorax. 2005;60:570–575. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu AH, Yu MC, Thomas DC, Pike MC, Henderson BE. Personal and family history of lung disease as risk factors for adenocarcinoma of the lung. Cancer Res. 1988;48:7279–7284. [PubMed] [Google Scholar]
- 11.Cohen BH, Diamond EL, Graves CG, Kreiss P, Levy DA, Menkes HA, Permutt S, Quaskey S, Tockman MS. A common familial component in lung cancer and chronic obstructive pulmonary disease. Lancet. 1977;2:523–526. doi: 10.1016/s0140-6736(77)90663-8. [DOI] [PubMed] [Google Scholar]
- 12.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance -- united states, 1971–2000. MMWR Surveillance Summer. 2002;51:1–16. [PubMed] [Google Scholar]
- 13.Ben-Zaken Cohen S, Pare PD, Man SF, Sin DD. The growing burden of chronic obstructive pulmonary disease and lung cancer in women: Examining sex differences in cigarette smoke metabolism. American journal of respiratory and critical care medicine. 2007;176:113–120. doi: 10.1164/rccm.200611-1655PP. [DOI] [PubMed] [Google Scholar]
- 14.Ries LAG MD, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK, editors. Bethesda, MD: National Cancer Institute; 2007. Seer cancer statistics review, 1975–2004. [Google Scholar]
- 15.Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. Regular adult aspirin use decreases the risk of non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev. 2008;17:148–157. doi: 10.1158/1055-9965.EPI-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brownson RC, Alavanja MC, Caporaso N, Berger E, Chang JC. Family history of cancer and risk of lung cancer in lifetime non-smokers and long-term ex-smokers. International journal of epidemiology. 1997;26:256–263. doi: 10.1093/ije/26.2.256. [DOI] [PubMed] [Google Scholar]
- 17.Mayne ST, Buenconsejo J, Janerich DT. Familial cancer history and lung cancer risk in united states nonsmoking men and women. Cancer Epidemiol Biomarkers Prev. 1999;8:1065–1069. [PubMed] [Google Scholar]
- 18.Wu AH, Fontham ET, Reynolds P, Greenberg RS, Buffler P, Liff J, Boyd P, Henderson BE, Correa P. Previous lung disease and risk of lung cancer among lifetime nonsmoking women in the united states. Am J Epidemiol. 1995;141:1023–1032. doi: 10.1093/oxfordjournals.aje.a117366. [DOI] [PubMed] [Google Scholar]
- 19.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose ct of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 20.Dransfield MT, Bailey WC. Copd: Racial disparities in susceptibility, treatment, and outcomes. Clinics in chest medicine. 2006;27:463–471. doi: 10.1016/j.ccm.2006.04.005. vii. [DOI] [PubMed] [Google Scholar]
- 21.Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2007;175:712–719. doi: 10.1164/rccm.200608-1125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viegi G, Scognamiglio A, Baldacci S, Pistelli F, Carrozzi L. Epidemiology of chronic obstructive pulmonary disease (copd) Respiration; international review of thoracic diseases. 2001;68:4–19. doi: 10.1159/000050456. [DOI] [PubMed] [Google Scholar]
- 23.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 24.Brody JS, Spira A. State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proceedings of the American Thoracic Society. 2006;3:535–537. doi: 10.1513/pats.200603-089MS. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz AG, Prysak GM, Bock CH, Cote ML. The molecular epidemiology of lung cancer. Carcinogenesis. 2006 doi: 10.1093/carcin/bgl253. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz AG, Ruckdeschel JC. Familial lung cancer: Genetic susceptibility and relationship to chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2006;173:16–22. doi: 10.1164/rccm.200502-235PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engels EA, Wu X, Gu J, Dong Q, Liu J, Spitz MR. Systematic evaluation of genetic variants in the inflammation pathway and risk of lung cancer. Cancer Res. 2007;67:6520–6527. doi: 10.1158/0008-5472.CAN-07-0370. [DOI] [PubMed] [Google Scholar]
- 28.Molfino NA. Current thinking on genetics of chronic obstructive pulmonary disease. Current opinion in pulmonary medicine. 2007;13:107–113. doi: 10.1097/MCP.0b013e328013e97d. [DOI] [PubMed] [Google Scholar]
- 29.Spira A, Beane J, Pinto-Plata V, Kadar A, Liu G, Shah V, Celli B, Brody JS. Gene expression profiling of human lung tissue from smokers with severe emphysema. American journal of respiratory cell and molecular biology. 2004;31:601–610. doi: 10.1165/rcmb.2004-0273OC. [DOI] [PubMed] [Google Scholar]
- 30.Pierrou S, Broberg P, O'Donnell RA, Pawlowski K, Virtala R, Lindqvist E, Richter A, Wilson SJ, Angco G, Moller S, et al. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2007;175:577–586. doi: 10.1164/rccm.200607-931OC. [DOI] [PubMed] [Google Scholar]
- 31.Borczuk AC, Powell CA. Expression profiling and lung cancer development. Proceedings of the American Thoracic Society. 2007;4:127–132. doi: 10.1513/pats.200607-143JG. [DOI] [PubMed] [Google Scholar]


