Abstract
Background
Regular aerobic exercise improves large artery compliance in middle-aged and older humans. However, the underlying mechanisms are unknown. We tested the hypothesis that the improved central arterial compliance with endurance training is mediated by decreased α-adrenergic tone and/or increased endothelial function.
Methods
Seven sedentary healthy adults (60±3 yr) underwent systemic α-adrenergic blockade (phentolamine) and nitric oxide synthase (NOS) inhibition using NG-monomethyl-L-arginine in sequence before and after a 3-month moderate endurance training (walk/jog, 4–5 days/week). Phentolamine was given first to isolate the contribution of nitric oxide to arterial compliance by minimizing reflex suppression of sympathetic tone resulting from systemic NOS inhibition as well as to assess the α-adrenergic receptor-mediated modulation of arterial compliance.
Results
Baseline arterial compliance (via simultaneous ultrasound and applanation tonometry on the carotid artery) increased 34±12% after exercise training (P<0.01). When α-adrenergic blockade was performed, arterial compliance increased 37±6% (P<0.01) before the exercise training but did not change significantly after the training. Decreases in arterial compliance from the α-adrenergic blockade to the subsequent additional NOS blockade were not different before and after exercise training.
Conclusion
Our results suggest that the reduction in α-adrenergic receptor-mediated vascular tone contributes to the improved central arterial compliance with endurance training.
Keywords: arterial stiffness, endothelial function, sympathetic nervous system
Introduction
With advancing age, large conductance vessels (e.g., the aorta and carotid arteries) lose their ability to distend in response to fluctuations in pressure [1]. The impaired buffering or compliance function of arteries contributes to a number of cardiovascular disorders, including elevated systolic blood pressure, augmented left ventricular afterload, decreased coronary blood flow [2].
We have demonstrated that the diminished arterial compliance can be improved by even several months of regular aerobic exercise in healthy middle-aged and older adults [3,4]. However, physiological mechanisms by which regular endurance exercise increases central arterial compliance have never been established. The elements of the arterial wall that determine its compliance are the composition of elastin and collagen (structural elements) and the vasoconstrictor tone exerted by its smooth muscle cells (functional elements). The elastin-collagen composition of the arterial wall is a more slowly changing component that contributes to arterial compliance [1]. As such, it is unlikely that this may be a physiological mechanism underlying increases in arterial compliance induced by short-term exercise training. Indeed, using an animal experiment, we demonstrated that the beneficial influence of regular aerobic exercise on arterial stiffness was not mediated by the quantitative changes in arterial wall elastin and collagen [5]. The other determinants of arterial compliance, functional elements, are difficult to address experimentally as numerous local and neuro-humoral factors could influence the vasoconstrictor tone exerted by its smooth muscle cells. In order to identify and confine relevant functional factors responsible for exercise training-induced increases in arterial compliance, we relied on the gene microarray technique and found that genes associated with nitric oxide synthase (NOS) (along with prostaglandins and C-type natriuretic peptide) were differentially expressed in the aorta of exercise-trained rats [6]. Yet, whether the improved endothelial function is involved in augmenting central arterial compliance in the endurance-trained states has not been addressed in humans. Another important functional element that has been implicated in the pathogenesis of arterial stiffening is the sympathetic vasoconstrictor tone [7]. The sympathetic nervous system exerts a tonic restraint on the compliance of the common carotid artery, and removal of that restraint produces an immediate increase in its compliance [8]. Thus, exercise training might modulate arterial compliance via this mechanism.
Accordingly, the primary aim of the present study was to test the hypothesis that increases in central artery compliance with regular aerobic exercise are mediated by the improvement of endothelial function and/or reductions in sympathetic vasoconstrictor tone. To address this aim, we assessed effects of systemic inhibition of α-adrenergic receptors and NOS on arterial compliance before and after 3 months of aerobic exercise training in middle-aged and older adults. Systemic, rather than local, administration of drugs was used in this study in order to target the compliance of “central” (cardiothoracic) arteries, which makes the dominant contribution to the elastic reservoir function of the arterial system.
Material and Methods
Subjects
Seven sedentary but apparently healthy middle-aged and older adults [47–69 yr; mean age: 60±3 yr; 2 men and 5 postmenopausal (i.e., estrogen deficient) women] were studied. Subjects who were current smokers or smoked within the past two years, who took medications {including hormone (estrogen) replacement therapy}, or who had significant intima-media thickening (>1.0 mm), plaque formation, and/or other characteristics of atherosclerosis (e.g., ankle-brachial index <0.9, [9]) were excluded. None of the subjects had engaged in regular physical activity (>twice/week) or exercise training program in the past one year. All subjects were free of overt cardiovascular disease as assessed by medical history, metabolic risk factor analyses, and physical examination (electrocardiogram during graded exercise test). This study was reviewed and approved by the Institutional Review Board at the University of Tsukuba. The study conformed with the principles outlined in the Helsinki Declaration. All potential risks and procedures of the study were explained to the subjects, and they gave their written informed consent to participate in the study.
Experimental Protocol
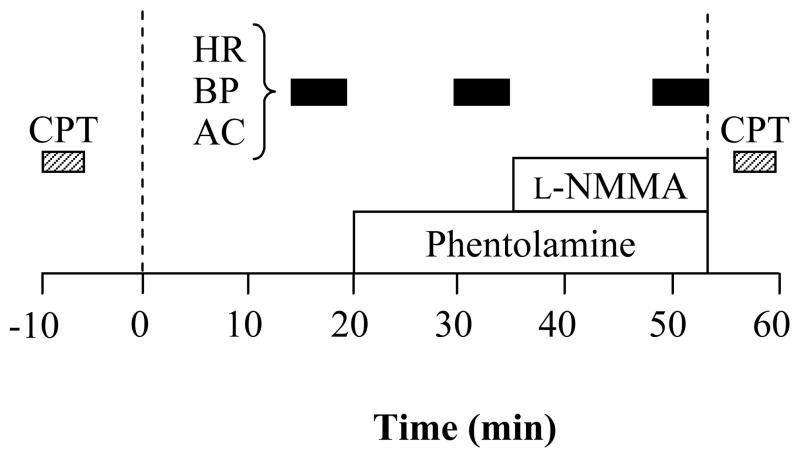
All measurements were performed after an abstinence of caffeine and an overnight fast. Experimental protocol before and after the exercise intervention period was conducted around the same time of the day to avoid any diurnal changes. Subjects underwent systemic blockade of α-adrenergic receptors (phentolamine mesylate) and NOS [NG-monomethyl-L-arginine (L-NMMA)]. Before and after the exercise intervention period, subjects underwent measurements of arterial compliance and other hemodynamic functions 3 times: baseline, with systemic α-adrenergic blockade, and with combined systemic α-adrenergic and NOS blockade. A timeline for experimental trial was displayed in Figure 1. In brief, after 15 min of supine rest in a temperature-controlled room (24–26°C), baseline measurements were made. Then, subjects received systemic α-adrenergic blockade [phentolamine, 0.1428 mg·kg−1 IV bolus over 2 min and a subsequent continuous (0.01428 mg·kg−1·min−1) IV infusion] as previously described [10]. The second set of measurements was performed 8–10 min after the commencement of continuous phentolamine infusion. Subsequently, subjects underwent systemic NOS inhibition [L-NMMA, 3 mg·kg−1 IV bolus over 5 min and a subsequent continuous (0.05 mg·kg−1·min−1) IV infusion] under α-adrenergic blockade condition [11–13]. The last set of measurements was performed 8–10 min after the commencement of continuous L-NMMA infusion. The procedure of infusions and dose of the systemic drug infusion were consistent with previous studies [10–13] that have reported that the dosage of blockade was adequate to block α-adrenergic system activity and nitric oxide (NO) production.
Figure 1.
Experimental protocol. A 20 min baseline period was followed by successive 13 min α-adrenergic blockade (phentolamine) and 18 min combined α-adrenergic blockade and nitric oxide synthase inhibition [phentolamine and NG-monomethyl-L-arginine (L-NMMA)]. Hemodynamic measurements were performed 3 times: at baseline, with systemic α-adrenergic blockade, and with the combined systemic α-adrenergic blockade and systemic NOS inhibition. Cold pressor tests (CPT) were performed before the baseline and immediately after the combined blockade. HR=heart rate, BP=brachial blood pressure, AC=arterial compliance.
To confirm effective systemic α-adrenergic blockade, subjects performed two cold pressor tests (hand immersion in ice water for 3 min) prior to and at the completion of the main protocol as shown in Figure 1. Before and 2 min after the start of submerging the hand, responses of heart rate, blood pressure, cardiac output, and total peripheral resistance were measured. Cardiac output was calculated from the blood pressure waveform using the Model Flow method with Portapres (Model 2 and BeatScope 1.0, TNO TPD Biomedical Instruments, The Netherlands). Total peripheral resistance was calculated as mean arterial pressure/cardiac output. We have previously demonstrated that the Model Flow method provides a reliable estimation of “relative” or % changes in cardiac output [14].
Carotid Arterial Compliance
The combination of ultrasound imaging of the common carotid artery with simultaneous applanation tonometry of the contralateral carotid artery was used to obtain arterial compliance as previously described [4]. A longitudinal image of the carotid artery was measured with an ultrasound machine (SonoSite 180II, SonoSite, Bothell, WA) equipped with a high-resolution multi-frequency linear-array transducer. Arterial diameter was determined by a perpendicular measurement from the media/adventitia interface of the near wall to the lumen/intima interface of the far wall of the vessel. Carotid arterial pressure waveforms were obtained with arterial applanation tonometry incorporating an array of 15 micropiezoresistive transducers (VP-2000, Colin Medical Instrument, San Antonio, TX) [9], and were calibrated by equating the carotid mean arterial and diastolic blood pressure to the brachial mean arterial and diastolic blood pressure [15]. Arterial compliance was calculated using the equations: [(D1−D0)/D0]/[2(P1−P0)] ×π ×D02, where D1 and D0 are the maximal and minimum arterial diameters and P1 and P0 are the highest and lowest blood pressures [4]. In order to exclude inter-investigator variability, one investigator, who was blinded to the pre-post measurements, analyzed all ultrasound images. The day-to-day coefficients of variation for carotid artery diameter, pulse pressure, and arterial compliance measurements are 2±1%, 7±3%, and 5±2% respectively.
Arterial Blood Pressure and Ankle-Brachial Index
Arterial blood pressure and ankle-brachial index were measured with the automated oscillometric pressure sensor method (VP-2000, Colin Medical Instrument, San Antonio, TX).
Body Fatness
Percent body fat was determined by the bioelectric impedance method.
Blood Samples
A blood sample was collected from the antecubital vein using venipuncture after an overnight fast. Plasma concentrations of glucose, lipids, and lipoproteins were determined by use of the standard enzymatic technique. Plasma samples from each subject before and after the training intervention were assayed in the same assay run.
Aerobic Fitness
All subjects underwent a submaximal graded cycle exercise test (after 2 min at 20 W, with 15 W increases every 1 min) until they reached 85% of the age-predicted maximal heart rate (208-age×0.7) [16]. Heart rate (via electrocardiogram) was measured throughout the protocol. Work rate corresponding to 85% of the age-predicted maximal heart rate (PWC85%) was used as an index of aerobic capacity. PWC85% has been shown to have a good correlation with directly measured maximal oxygen consumption [17].
Aerobic Exercise Training Intervention
Subjects underwent aerobic exercise training 4–5 days/week (walk/jog, 2 supervised and additional home-based trainings) for 12 weeks. The duration and intensity of the training were 30–45 min/day and 65–75% of their individual maximum heart rate. Adherence to the exercise prescription was documented through the use of Polar heart rate monitors and physical activity logs. The actual average heart rate during the training was 70±1% of their individual maximal heart rate, and the average duration and frequency of the exercise training were 44±2 min/day and 4.4±0.1 days/week, respectively.
Statistical Analyses
MANOVA and ANCOVA with repeated measures were used to determine the effects of exercise training intervention, as well as systemic α-adrenergic and NOS blockades on carotid arterial compliance and other hemodynamic measures. In the case of a significant F value, a post hoc test using the Newman-Keuls method identified significant differences among mean values. Changes in arterial compliance from baseline to the phentolamine administration were used as indices of “α-adrenergic receptor-dependent vascular tone”, as previously described by Halliwill et al[10]. Changes in arterial compliance from the phentolamine administration to the combined phentolamine and L-NMMA administration were used as indices of “NO-dependent vascular tone” [10]. Paired t-test used to compare these values before and after exercise. All data are reported as the mean±SEM. Statistical significance was set a priori at P<0.05.
Results
As shown in Table 1, there were no significant changes in body mass, adiposity, plasma concentrations of lipids, cholesterol, and glucose, or heart rate and blood pressure at rest with the exercise training intervention. PWC85%, an index of aerobic fitness, increased significantly after the exercise training intervention.
TABLE 1.
Selected Subject Characteristics
| Before Training | After Training | |
|---|---|---|
| Height, cm | 161 ± 2 | … |
| Body mass, kg | 59.7 ± 3.8 | 60.2 ± 3.8 |
| Body mass index, kg/m2 | 22.9 ± 1.2 | 23.1 ± 1.2 |
| Fat mass, % | 28 ± 2 | 28 ± 2 |
| Heart rate at rest, bpm | 59 ± 4 | 57 ± 4 |
| Systolic BP, mmHg | 123 ± 6 | 122 ± 6 |
| Diastolic BP, mmHg | 73 ± 3 | 71 ± 4 |
| Total cholesterol, mmol/L | 5.8 ± 0.3 | 5.4 ± 0.2 |
| HDL cholesterol, mmol/L | 1.6 ± 0.1 | 1.6 ± 0.2 |
| LDL cholesterol, mmol/L | 3.6 ± 0.2 | 3.2 ± 0.2 |
| Triglyceride, mmol/L | 1.3 ± 0.3 | 1.3 ± 0.3 |
| Blood glucose, mmol/L | 5.2 ± 0.1 | 5.4 ± 0.2 |
| PWC85%, watt | 104 ± 11 | 114 ± 11* |
Data are mean±SEM.
P<0.05 vs. before training. BP=blood pressure, PWC85%=Physical work capacity corresponding to 85% of the age-predicted maximal heart rate.
Brachial and carotid blood pressure decreased after α-adrenergic blockade, and then increased after combined α-adrenergic and NOS blockade (Table 2). Conversely, heart rate increased after α-adrenergic blockade and then decreased after the combined blockade. Brachial and carotid pulse pressure did not change significantly with these pharmacological blockades. The overall trend of changes in brachial and carotid blood pressure and pulse pressure were similar before and after the exercise training.
TABLE 2.
Hemodynamic measures in response to α-adrenergic and nitric oxide synthase (NOS) blockade before and after exercise training
| Baseline | α-adrenergic blockade | Combined α-adrenergic and NOS blockade | ||
|---|---|---|---|---|
| Heart rate, beats/min | Before | 59 ± 4 | 70 ± 5† | 61 ± 4† |
| After | 57 ± 4 | 71 ± 5† | 54 ± 3*†‡ | |
| Brachial SBP, mmHg | Before | 124 ± 6 | 109 ± 5† | 133 ± 9‡ |
| After | 121 ± 6 | 103 ± 6† | 127 ± 6‡ | |
| Brachial MAP, mmHg | Before | 94 ± 6 | 79 ± 5† | 96 ± 5‡ |
| After | 93 ± 4 | 76 ± 4† | 97 ± 5‡ | |
| Brachial DBP, mmHg | Before | 72 ± 3 | 65 ± 3† | 79 ± 5†‡ |
| After | 70 ± 4 | 59 ± 4*† | 75 ± 3‡ | |
| Brachial PP, mmHg | Before | 52 ± 4 | 44 ± 3 | 54 ± 5 |
| After | 50 ± 4 | 47 ± 3 | 53 ± 4 | |
| Carotid SBP, mmHg | Before | 114 ± 7 | 100 ± 5† | 122 ± 9‡ |
| After | 112 ± 7 | 95 ± 6† | 117 ± 6‡ | |
| Carotid PP, mmHg | Before | 42 ± 5 | 35 ± 5 | 43 ± 5 |
| After | 42 ± 5 | 36 ± 4 | 42 ± 5 |
Data are mean±SEM. SBP=systolic blood pressure, MAP=mean arterial pressure, DBP=diastolic blood pressure, PP=pulse pressure.
P<0.05 vs. before training
P<0.05 vs. baseline
P<0.05 vs. α-adrenergic blockade.
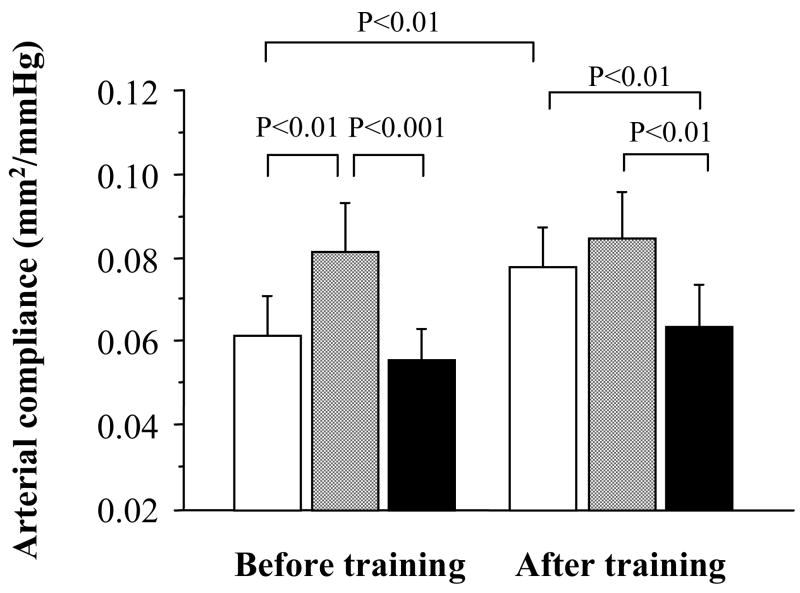
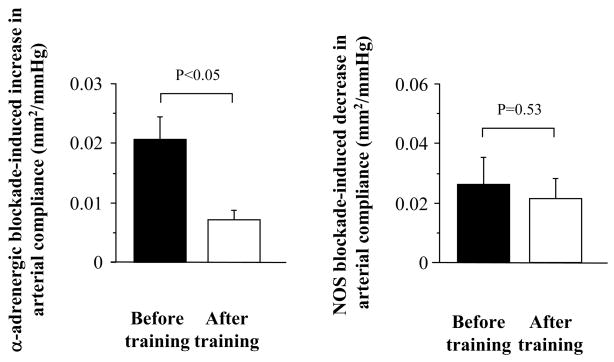
Basal (resting) carotid arterial compliance increased 34% in the exercise intervention group (P<0.01, Figure 2). Carotid arterial compliance increased with α-adrenergic blockade (P<0.01) before the training intervention, but did not change significantly after the training (P=0.72). Thus, the α-adrenergic receptor-dependent change in arterial compliance was greater before training compared with after training (P<0.05, Figure 3). This difference remained significant when the influences of corresponding changes in blood pressure or heart rate were account for ANCOVA. The subsequent NOS blockade induced significant reductions in carotid arterial compliance before and after the training. The NO-dependent changes in arterial compliance were not significantly different before and after training (P=0.53, Figure 3). This difference remained non-significant even after eliminating influences of corresponding changes in blood pressures (P=0.35~0.44) or heart rate (P=0.92).
Figure 2.
Carotid arterial compliance in response to α-adrenergic and nitric oxide synthase (NOS) blockades. Opened bars are the baseline, gray bars are α-adrenergic blockade condition, and solid (black) bars are the combined systemic α-adrenergic blockade and systemic NOS inhibition condition.
Figure 3.
Effects of endurance training on α-adrenergic receptor-dependent” and “nitric oxide-dependent” changes in carotid arterial compliance. Increases in arterial compliance from the baseline to the phentolamine administration were used as indices of α-adrenergic receptor-dependent vascular tone (left). Deceases in arterial compliance from the phentolamine administration to the combined phentolamine and L-NMMA administration were used as indices of systemic and local nitric oxide-dependent vascular tone (right).
The cold pressor test evoked significant increases in mean arterial pressure (before training: +29±5%; after training: +20±5%) and total peripheral resistance (before training: +45±11%; after training: +24±9%) before α-adrenergic and NOS blockade. After the combined blockade, however, significant vasoconstrictions were diminished as seen in no significant changes in mean arterial pressure (before training: +6±7%; after training: +12±6%) and total peripheral resistance (before training: +5±8%; after training: −5±4%).
Discussion
The salient findings of the present study were as follows. Endurance training mainly composed of walking/jogging induced a 34% increase in basal carotid artery compliance, confirming previous studies reporting beneficial impacts of regular aerobic exercise on central arterial compliance in middle-aged and elderly people [3,4]. The α-adrenergic receptor tone on carotid artery significantly decreased following the aerobic exercise intervention, as evidenced by a diminished increase in arterial compliance from baseline to the phentolamine administration. The NO-dependent vascular tone, however, did not change significantly after the endurance training, as the magnitude of decreases in arterial compliance from the phentolamine administration to the combined phentolamine and L-NMMA administration was similar before and after exercise training. These results suggest that endurance training-induced increases in arterial compliance are mediated through α-adrenergic receptor-dependent, rather than NO-dependent, reductions in vasoconstrictor tone.
Prior to the exercise training intervention, arterial compliance increased significantly with systemic α-adrenergic blockade, suggesting a substantial chronic restraint placed on the elastic artery by the sympathetic vascular tone. After the exercise training, however, arterial compliance was not affected by α-adrenergic blockade. These results suggest that the sympathetic vascular tone affecting central elastic arteries decreased with endurance training, thereby removing a tonic restraint placed on the arterial compliance and enhancing its elasticity. To our knowledge, there have been no other studies to determine exercise training-induced changes in sympathetic nerve activity to the aorta or other cardiothoracic arteries. Changes in sympathetic nervous system activity with exercise training remain controversial and appear to be very heterogeneous. Regular aerobic exercise training is in general associated with augmented basal sympathetic nervous system activity to the peripheral tissues (e.g., skeletal muscle) in healthy middle-aged and older adults [18,19]. However, sympathetic nerve activity to more central tissues may be reduced or unchanged with exercise training. For example, Meredith et al. [20] reported that endurance training reduced renal but not cardiac plasma norepinephrine spillover in young adult males. Our present results are consistent with the idea that sympathetic nervous system activity to the vascular smooth muscle surrounding the cardiothoracic arteries is reduced with exercise training.
The impact of endurance training on arterial compliance has been shown to manifest more clearly in central (elastic) vs. peripheral (muscular) arteries [21,22]. As the effect of vasoconstrictor tone exerted by smooth muscle cells is expected to be greater in more peripheral arteries in virtue of greater smooth muscle cells [23], it may argue against the possibility that reduced sympathetic vasoconstrictor tone would be a mechanism underlying the effect of exercise training on central arterial compliance. However, it is possible that some mechanical/physical factors may have interacted with functional elements to enhance arterial compliance, as beat-by-beat arterial distension is significantly greater in central vs. peripheral arteries. In this context, the lower number of vascular smooth muscle cells and their differential geometry in central vs. peripheral arteries allows only a mild reduction in lumen diameter even when maximally stimulated by the sympathetic nervous system [24]. In the central elastic arteries, the vasoconstrictor tone exerted by its smooth muscle cells appear to modulate arterial compliance through redistribution of tensile force between elastin and collagen [25].
From the experimental standpoint, it is ideal to “locally” manipulate factors influencing vasoconstrictor states of the smooth muscle cells. However, such local manipulation is difficult to perform when the experimental target is central arteries (e.g., aorta and carotid artery). Accordingly, we chose to perform “systemic” infusion of NOS blockers. Systemic infusion of NOS inhibitors, however, would induce sustained increases in arterial pressure, which would be a potent stimulus to activate arterial baroreceptors resulting in a reflex suppression of sympathetic nervous system activity. Although phentolamine was used primarily to block α-adrenergic receptor in the present study, it was also used to minimize the influences of baroreflex-induced systemic changes that could confound the interpretation of NOS blockers. Contrary to our hypothesis, we found that the administration of L-NMMA induced similar depressions of arterial compliance before and after the exercise intervention. Therefore, the association between changes in the NO-dependent vascular tone and arterial compliance with the aerobic exercise training was not supported in the present study. However, several previous studies demonstrated that regular endurance training is associated with enhanced endothelial function in older adults [26,27]. For example, resting plasma concentrations of nitrite/nitrate (NOx), the stable end product of NO, and cyclic guanosine monophosphate (cGMP), a second messenger of NO, significantly increased after 3 months of moderate aerobic exercise training in sedentary postmenopausal women [27]. As shown in isolated blood vessel preparation, NO is capable of acting directly on vascular smooth muscle to attenuate sympathetic vasoconstrictor tone [28]. Release of norepinephrine from post-ganglionic sympathetic nerve endings is modulated by NO [29]. Furthermore, α-adrenergic receptor sensitivity of rat abdominal aorta to norepinephrine is blunted by regular aerobic exercise training through the endothelium-dependent mechanism [30]. Thus, increased NO production and/or bioavailability may have exerted “sympatholysis”-like effects that have been observed during a bout of aerobic exercise [31] and acted to suppress tonic sympathetic vasoconstrictor tone, resulting in an increased arterial compliance.
A few limitations in this study should be noted and emphasized. First, no test was conducted to confirm completeness of NOS blockade. Dose of systemic L-NMMA administration was, however, confirmed to be effective to block NO production by previous studies [11–13]. Importantly, hemodynamic responses (i.e., increase in mean arterial pressure and decrease in cardiac output) to pharmacological interventions were similar before and after the training (data were not shown). Second, there was no control group that remained sedentary during the intervention period, and a sample size was small. Third, we have included both sexes in this study, and a previous study has demonstrated different vasoconstrictor responses to L-NMMA in men and women [32]. However, these sex-related effects seem to be mediated through the influence of estrogen on locally-produced vasoactive factors such as NO [33], and we studied postmenopausal (i.e., estrogen deficient) women who did not take hormone replacement therapy.
In summary, our findings support the idea that endurance training-induced improvements in arterial compliance may be mediated, at least in part, through reductions in α-adrenergic receptor-dependent vasoconstrictor tone.
Acknowledgments
Grant Support: This work was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (18300215, 18650186) and a grant from the National Institutes of Health (AG20966).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichols W, O’Rourke MF. McDonald’s Blood Flow in Arteries. London: Arnold; 1998. [Google Scholar]
- 2.Safar ME, London GM. Therapeutic studies and arterial stiffness in hypertension: recommendations of the European Society of Hypertension. The Clinical Committee of Arterial Structure and Function. Working Group on Vascular Structure and Function of the European Society of Hypertension. J Hypertens. 2000;18:1527–1535. doi: 10.1097/00004872-200018110-00001. [DOI] [PubMed] [Google Scholar]
- 3.Sugawara J, Otsuki T, Tanabe T, Hayashi K, Maeda S, Matsuda M. Physical activity duration, intensity, and arterial stiffening in postmenopausal women. Am J Hypertens. 2006;19:1032–1036. doi: 10.1016/j.amjhyper.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 5.Nosaka T, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol. 2003;28:204–212. doi: 10.1139/h03-016. [DOI] [PubMed] [Google Scholar]
- 6.Maeda S, Iemitsu M, Miyauchi T, Kuno S, Matsuda M, Tanaka H. Aortic stiffness and aerobic exercise: mechanistic insight from microarray analyses. Med Sci Sports Exerc. 2005;37:1710–1716. doi: 10.1249/01.mss.0000175052.37087.f8. [DOI] [PubMed] [Google Scholar]
- 7.Failla M, Grappiolo A, Emanuelli G, Vitale G, Fraschini N, Bigoni M, Grieco N, Denti M, Giannattasio C, Mancia G. Sympathetic tone restrains arterial distensibility of healthy and atherosclerotic subjects. J Hypertens. 1999;17:1117–1123. doi: 10.1097/00004872-199917080-00011. [DOI] [PubMed] [Google Scholar]
- 8.Mangoni AA, Mircoli L, Giannattasio C, Mancia G, Ferrari AU. Effect of sympathectomy on mechanical properties of common carotid and femoral arteries. Hypertension. 1997;30:1085–1088. doi: 10.1161/01.hyp.30.5.1085. [DOI] [PubMed] [Google Scholar]
- 9.Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol. 2003;91:1519–1522. doi: 10.1016/s0002-9149(03)00416-8. [DOI] [PubMed] [Google Scholar]
- 10.Halliwill JR, Minson CT, Joyner MJ. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol. 2000;89:1830–1836. doi: 10.1152/jappl.2000.89.5.1830. [DOI] [PubMed] [Google Scholar]
- 11.Mayer BX, Mensik C, Krishnaswami S, Derendorf H, Eichler HG, Schmetterer L, Wolzt M. Pharmacokinetic-pharmacodynamic profile of systemic nitric oxide-synthase inhibition with L-NMMA in humans. Br J Clin Pharmacol. 1999;47:539–544. doi: 10.1046/j.1365-2125.1999.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–2040. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- 13.Sugawara J, Maeda S, Otsuki T, Tanabe T, Ajisaka R, Matsuda M. Effects of nitric oxide synthase inhibitor on decrease in peripheral arterial stiffness with acute low-intensity aerobic exercise. Am J Physiol Heart Circ Physiol. 2004;287:H2666–2669. doi: 10.1152/ajpheart.00077.2004. [DOI] [PubMed] [Google Scholar]
- 14.Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand. 2003;179:361–366. doi: 10.1046/j.0001-6772.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 15.Armentano R, Megnien JL, Simon A, Bellenfant F, Barra J, Levenson J. Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension. 1995;26:48–54. doi: 10.1161/01.hyp.26.1.48. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 17.Miyashita M, Mutoh Y, Yoshioka N, Sadamoto T. PWC75%HRmax: a measure of aerobic work capacity. Sports Med. 1985;2:159–164. doi: 10.2165/00007256-198502030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Ng AV, Callister R, Johnson DG, Seals DR. Endurance exercise training is associated with elevated basal sympathetic nerve activity in healthy older humans. J Appl Physiol. 1994;77:1366–1374. doi: 10.1152/jappl.1994.77.3.1366. [DOI] [PubMed] [Google Scholar]
- 19.Poehlman ET, Danforth E., Jr Endurance training increases metabolic rate and norepinephrine appearance rate in older individuals. Am J Physiol. 1991;261:E233–239. doi: 10.1152/ajpendo.1991.261.2.E233. [DOI] [PubMed] [Google Scholar]
- 20.Meredith IT, Friberg P, Jennings GL, Dewar EM, Fazio VA, Lambert GW, Esler MD. Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension. 1991;18:575–582. doi: 10.1161/01.hyp.18.5.575. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol. 2005;55:235–239. doi: 10.2170/jjphysiol.S2116. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- 23.Bevan JA. Some bases of differences in vascular response to sympathetic activity. Circ Res. 1979;45:161–171. doi: 10.1161/01.res.45.2.161. [DOI] [PubMed] [Google Scholar]
- 24.Greenwald SE, Newman DL, Denyer HT. Effect of smooth muscle activity on the static and dynamic elastic properties of the rabbit carotid artery. Cardiovasc Res. 1982;16:86–94. doi: 10.1093/cvr/16.2.86. [DOI] [PubMed] [Google Scholar]
- 25.Rachev A, Hayashi K. Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann Biomed Eng. 1999;27:459–468. doi: 10.1114/1.191. [DOI] [PubMed] [Google Scholar]
- 26.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 27.Maeda S, Tanabe T, Otsuki T, Sugawara J, Iemitsu M, Miyauchi T, Kuno S, Ajisaka R, Matsuda M. Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res. 2004;27:947–953. doi: 10.1291/hypres.27.947. [DOI] [PubMed] [Google Scholar]
- 28.Topouzis S, Schott C, Stoclet JC. Participation of endothelium-derived relaxing factor and role of cyclic GMP in inhibitory effects of endothelium on contractile responses elicited by alpha-adrenoceptor agonists in rat aorta. J Cardiovasc Pharmacol. 1991;18:670–678. doi: 10.1097/00005344-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg SS, Diecke FP, Peevy K, Tanaka TP. Release of norepinephrine from adrenergic nerve endings of blood vessels is modulated by endothelium-derived relaxing factor. Am J Hypertens. 1990;3:211–218. doi: 10.1093/ajh/3.3.211. [DOI] [PubMed] [Google Scholar]
- 30.Spier SA, Laughlin MH, Delp MD. Effects of acute and chronic exercise on vasoconstrictor responsiveness of rat abdominal aorta. J Appl Physiol. 1999;87:1752–1757. doi: 10.1152/jappl.1999.87.5.1752. [DOI] [PubMed] [Google Scholar]
- 31.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- 32.Kneale BJ, Chowienczyk PJ, Cockcroft JR, Coltart DJ, Ritter JM. Vasoconstrictor sensitivity to noradrenaline and NG-monomethyl-L-arginine in men and women. Clin Sci (Lond) 1997;93:513–518. doi: 10.1042/cs0930513. [DOI] [PubMed] [Google Scholar]
- 33.Majmudar NG, Robson SC, Ford GA. Effects of the menopause, gender, and estrogen replacement therapy on vascular nitric oxide activity. J Clin Endocrinol Metab. 2000;85:1577–1583. doi: 10.1210/jcem.85.4.6530. [DOI] [PubMed] [Google Scholar]