Abstract
Cobalamins (Cbl) are important co-factors for methionine synthase and methylmalonyl-coA mutase. Certain corrins also bind nitric oxide (NO), quenching its bioactivity. To determine if corrins would inhibit NO synthase (NOS), we measured their effects on 14-C-L-arginine-to-14-C-L-citrulline conversion by NOS1, NOS2, and NOS3. Hydroxocobalamin (OH-Cbl), cobinamide (Cbi), and dicyanocobinamide (CN2-Cbi) potently inhibited all isoforms, whfile cyanocobalamin, methylcobalamin, and adenosylcobalamin had much less effect. OH-Cbl and CN2-Cbi prevented binding of the oxygen analog carbon monoxide (CO) to the reduced NOS1 and NOS2 heme active site. CN2-Cbi did not react directly with NO or CO. Spectral perturbation analysis showed that CN2-Cbi interacted directly with the purified NOS1 oxygenase domain. NOS inhibition by corrins was rapid and not reversed by dialysis with L-arginine, tetrahydrobiopterin. Molecular modeling indicated that corrins could access the unusually large heme and substrate-binding pocket of NOS. Best fits were obtained in the “base-off” conformation of the lower axial dimethylbenzimidazole ligand. CN2-Cbi inhibited interferon-γ-activated Raw264.7 mouse macrophage NO production. We show for the first time that certain corrins directly inhibit NOS, suggesting that these agents (or their derivatives) may have pharmacological utility. Endogenous cobalamins and cobinamides might play important roles regulating NOS activity in normal and pathological conditions.
Keywords: cobalamin, cobinamide, vitamin B12, nitric oxide, nitric oxide synthase, arginine, macrophage
Introduction
Nitric oxide (NO) synthase (NOS) converts L-arginine to L-citrulline and NO [1, 2]. NOS1 (“neural” NOS) and NOS3 (“endothelial” NOS) generally produce low levels of NO and are constitutively active. Inducible NOS (NOS2) is induced by cytokines and microbial factors and produces high levels of NO. NO plays very important roles in normal physiology and in various pathologic processes [3–5]. NO serves as a signal-transducing molecule, an effector molecule for the stasis and killing of microbes (e.g., certain viruses, fungi, bacteria, and protozoa), and neoplastic cells. NO can also block apoptosis by S-nitrosylating caspases [6]; in resting, normal B lymphocytes, the active site cysteine of caspase 3 is inhibited by nitrosylation, and undergoes denitrosylation upon fas activation and apoptosis [7]. NO controls smooth muscle contraction and thus influences vessel, bowel, bronchial, uterine, ureteral, and ductal tension and contractions [3].
There are three major isoforms of NOS (NOS1, NOS2, and NOS3) encoded by three separate genes [1]. While these isoforms are expressed in several cell types and tissues, NOS1 is found mainly in neural and muscle tissues, NOS2 in monocytes/macrophages, hepatocytes, and chondrocytes, and NOS3 in endothelial cells. A variety of agents inhibit the enzymatic function of NOS [1, 8]. Compounds that bind, quench, or scavenge NO have been used to negate the effects of NO [9]. These scavengers include heme compounds and cobalt-containing cobalamins and associated molecules. Certain cobalamins bind NO and thus quench their effects [10–21]. We hypothesized that in addition to binding NO, certain corrin derivatives would also directly interact with NOS and inhibit NOS activity, and that these NOS inhibitor effects would be separate and different from their scavenging effects. The biochemical and molecular modeling analyses presented here suggest a promising role of isoform-selective NOS inhibition by corrins. To our knowledge, no one has previously reported that corrins inhibit NOS.
Materials and methods
We prepared highly purified, recombinant rat NOS1 and human NOS2 (holoprotein and oxygenase domain) as previously described [22, 23]. Recombinant bovine NOS3 was purchased from Cayman (Ann Arbor, MI). Tetrahydrobiopterin and carboxy-PTIO [2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (potassium salt)] were from Alexis (San Diego, CA). 14-C-labeled L-arginine and 14-C-labeled citrulline were from Perkin-Elmer Life and Analytical Sciences (Boston, MA). All other reagents were from Sigma-Aldrich (St. Louis). Glutathionylcobalamin was synthesized as described before [24].
The Raw 264.7 mouse macrophage cell line was from the ATCC (Manassas, VA). Cells were maintained by serial passage in RPMI-1640 in 10% fetal bovine serum as noted earlier [25]. NO was measured using diaminofluorescein-FM (4-amino-5-methylamino-2′, 7′-difluro-fluorescein) from Invitrogen-Molecular Probes. Briefly, cells were treated with 500 units/ml of murine interferon-gamma for 24 hours, incubated with 10 μM DAF-FM for 30 minutes, and washed. The cells were then assessed for fluorescence in a platereader fluorimeter (excitation/emission 495/515 nm); NO formation is proportional to fluorescence. To determine NOS activity, we incubated 2.9 μM 14-C-labeled-L-arginine at 37°C with 5 nM recombinant NOS1 or NOS2 in 50 mM HEPES buffer (pH 7.5), 10 μM unlabeled L-arginine, 10 μM flavin adenine dinucleotide, 1 mM dithiothreitol, 100 μM tetrahydrobiopterin, and 200 μM NADPH in the absence or presence of different concentrations of the compound in question for 60 minutes at 37° C [26, 27]. The reaction was stopped by addition by adding 0.1 M HEPES, pH 5.5, 1 mM L-citrulline, and 5 mM EDTA, and 14-C-labeled L-arginine was separated from 14-C-labeled citrulline using a Dowex 50W-X8 cation exchange column. 14-C-labeled L-arginine binds to the Dowex, and 14-C-labeled citrulline elutes free from the column [26]. Radioactivity was determined with a scintillation counter, and percentage NOS inhibition was calculated. None of the corrin compounds quenched detection of the radioactivity. Spectroscopic measurement for NO and CO binding were determined using a Hitachi U2010 spectrometer (San Jose, CA) using computer-assisted data collection software as reported before [15, 22, 23, 28].
Molecular modeling was used to investigate the feasibility of docking cobalamins in the catalytic sites of NOS1 and NOS2. We used the InsightII/Discover suite of programs (Accelrys) with structures from PDB entries P_1DWW (NOS2) and P_1ZVL (NOS1). Side chains were individually torsioned to remove intramolecular clashes while monitoring local energies (Van der waals and Coulomb) to eliminate unrealistic conformations. Single bonds in cobalamin were torsioned to reposition dimethylbenzimidazole for reduced steric effects. No attempt to estimate actual binding energies was made because of the lack of a force field for corrins, and because the estimate required several layers of side chain adjustment.
Results
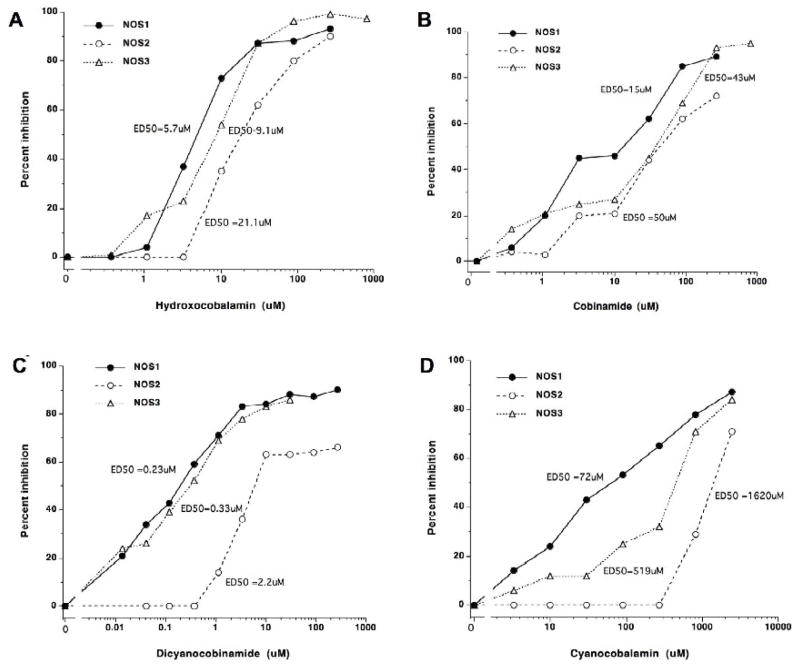
We first examined the effects of various corrin-related compounds on the catalytic activity of NOS. Using 14-C-labeled L-arginine, purified recombinant NOS1, NOS2, or NOS3, and NOS co-factors, we tested the compounds for their abilities to influence conversion of 14-C-L-arginine to 14-C-L-citrulline. The general structures of corrins/cobalamins are shown in Figure 1. Hydroxocobalamin (OH-Cbl), cobinamide (Cbi), and dicyanocobinamide (CN2-Cbi) were potent NOS1, NOS2, and NOS3 inhibitors, while cyanocobalamin had much less activity (Figure 2). In general, the constitutive NOS isoforms NOS1 and NOS3 were inhibited more than was NOS2, but all were inhibited by the agents. Methylcobalamin and adenosylcobalamin had very little ability to inhibit NOS. Illumination of these compounds liberates the methyl and adenosyl groups from cobalamin, generating OH-Cbl [15]. The photoactivated derivatives had inhibitory potency comparable to authentic OH-Cbl (Figure 3). Wheatley hypothesized that a glutathionylcobalamin might be able to directly modulate NOS activity but reported no data to support this hypothesis [29]. Our studies show that glutathionylcobalamin inhibited NOS1, NOS2, and NOS3 with comparable potencies [effective doses to inhibit 50% activity (ED50s)] to those of OH-Cbl. Table 1 summarizes the inhibition data.
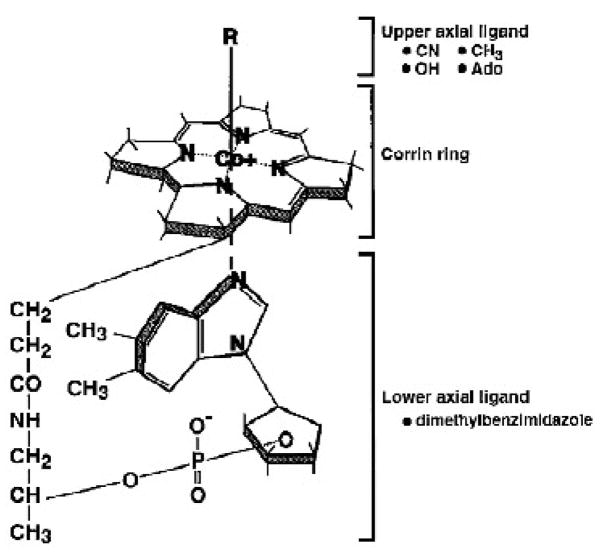
Figure 1. General structure of corrins/cobalamins.
The figure demonstrates various upper axial ligands, the corrin ring, and a lower axial ligand. Hydroxocobalamin has a hydroxyl group at the R position. Cyanocobalamin has a cyano group at the R position. Methylcobalamin and adenosylcobalamin have a methyl and adenosyl group, respectively, at the R position. Cobinamide lacks the lower axial ligand and has a hydroxyl group at the R position. Dicyanocobinamide has a cyano group as the lower axial ligand and at the R position.
Figure 2. A–D. Inhibition of NOS1, NOS2, and NOS3 enzymatic by cobinamides and cobalamins.
The enzymatic activity of purified NOS1, NOS2, or NOS3 was assessed in presence or absence of different concentrations of (A) hydroxocobalamin, (B) cobinamide, (C) dicyanocobinamide, or (D) cyanocobalamin. Results are expressed as percent inhibition. The estimated effective dose for 50% inhibition (ED50) is displayed. Note the differing scales of the x axes.
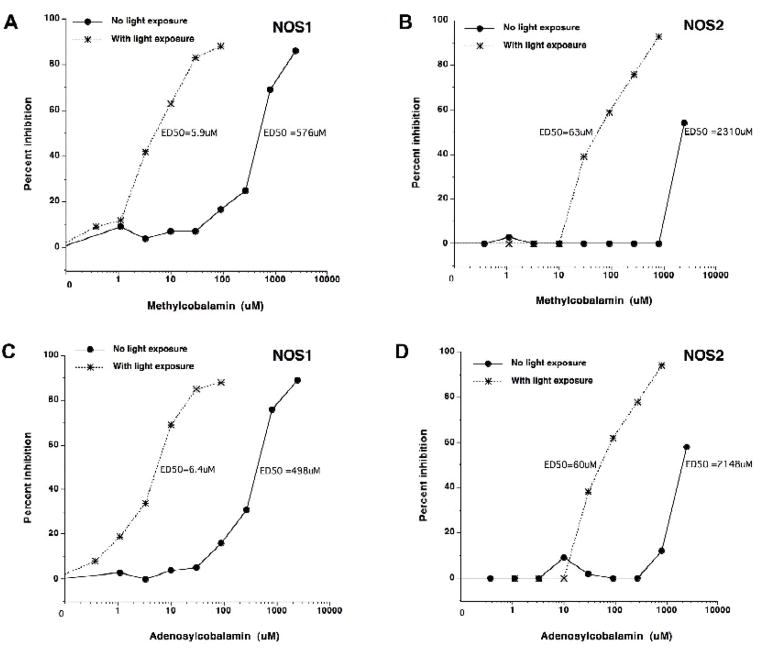
Figure 3. A–D. Influence of light on inhibition of purified, recombinant human NOS1 and NOS2 by methylcobalamin and adenosylcobalamin.
The effect of methylcobalamin (not exposed to light or exposed to light) and adenosylcobalamin (not exposed to light or exposed to light) on NOS1 (A and C) or NOS2 (B and D) activity is displayed. The agents were protected from light or exposed to light for 60 minutes. We performed the enzyme assays in near-dark conditions (as low light as possible). Results are expressed as percent inhibition. The effective dose for 50% inhibition (ED50) is displayed.
Light dissociates the methyl- or adenosyl- group from the agent resulting in hydroxocobalamin. Methylcobalamin and adenosylcobalamin have very little NOS inhibitory activity, but after light-induced change to hydroxocobalamin, they actively inhibit NOS1 and NOS2 activity.
Table 1. Inhibition of nitric oxide synthases by cobalamins and cobinamides.
The effects of the indicated agents on the enzymatic activity of NOS1 and NOS2 were determined. Methyl-cobalamin and adenosylcobalamin were tested either having been exposed to light or not exposed to light. Results are displayed as the ED50 (μM).
| Estimated ED50 (μM) | |||
|---|---|---|---|
| NOS1 | NOS2 | NOS3 | |
| Hydroxocobalamin | 5.7 | 21.1 | 9.1 |
| Cobinamide | 15.0 | 50.0 | 43.0 |
| Dicyanocobinamide | 0.23 | 2.29 | 0.33 |
| Cyanocobalamin | 72.0 | 1620.0 | 519.0 |
| Glutathionylcobalamin | 9.6 | 29.0 | 41.0 |
| Carboxy-PTIO | 1360.0 | 1359.0 | - |
| Methylcobalamin (no light) | 576.0 | 231.0 | - |
| Methylcobalamin (with light) | 5.9 | 63.0 | - |
| Adenosylcobalamin (no light) | 498.0 | 2148.0 | - |
| Adenosylcobalamin (with light) | 6.4 | 60.0 | - |
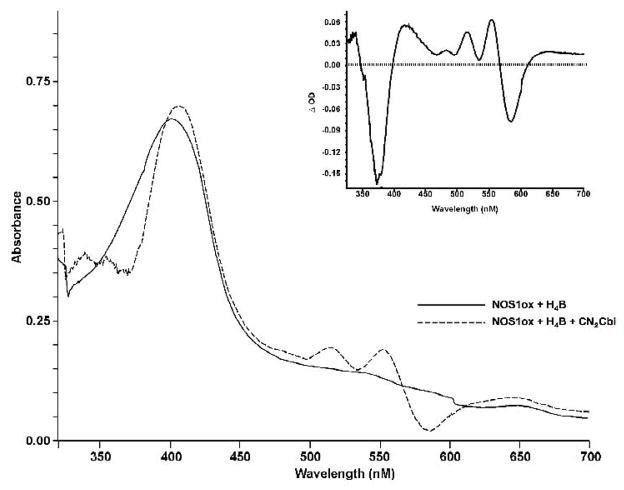
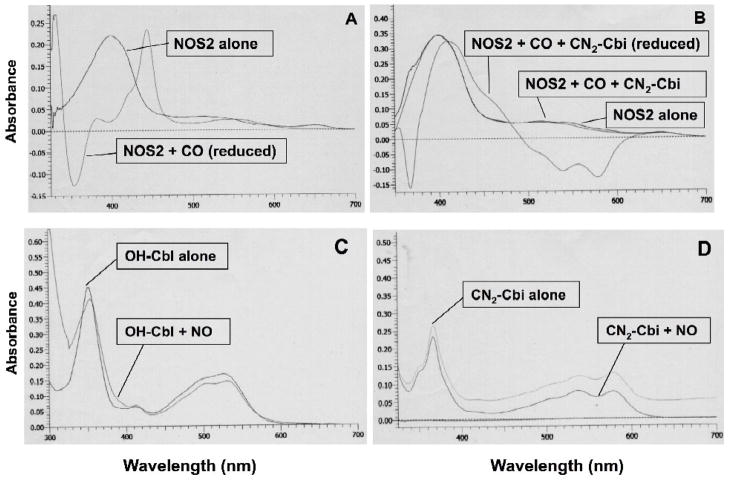
Spectroscopic studies of cobalamins and cobinamides binding to NOS provide further details of the interaction of the enzymes with these inhibitors. We analyzed binding of CN2Cbi to the H4B-replete NOS1 oxygenase domain (NOS1ox) by examining the effects of CN2Cbi binding on the heme spectrum at room temperature (Figure 4). Binding produced a significant red shift in the high spin heme Soret peak of NOS1ox (395 nm to 412 nm), indicative of conversion to the low spin state. The binding spectrum is displayed in Figure 4, with an insert showing the difference spectrum. These results suggest that CN2Cbi interacts directly with the NOS heme binding pocket converting high spin ferric to a low spin ferric adduct. The low spin species formed could be the result of direct interaction between a corrin-associated group and heme, but it could also be the result of corrin displacement of arginine, allowing the interaction of an active site water molecule with heme iron. Arginine displacement would also require close approach of the corrin to the active site, resulting in binding domain overlap. Our molecular modeling analysis (see below) further supports the possibility of corrin penetration into the heme pocket. These data are the first to demonstrate a direct interaction between a corrin and a NOS heme protein.
Figure 4. Effect of dicyanocobinamide on the UV-visible spectra of purified, recombinant NOS1 oxygenase domain.
Binding of 40 μM CN2Cbi to NOS1 oxygenase domain (NOS1ox; ~ 10 μM) in 40 mM bis- tris-propane buffer (pH 7.5) containing 5% glycerol and 10 μM H4B with at room temperature. The solid line represents NOS1ox alone, and the dashed line NOS1ox with CN2Cbi after 200 minutes’ interaction. The inset displays the difference spectrum generated by subtracting NOS1ox initial spectrum from the spectrum after 200 minutes with CN2Cbi.
Oxygen binding to reduced heme is an obligatory step in the catalytic cycle of NOS. Binding of the oxygen analog carbon monoxide to ferroheme shifts the Soret band of the heme UV-visible absorbance spectrum to 446 nm [30], analogous to the well known CO ferroheme complex of P450 cytochromes (Figure 5A). As shown in Figure 5B, CN2-Cbi markedly diminished CO-induced spectral changes in NOS2 oxygenase domain, indicating that CN2-Cbi blocks formation of the ferrous-CO adduct. This suggests that CN2-Cbi-mediated inhibition of NOS activity is due in part to blocking of oxygen binding to heme heme, along with the arginine displacement implied by conversion to the low spin state. To our knowledge, there is no prior report of an interaction of a cobalamin or a cobinamide with NOS.
Figure 5. A–D. Spectroscopy of dicyanocobinamide interactions with purified, recombinant NOS2 oxygenase domain, or NO.
Dicyanocobinamide reacts directly with purified, recombinant NOS2 oxygenase domain (A & B): Oxygen reacts directly with the reduced iron of the heme group in NOS. We used carbon monoxide (CO) (that simulates O2 in binding to the reduced iron) to determine the influence of CN2-Cbi on oxygen binding to NOS. The reaction of purified NOS2 oxygenase domain (approximately 3 μM final concentration) with CO in a reduced state (with dithionite) markedly changed the UV/vis spectrum, with appearance of the typical CO-bound Fe+2-heme peak at 445 nm (A). However, in the presence of CN2-Cbi, there was no appearance of the 445 nm peak induced by CO in a reduced state using NOS2 oxygenase domain at a final concentration of approximately 4.5 μM (B). Similar findings were noted with OH-Cbl instead of CN2-Cbi.
Hydroxocobalamin binds NO, but dicyanocobinamide does not (C & D). NO reacts with OH-Cbl (C) as indicated by the change in the UV/Vis spectrum (shift of peak from 352 nm to 354–355 nm, and alteration in the 520 nm region). However, NO does not react with CN2-Cbi (D) (no change in spectrum).
Inhibition of NOS1 by CN2-Cbi was rapid with 56% inhibition by 2 μM at 7 seconds, 57% at 15 seconds, 61% at 30 seconds, and 68% at 60 seconds. The inhibitory effect of CN2-Cbi for NOS1 was not blocked by tetrahydrobiopterin (100–800 μM) or NADPH (100–400 μM) (assessed by the 14-C-L-arginine to 14-C-L-citrulline assay). Also, L-arginine (0.5 to 10.0 mM) did not reverse CN2-Cbi inhibition of NOS1 (assessed by spectroscopic ligand binding). NOS1 inhibition by CN2-Cbi could not be reversed. We incubated 60 μM CN2-Cbi with 3.8 μM NOS1 in 0.1 ml for 30 minutes at room temperature and dialyzed the solution for 18 hours against 40 mM Bis Tris propane buffer (BTP, pH 7.5) supplemented with 10 μM tetrahydrobiopterin and 1 mM L-arginine. NOS1 was still fully inhibited after dialysis (78% inhibition versus 60% inhibition without the dialysis). CN2-Cbi inhibition of NOS cannot be described as competitive for tetrahydrobiopterin, NADPH, or L-arginine. Taken together, the results suggest that the binding domain of CN2-Cbi overlaps the binding site for substrate oxygen and arginine. It is not feasible for the bulky corrin moiety to overlap the CO binding site without also blocking the arginine-binding site.
As noted above, OH-Cbl can directly bind NO to its cobalt and quench or scavenge NO actions [10–21]. We tested the ability of CN2-Cbi to interact with NO (Figure 5C and 5D). NO reacted with OH-Cbl as indicated by the change in the UV/visible spectrum (Figure 5C; shift of peak from 352 nm to 354–355 nm, and alteration in the 520 nm region). However, NO did not react with CN2-Cbi (Figure 5D); there was essentially no change in its spectrum. CN2-Cbi does not bind/quench NO, and carboxy-PTIO (a known NO binding/quenching compound) does not inhibit NOS (Table 1). Therefore, we conclude that NO binding/quenching does not influence NOS activity and that the NOS inhibitory actions of cobalamins and cobinamide are not related to NO binding/quenching. Instead, direct binding of cobalamins and cobinamide to the NOS active site causes the inhibition.
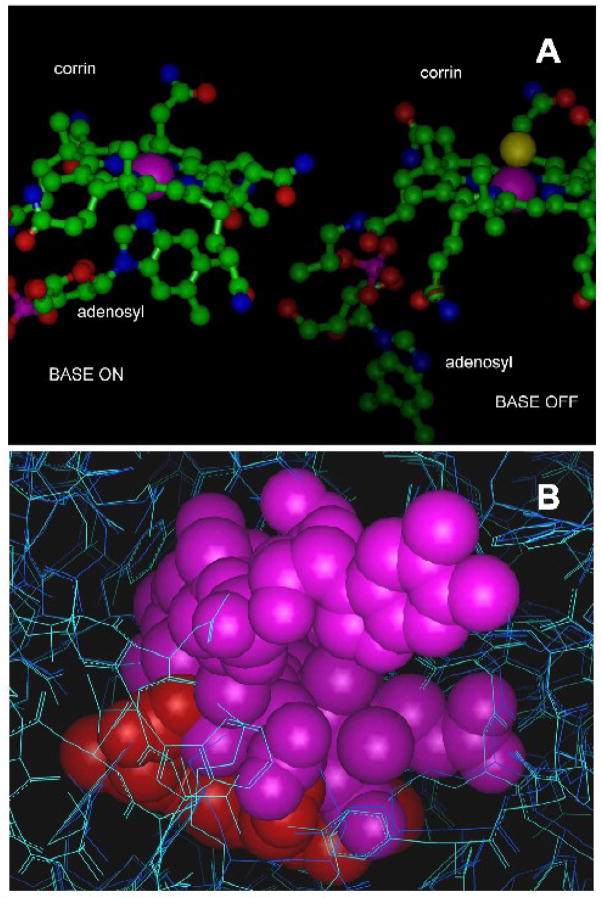
To explore potential mechanisms of the NOS-corrin interactions, we used molecular modeling investigations with manual computer-assisted docking analysis (Figure 6) to examine the feasibility of corrin species access to the catalytic site. Cobalamins exist in aqueous solution in two main conformations, “base-on” and “base-off.” In the “base-on” conformation, the dimethylbenzimidazole moiety provides an axial ligand to the cobalt atom (Figure 6A). As shown in Figure 6B, there is enough space in the heme and substrate binding pocket of NOS1 and NOS2 to accommodate cobalamin in the “base-off” conformation. It was necessary to adjust the conformation of binding site residues and ligand to allow ligand binding, but these adjustments do not come at the expense of large amounts of energy. A critical point revealed by docking exercises is that a very large interaction surface between base-off cobalamin and NOS (~ 800 Angstrom) can be obtained despite the high degree of exposure of the dimethylbenzimidazole moiety to solvent water. The complexity and scale of the binding region and the need for adjustments in side chain orientation to allow the cobalamin to fit prevented us from confidently defining specific interactions within the pocket. However, our analyses confirm that access of corrins to the active site is feasible. The large interaction surface accounts for the high affinity of NOS1 for cobalamins. Small differences in pocket geometry provide slightly more space in NOS1 than in NOS2 (Figure 6B). This may account for the generally higher affinity of NOS1 for cobalamins.
Figure 6. Structural studies of cobalamin-NOS interactions.
6A. Structures of adenosylcobalamin in the “base-on” and “base-off” configurations showing the positions of the corrin ring and the dimethylbenzimidazole moiety. The purple spheres represent the Van der Waals surface of the cobalt atom. The structure of the “base-off” form was taken from the structure of glutamate mutase reported by Reitzer et al (PDB entry 1cb7) [44]. In this structure, the cobalt ligand trans to the methyl axial ligand is a histidyl residue (not shown) supplied by the protein. The “base-on” form shown was taken from the structure of cobalamin bound to transcobalamin reported by Wuerges et al (PDB entry 2bb5) [45]. The axial ligand trans to dimethylbenzimidazole is again a histidyl residue (not shown) supplied by the protein. Slight differences in the planarity of the corrins include movement of the cobalt towards dimethylbenzimidazole in the “base-on” configuration. Considerable flexibility in the molecule exists, making many other orientations of the base possible.
6B. Docking of the “base-off” form of cobalamin (as in Figure 5A) in the heme pockets of NOS2 (dark blue) and NOS1 (cyan), illustrating the extent of the ligand-binding cavity relative to the size of cobalamin. Heme is shown in red and cobalamin in purple. The very large substrate-binding pocket accommodates surprisingly large ligands, including porphyrins (similar in size and shape to the corrin shown here). The insertion of cobalamin into the heme pocket is ultimately limited by a beta sheet near V567 (NOS1). The configuration shown generates a 0.4Angstrom clash with the valine side chain in the crystal structure from PDB P_1ZVL, and represents the approximate limit of insertion of cobalamin, assuming slight rearrangements in the backbone are possible. A similar configuration with approximately 80% overlap of the corrin and heme can be obtained without backbone or unrelaxable side chain clashes. Slight torsioning of some active site residue side chains and repositioning of the DMBzI moiety of the cobalamin (visible as rings in solid rendering at the upper right) was necessary. The aromatic side chains visible at the bottom of the figure at the edge of the access channel are W306 and Y706 in NOS1.
The effect of axial ligands on affinity suggests strongly that specific interactions within the pocket are important, and that these interactions account for part of the specificity for cobalamins based on axial ligation. Hydrogen bonds and metal-ligand bonds may both contribute—numerous groups are available for hydrogen bond formation in the pocket, including the conserved residues involved in binding arginine. There are no active site histidyl residues available to bond to cobalt. However, other residues may supply a ligand, including a group of tryptophan residues surrounding the heme pocket.
The inhibitory potency of CN2-Cbi relative to CN-Cbl corresponds with results of our docking exercises and strongly suggests that the ‘base-off’ state is responsible for inhibition. The cobalamin derivatives that are primarily in the “base-on” state in solution must overcome the energetic cost of removal of the ligand by making strong interactions in the binding site [31]. If only the “base-on”/“base-off” ratio were significant, methylcobalamin would be the best inhibitor other than CN2-Cbi. Clearly, it is also necessary to have an axial group capable of making strong specific interactions. OH-Cbl is able to overcome a large energy cost corresponding to a factor of 10−7–10−8 in the “base-on”/“base-off” equilibrium constant, while Me-Cbl, with a much smaller cost, is a very poor inhibitor. It is unlikely, however, that cobalamins bind to NOS initially in the “base-off” state. The fraction of “base-off” molecules in a solution of OH-Cbl is so low (a few parts in 108) that binding would be too slow to account for the observed results. It is likely that initial weak corrin binding occurs in the “base-on” state, a condition in which the corrin can be docked partially into the heme pocket with the corrin at an approximate 45° angle to the heme (not shown). Intermediate, weakly bound states could provide a mechanism to reach the “base-off” state, allowing reasonably rapid binding of cobalamins that are essentially all in the “base-on” state in solution.
The total “base-off” structure of CN2-Cbi and the great potential of OH-Cbl to hydrogen-bond in the pocket help explain their low ED50s. The slightly larger active site pocket of NOS1 compared to NOS2 and NOS3 likely explains the lower ED50s for NOS1.
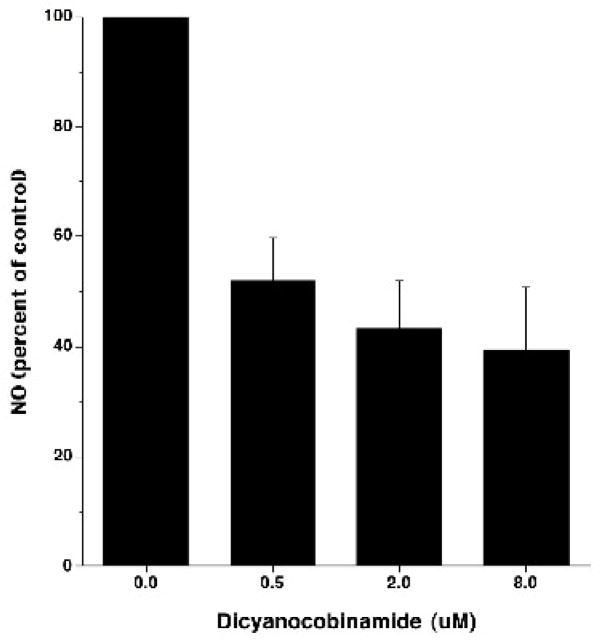
We also examined the ability of CN2-Cbi to influence NO production by cells expressing NOS. When we incubated CN2Cbi with mouse macrophage Raw 264.7 cells activated with interferon-gamma to produce NO in vitro, there was a dose-dependent inhibition of NO production (Figure 7). This indicates that compounds of this class can inhibit NO production by NOS in living cells.
Figure 7. Dicyanocobinamide inhibition of NO formation by mouse macrophages.
Cells of the mouse macrophage cell line Raw 264.7 were incubated 24 hours with 500 units/ml murine interferon gamma to activate them for NO production. The cultures also contained 0, 0.5, 2.0, or 8.0 μM dicyanocobinamide for the entire culture period. At the end of the culture period, the cells were assessed for NO production using DAF-FM and a fluorimeter. Results are expressed as NO production (percent of control). The error bars show the SEM. This is from 6 separate experiments.
Discussion
While cobalamins have been previously noted to bind and quench the actions of NO, no one has shown that they directly inhibit NOS. This is the first full report that OH-Cbl, Cbi, and CN2Cbi can potently inhibit the enzymatic function of NOS and thus block formation of NO [we earlier reported preliminary findings of this in abstract form [32]]. OH-Cbl and Cbi can both bind and scavenge NO. Thus, these two agents are able to bifunctionally inhibit the NOS/NO system by decreasing NOS activity and NO synthesis and quenching existing NO. Unlike OH-Cbl and Cbi, we show that CN2Cbi does not bind NO, but it directly interacts with NOS and potently inhibits NOS activity.
Our structural modeling and uv-visible spectroscopy studies indicate that NOS inhibition by these cobalamins and cobinamides is likely mediated by approximation of the agent to the NOS heme in active site. The active site pocket is large and accommodates the agents. The greater ability to inhibit NOS1 than NOS2 appears to be structurally based—the NOS1 active site pocket is larger than the NOS2 pocket. As noted, CN2Cbi is the most potent inhibitor. Absence of the lower axial ligand (essentially having a continuous “base-off” configuration) enhances access to the NOS heme. Based on inhibitory ability of OH-Cbl and structural modeling, it appears that hydrogen bonding or other specific interactions between the polar hydroxyl group of OH-Cbl and NOS contribute significantly to the OH-Cbl-NOS binding. Absence of a comparably polar upper axial ligand in CN-Cbl and a majority “base-on” configuration likely explains the poor ability of CN-Cbl to inhibit NOS. Other researchers have reported inhibition of NOS by certain heme derivatives such as ferriprotoporphyrin IX or cobalt protoporphyrin [33–35].
Me-Cbl and Ado-Cbl have very poor inhibitory activity. However, if they are exposed to light (a process that dissociates the methyl and adenosyl groups from the molecules, converting them to OH-Cbl), they inhibit at concentrations similar to OH-Cbl. It is possible that this property of light-activation for NOS inhibition could be useful for site- and time-selective delivery of the agents. Large amounts of Me-Cbl or Ado-Cbl could be administered intravenously, and then accessible areas could be illuminated to selectively release the NOS inhibitor/NO quencher in a local area.
During NO synthesis, NOS flavins transfer NADPH-derived electrons to heme iron. This process enables heme to bind and activate oxygen in both steps of NO synthesis. Tetrahydrobiopterin, a required NOS cofactor, binds to NOS tightly near the heme and is necessary for the homodimer formation and interaction with L-arginine [2]. NOS usually produces reduced oxygen species as products of leak reactions, but in the absence of L-arginine or BH4, the oxidase function of NOS has a greatly increased rate of generation of superoxide from oxygen. Superoxide can contribute to inflammation and tissue damage in disorders such as arthritis, vasculitis, nephritis, and reperfusion injury [36]. We expect that these corrin NOS inhibitors would inhibit both NO and superoxide generation by NOS.
Cobalamins (e.g., OH-Cbl) have been used to treat humans with vitamin B12 deficiency and cyanide poisoning successfully with no or few toxicities [37, 38]. Small doses of OH-Cbl (e.g., 1 mg daily to monthly given orally, intravenously, or intramuscularly) are used to treat vitamin B12 deficiency, while much larger doses (e.g., 5 to 15 gm intravenously) are used to treat cyanide poisoning [37, 38]. The gram doses used for cyanide poisoning produce transient, mild hypertension as a side effect. This hypertension is likely due to in vivo OH-Cbl inhibition of NOS and quenching of NO.
We speculate that corrins/cobalamins may play a role in modulating NOS enzymatic function (and NO formation) in vivo. Physiological concentrations of OH-Cbl, Me-Cbl, and Ado-Cbl are very low in vivo (pM to low nM range) and thus might not be in concentrations high enough to modulate NOS activity. However, a variety of unique cobalamins, cobinamides, and corrins are found in vivo [39, for example] that might have high higher capacity to function as NOS inhibitors. Wheatley hypothesized that a cobalamin might be able to directly modulate NOS activity, but reported no data regarding this hypothesis [29]. Patients with severe vitamin B12 deficiency have neurologic and psychiatric abnormalities, and white matter lesions [40, 41]. It is possible that lack of nervous system cobalamins or cobinamides could play a role in the pathophysiology of these disorders—the low levels and lack of NOS inhibition and NO quenching might disturb neurotransmission or allow neuronal damage. Some patients with vitamin B12 deficiency report subjective improvement by vitamin B12 administration before there are any improvements in hemoglobin levels [40, 42]. This subjective improvement could be related to cobalamin/cobinamide effects on NOS1 in brain, with resultant changes in neuropsychiatric sensations. Administration of various cobalamins has been reported to modulate neuropsychiatric function in humans (e.g., circadian body temperatures and alertness [43]).
Cobalamins and cobinamides could potentially be useful agents for inhibiting NOS and NO generation in vivo in humans with certain diseases in which NO acts in a deleterious fashion (e.g., inflammatory diseases). These agents could be given in high doses, comparable to high dose OH-Cbl administered to patients with cyanide poisoning [37, 38]. With intravenous administration of gram amounts of OH-Cbl, peak blood levels over 1000 micromolar are achieved with minimal side effects (red urine and mild, reversible hypertension). Cobalamins or cobinamides could also be administered acutely, subacutely, or chronically in lower amounts orally, parenterally, topically, or by inhalation. With an agent such as CN2Cbi, one might be able to achieve anti-inflammatory effects at much lower doses with very few side effects.
Acknowledgments
The work was supported in part by the National Institutes of Health, the Leukemia & Lymphoma Society, and the V.A. Research Service (JBW).
Abbreviations
- Ado-Cbl
adenosylcobalamin
- ATCC
American Tissue Culture Collection
- CO
carbon monoxide
- carboxy-PTIO
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (potassium salt)
- Cbl
cobalamin(s)
- Cbi
cobinamide
- Cbi
cobinamide
- CN-Cbl
cyanocobalamin
- diaminofluorescein-FM
4-amino-5-methylamino-2′, 7′-diflurofluorescein
- CN2-Cbi
dicyanocobinamide
- OH-Cbl
hydroxocobalamin
- OH-Cbl
hydroxocobalamin
- Me-Cbl
methyl-cobalamin
- NOS
nitric oxide synthase
- NO
nitric oxide
- NOS1ox
NOS1 oxygenase domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 3.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 4.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg JB. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes - a review [Review] Mol Med. 1998;4:557–591. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li JR, Billiar TR, Talanian RV, Kim YM. Nitric Oxide Reversibly Inhibits Seven Members Of the Caspase Family Via S-Nitrosylation. Biochem Biophys Res Commun. 1997;240:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 7.Mannick JB, Hausladen A, Liu LM, Hess DT, Zeng M, Miao QX, Kane LS, Gow AJ, Stamler JS. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 8.Babu BR, Griffith OW. Design of isoform-selective inhibitors of nitric oxide synthase [Review] Curr Opin Chem Biol. 1998;2:491–500. doi: 10.1016/s1367-5931(98)80125-7. [DOI] [PubMed] [Google Scholar]
- 9.Fricker SP. Nitrogen monoxide-related disease and nitrogen monoxide scavengers as potential drugs. In: Sigel A, Sigel H, editors. Met Ions Biol Syst. Basel: Marcel Dekker, Inc.; 1999. pp. 665–721. [PubMed] [Google Scholar]
- 10.Greenberg SS, Xie JM, Zatarain JM, Kapusta DR, Miller MJS. Hydroxocobalamin (Vitamin B12a) Prevents and Reverses Endotoxin-Induced Hypotension and Mortality In Rodents - Role Of Nitric Oxide. J Pharmacol Exp Ther. 1995;273:257–265. [PubMed] [Google Scholar]
- 11.Broderick KE, Singh V, Zhuang S, Kambo A, Chen JC, Sharma VS, Pilz RB, Boss GR. Nitric oxide scavenging by the cobalamin precursor cobinamide. J Biol Chem. 2005;280:8678–8685. doi: 10.1074/jbc.M410498200. [DOI] [PubMed] [Google Scholar]
- 12.van der Kuy PH, Merkus FW, Lohman JJ, ter Berg JW, Hooymans PM. Hydroxocobalamin, a nitric oxide scavenger, in the prophylaxis of migraine: an open, pilot study. Cephalalgia. 2002;22:513–519. doi: 10.1046/j.1468-2982.2002.00412.x. [DOI] [PubMed] [Google Scholar]
- 13.Kruszyna H, Magyar JS, Rochelle LG, Russell MA, Smith RP, Wilcox DE. Spectroscopic studies of nitric oxide (NO) interactions with cobalamins: reaction of NO with superoxocobalamin(III) likely accounts for cobalamin reversal of the biological effects of NO. J Pharmacol Exp Ther. 1998;285:665–671. [PubMed] [Google Scholar]
- 14.Kikuchi M, Kashii S, Honda Y, Tamura Y, Kaneda K, Akaike A. Protective effects of methylcobalamin, a vitamin B12 analog, against glutamate-induced neurotoxicity in retinal cell culture. Invest Ophthalmol Vis Sci. 1997;38:848–854. [PubMed] [Google Scholar]
- 15.Brouwer M, Chamulitrat W, Ferruzzi G, Sauls DL, Weinberg JB. Nitric oxide interactions with cobalamins: biochemical and functional consequences. Blood. 1996;88:1857–1864. [PubMed] [Google Scholar]
- 16.Akaike A, Tamura Y, Sato Y, Yokota T. Protective effects of a vitamin B12 analog, methylcobalamin, against glutamate cytotoxicity in cultured cortical neurons. Eur J Pharmacol. 1993;241:1–6. doi: 10.1016/0014-2999(93)90925-8. [DOI] [PubMed] [Google Scholar]
- 17.Li CG, Rand MJ. Effects of hydroxocobalamin and haemoglobin on NO-mediated relaxations in the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1993;20:633–640. doi: 10.1111/j.1440-1681.1993.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 18.Jenkinson KM, Reid JJ, Rand MJ. Hydroxocobalamin and Haemoglobin Differentiate Between Exogenous and Neuronal Nitric Oxide In the Rat Gastric Fundus. Eur J Pharmacol. 1995;275:145–152. doi: 10.1016/0014-2999(94)00762-v. [DOI] [PubMed] [Google Scholar]
- 19.Rochelle LG, Morana SJ, Kruszyna H, Russell MA, Wilcox DE, Smith RP. Interactions between hydroxocobalamin and nitric oxide (NO) - Evidence for a redox reaction between NO and reduced cobalamin and reversible NO binding to oxidized cobalamin. J Pharmacol Exp Ther. 1995;275:48–52. [PubMed] [Google Scholar]
- 20.Akaike A, Tamura Y, Sato Y, Yokota T. Protective effects of a vitamin B12 analog, methylcobalamin, against glutamate cytotoxicity in cultured cortical neurons. Eur J Pharmacol. 1993;241:1–6. doi: 10.1016/0014-2999(93)90925-8. [DOI] [PubMed] [Google Scholar]
- 21.Sharma VS, Pilz RB, Boss GR, Magde D. Reactions of nitric oxide with vitamin B12 and its precursor, cobinamide. Biochemistry (Mosc) 2003;42:8900–8908. doi: 10.1021/bi034469t. [DOI] [PubMed] [Google Scholar]
- 22.Gao YT, Smith SM, Weinberg JB, Montgomery HJ, Newman E, Guillemette JG, Ghosh DK, Roman LJ, Martasek P, Salerno JC. Thermodynamics of oxidation-reduction reactions in mammalian nitric oxide synthase isoforms. J Biol Chem. 2004;279:18759–18766. doi: 10.1074/jbc.M308936200. [DOI] [PubMed] [Google Scholar]
- 23.Newman E, Spratt DE, Mosher J, Cheyne B, Montgomery HJ, Wilson DL, Weinberg JB, Smith SM, Salerno JC, Ghosh DK, Guillemette JG. Differential activation of nitric-oxide synthase isozymes by calmodulin-troponin C chimeras. J Biol Chem. 2004;279:33547–33557. doi: 10.1074/jbc.M403892200. [DOI] [PubMed] [Google Scholar]
- 24.Xia L, Cregan AG, Berben LA, Brasch NE. Studies on the formation of glutathionylcobalamin: any free intracellular aquacobalamin is likely to be rapidly and irreversibly converted to glutathionylcobalamin. Inorg Chem. 2004;43:6848–6857. doi: 10.1021/ic040022c. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh DK, Misukonis MA, Reich C, Pisetsky DS, Weinberg JB. Host response to infection: the role of CpG DNA in induction of cyclooxygenase 2 and nitric oxide synthase 2 in murine macrophages. Infect Immun. 2001;69:7703–7710. doi: 10.1128/IAI.69.12.7703-7710.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, Haney AF, Granger DL. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS). Analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 27.Sharara AI, Perkins DJ, Misukonis MA, Chan SU, Dominitz JA, Weinberg JB. Interferon (IFN)-alpha activation of human blood mononuclear cells in vitro and in vivo for nitric oxide synthase (NOS) type 2 mRNA and protein expression - possible relationship of induced NOS2 to the anti-hepatitis C effects of IFN-alpha in vivo. J Exp Med. 1997;186:1495–1502. doi: 10.1084/jem.186.9.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh DK, Rashid MB, Crane B, Taskar V, Mast M, Misukonis MA, Weinberg JB, Eissa NT. Characterization of key residues in the subdomain encoded by exons 8 and 9 of human inducible nitric oxide synthase: a critical role for Asp-280 in substrate binding and subunit interactions. Proc Natl Acad Sci U S A. 2001;98:10392–10397. doi: 10.1073/pnas.181251298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheatley C. Cobalamin in inflammation III - glutathionylcobalamin and methylcobalamin/adenosylcobalamin coenzymes: the sword in the stone? How cobalamin may directly regulate the nitric oxide synthases. J Nutr Environ Med. 2007;16:212–226. doi: 10.1080/13590840701791863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMillan K, Bredt DS, Hirsch DJ, Snyder SH, Clark JE, Masters BS. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci U S A. 1992;89:11141–11145. doi: 10.1073/pnas.89.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamza MSA, Zou X, Brown KL, van Eldik R. Thermodynamic and kinetic data for the base-on/base-off equilibration of alkylcobalamins. Eur J Inorg Chem. 2003:268–276. [Google Scholar]
- 32.Weinberg JB, Chen Y, Beasley BE, Ghosh DK. Cobalamins and cobinamides inhibit nitric oxide synthase enzymatic activity. Blood. 2005;106(Suppl 1):627a. [Google Scholar]
- 33.Jozkowicz A, Dulak J. Effects of protoporphyrins on production of nitric oxide and expression of vascular endothelial growth factor in vascular smooth muscle cells and macrophages. Acta Biochim Pol. 2003;50:69–79. [PubMed] [Google Scholar]
- 34.Lim MD, Lorkovic IM, Ford PC. NO and NO(x) interactions with group 8 metalloporphyrins. J Inorg Biochem. 2005;99:151–165. doi: 10.1016/j.jinorgbio.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Wolff DJ, Naddelman RA, Lubeskie A, Saks DA. Inhibition Of Nitric Oxide Synthase Isoforms By Porphyrins. Arch Biochem Biophys. 1996;333:27–34. doi: 10.1006/abbi.1996.0360. [DOI] [PubMed] [Google Scholar]
- 36.Kumar V, Abbas AK, Fausto N. Pathologic basis of disease. Philadelphia: Elsevier Saunders; 2005. [Google Scholar]
- 37.Forsyth JC, Mueller PD, Becker CE, Osterloh J, Benowitz NL, Rumack BH, Hall AH. Hydroxocobalamin as a cyanide antidote: safety, efficacy and pharmacokinetics in heavily smoking normal volunteers. Journal of Toxicology and Clinical Toxicology. 1993;31:277–294. doi: 10.3109/15563659309000395. [DOI] [PubMed] [Google Scholar]
- 38.Houeto P, Borron SW, Sandouk P, Imbert M, Levillain P, Baud FJ. Pharmacokinetics of hydroxocobalamin in smoke inhalation victims. Journal of Toxicology - Clinical Toxicology. 1996;34:397–404. doi: 10.3109/15563659609013809. [DOI] [PubMed] [Google Scholar]
- 39.Stabler SP, Brass EP, Marcell PD, Allen RH. Inhibition of cobalamin-dependent enzymes by cobalamin analogues in rats. J Clin Invest. 1991;87:1422–1430. doi: 10.1172/JCI115148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kass L. Pernicious Anemia. Philadelphia: W.B. Saunders Company; 1976. [Google Scholar]
- 41.Savage DG, Lindenbaum J. Neurological complications of acquired cobalamin deficiency: clinical aspects. Baillieres Clin Haematol. 1995;8:657–678. doi: 10.1016/s0950-3536(05)80225-2. [DOI] [PubMed] [Google Scholar]
- 42.Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood. 2008 doi: 10.1182/blood-2008-03-040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchiyama M, Mayer G, Okawa M, Meier-Ewert K. Effects of vitamin B12 on human circadian body temperature rhythm. Neurosci Lett. 1995;192:1–4. doi: 10.1016/0304-3940(95)11591-j. [DOI] [PubMed] [Google Scholar]
- 44.Reitzer R, Gruber K, Jogl G, Wagner UG, Bothe H, Buckel W, Kratky C. Glutamate mutase from Clostridium cochlearium: the structure of a coenzyme B12-dependent enzyme provides new mechanistic insights. Structure. 1999;7:891–902. doi: 10.1016/s0969-2126(99)80116-6. [DOI] [PubMed] [Google Scholar]
- 45.Wuerges J, Garau G, Geremia S, Fedosov SN, Petersen TE, Randaccio L. Structural basis for mammalian vitamin B12 transport by transcobalamin. Proc Natl Acad Sci U S A. 2006;103:4386–4391. doi: 10.1073/pnas.0509099103. [DOI] [PMC free article] [PubMed] [Google Scholar]