Abstract
Although volume excess causes hypertension whether it also affects circadian patterns of arterial pressures among hemodialysis patients remains unknown. To test the notion whether volume overload is associated with a unique BP “signature” a post-hoc analysis was performed among 145 patients participating in the dry-weight reduction in hypertensive hemodialysis patients (DRIP) randomized controlled trial. Using 400 ambulatory BP recordings over 8 weeks comprising 35,302 measurements the trended cosinor model was found to be the best descriptor of BP chronobiology. The trended cosinor model may be described as a pattern of sinusoidal oscillation around a straight line with an upward trend during the interdialytic period and which has an intercept at the postdialysis time. Augmented volume removal therapy (AVRT) reduced the intercept systolic BP and increased the rate of rise in systolic BP over the interdialytic interval but had no effect on the systolic BP fluctuation (amplitude). Thus an elevated intercept and blunted slope pattern characterizes the “volume overload BP pattern” on ambulatory BP monitoring. Similar changes were seen for diastolic BP. AVRT neither restored dipping nor was associated with a lag-phenomenon for either the wake or sleep systolic BP. Lowering of systolic BP was greater than diastolic BP such that pulse pressure was reduced. An observational cohort of 37 patients followed for 6 months confirmed these findings. Randomized trials are now needed to evaluate the clinical impact of AVRT on hard outcomes since reduction of pulse pressure with this simple expedient has the potential to improve survival in hemodialysis patients.
Keywords: hypertension, hemodialysis, ambulatory blood pressure monitoring, blood volume, chronobiology phenomena
Introduction
Hypertension is a cardiovascular risk-factor common among hemodialysis patients 1 and one which that may be better assessed using out-of-office blood pressure (BP) monitoring either by home BP measurements or automatic interdialytic ambulatory BP recordings 2–5. Interdialytic ambulatory BP monitoring more closely correlates with left ventricular hypertrophy 6 and all-cause mortality 3 compared to BP recorded in the hemodialysis unit and, in contrast to home BP recordings, offers greater insights into circadian rhythms 2.
Chronobiologic analysis reveals that arterial BP rhythms are markedly altered in patients on hemodialysis 7. BP steadily increases over the interdialytic interval 8 and the rate of change in BP is proportional to the interdialytic weight gain 9. Most hemodialysis patients have absence of nocturnal decline in BP, a phenomenon known as non-dipping 10. Augmented volume removal therapy (AVRT) and restricted dialysate sodium delivery effectively treats hypertension in hemodialysis patients 11. In patients with normal kidney function, diuretics and salt-restriction can restore the dipping-phenomenon 12, 13. However, the effect of AVRT on dipping phenomenon and arterial pressure rhythms in hemodialysis patients remains of great interest but currently unknown 14. Furthermore, studies have suggested that studying the chronobiology of arterial rhythms may be more sensitive in detecting changes in BP patterns compared to studying the dipping phenomenon 15, 16. Thus, volume overload may have a specific pattern in hypertensive hemodialysis patients.
The “lag phenomenon” refers to a fall in blood pressure that occurs weeks to months after reducing dry-weight 17, 18. In the dry-weight reduction in hypertensive hemodialysis patients (DRIP) trial we demonstrated a reduction of ~7/3 mmHg in response to probing dry weight measured by interdialytic ambulatory BP at 4 weeks and no further reduction at 8 weeks despite maintaining dry-weight. We interpreted these findings to mean that the lag-phenomenon does not exist in long-term prevalent hemodialysis patients. An alternative explanation may be that the lag-phenomenon may be evident for day-time and not night time BP. After all the lag-phenomenon was described using pre- and post-dialysis BP recordings which may correlate more with day-time recordings. If so, then the absence of lag-phenomenon in the DRIP trial may be due to the disparate effects of AVRT on day-time and sleep BP.
The purpose of this report was to evaluate the effect of volume on the patterns of hemodynamic rhythms. Specifically, we investigated whether volume removal can alter the chronobiology of arterial pressure rhythms such that it would provide a unique signature that would be attributable to volume overload. Using a randomized controlled trial design we also tested whether AVRT can restore the dipping phenomenon and whether AVRT is associated with the lag-phenomenon by measuring the contribution of volume on sleep and wake ambulatory BP.
Methods
The trial results and methods have been previously published 19. Briefly, we recruited patients 18 years of age or older on long-term hemodialysis for at least 3 months, who had hypertension defined as mean interdialytic ambulatory BP of 135/85 mm Hg or more. Morbidly obese patients (body mass index 40 kg/m2 or more) were excluded because BP is often difficult to obtain in such patients. BP medications were withdrawn in some patients in order that they might become hypertensive and therein participate in this study. After a six hemodialysis run-in phase, during which baseline data were collected, patients were randomized in 1:2 proportion into control group vs. ultrafiltration trial group for 8 weeks. During this 24 dialysis treatment phase (8 weeks × 3 dialysis per week), patients were seen at each dialysis visit and had dry-weight probed as assessed by symptoms and signs related to hypovolemia 20, 21. In the ultrafiltration group an initial additional weight loss of 0.1 kg/10 kg body-weight was prescribed per dialysis without increasing the time or frequency of dialysis. This additional weight loss was combined with the ultrafiltration volume required to remove interdialytic weight gain to achieve the desired reduction in dry weight. If ultrafiltration was not tolerated based on symptoms and signs such as muscle cramps, need for excessive saline or symptomatic hypotension, the additional prescribed weight loss was reduced by 50% till even 0.2 kg incremental weight loss per dialysis was not tolerated. At this point, the patient was said to be at his or her dry weight.
Blood Pressure Monitoring
Ambulatory BP monitoring was performed after the mid-week hemodialysis session for 44 hours. Blood pressures were recorded every 20 minutes during the day (6 AM to 10 PM) and every 30 minutes during the night (10 PM to 6 AM) using a validated Spacelab 90207 ABP monitor (SpaceLabs Medical Inc, Redmond, WA, USA) in the non-access arm 22. Recordings began immediately after hemodialysis and terminated immediately before the subsequent dialysis. Accuracy of ambulatory BP recordings was confirmed against auscultated blood pressure at baseline. We excluded those patients who had less than 22 hours of ambulatory BP recording and also those who had long gaps in measurement.
Patients kept diaries of wake and sleep periods which were used to calculate wake and sleep BP. If no diary was kept, the period from midnight to 5 AM was used as the sleep period and from 8AM to 10PM as wake period.
The study protocol was approved by the Institutional Review Boards (IRB) and the VA Research and Development Committee and all patients provided written informed consent. The trial was registered at ClinicalTrials.gov (NCT00067665).
Validation cohort
In a group of 37 ESRD patients on long-term hemodialysis we performed interdialytic ambulatory BP monitoring as described above at baseline and at 6 months. At each of these time points we performed continuous blood volume monitoring to assess volume status as described previously 23. Those who were in the top one-third of volume index at baseline (volume index of ≥−0.198 %/hr/mL/h ultrafiltration/kg body weight) were said to have hypervolemia. At 6 months if the volume index exceeded this threshold hypervolemia was diagnosed.
Statistical Analysis
The mean level, trend and diurnal pattern in arterial pressure pattern in an interdialytic interval was modeled using the trended cosinor change model 8. This method entails fitting an oscillating curve to temporal hemodynamic variables using a 24 hour periodicity. The trended cosinor model describes the rhythmic cycle as y = b0 + b1 × Cos[(2π/24)t1] + b2 × Sin[(2π/24)t1] + b3t, where y represents the observed systolic BP, diastolic BP, or pulse pressure; b0, b1, b2 and b3 are regression coefficients; t represents time elapsed after dialysis and t1 the time elapsed since midnight on a 24 hour clock. The constant 2π/24 represents the 24 hour periodicity of BP. The coefficient b0 represents the 24-hour rhythm-adjusted average intercept value of arterial pressure. The regression coefficients b1 and b2 are the coefficients for the cosine and sine component, respectively, and collectively describe the amplitude of the cosine curve which is defined as The amplitude represents half the extent of rhythmic change in a cycle approximated by the fitted curve, which implies it can be interpreted as the mean deviation across the time span. The coefficient b3 represents the linear trend in arterial pressure in the interdialytic period. Thus, the model considers the intercept and 2 types of change in a unified manner: a change that has a systematic linear component and another change that oscillates.
Using the above trended cosinor model we examined the fixed effects of the intervention (control versus ultrafiltration), weeks of measurement (week 4 and week 8) and their interaction on the intercept, slope and amplitude of the variation in arterial pressure and pulse rate.
The mean change within subjects and between treatments in sleep and wake ambulatory BP was compared using a mixed effects model to account for repeated observations 24. A similar mixed effects model was used to analyze the validation cohort. The nominal level of significance was set at two sided p of <0.05 and all statistical analyses were performed with Stata version 10.1 (StataCorp LP, College Station, TX).
Results
Of the 50 possible control and 100 possible ultrafiltration ambulatory BP recordings, 2 patients in the control and 3 in the ultrafiltration group had inadequate ambulatory BP recordings for this analysis. At 4 weeks, 43 patients in the control group and 85 in the ultrafiltration group had adequate ambulatory BP recordings. At 8 weeks, 43 patients in the control group and 84 in the ultrafiltration group had complete data. In all, 400 ambulatory BP recordings comprising 35,302 measurements form the basis of the current analysis.
Interdialytic weight gain averaged 2.9 kg in the ultrafiltration group and 2.8 kg in the control group at baseline and did not change significantly within or between groups over time. AVRT resulted in a mean change of 0.9 kg weight loss at 4 weeks and 1 kg at 8 weeks. Most patients were dialyzed with dialysate Na of 140 mEq/L. In the control group 46% of the patients had Na modeling vs 45% in the AVRT group. We did not change the dialysate Na, UF profiling prescription, or antihypertensive medications during the trial.
The baseline characteristics of the eligible patients are shown in Table 1. The two treatment groups were well balanced with respect to the baseline characteristics of the patients. At baseline the average number of BP measurements with each ambulatory recording was was 91 ± 20 (range 31–130) in the control group and 91 ± 19 (range 40–120) in the ultrafiltration group. Over the course of the trial, the average number of BP measurements was 88 ± 21 per ambulatory recording.
Table 1.
Clinical characteristics of the study population
| Clinical Characteristic | Control (n=48) | Ultrafiltration (n=97) |
|---|---|---|
| Age (years) | 55.0 ± 11.3 | 54.3 ± 12.8 |
| Men | 37 (77%) | 63 (65%) |
| Race | ||
| White | 3 (6%) | 12 (12%) |
| Black | 44 (92%) | 82 (85%) |
| Other | 1 (2%) | 3 (3%) |
| Pre-dialysis BP | 158.5 ± 15.1/87.6 ± 12.1 | 160.3 ± 16.1/86.4 ± 10.5 |
| Post-dialysis BP | 142.8 ± 19.3/78.5 ± 12.8 | 143.9 ± 17.4/78.0 ± 10.3 |
| Pre-dialysis weight (kg) | 84.8 ± 20.1 | 83.7 ± 19.8 |
| Post-dialysis weight (kg) | 82.0 ± 19.2 | 80.7 ± 19.2 |
| Body Mass Index (kg/m2) | 27.2 ± 6.5 | 27.3 ± 5.9 |
| Years of dialysis | 4.5 ± 5.8 | 3.9 ± 4.8 |
| Etiology of end-stage renal disease | ||
| Diabetes Mellitus | 17 (35%) | 38 (39%) |
| Hypertension | 24 (50%) | 46 (47%) |
| Glomerulonephritis | 2 (4%) | 4 (4%) |
| Polycystic Kidney Disease | 0 (0%) | 3 (3%) |
| Other | 5 (10%) | 6 (6%) |
| Current Smoker | 19 (40%) | 32 (33%) |
| History of | ||
| Congestive Heart Failure | 4 (8%) | 17 (18%) |
| Myocardial Infarction | 6 (13 %) | 14 (14%) |
| Stroke | 5 (10%) | 9 (9%) |
| Urea reduction ratio | 73.3 ± 6.3 | 74.3 ± 7.0 |
| Albumin (g/dl) | 3.7 ± 0.4 | 3.7 ± 0.5 |
| Hemoglobin (g/dl) | 12.1 ± 1.3 | 12.2 ± 1.1 |
| Presence of Edema* | 8 (17%) | 19 (20%) |
| Statin use | 21 (44%) | 40 (41%) |
| Erythropoietin stimulating agents | 21 (44%) | 41 (43%) |
| Number receiving antihypertensive drugs | 36 (75%) | 85 (88%) |
| Number of antihypertensives in users | 2.6 ± 1.3 | 2.6 ± 1.4 |
| Nature of antihypertensive agent | ||
| Dihydropyridine calcium channel blockers | 19 (40%) | 46 (47%) |
| Non-dihydropyridine calcium-channel blockers | 2 (4%) | 4 (4%) |
| Beta-blockers | 30 (63%) | 68 (70%) |
| Alpha-blockers | 4 (8%) | 7 (7%) |
| Centrally acting agents | 9 (19%) | 25 (26%) |
| Vasodilators | 9 (19%) | 16 (16%) |
| ACE Inhibitors | 24 (50%) | 51 (53%) |
| Angiotension Receptor Blockers | 4 (8%) | 19 (20%) |
± indicates standard deviation. Parenthesis have percent of patients.
Missing in 2 patients in the ultrafiltration group.
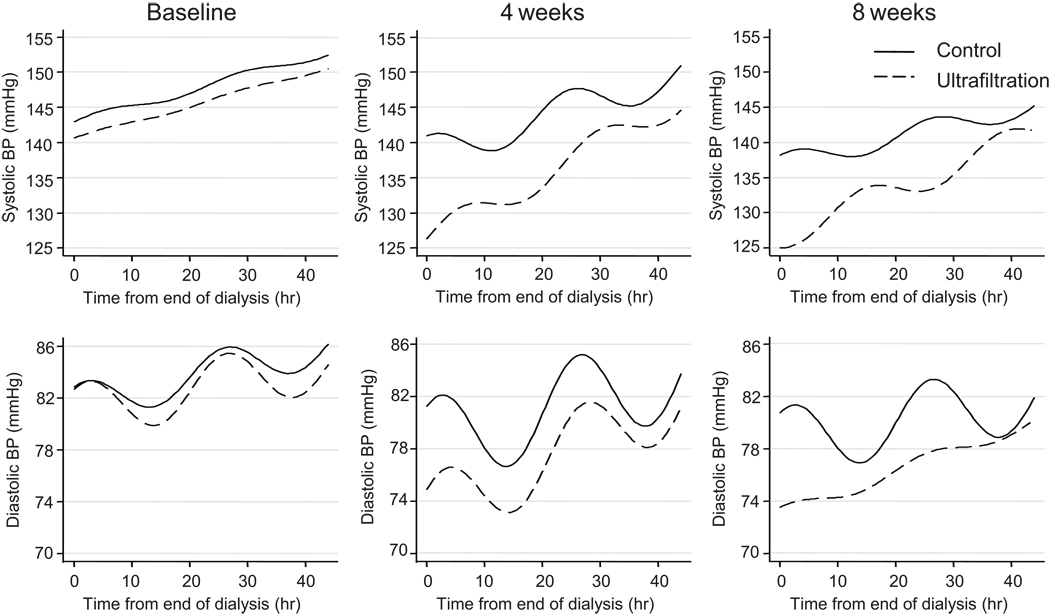
At baseline, the average ambulatory BP in the control group was 146.7 ± 10.6/83.6 ± 11.1 mmHg and 145.4 ± 10.2/82.7 ± 9.8 mmHg in the ultrafiltration group. Pulse rates were 78.9 ± 10.3 in the control group and 77.1 ± 10.1 in the ultrafiltration group. The patterns of systolic and diastolic BP over 44 hours of an interdialytic period at baseline and at 4 and 8 weeks after randomization are shown in Figure 1. Ultrafiltration (UF) caused a reduction in intercept systolic and diastolic BP but steepened the slope of change over time at 4 weeks and 8 weeks. The amplitude of variation increased in the control group compared to UF group at 4 weeks and 8 weeks in the case of diastolic but not systolic BP.
Figure 1.
Patterns of systolic and diastolic BP obtained using ambulatory BP monitoring over 44 hours during an interdialytic period and analyzed using the trended cosinor change model. The solid line is the control group and dotted line the ultrafiltration (UF) group. The left panel represent recordings at baseline, the center panel measurements at 4 weeks and the right panel 8 weeks following randomization.
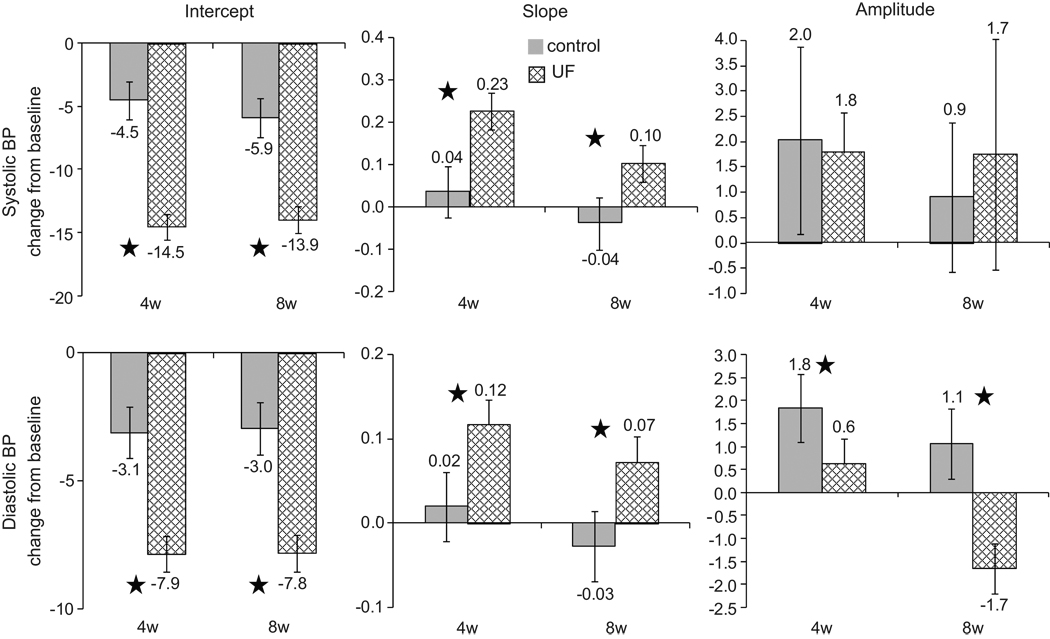
Figure 2 shows the change from baseline in the control group and the change from baseline in the UF in intercept, slope and amplitude of BP. Intercept systolic BP fell 10 mmHg more at 4 weeks (p<0.0001) and 8.1 mmHg more at 8 weeks (p<0.0001) in the UF group compared to the control group. Slopes of systolic BP steepened by 0.192 mmHg/hr more at 4 weeks (p<0.0001) and 0.141 mmHg/hr more at 8 weeks (p<0.0001) in the UF group compared to the control group. There was a greater variation in the amplitude of systolic BP at 4 weeks in both control and UF groups, but no between group differences emerged at 4 or 8 weeks. Diastolic BP fell 4.8 mmHg more in the UF group compared to control group at 4 weeks (p<0.0001) and 4.9 mmHg more at 8 weeks (p<0.0001). Slopes of diastolic BP steepened by 0.097 mmHg/hr more at 4 weeks (p<0.0001) and 0.100 mmHg/hr more at 8 weeks (p<0.0001) in the UF group compared to the control group. The amplitude of diastolic BP variation increased in the control group by 1.2 mmHg (p=0.01) compared to the UF group at 4 weeks. At 8 weeks a fall in the amplitude of diastolic BP variation in UF group revealed between group difference of −2.7 mmHg (p<0.0001).
Figure 2.
Solid bars show the change from baseline in the control group and hatched bars the change from baseline in the ultrafiltration (UF) group. Stars indicate p<0.05 for the difference between changes from baseline. Means are maximal likelihood estimates from the statistical model described in the text. The error bars represent the 95% confidence interval of the mean and when they cross zero, the mean change is not distinguishable from zero.
Figure S1 shows that UF caused a reduction in intercept pulse pressure (please see http://hyper.ahajournals.org). Pulse pressure slope was steepened with UF at 4 weeks compared to the control group. However, there were no differences between control and UF pulse pressure slopes at 8 weeks. Heart rate intercept fell from baseline to 4 weeks in the control group and remained low at 8 weeks such that differences between groups were significant at both time 4 and 8 weeks. There was flattening of the slope of heart rate in the UF group but no change in the control group at 4 weeks with no overall difference seen between group changes. At 8 weeks there was flattening of the slope of control group but no change in UF group. The amplitude of variation did not change for pulse pressure or heart rate between groups at any time point.
Figure S2 shows that intercept pulse pressure fell 5.2 mmHg more at 4 weeks (p<0.0001) and 3.1 mmHg more at 8 weeks (p<0.0001) in the UF group compared to the control group (please see http://hyper.ahajournals.org). Slopes of pulse pressure steepened by 0.091 mmHg/hr more at 4 weeks (p<0.0001) in the UF group compared to the control group. At 8 weeks, however, the slopes of pulse pressure were similar between groups. The amplitude of pulse pressure changes showed no differences within and between groups. Heart rate fell 1.2 more beats/min more in the control group at 4 weeks (p=0.006) and 4.6 beats/min more 8 weeks (p<0.0001) compared to the control group. Slopes of heart rates were similar at 4 weeks but showed steeper changes at 8 weeks (p=0.004) in the control group compared to the UF group. The amplitude of heart rate showed similar changes within and between groups at 4 and 8 weeks.
The extent of dipping (sleep minus wake systolic BP) is shown in Figure S3 (please see http://hyper.ahajournals.org). The 95% confidence interval of dipping at each time point included zero. Thus there was no evidence for dipping in the control or UF groups either at baseline or over time. The change in dipping pattern was opposite of what would be expected for the UF group (Figure S3, panel C). The change in dipping in the UF group was −1.2 mmHg (95% CI −9.6 to 7.1) compared to the control group at 4 weeks and 3.6 mmHg (95% CI −4.7, 11.9) at 8 weeks. Thus, AVRT did not restore dipping.
The results of the validation cohort are shown in Table 2. Volemia was defined by the volume index as previously described 23. Of the 37 patients, 19 remained euvolemic, 5 had incident hypervolemia, 4 incident euvolemia whereas 9 were persistently hypervolemic. The most notable finding was that incident hypervolemia was associated with increased intercept and blunted slope. Persistent hypervolemia was associated with further worsening of intercept but no further blunting of slope. There were only 4 patients who experienced incident euvolemia and compared to the control group of patients with persistent euvolemia changes in intercept and slope were small.
Table 2.
Parameters of the slope-intercept model in a validation cohort
| Volume state at baseline |
ABPM Intercept at baseline (mmHg) |
Volume state at 6 mo |
ABPM Intercept at 6 mo (mmHg) |
n | Intercept Change from baseline (mmHg) |
95% CI of change |
p for change |
|---|---|---|---|---|---|---|---|
| Euvolemia | 115.1 ± 3.9 | Euvolemia | 117.8 ± 3.9 | 19 | 2.7 | 0.7, 4.6 | 0.007 |
| Hypervolemia | 115.5 ± 7.5 | Euvolemia | 118.9 ± 7.5 | 4 | 3.4 | −0.04, 6.9 | 0.052 |
| Euvolemia | 106.5 ± 8.4 | Hypervolemia | 144.6 ± 8.4 | 5 | 38.2 | 34.3, 42.0 | <0.001 |
| Hypervolemia | 118.1 ± 5.6 | Hypervolemia | 136.9 ± 5.6 | 9 | 18.8 | 16.2, 21.3 | <0.001 |
| ABPM Slope at baseline (mmHg/hr) |
ABPM Slope at 6 mo (mmHg/hr) |
Slope Change from baseline (mmHg/hr) |
|||||
| Euvolemia | 0.23 ± 0.06 | Euvolemia | 0.18 ± 0.06 | 19 | −0.05 | −0.13, 0.02 | 0.18 |
| Hypervolemia | 0.23 ± 0.12 | Euvolemia | 0 ± 0.12 | 4 | −0.23 | −0.37,−0.09 | 0.002 |
| Euvolemia | 0.41 ± 0.14 | Hypervolemia | −0.08 ± 0.14 | 5 | −0.49 | −0.65, −0.33 | <0.001 |
| Hypervolemia | 0.23 ± 0.09 | Hypervolemia | 0.27 ± 0.09 | 9 | 0.04 | −0.07, 0.14 | 0.46 |
Volume state was determined by continuous blood volume monitoring. ± reflect standard error of the mean. Transition from euvolemia to hypervolumia was associated with increase in intercept BP and blunting of the slope as would be predicted from the parent cohort.
A per protocol analysis of the data confirmed the intent-to-treat analysis. A fall in weight at either month was associated with a drop in intercept BP of between 4 and 5 mmHg/kg and steepening of the slope by 0.04 to 0.05 mmHg/hr/kg without effects on amplitude.
Discussion
In this study we found that AVRT in prevalent, long-term, hypertensive hemodialysis patients with ESRD reduced the overall BP and also altered the chronobiology of hemodynamic rhythms. AVRT evoked these changes by reducing the intercept of systolic BP more than diastolic BP such that the pulse pressure intercept was also reduced. The systolic and diastolic slopes were steeper but there was no effect on the lability (amplitude) of BP variation over the interdialytic period, including a lack of change in the amplitude of pulse pressure variation. AVRT failed to restore dipping and was not associated with a lag-phenomenon for either the wake or sleep systolic BP. Thus an elevated intercept and blunted slope pattern best characterizes the “volume overload BP pattern” on ambulatory BP monitoring.
Short dialysis sessions are associated with increased mortality 25. Although the reason(s) for this observation remains elusive, shorter dialysis sessions may be associated with inadequate volume removal and therefore hypertension. AVRT caused a large and consistent drop in systolic BP more than diastolic BP such than pulse pressure was also reduced. Since pulse pressure has been associated with increased mortality in hemodialysis patients 26, 27, it is possible that AVRT may confer survival benefits in this population which has an astounding mortality rate. The fall in intercept BP was matched by a steeper increase in systolic and diastolic BP over the interdialytic period. However, by 8 weeks despite the increase in rate of change of BP during the interdialytic period, the BP at the end of the recording period was approximately what was seen at intercept at baseline. These changes are especially remarkable because they occurred while the patients received an average of 2.6 antihypertensive medications. The change in BP patterns in the control group may have occurred due to better compliance with diet or antihypertensive drugs due to participation in a clinical trial.
These effects on intercept systolic, diastolic and pulse BP occurred within 4 weeks and persisted at 8 weeks which is evidence against the lag-phenomenon 17, 18. Treatment of essential hypertension with thiazide diuretics is analogous to AVRT in patients on hemodialysis. Although the exact mechanism of reduction in BP with thiazide diuretics remains elusive, since BP reduction is not seen in anephric patients, it appears that volume contraction is a central component that triggers the antihypertensive effect of these drugs. The BP reduction with thiazide diuretics also occurs rapidly and shows no lag-phenomenon 17, 28. The lag-phenomenon as reported previously may be specific to incident patients on dialysis who may require longer to achieve dry-weight 17. It is also possible, that the lag-phenomenon may be related to the poor assessment of BP surrounding the dialysis treatment 29. Since we used a sensitive technique to monitor BP we may have detected significant changes in BP earlier and more precisely. Restricting the analysis to wake BP also did not reveal the presence of the lag-phenomenon.
The lack of sleep-related fall in BP—non-dipping— occurs early in the course of CKD 30, 31. Non-dipping in patients with CKD not on dialysis is associated with older age, greater proteinuria, and lower serum albumin risk factors common to inflammatory and atherosclerotic processes 31. Amar et al have reported poor cardiovascular outcomes among hemodialysis patients who were non-dippers 26. We did not find evidence of restoration of dipping in our patients treated with augmented volume removal. We did not find support for dipping when data were analyzed either with the cosinor model or with the more conventional sleep-wake model. The lack of restoration of dipping may not be surprising if factors such as autonomic activation play a more important role in causing non-dipping. For example, if renalase deficiency, an enzyme that breaks down catecholamines is important in the pathogenesis of non-dipping, then replacing this enzyme—rather than removing volume—may be more important for restoring the nocturnal dip in BP 32. In defense of this hypothesis, there is some evidence that renal transplantation may restore the dipping phenomenon 33, 34. Sympathetic activation which is common in hemodialysis patients may be another mechanism mediating non-dipping but may not be mitigated by volume removal 35.
Some limitations of our study should be recognized. The majority of the participants in our study were black hemodialysis patients. Whether black participants have a different chronobiology than non-black participants in the interdialytic period is not known. Our study lasted only 8 weeks. Whether longer term study would have revealed restoration of dipping cannot be answered by our short-term trial.
Perspective
AVRT evoked parallel reductions in wake and sleep BP. There was no additional reduction in sleep BP compared to wake BP. Confirmed by cosinor rhythmometric analysis, lack of evidence of change in circadian amplitudes of systolic or diastolic BP does not support the notion that augmented volume removal can restore nocturnal dipping in hemodialysis patients. This is an important negative finding of our randomized trial. However, AVRT substantially altered arterial pressure and heart rate rhythm patterns in hemodialysis patients that were characterized by a reduction in BP intercept, an increase in the slope but no increase in lability of BP. Thus an elevated intercept and blunted slope pattern characterizes the “volume overload BP pattern” on ambulatory BP monitoring. Reduction of systolic BP exceeded that of diastolic BP; the pulse pressure reduction of 3 to 5 mmHg and the reduced BP load has the potential to impact survival in hemodialysis patients 36. Outcome trials are now needed to translate these findings to clinical practice.
Acknowledgments
Source of Funding
Grant Support: NIH-NIDDK 5RO1-063020-05
Footnotes
Disclosures
None
Reference List
- 1.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115:291–297. doi: 10.1016/s0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 2.Thompson AM, Pickering TG. The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int. 2006;70:1000–1007. doi: 10.1038/sj.ki.5001695. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R. How should hypertension be assessed and managed in hemodialysis patients? Home BP, not dialysis unit BP, should be used for managing hypertension. Semin Dial. 2007;20:402–405. doi: 10.1111/j.1525-139X.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Andersen MJ. Blood pressure recordings within and outside the clinic and cardiovascular events in chronic kidney disease. Am J Nephrol. 2006;26:503–510. doi: 10.1159/000097366. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R. Hypertension diagnosis and prognosis in chronic kidney disease with out-of-office blood pressure monitoring. Curr Opin Nephrol Hypertens. 2006;15:309–313. doi: 10.1097/01.mnh.0000222700.14960.18. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47:62–68. doi: 10.1161/01.HYP.0000196279.29758.f4. [DOI] [PubMed] [Google Scholar]
- 7.Rodby RA, Vonesh EF, Korbet SM. Blood pressures in hemodialysis and peritoneal dialysis using ambulatory blood pressure monitoring. Am J Kidney Dis. 1994;23:401–411. doi: 10.1016/s0272-6386(12)81003-1. [DOI] [PubMed] [Google Scholar]
- 8.Kelley K, Light RP, Agarwal R. Trended cosinor change model for analyzing hemodynamic rhythm patterns in hemodialysis patients. Hypertension. 2007;50:143–150. doi: 10.1161/HYPERTENSIONAHA.107.091579. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Light RP. Arterial stiffness and interdialytic weight gain influence ambulatory blood pressure patterns in hemodialysis patients. Am J Physiol Renal Physiol. 2007;294:F303–F308. doi: 10.1152/ajprenal.00575.2007. [DOI] [PubMed] [Google Scholar]
- 10.Hermida RC, Smolensky MH. Chronotherapy of hypertension. Curr Opin Nephrol Hypertens. 2004;13:501–505. doi: 10.1097/00041552-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 11.de Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJ, Santos SF. Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int. 2004;66:1232–1238. doi: 10.1111/j.1523-1755.2004.00876.x. [DOI] [PubMed] [Google Scholar]
- 12.Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. doi: 10.1161/01.cir.100.15.1635. [DOI] [PubMed] [Google Scholar]
- 13.Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997;96:1859–1862. doi: 10.1161/01.cir.96.6.1859. [DOI] [PubMed] [Google Scholar]
- 14.Peixoto AJ, White WB. Circadian blood pressure: clinical implications based on the pathophysiology of its variability. Kidney Int. 2007;71:855–860. doi: 10.1038/sj.ki.5002130. [DOI] [PubMed] [Google Scholar]
- 15.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
- 16.Cornelissen G, Halberg F, Otsuka K, Singh RB, Chen CH. Chronobiology predicts actual and proxy outcomes when dipping fails. Hypertension. 2007;49:237–239. doi: 10.1161/01.HYP.0000250392.51418.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charra B, Bergstrom J, Scribner BH. Blood pressure control in dialysis patients: Importance of the lag phenomenon. Am J Kidney Dis. 1998;32:720–724. doi: 10.1016/s0272-6386(98)70147-7. [DOI] [PubMed] [Google Scholar]
- 18.Khosla UM, Johnson RJ. Hypertension in the hemodialysis patient and the "lag phenomenon": insights into pathophysiology and clinical management. Am J Kidney Dis. 2004;43:739–751. doi: 10.1053/j.ajkd.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-Weight Reduction in Hypertensive Hemodialysis Patients (DRIP). A Randomized, Controlled Trial. Hypertension. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charra B, Laurent G, Chazot C, Calemard E, Terrat JC, Vanel T, Jean G, Ruffet M. Clinical assessment of dry weight. Nephrol Dial Transplant. 1996;11 Suppl 2:16–19. doi: 10.1093/ndt/11.supp2.16. [DOI] [PubMed] [Google Scholar]
- 21.Jaeger JQ, Mehta RL. Assessment of dry weight in hemodialysis: an overview. J Am Soc Nephrol. 1999;10:392–403. doi: 10.1681/ASN.V102392. [DOI] [PubMed] [Google Scholar]
- 22.Peixoto AJ, Gray TA, Crowley ST. Validation of the SpaceLabs 90207 ambulatory blood pressure device for hemodialysis patients. Blood Press Monit. 1999;4:217–221. [PubMed] [Google Scholar]
- 23.Agarwal R, Kelley K, Light RP. Diagnostic utility of blood volume monitoring in hemodialysis patients. Am J Kidney Dis. 2008;51:242–254. doi: 10.1053/j.ajkd.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 24.Holden JE, Kelley K, Agarwal R. Analyzing Change: A Primer on Multilevel Models with Applications to Nephrology. Am J Nephrol. 2008;28:792–801. doi: 10.1159/000131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 26.Amar J, Vernier I, Rossignol E, Bongard V, Arnaud C, Conte JJ, Salvador M, Chamontin B. Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int. 2000;57:2485–2491. doi: 10.1046/j.1523-1755.2000.00107.x. [DOI] [PubMed] [Google Scholar]
- 27.Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, Lazarus JM, Owen WF., Jr Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA. 2002;287:1548–1555. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- 28.Carlsen JE, Kober L, Torp-Pedersen C, Johansen P. Relation between dose of bendroflumethiazide, antihypertensive effect, and adverse biochemical effects. Br Med J. 1990;300:975–978. doi: 10.1136/bmj.300.6730.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Pre and post dialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1:389–398. doi: 10.2215/CJN.01891105. [DOI] [PubMed] [Google Scholar]
- 30.Baumgart P, Walger P, Gemen S, von Eiff M, Raidt H, Rahn KH. Blood pressure elevation during the night in chronic renal failure, hemodialysis and after renal transplantation. Nephron. 1991;57:293–298. doi: 10.1159/000186278. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension. 2005;46:514–520. doi: 10.1161/01.HYP.0000178102.85718.66. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, Wu Y, Peixoto A, Crowley S, Desir GV. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115:1275–1280. doi: 10.1172/JCI24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatzka CD, Schobel HP, Klingbeil AU, Neumayer HH, Schmieder RE. Normalization of circadian blood pressure profiles after renal transplantation. Transplantation. 1995;59:1270–1274. [PubMed] [Google Scholar]
- 34.Covic A, Gusbeth-Tatomir P, Mardare N, Buhaescu I, Goldsmith DJ. Dynamics of the circadian blood pressure profiles after renal transplantation. Transplantation. 2005;80:1168–1173. doi: 10.1097/01.tp.0000167003.97452.a8. [DOI] [PubMed] [Google Scholar]
- 35.Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cateliotti A, Stancanelli B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 36.Hermida RC, Ayala DE, Calvo C, Portaluppi F, Smolensky MH. Chronotherapy of hypertension: administration-time-dependent effects of treatment on the circadian pattern of blood pressure. Adv Drug Deliv Rev. 2007;59:923–939. doi: 10.1016/j.addr.2006.09.021. [DOI] [PubMed] [Google Scholar]