Abstract
Williams syndrome (WS) is a neurodevelopmental disorder associated with impaired visuospatial representations subserved by the dorsal stream and relatively strong object recognition abilities subserved by the ventral stream. There is conflicting evidence on whether this uneven pattern extends to working memory (WM) in WS. The present studies provide a new perspective, testing WM for a single stimulus using a delayed recognition paradigm in individuals with WS and typically developing children matched for mental age (MA matches). In three experiments, participants judged whether a second stimulus ‘matched’ an initial sample, either in location or identity. We first examined memory for faces, houses and locations using a 5 s delay (Experiment 1) and a 2 s delay (Experiment 2). We then tested memory for human faces, houses, cat faces, and shoes with a 2 s delay using a new set of stimuli that were better controlled for expression, hairline and orientation (Experiment 3). With the 5 s delay (Experiment 1), the WS group was impaired overall compared to MA matches. While participants with WS tended to perform more poorly than MA matches with the 2 s delay, they also exhibited an uneven profile compared to MA matches. Face recognition was relatively preserved in WS with friendly faces (Experiment 2) but not when the faces had a neutral expression and were less natural looking (Experiment 3). Experiment 3 indicated that memory for object identity was relatively stronger than memory for location in WS. These findings reveal an overall WM impairment in WS that can be overcome under some conditions. Abnormalities in the parietal lobe/dorsal stream in WS may damage not only the representation of spatial location but also may impact WM for visual stimuli more generally.
Keywords: Williams syndrome, working memory, dorsal stream, parietal lobe, development, developmental disorder, spatial representation, visuospatial, frontoparietal
Williams syndrome (WS) is a rare genetic disorder characterized by physical anomalies, a friendly personality, and an uneven cognitive profile (Bellugi, Lichtenberger, Jones, & Lai, 2000; Mervis et al., 2000; Meyer-Lindenberg, Mervis, & Berman, 2006). This uneven profile consists of relatively strong skills on tasks related to social interaction, including visual tasks such as face recognition and the perception of biological motion, and severe deficits on other types of visual-spatial processing, most often in block construction and drawing tasks (Atkinson et al., 1997; Bellugi, Lichtenberger, Jones, Lai, & George, 2001; Hoffman, Landau, & Pagani, 2003; Wang, Doherty, Rourke, & Bellugi, 1995; Jordan, Reiss, Hoffman, & Landau, 2002; Landau et al., 2005; Reiss, Hoffman, & Landau, 2005; Tager-Flusberg, Plesa-Skwerer, Faja, & Joseph, 2003). Previous evidence has indicated that the pattern of performance on visual tasks in WS may reflect selective damage to dorsal stream areas that subserve visual processing of ‘where’ or ‘how’ information (i.e, spatial location) with relative sparing of ventral stream areas in the temporal lobe that subserve visual processing of ‘what’ information (i.e., object recognition: Atkinson et al., 1997; Dilks, Hoffman, & Landau, 2008; Meyer-Lindenberg et al., 2004).
The present paper explores whether the uneven deficit in visual-spatial tasks evident in WS extends to working memory (WM), with memory for location more impaired than memory for object identity, including face identity/recognition. We tested individuals with WS and typically developing children matched for mental age in three experiments using a delayed match to sample paradigm. Participants remembered either the location or the identity of the stimulus, similar to tasks used previously to examine perceptual processing in WS (Paul, Stiles, Passarotti, Bavar, & Bellugi, 2002). We tested memory for a single stimulus, to simplify task demands for testing younger and lower functioning participants. To examine whether the uneven pattern was evident across memory demands, we used both a 5 s (Experiment 1) and a 2 s (Experiments 2 and 3) delay. To test the generality and specificity of the previously proposed expertise for faces in WS, we changed the set of stimuli used between Experiments 1/2 and Experiment 3. The new stimuli used in Experiment 3 included a wider range of stimulus types and face stimuli that were better controlled for hairline, orientation, and expression.
Paul and colleagues (2002) did a behavioral study using the well-established ‘face/place’ paradigm to examine the perception of human faces compared to locations in children, adults, and a group of individuals with WS. Since this paradigm is known to differentially activate dorsal and ventral streams in typical adults and children (Haxby et al., 1994; Passarotti et al., 2003), Paul and colleagues hypothesized that, if dorsal stream function was more affected by WS than ventral stream function, perception of location would be relatively more impaired in WS than the perception of human faces. Participants saw two stimuli and then, after a 0.5 s delay, judged whether the identity or the location of the third stimulus matched either of the previous ones. Their results suggested that the representation of location was more impaired than face recognition in WS, consistent with their hypothesis. While suggestive, there were several limitations in this experiment: the faces included hair which could be used for recognizing the faces without processing the internal features; it tested only face recognition, not other kinds of objects; and the comparison to the control group was difficult to interpret, as the controls were not individually matched to the WS group on intellectual ability and were older (M age of 9-years-old) than controls in other studies (generally mental age matches around 6 years of age – Vicari, Bellucci, & Carlesimo, 2006; O'Hearn, Landau, & Hoffman, 2005; Jarrold, Phillips, & Baddeley, 2007b).
Evidence on the structure and function of the brain in WS supports the proposal of dorsal stream dysfunction (Reiss et al., 2000; Meyer-Lindenberg et al., 2004; Eckert et al., 2005; Meyer-Lindenberg et al., 2006). Studies of WS have reported decreased grey matter volume (Eckert et al., 2005; Meyer-Lindenberg et al., 2004; Reiss et al., 2000), sulcal depth (Kippenhan et al., 2005; Van Essen et al., 2006), and functional activation (Meyer-Lindenberg et al., 2004; Mobbs et al., 2004) in the parietal and dorsal occipital regions. For instance, a neuroimaging study of WS (Meyer-Lindenberg et al., 2004) used a face/place task to compare functional activation in the dorsal and ventral streams in adults with WS, who had IQs in the normal range, and controls matched on both age and IQ. This study used stimuli similar to that of Paul and colleagues, but with minimal hair included (same stimuli as those used in the present Experiments 1 and 2, from Haxby et al., 1994). Participants reported whether two sequential stimuli were at the same vertical position (i.e., location task) or were the same object (i.e., identity task) while in the scanner. No behavioral differences were found on either task. However, there were differences in the level of functional activation during the location task. The group with WS displayed decreased activation in bilateral parietal lobe when compared to controls during the location > identity contrast. In addition, controls exhibited significant activation in the parietal lobe during the location task relative to the identity task but individuals with WS did not. In contrast to these effects during the location task, the level of activation in ventral stream areas during the identity > location contrast did not differ between groups. In particular, there were similar patterns of activation across groups in the temporal lobe for both face recognition (e.g., in fusiform face area, FFA) and house recognition (e.g., parahippocampal place area, PPA). A path analysis associated the differences in functional activation in the parietal lobe found in WS with decreased gray matter volume in the intraparietal sulcus (IPS), a dorsal stream area posterior to where significant functional activation was evident in controls.
Meyer-Lindenberg and colleagues' (2004) results suggest intact ventral stream function in people with WS for faces and houses, raising the possibility that the strength for faces in WS extends to houses and possibly other objects. A recent study using passive viewing with the same set of stimuli (Sarpal et al., 2008) also found that the pattern of activation in the ventral stream areas was comparable for individuals with WS and matched controls. However, Sarpal and colleagues also found differences between the two groups. People with WS showed decreased activation in dorsal stream areas (IPS) when they viewed houses, relative to viewing scrambled pictures (house > scrambled contrast), compared to controls. In addition, there were group differences in the functional connectivity between ventral stream and other regions, with the WS group exhibiting increased connectivity among temporal lobe regions and decreased connectivity between temporal regions and parietal and prefrontal cortex, compared to controls. While the group differences in functional connectivity was evident for both house and face stimuli, differences between the WS group and controls in the magnitude of activation was specific to IPS and the house stimuli, as face stimuli did not activate this area even in controls. This result suggests that individuals with WS might show uneven ability across object types, with specific deficits evident with object types that activate the parietal lobe.
Vicari and colleagues propose that uneven performance is also evident across working memory (WM) tasks in individuals with WS, with WM for location more impaired than WM for identity (Vicari, Bellucci, & Carlesimo, 2003; Vicari et al., 2006). However, this claim is controversial (Jarrold et al., 2007b). WM provides short-term maintenance of immediately pertinent information, and is thought to be subserved by limited capacity storage buffers that are specialized for different types of information (Baddeley, 1993), including distinct visual WM stores for spatial location and object identity (Carlesimo, Perri, Turriziani, Tomaiuolo, & Caltagirone, 2001; Riddoch, Humphreys, Blott, Hardy, & Smith, 2003; Humphreys & Riddoch, 2001; Wilson, Clare, Young, & Hodges, 1997). One possibility is that uneven WM performance reflects uneven perceptual processes rather than working memory per se, which may help account for the inconsistent evidence as perceptual demands differ across the studies. For instance, parietal lobe dysfunction in WS could impair the encoding of location information used for subsequent memory maintenance more than it affects the encoding of other types of information (Smith et al., 1995; Ungerleider & Haxby, 1994; Picchioni et al., 2007). Alternatively, the uneven impairment could extend to WM per se, possibly reflecting particular damage to dorsal frontal regions representing spatial WM and/or to frontoparietal circuitry (Courtney, Ungerleider, Keil, & Haxby, 1996; Sala, Rama, & Courtney, 2003; van Leijenhorst, Crone, & van der Molen, 2007; Haxby, Petit, Ungerleider, & Courtney, 2000; Courtney, Petit, Maisog, Ungerleider, & Haxby, 1998; Munk et al., 2002). Impaired frontoparietal circuitry in WS could lead to decreased communication from parietal lobe to dorsal frontal regions required for spatial WM, consistent with previous proposals that frontoparietal connectivity subserving visuospatial WM is impaired (Atkinson et al., 2003).
In the current study we tested whether, in a face/place task, WM for location would be relatively poorer than WM for object identity in individuals with WS, as previously indicated by the work of Vicari and colleagues. To examine whether this profile was different across memory demands, indicating a WM impairment rather than perceptual encoding impairments affecting WM task performance, we varied the delay between Experiments 1 (5 s) and 2 (2 s). Also, on the basis of relatively normal performance with houses in Meyer-Lindenberg et al. (2004), we hypothesized that the relatively strong performance in WS on face recognition would extend to non-face objects. We first used faces and houses to examine this proposal because houses seemed the strongest test of our hypothesis: Houses are more likely to be impaired in WS than other objects because they are particularly spatial objects (Sala et al., 2003) and they engage hippocampal or parietal areas that are abnormal in WS (Meyer-Lindenberg et al., 2005). We expanded the object types used in Experiment 3 to include shoes (a man-made object like houses, but also very different from houses) and cat faces (to examine whether expertise with faces included non-human faces). In Experiment 3, we also used face and house stimuli in which orientation, expression and hairline were more tightly controlled and internally consistent, to test whether the performance profile in Experiment 2 generalized to other face and house stimuli. In particular, the faces in Experiment 3 had less engaging expressions than those in Experiment 2; since people with WS are particularly interested in the social aspects of faces, we hypothesized that reducing this dimension (i.e., the friendliness) of the stimuli might influence the results.
Experiment 1: Five second delay
Participants
The WS group included 8 males and 6 females (M age16 years, 2 months: range 10;5 – 22;7), positively diagnosed by a geneticist and the fluorescence in situ hybridization (FISH) test. The FISH test is a blood test identifying whether a region on chromosome 7, associated with the protein elastin, is missing from one of the two copies of chromosome 7, which occurs in approximately 95% of individuals diagnosed with WS (Ewart et al., 1993; Meyer-Lindenberg et al., 2006). Typically developing controls were 7 males and 7 females (M age 6 years, 1 month: range 4;11 - 7;11). They were individually matched to the WS participants on mental age (MA matches), using the raw scores on the matrices subtest of the Kaufman Brief Intelligence Test (KBIT; Kaufman & Kaufman, 1990). The Matrices subtest requires picture-based category matching and has few spatial items; therefore it does not overly penalize WS people for their spatial deficit (M: WS = 21.5 ± 3.9; controls = 21.2 ± 4.5; t(26) = .18, p = .86). The two groups also performed similarly on the verbal subtest of the KBIT which tests picture naming, a relative strength in WS (M: WS = 39.9 ± 7.8; controls = 36.3 ± 5.1; t (26) = 1.44, p = .16). The WS profile was typical (Mervis, et al., 2000), with a mean IQ of 64 ± 15.6 (KBIT), and a median block construction score at the 1st percentile for age (M at the 2nd percentile; Differential Abilities Scale, DAS, Elliot, 1990). People with WS were recruited through the Williams Syndrome Association; controls had previously participated in our studies or were recruited through local preschools.
Participation across experiments
Experiments 1 and 2 were most often done on the same day with a break in between (each study took approximately 45 minutes), but with at most 3 months between Experiments (due to the spacing of multiple visits by people with WS to our lab). The order of these experiments was counterbalanced. Fourteen participants with WS participated in both experiments, and one individual with WS did only Experiment 2 (Exp. 1, N=14; Exp. 2, N=15). Twelve of these participants, along with 6 additional participants with WS, participated in Experiment 3 between 1 and 1.5 years later.
Stimuli & Procedure
As in previous studies (Paul et al., 2002; Meyer-Lindenberg et al., 2004; Sarpal et al., 2008), we used naturalistic photos of real-life objects known to differentially activate dorsal and ventral visual processing regions (Ungerleider et al., 1994; Haxby et al., 1994; Courtney, Ungerleider, Keil, & Haxby, 1996; Sala et al., 2003). Stimuli were 24 black and white photos of each of 3 types (faces, houses, or scrambled faces) presented in 1 of 24 possible locations on the computer screen. The photos were presented against a gray background using Superlab software (Cedrus, USA) on a MacIntosh computer. Of the faces, half were male and half were female: of the houses, half were wooden beach houses and half were brick colonial houses (See Sala et al., 2003). The same sort of face or house (e.g., male face, beach house) was used within a trial. Scrambled faces were the same faces phase-scrambled; thus, they had the same luminance, spatial frequency content, and contrast as the faces but were unidentifiable. The location condition with scrambled faces (i.e., scrambled location condition) was a control to ensure that attention to the objects themselves did not enhance or impair accurate location judgments.
For each trial, one photo was presented, followed by a 5 s delay, and then a second photo was presented (Figure 1). There were 16 consecutive trials in each of the five conditions (face location, face identity, scrambled location, house location, and house identity: Figure 2). The same trials were used in both the face identity and the face location condition (and these locations were also used in the scrambled face condition), and in the house identity and the house location condition. Thus, the only difference between the two tasks was the information that was remembered. Since the stimuli in these conditions were identical, two other conditions were always presented in between them creating 4 possible orders for the conditions (e.g., face identity, face location, scrambled location, house identity, house location). For each condition, there were 8 possible trial orders – these were sets of 16 trials using a random order of stimuli and locations, with the constraint that the same stimuli or locations could not be used in consecutive trials. Both the condition order and the trial order were counterbalanced between subjects and matched between groups (i.e., each subject and his/her match saw the same condition order and trial order).
Figure 1.
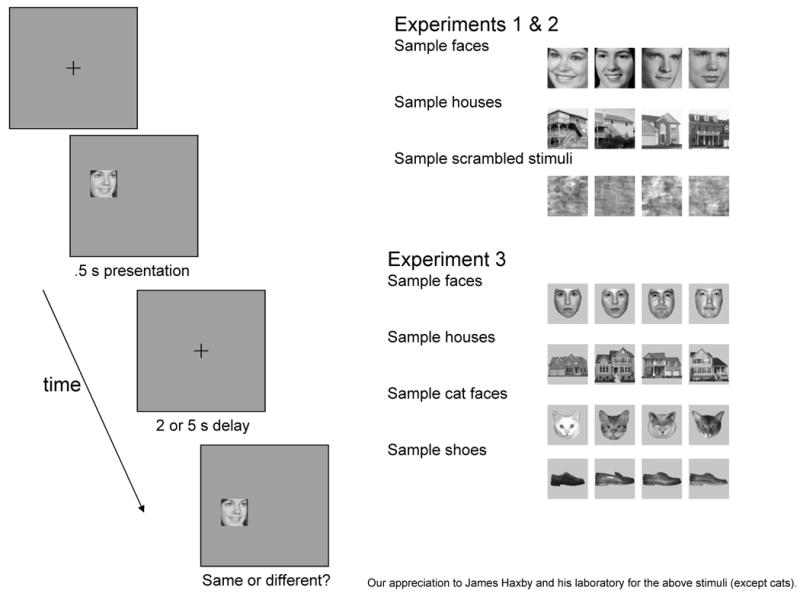
Illustration of task and the stimuli used.
Figure 2.
Task Design.
Participants reported whether the location or identity (depending on the task instructions for that block of trials) of the second photo was the same or different from the first one. The experimenter entered the participant's verbal response. All subjects were encouraged to ‘remember the location’ or ‘remember the face/house’ throughout the delay. At least 2 practice trials were done before each condition, and breaks were taken as needed between the conditions.
Statistical analyses
To examine differences in percent correct between groups, we used two mixed model analyses of variance (ANOVAs) in each experiment, followed by post-hoc tests when appropriate. Group (WS or controls) was always a between-subjects factor. The first ANOVA used Condition as a within-subject factor (5 conditions in Experiment 1/2, 9 conditions in Experiment 3: See Figure 2), including the scrambled location condition. The goal of this ANOVA was to identify Group by Condition interactions, indicating that the two groups performed differently across the conditions, as well as main effects of Group indicating an overall impairment in WS. In the second ANOVA, we excluded the scrambled condition, and used Task (2 Tasks – location or identity -- in all Experiments) and Object Type (2 Types – face and house – in Experiments 1/2: 4 Types – face, house, shoe and cat face – in Experiment 3) as within-subject factors. This second analysis had two benefits: 1) it increased our power to detect group differences common across Task or Object Types, and 2) without the scrambled location condition, the stimuli were identical, thus perfectly matched, across the two Tasks.
Results
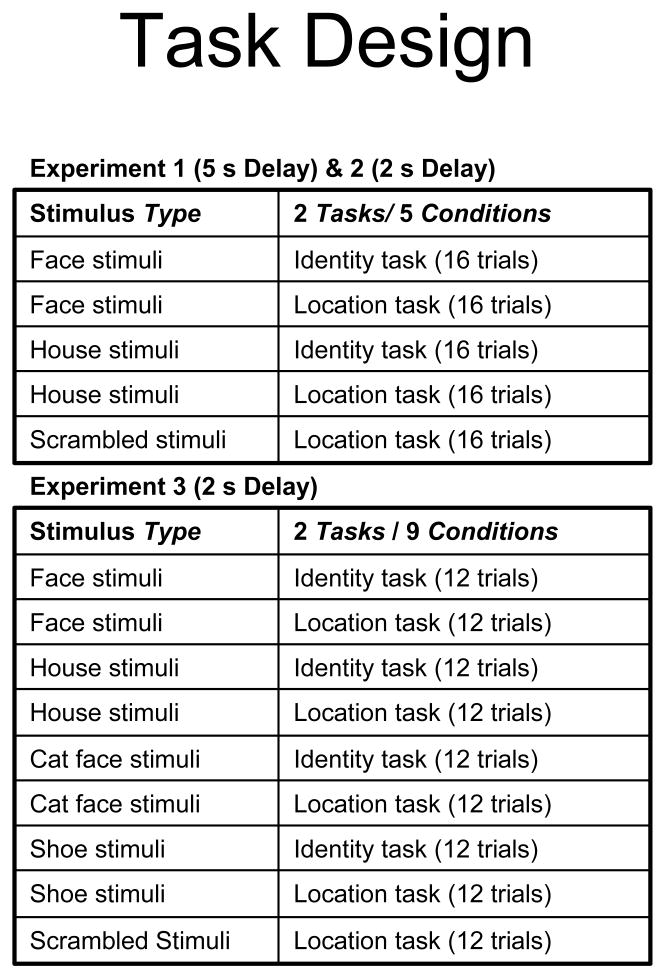
People with WS performed more poorly than MA matches in this experiment regardless of the task or the type of object (Figure 3). A mixed model repeated measures analysis of variance with Group (WS, MA matches) and Condition (face identity, face location, scrambled location, house identity, house location) on percent correct revealed a main effect of Group [F(1,26) = 4.23, p =.05], but no effect of Condition [F(4, 104) = .85, p = .50] nor an interaction between Group and Condition [F(4, 104) = .80, p = .53]. To further examine this, we did another repeated measures analysis of variance excluding the scrambled location condition, with the factors of Group (WS, MA matches), Task (Identity, Location) and Object Type (Face, House). If identity was remembered more accurately than spatial location, as we hypothesized, a Group × Task interaction would be expected. However, like the first analysis, this analysis also failed to reveal an interaction between Group and Task, or Object Type. The only significant effect was the main effect of Group (Group [F(1,26) = 4.16, p = .05]; Task [F(1,26) = 2.37, p = .14]; Object Type [F(1,26) = .84, p = .37]; Group × Task [F(1,26) = .85, p = .37]; Group × Object Type [F(1,26) = .84, p = .37]; Object Type × Task [F(1,26) = .88, p = .36]; Group × Task × Object Type [F(1,26) = 1.24, p = .28]). Thus, the WS group was similarly impaired across WM for identity and location with a 5 s delay, though they were above chance – 50% - on all conditions.
Figure 3.
Percent correct in all conditions in Experiment 1, with a 5 s delay. All error bars are the standard error of the mean.
Experiment 2: Two second delay
Participants
The group with WS included 8 males and 7 females (M age, 17 years, 8 months: range 10;5 – 38;10), including all 14 participants from Experiment 1 plus one additional participant. Typically developing controls were 8 males and 7 females (M age, 6 years, 3 months: range 4;4 -8;10: ten participated in Experiment 1) who were matched individually to controls on the basis of raw score on the matrices subtest of the KBIT. These groups scored similarly on both the Verbal and the Matrices subtests of the KBIT (Verbal: M: WS = 39.3 ± 7.9; controls = 36.0 ± 6.8; t(28) = 1.22, p = .23 – Matrices: M: WS = 21.3 ± 3.8; controls = 21.5 ± 3.5; t(28) = -.01, p = .92). The WS group had a mean IQ of 63 ± 15.2 (KBIT), and a median block construction score at the 1st percentile for age (M at 2nd percentile; Elliot, 1990).
Stimuli, & Procedure
This experiment was identical to Experiment 1 (Figure 1 and 2), except that the delay was 2 seconds instead of 5 seconds.
Results
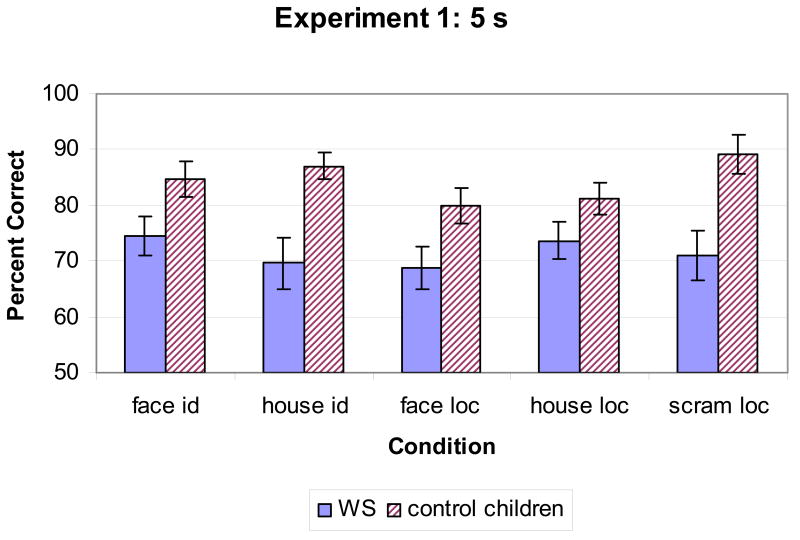
In this experiment, people with WS performed relatively better on the face identity condition than on the other conditions. We again carried out a repeated measures analysis of variance on percent correct (Figure 4), with the between-subject factor of Group (WS, MA matches) and the within-subject factor of Condition (face identity, face location, scrambled location, house identity, house location). This analysis revealed a main effect of Condition [F(4,112) = 4.28, p = .003] and Group [F(1,28) = 6.78, p = .01]. Importantly, and in contrast to Experiment 1, there was also an interaction between Group and Condition [F(4,112) = 3.34, p =.01].1 To examine whether this interaction reflected differences between groups specific to a Task or Object Type, we did a second repeated measures ANOVA with Group, Task (location, identity) and Object Type (face, house).This analysis found only a main effect of Task, but no Group × Task interaction, with both groups performing better on the identity than on the location conditions, nor a Group × Object Type interaction (Group [F(1,28) = 3.26, p =.08]; Task [F(1,28) = 14.71, p =.001]; Object Type [F(1,28) = .53, p = .47]); Group × Task [F(1,28) = 1.55, p = .22]; Group × Object Type [F(1,28) = 2.75, p = .11]; Task × Object Type [F(1,28) = .0, p = 1.0]; Group × Task × Object Type [F(1,28) = .10, p = .76]). To examine the Condition × Group interaction further, post-hoc comparisons using t-tests on each condition separately were performed. These revealed that people with WS performed more poorly than MA matches on the scrambled location [t(28) = -4.59, p = .001], house location [t(28) = -2.38, p=.02] tasks, and there was also a trend for poorer performance on the house identity condition [t(28) = -1.82, p = .08]. Faces appeared to be better represented than other stimuli in WS, and this was particularly true of the face identity task (see Figure 4).
Figure 4.
Percent correct in all conditions in Experiment 2, with a 2 s delay.
In contrast to the general impairment found in Experiment 1 with a 5 s delay, people with WS showed an uneven pattern across conditions with a 2 s delay. In Experiment 2 with a 2 s delay, people with WS performed more poorly than MA matches on most WM tasks, including location memory and house recognition, but were comparable to MA matches on face recognition. Since we only used faces and houses, we were unable to distinguish whether the WS group performed better with faces than with other objects, or more poorly with houses than with other objects. If the highly social personality found in WS leads to increased attention to faces (Meyer-Lindenberg et al., 2006), this increased attention might aid encoding of and memory for faces but not other objects. Alternatively, houses might be remembered more poorly than other objects because they are particularly spatial objects (Sala et al., 2003), requiring activation in hippocampal and parietal areas that may be abnormal in WS (Meyer-Lindenberg et al., 2005). To distinguish these possibilities, we carried out another experiment with a new set of stimuli that included shoes and cat faces in addition to houses and human faces. Using these additional types of objects allowed us to distinguish among the two possible explanations for the results in Experiment 2: that human faces are remembered better than other objects in WS or that houses are remembered more poorly than other objects. Since we used cat faces in addition to human faces, we could also investigate whether the strong performance with human faces extends to cat faces (but not houses or shoes).
Experiment 3 utilized a new set of stimuli that has been recently used to examine ventral stream activation (used in Haxby et al., 2001, while those in Experiment 1 and 2 were used in Haxby et al., 1994; Meyer-Lindenberg et al., 2004; Sarpal et al., 2008). These new stimuli were more internally consistent in expression (neutral), orientation (front-facing), and did not include any hair (Figure 1). Since these dimensions may influence face recognition (Duchaine & Nakayama, 2005; Lander & Metcalfe, 2007; Pascalis, de Schonen, Morton, & Deruelle, 1995; Troje & Bulthoff, 1996), we wanted to examine whether the results from Experiment 2 generalized to this new set stimuli in Experiment 3. Whether the pattern of results is specific to the stimuli used (i.e., one set of faces), or general to the object type examined (i.e., all faces), provides crucial information about what aspect of the stimuli is influencing performance in WS.
Experiment 3: 2 second delay with new stimuli
Participants
This study included 18 participants with WS (9 M: M age, 20; 8: range, 10;5 - 48;6) and 18 typically developing controls (8 M; M age, 6; 4: range, 5;2 - 7;11). While we tested 12 of the same individuals with WS in Experiment 1 and 2 (M age = 16;7: M verbal score = 40.5, SD = 4.9: M matrices score = 20.5, SD = 4.1), we also included 6 new WS participants to increase our power to detect differences in WM (M age = 28;11: M verbal score = 41.5, SD = 4.0: M matrices score = 19.0, SD = 2.8). Groups were again matched on the Matrices subtest of the KBIT and had comparable scores on this section (M: WS = 20.0 ± 3.7; controls = 20.56 ± 4.3; t(34) = -.41, p = .68). In contrast to the previous experiment, these two groups differed significantly on the Verbal subtest (M: WS = 40.8 ± 4.6; controls = 34.7 ± 4.6; t(34) = 4.05, p < .01). Overall, the WS group had a mean IQ of 62 ± 14.6, and their median DAS block construction score was in the 1st percentile for their age, typical for this syndrome (Mervis et al., 2000).
Stimuli & Procedure
This experiment was identical to Experiment 2, except for the photos used as stimuli (See Figure 1), the number of conditions (9 instead of 5: see Figure 2), and the number of trials (12/condition instead of 16/condition). The faces faced forward, were unsmiling and slightly smaller than in Experiments 1 and 2, and had all traces of a hairline removed. Again, the stimuli consisting of human faces were half male and half female. House stimuli were now shown directly from the front. New object types included cat faces (images collected and cropped from the web to include only frontal views of cat faces) and men's shoes from the side (Haxby et al., 2001). All stimuli had background information removed.
Results
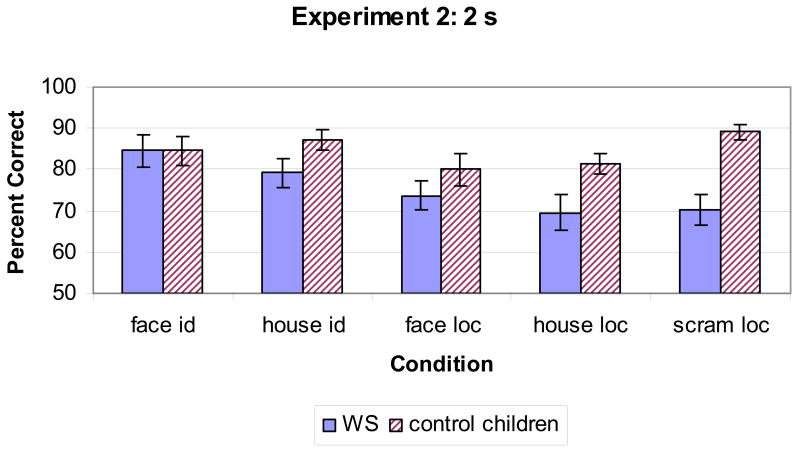
Figure 5 shows the percent correct by condition in Experiment 3. Since raw score on the verbal subtest of the KBIT differed between groups, this factor was used as a covariate in all analyses. Our first question was whether performance differed across conditions for WS, compared to MA matches, leading to an interaction between Condition and Group. A mixed model repeated measures ANOVA, with the between subject factor of Group (WS, MA Matches) and within subject factor of Condition (face identity, house identity, cat face identity, shoe identity, scrambled location, face location, house location, cat face location, shoe location), suggested that people with WS were impaired across all conditions. This analysis showed a main effect of Group [F(1,33) = 6.46, p = .02], but no effect of Condition [F(8,264) = 1.22, p = .29] nor a Condition by Group interaction [F(8,264) = 1.31, p = .24].
Figure 5.
Percent correct in all conditions in Experiment 3, with a 2 s delay. Top graph shows the identity conditions and bottom graph shows the location conditions.
We furthered examined performance across group using a repeated measures ANOVA that included within subject factors of Task (Location, Identity) and Object Type (Human face, house, cat face, shoe), in addition to the between subject factor of Group. This analysis effectively collapsed across object type to increase power to detect a difference in performance between the identity and location tasks. This analysis resulted in a significant Group by Task interaction ([F(1,33) = 3.99, p = .05]), indicating relatively poorer performance in the WS group than in MA Matches on location tasks compared to identity tasks, as well as a main effect of Group [F(1,33) = 6.91, p = .01].2 There were no other significant main effects or interactions. Importantly, there was not a significant Group × Object Type ([F(3,99) = .35, p = .78]), nor Group × Object Type × Task interaction ([F(3,99) = .30, p = .82]). Additional repeated measures ANOVAs of the identity conditions compared human faces to all other stimuli (houses, cats, shoes), houses to all other stimuli (faces, cats, shoes), all faces (human/cat) to all other objects (houses/shoes), and human faces to houses; these analyses failed to reveal any significant interactions involving Group and Object Type (all p's > .25). With these stimuli, people with WS did not represent faces better than other objects, or houses worse than other objects.
Since the pattern of results differed from Experiment 2, we wanted to examine whether this difference was due to the slightly older sample of individuals with WS (see participants section), compared to the previous Experiments. To examine this, we did the same analyses in the 12 individuals with WS who had participated in the previous studies and their MA Matches. We found the same pattern of results in this smaller sample. There was again an interaction between Task and Group, with the WS group performing more poorly on the Location Tasks than on Identity Tasks, but no effect of Object Type (Group [F(1,22) = 3.64, p = .07]; Task [F(1,22) = .07, p = .79]; Object Type [F(1,22) = 1.05, p = .39]; Group × Task [F(1,22) = 6.53, p = .02]; Group × Object Type [F(1,22) = .86, p = .47]; Object Type × Task [F(1,22) = 1.44, p = .24]; Group × Task × Object Type [F(1,22) = .50, p = .69]).3
Discussion & Conclusions
The present studies examined the proposal that people with WS have a specific impairment in location WM, which has received mixed support in previous studies (Vicari et al., 2006; Jarrold et al., 2007b). Vicari and colleagues argue that WS is associated with uneven WM ability, with location memory relatively more impaired than memory for identifying a set of features (Vicari et al., 2006; Vicari et al., 2003), while Jarrold and colleagues find that the WS group in their studies perform like MA matches both when remembering locations and when remembering features (Jarrold et al., 2007b). However, the experimental methods supporting both these proposals had complex task demands, requiring memory for multiple stimuli and using abstract patterns for the identity conditions. Furthermore, the conflicting results are less surprising considering that Vicari and colleagues examined memory for sequential stimuli, while Jarrold examined memory for multiple simultaneously shown stimuli. These two types of spatial WM may be dissociable (Mammarella et al., 2006; Finke, Bublak, & Zihl, 2006). In addition, using multiple stimuli increases the likelihood that participants are using alternate strategies, which may differ across sequential and simultaneous presentations.
The present studies provide a new perspective, testing WM for a single stimulus with a delayed recognition paradigm. To discriminate the nature of working memory maintenance in people with WS, Experiments 1 and 2 differed only in the length of the delay. To examine the effects of object type, Experiments 2 and 3 differed only in the stimuli used. This allowed us to examine proposals that people with WS are particularly skilled with human faces, compared to other types of objects, using two distinct sets of stimuli. Our results indicate that there is an overall WM impairment in people with WS, with the severity of the deficit modulated by the length of delay, the type of information, and the details of the stimuli.
Experiment 1 demonstrated that, with a 5 s delay, individuals with WS have a broad WM impairment, regardless of task (identity, location) or object type (houses, faces). Experiments 2 and 3 indicated that, with a 2 s delay, this impairment was uneven. In Experiment 2, memory for human faces was relatively preserved in WS, compared to location and houses. This is consistent with recent results from Sarpal and colleagues (2008) indicating that house stimuli are associated with decreased activation in IPS that could affect WM performance. Experiment 3 revealed that the strong performance on face identity in WS did not hold when the face stimuli were more tightly controlled for expression, orientation and hairline. With this set of stimuli, people with WS did not remember faces better – nor houses more poorly – than other objects. In Experiment 3, an uneven pattern was evident across identity and location tasks, with memory for identity stronger than memory for location in WS. While this pattern was not evident in Experiment 2, this difference between experiments may reflect the increased number of trials, and therefore power to detect a difference, in Experiment 3 (i.e., 48 trials instead of 32).
A generalized WM impairment in WS is not surprising considering that abnormalities in the parietal lobe, and possibly impaired frontoparietal connectivity (Sarpal et al., 2008; Atkinson et al., 2003), could affect not only location memory but also WM more broadly. In addition to representing spatial location (Burgess, Jeffery, & O'Keefe, 1999), parietal areas are also crucial for attentional selection and WM updating for both location and identity information (Roth, Serences, & Courtney, 2006; Roth & Courtney, 2007; Serences, Schwarzbach, Courtney, Golay, & Yantis, 2004; Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006; Klingberg, 2006). Thus, the parietal abnormalities in WS could contribute not only to impairments in spatial WM but also to impairments in remembering identity information. The possibility of a general WM impairment is supported by studies showing a memory deficit in people with WS compared to mental age matches on a wide range of tasks, including long-term memory (Jarrold, Baddeley, & Phillips, 2007a; Sampaio, Sousa, Fernandez, Henriques, & Goncalves, 2008); verbal memory (Jarrold et al., 2007a; Sampaio et al., 2008); binding of location and featural information (Jarrold et al., 2007b); and implicit memory (Vicari, Verucci, & Carlesimo, 2007). Some of these WM deficits seem to be related to mental retardation or learning disabilities more generally (Kittler, Krinsky-McHale, & Devenny, 2008; Jarrold et al., 2007b; Jarrold et al., 2007a).
While our results indicate a general impairment in WM in WS, we also found evidence that this deficit was mitigated by delay, stimuli, and whether the task was to remember location or identity. That the WM deficit would be more generalized at longer delays makes sense, as longer delays require increased activation in posterior parietal cortex (as well as other areas) during both location and identity judgments (Picchioni et al., 2007). Uneven impairments may reflect impaired perceptual representation of location, compared to identity, in individuals with WS. However, with longer delays, this uneven functioning in perceptual processing may be overwhelmed by a general WM impairment, reflecting parietal abnormalities and dysfunctional frontoparietal circuitry.
The results from Experiment 2 suggest that object type influences WM ability in WS, with face recognition an area of particular strength, while the results from Experiment 3 indicates that this pattern does not generalize to all face stimuli. That this pattern was only evident with the more inviting faces in Experiment 2 indicates that strengths in face processing in WS do not reflect the intrinsic visual properties of a face (e.g., the internal configuration of eyes, nose and mouth). We hypothesize that the strong performance with faces in Experiment 2, and the discrepancy between the results with faces in Experiment 2 and 3, reflects enhanced attention to smiling faces (over neutral faces and other objects) in individuals with WS, consistent with the high sociability found in this disorder. Enhanced attention has been shown to increase activation in ventral visual stream areas in the temporal lobe (e.g., Lepsien & Nobre, 2008), and frontoparietal areas (Astafiev et al., 2003; Serences et al., 2004). Thus, the increase in attention with friendly faces may help compensate for brain abnormalities in WS. Alternately, other circuits for face processing (Haxby, Hoffman, & Gobbini, 2002) may support the strong performance in face recognition including neural systems associated with facial or emotional expression (but see Plesa-Skwerer, Faja, Verbalis, & Tager-Flusberg, 2008).
Results from Experiment 3 also indicated that, with a 2 s delay, people with WS remember location more poorly than they remember identity, consistent with previous claims (Vicari et al., 2006). However, this pattern was not evident in Experiments 1 and 2. While this may reflect that Experiments 1 and 2 had fewer trials, and thus less power to detect an uneven pattern, it also suggests that the effect may be weaker than previously claimed, helping to explain the inconsistent results from other studies (Vicari et al., 2006; Jarrold et al, 2007b). We think the poorer performance on the location task reflects the initial encoding of the stimuli, which may be worse for location than for identity, consistent with previous behavioral and neurophysiological evidence on perceptual processing (Meyer-Lindenberg et al., 2004; Paul et al., 2002).
The present paper reveals that, with a simpler paradigm, WM performance is generally impaired in WS, especially with longer delays (Experiment 1). In addition, we found some evidence of uneven WM ability. Face recognition was strong in Experiment 2, but not with the neutral faces in Experiment 3, suggesting that additional attention to social stimuli (i.e., smiling faces) might help individuals with WS to compensate for WM deficits under some circumstances. The greater impairment in location than in identity judgments in Experiment 3 may reflect difficulties with the initial encoding of spatial information. However, these uneven patterns of performance on WM tasks depended crucially on the characteristics of the stimuli, and appear to be overwhelmed at longer delays by a generalized WM deficit in WS.
Acknowledgments
This work was done at Johns Hopkins University and supported by grants from NICHD (F32 HD42346 to KO), NINDS (RO1 NS 050876 to BL), NSF (BCS 0117744 and 9808585 to BL) and March of Dimes (12-01-0087 to BL). We thank Gitana Chunyo, Elizabeth Crowe, Eric Hsiao, Leslie Huang, and Joe Sala for their assistance, as well as James Haxby and his laboratory for the stimuli. We gratefully acknowledge our participants and the Williams Syndrome Association. Preliminary results were presented at the 4th Annual Conference for the Vision Sciences Society, 2004, Sarasota, FL.
Footnotes
To ensure that the pattern of performance found in individuals with WS did not simply reflect immature performance (i.e., face recognition reaching mature levels earlier than the other conditions), we compared the WS group to 12 younger typically developing children (M age 4 years, 10 months: 4;4-5;2). Again, we found an interaction between Condition and Group [F(4,100) = 5.49, p < .01], but no main effects of Condition [F(4,100) = 1.79, p = .14] or Group [F(1,25) = .21, p = .65]. Post-hoc comparisons (t-tests) revealed that the WS group performed significantly better at face identity than the younger group [t(25) = 2.09, p < .05], while the younger children performed significantly better at scrambled location [t(25) = -2.91, p < .05].
Without the covariate of verbal score, there was only a main effect of Group (Group [F(1,34) = 8.78, p < .01]; Condition [F(1,34) = .46, p = .50]; Group × Condition [F(1,34) = 2.29, p = .14]). Inclusion of this covariate did not affect the results in Experiment 1 or 2.
Practice effects could be a concern with these experiments. Experiment 1 and 2 were counterbalanced, and thus practice effects could not explain the different results in these 2 experiments (i.e., practice with Experiment 1 could not have led to the improved performance in Experiment 2, thus delay must have been the crucial variable). Experiment 3 was done between 1 and 1.5 years after Experiment 1 and 2, so practice is unlikely to have influenced the results. Nonetheless, we compared performance on Experiment 2 and Experiment 3 in the 12 participants with WS and 11 controls who participated in both Experiments (only 4 were MA matches in both). There were no significant differences in performance between the two Experiments (all p's > .10), despite the fact that all the participants were at least 1 year older, except for the scrambled location condition in the WS group (t[11]=3.0, p=.01). We are not sure why this occurred, possibly by chance or because they developed a new strategy with increasing age. However, the fact that there was no improvement in 9 out of 10 analyses suggests that there was not a general effect of practice. In addition, the scrambled location condition was not included in the ANOVAs with Task and Type, so this improvement could not have affected the pattern of significant results in Experiment 3.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. The Journal of Neuroscience. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Anker S, Curran W, Andrew R, Wattam-Bell J, et al. Neurobiological models of visuospatial cognition in children with Williams syndrome: Measures of dorsal-stream and frontal function. Developmental Neuropsychology. 2003;23:139–172. doi: 10.1080/87565641.2003.9651890. [DOI] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in William's syndrome. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience. 1997;8:1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Verbal and visual subsystems of working memory. Current Biology. 1993;3:563–565. doi: 10.1016/0960-9822(93)90059-w. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Jones W, Lai Z. I. The neurocognitive profile of Williams syndrome: A complex pattern of strengths and weaknesses. Journal of Cognitive Neuroscience. 2000;12:7–29. doi: 10.1162/089892900561959. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Jones W, Lai Z, St George M. The neurocognitive profile of Williams syndrome: A complex pattern of strengths and weaknesses. In: Bellugi U, St George M, editors. Journey from cognition to brain to gene: Perspectives from Williams syndrome. The MIT Press; 2001. pp. 1–41. [Google Scholar]
- Burgess N, Jeffery KJ, O'Keefe J. The hippocampal and parietal foundations of spatial cognition. Oxford University Press; 1999. [Google Scholar]
- Carlesimo GA, Perri R, Turriziani P, Tomaiuolo F, Caltagirone C. Remembering what but not where: independence of spatial and visual working memory in the human brain. Cortex. 2001;37:519–34. doi: 10.1016/s0010-9452(08)70591-4. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cerebral Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks DD, Hoffman JE, Landau B. Vision for perception and vision for action: Normal and unusual development. Developmental Science. doi: 10.1111/j.1467-7687.2008.00693.x. in press. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2005;44:576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Hu D, Eliez S, Bellugi U, Galaburda A, Korenberg J, et al. Evidence for superior parietal impairment in Williams syndrome. Neurology. 2005;64:152–153. doi: 10.1212/01.WNL.0000148598.63153.8A. [DOI] [PubMed] [Google Scholar]
- Finke K, Bublak P, Zihl J. Visual spatial and visual pattern working memory: neuropsychological evidence for a differential role of left and right dorsal visual brain. Neuropsychologia. 2006;44:649–661. doi: 10.1016/j.neuropsychologia.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–30. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. Journal of Neuroscience. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. NeuroImage. 2000;11:145–156. doi: 10.1006/nimg.1999.0527. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Landau B, Pagani B. Spatial breakdown in spatial construction: Evidence from eye fixations in children with Williams syndrome. Cognitive Psychology. 2003;46:260–301. doi: 10.1016/s0010-0285(02)00518-2. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Riddoch MJ. The neuropsychology of visual object and space perception. In: Goldstein EB, editor. Blackwell handbook of perception. Blackwell Publishers; 2001. pp. 204–236. [Google Scholar]
- Jarrold C, Baddeley AD, Phillips C. Long-term memory for verbal and visual information in Down syndrome and Williams syndrome: performance on the Doors and People test. Cortex. 2007a;43:233–247. doi: 10.1016/s0010-9452(08)70478-7. [DOI] [PubMed] [Google Scholar]
- Jarrold C, Phillips C, Baddeley AD. Binding of visual and spatial short-term memory in Williams syndrome and moderate learning disability. Developmental Medicine & Child Neurology. 2007b;49:270–273. doi: 10.1111/j.1469-8749.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- Jordan H, Reiss JE, Hoffman JE, Landau B. Intact perception of biological motion in the face of profound spatial deficits: Williams syndrome. Psychological Science. 2002;13:162–167. doi: 10.1111/1467-9280.00429. [DOI] [PubMed] [Google Scholar]
- Kippenhan JS, Olsen RK, Mervis CB, Morris CA, Kohn P, Meyer-Lindenberg A, et al. Genetic contributions to human gyrification: sulcal morphometry in Williams syndrome. J Neurosci. 2005;25:7840–7846. doi: 10.1523/JNEUROSCI.1722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler PM, Krinsky-McHale SJ, Devenny DA. Dual-task processing as a measure of executive function: a comparison between adults with williams and down syndromes. American Jouranal of Mental Retardation. 2008;113:117–132. doi: 10.1352/0895-8017(2008)113[117:DPAAMO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuospatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Landau B, Hoffman JE, Reiss JE, Dilks DD, Lakusta L, Chunyo G. Specialization and breakdown in spatial cognition: Lessons from Williams syndrome. In: Morris CA, Lenhoff H, Wang P, editors. Williams-Beuren Syndrome: Research and Clinical Perspectives. Baltimore, MD: Johns Hopkins University Press; 2005. [Google Scholar]
- Lander A, Metcalfe S. The influence of positive and negative facial expressions on face familiarity. Memory. 2007;15:63–69. doi: 10.1080/09658210601108732. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Nobre A. Attentional modulation of object representations in working memory. Cerebral Cortex. 2008;17:2072–2083. doi: 10.1093/cercor/bhl116. [DOI] [PubMed] [Google Scholar]
- Mammarella IC, Cornoldi C, Pazzaglia F, Toso C, Grimoldi M, Vio C. Evidence for a double dissociation between spatial-simultaneous and spatial-sequential working memory in visuospatial (nonverbal) learning disabled children. Brain & Cognition. 2006;62:58–67. doi: 10.1016/j.bandc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Bertrand J, Morris CA, Klein-Tasman BP, Armstrong SC. The Williams syndrome cognitive profile. Brain Cogn. 2000;44:604–28. doi: 10.1006/brcg.2000.1232. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen RK, Morris CA, et al. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–31. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci. 2006;7:380–393. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Sarpal D, Koch P, Steele S, Kohn P, et al. Functional, structural, and metabolic abnormalities of the hippocampal formation in Williams syndrome. The Journal of Clinical Investigation. 2005;115:1888–1895. doi: 10.1172/JCI24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Garrett AS, Menon V, Rose FE, Bellugi U, Reiss AL. Anomalous brain activation during face and gaze processing in Williams syndrome. Neurology. 2004;62:2070–6. doi: 10.1212/01.wnl.0000129536.95274.dc. [DOI] [PubMed] [Google Scholar]
- Munk MHJ, Linden DEJ, Muckli L, Lanfermann H, Zanella FE, Singer W, et al. Distributed cortical systems in visual short-term memory revealed by event-related functional magnetic resonance imaging. Cerebral Cortex. 2002;12:866–876. doi: 10.1093/cercor/12.8.866. [DOI] [PubMed] [Google Scholar]
- O'Hearn K, Landau B, Hoffman JE. Multiple object tracking in people with Williams syndrome and in normally developing children. Psychological Science. 2005;16:905–912. doi: 10.1111/j.1467-9280.2005.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascalis O, de Schonen S, Morton J, Deruelle C. Mother's face recognition by neonates: A replication and an extension. Infant Behavior & Development. 1995;18:79–85. [Google Scholar]
- Passarotti AM, Paul BM, Bussiere JR, Buxton RB, Wong EC, Stiles J. The development of face and location processing: An fMRI study. Developmental Science. 2003;6:100–117. [Google Scholar]
- Paul BM, Stiles J, Passarotti A, Bavar N, Bellugi U. Face and place processing in Williams syndrome: Evidence for a dorsal-ventral dissociation. Neuroreport: For Rapid Communication of Neuroscience Research. 2002;13:1115–1119. doi: 10.1097/00001756-200207020-00009. [DOI] [PubMed] [Google Scholar]
- Picchioni M, Matthiasson P, Broome M, Giampietro V, Brammer M, Mathes B, et al. Medial temporal lobe activity at recognition increases with the duration of mnemonic delay during an object working memory task. Hum Brain Mapp. 2007;28:1235–1250. doi: 10.1002/hbm.20357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesa-Skwerer D, Faja S, Verbalis A, Tager-Flusberg H. Perceiving facial and vocal expressions of emotion in individuals with Williams syndrome. American Journal of Mental Retardation. 2008;111:15–26. doi: 10.1352/0895-8017(2006)111[15:PFAVEO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, et al. IV. Neuroanatomy of Williams syndrome: a high-resolution MRI study. J Cogn Neurosci. 2000;12 1:65–73. doi: 10.1162/089892900561986. [DOI] [PubMed] [Google Scholar]
- Reiss JE, Hoffman JE, Landau B. Motion processing specialization in Williams syndrome. Vision Research. 2005;45:3379–3390. doi: 10.1016/j.visres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Riddoch MJ, Humphreys GW, Blott W, Hardy E, Smith AD. Visual and Spatial Short-term Memory in Integrative Agnosia. Cognitive Neuropsychology. 2003;20:641–671. doi: 10.1080/02643290342000078. [DOI] [PubMed] [Google Scholar]
- Roth JK, Courtney SM. Neural system for updating object working memory from different sources: sensory stimuli or long-term memory. Neuroimage. 2007;38:617–630. doi: 10.1016/j.neuroimage.2007.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JK, Serences JT, Courtney SM. Neural system for controlling the contents of object working memory in humans. Cerebral Cortex. 2006;16:1595–1603. doi: 10.1093/cercor/bhj096. [DOI] [PubMed] [Google Scholar]
- Sala JB, Rama P, Courtney SM. Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia. 2003;41:341–56. doi: 10.1016/s0028-3932(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Sampaio A, Sousa N, Fernandez N, Henriques M, Goncalves OF. Memory abilities in Williams syndrome: dissociation or developmental delay hypothesis? Brain & Cognition. 2008;66:290–297. doi: 10.1016/j.bandc.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Sarpal D, Buchsbaum BR, Kohn PD, Kippenhan JS, Mervis CB, Morris CA, et al. A genetic model for understanding high-order visual processing: Functional interactions of the ventral visual stream in Williams Syndrome. Cerebral Cortex. doi: 10.1093/cercor/bhn004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Schwarzbach J, Courtney SM, Golay X, Yantis S. Control of object-based attention in human cortex. Cerebral Cortex. 2004;14:1346–1357. doi: 10.1093/cercor/bhh095. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher EH, Minoshima S. Spatial versus object working memory: PET investigations. Journal of Cognitive Neuroscience. 1995;7:337–356. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Plesa-Skwerer D, Faja S, Joseph RM. People with Williams syndrome process faces holistically. Cognition. 2003;89:11–24. doi: 10.1016/s0010-0277(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Troje NF, Bulthoff HH. Face recognition under varying poses: the role of texture and shape. Vision Research. 1996;36:1761–1771. doi: 10.1016/0042-6989(95)00230-8. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–65. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker D, Snyder AZ, Raichle ME, Reiss AL, Korenberg J. Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. J Neurosci. 2006;26:5470–5483. doi: 10.1523/JNEUROSCI.4154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EA, van der Molen MW. Developmental trends for object and spatial working memory: a psychophysiological analysis. Child Development. 2007;78:987–1000. doi: 10.1111/j.1467-8624.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- Vicari S, Bellucci S, Carlesimo GA. Visual and spatial working memory dissociation: evidence from Williams syndrome. Dev Med Child Neurol. 2003;45:269–73. doi: 10.1017/s0012162203000513. [DOI] [PubMed] [Google Scholar]
- Vicari S, Bellucci S, Carlesimo GA. Evidence from two genetic syndromes for the independence of spatial and visual working memory. Developmental Medicine & Child Neurology. 2006;48:126–131. doi: 10.1017/S0012162206000272. [DOI] [PubMed] [Google Scholar]
- Vicari S, Verucci L, Carlesimo GA. Implicit memory is independent from IQ and age but not from etiology: evidence from Down and Williams syndromes. Journal of Intellectual Disability Research. 2007;51:932–941. doi: 10.1111/j.1365-2788.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- Wang PP, Doherty S, Rourke SB, Bellugi U. Unique profile of visuo-perceptual skills in a genetic syndrome. Brain & Cognition. 1995;29:54–65. doi: 10.1006/brcg.1995.1267. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Clare L, Young AW, Hodges JR. Knowing where and knowing what: a double dissociation. Cortex. 1997;33:529–541. doi: 10.1016/s0010-9452(08)70234-x. [DOI] [PubMed] [Google Scholar]