Abstract
Background
Phenylephrine bolus injection is an established technique to measure baroreflex sensitivity (BRS). This study quantified the relationship between the phenylephrine method and noninvasive measures of BRS and examined the effects of aging and hypertension on BRS. We also examined whether heart rate variability (HRV) provides as much information as does BRS.
Methods
BRS was determined by phenylephrine bolus (BRSphe), amyl nitrite inhalation (BRSamyl), Valsalva maneuver (BRSVals) and by time (BRS(+)) and spectral domain analysis (BRSLFα, 004–015Hz) of spontaneous blood pressure and R—R interval changes over the 5-min time period.
Results
The phenylephrine method significantly correlated with other methods (BRSLFα R=0.54, BRS(+) R=0.55, BRSVals R=0.43 and BRSamyl R=0.39; P≤0.001). Each method underestimated the BRSphe by the factors 0.62, 0.64, 0.59 and 0.33, respectively; P value less than 0.001. Only BRSLFα was significantly different between normotensive and hypertensive patients in young [24.3±1.4 (n=40) vs. 12.2±2.3 (n=7)] and middle-aged [16.5±1.1 (n=71) vs. 10.8±1.1 (n=31) groups, respectively]. HRV in the high frequency band (0.15–0.40Hz) was significantly lower in young hypertensive patients than in normal controls (26±6.0 vs. 50±2.4, P<0.05).
Conclusion
Although all methods correlated with the phenylephrine technique, none of them could be used interchangeably with that technique. BRSLFα detected the baroreflex loss of hypertension most clearly, and BRSamyl did not differ among groups.
Keywords: aging, baroreflex sensitivity, heart rate variability, hypertension, phenylephrine technique
Introduction
The baroreflex is initiated by stretch receptors in the carotid sinuses, large arteries in the neck and aortic arch. Baroreflex sensitivity (BRS) quantifies the ability of the baroreflex to change heart rate (HR) in response to change in arterial pressure. Sympathetic and parasympathetic modulations of HR can be quantitatively analyzed by measures of HR variability (HRV) in the time and frequency domain [1]. Conditions such as age, hypertension and radiation therapy to the neck diminish both arterial compliance and the baroreflex, and impaired baroreflex responses in these conditions are thought to reflect vascular stiffness. BRS and HRV are helpful in the diagnosis and prognosis of cardiac diseases [2,3]. The ‘Oxford Method’ [4] is the standard and best-established technique to measure baroreflex gain as an estimate of the slope of the electrocardiographic R—R interval prolongation induced by the pressor effect of a phenylephrine injection and to demonstrate baroreflex deficits in a number of diseases. In contrast to this invasive pharmacological method, spontaneous fluctuations of arterial blood pressure (BP) and R—R intervals can be used to assess baroreflex gain without a drug injection [5,6]. However, Pitzalis et al. [7] concluded that the noninvasive BRS methods should not be used in clinical practice as an alternative to the phenylephrine method. We wished to compare the phenylephrine baroreflex with noninvasive measures.
The phenylephrine method to measure BRS relies upon a moderate increase in BP induced by a pressor drug. It is also possible to measure the baroreflex response to hypotension (e.g. induced by amyl nitrite) [8], but there has been little attention paid to whether this hypotensive response is simply the converse of the pressor response or if it is affected by hypertension and age. Spontaneous baroreflex measurements rely upon minor changes in BP and R—R interval around the set point of the baroreflex control system. During most cycles, BP and R—R interval do not change in concert. However, an analysis of cycles when they do change together provides a measure of BRS. As both the spontaneous and invasive methods of determining BRS rely so heavily on R—R interval, it is possible that HRV might provide much of the same information as BRS.
We carried out this study to determine the relationship between the phenylephrine method and other less invasive measures of BRS to see if the less invasive techniques can be substituted for the phenylephrine technique. We also examined which measure of BRS best reflects the cardiovascular changes that accompany aging and hypertension. Finally, we determined whether changes in HRV provide as much information about common cardiovascular changes as does BRS. Our findings suggest that simpler, safer methods best elucidate the BRS deficits induced by hypertension and aging. Our studies advance prior investigations by determining whether different BRS measures are interchangeable, which best reveal the effects of age and hypertension, whether there is an interaction between age and hypertension in terms of BRS and whether simple HRV measures can detect the effects of age and hypertension as well as measures of BRS.
Methods
Participants
We contacted 551 people by notice, word of mouth or physician referral. Two hundred seventy-nine people agreed to an initial interview; 178 were willing to be study participants and 165 of those met all inclusion criteria. All participants gave written consent to a protocol approved by the Institutional Review Board (IRB) of the University of California San Diego (UCSD) and performed in the General Clinical Research Center (GCRC) of the UCSD Medical Center. Pregnant women and volunteers who could not fully understand the protocol procedures were excluded. Participants taking any medication that might affect blood pressure or HR had that medication slowly tapered with frequent monitoring and daily blood pressure measurements. Those medications were withheld for 2 weeks prior to testing. If diastolic BP was consistently below 105mmHg, the patient remained eligible for the study. Participants with BP of at least 140/90mmHg (average of three measurements in a sitting position 1min apart) were considered hypertensive. Prior to testing, prospective participants had a medical history and physical examination, urine analysis and 12-lead ECG and blood taken for chemistry panel and hematology. Women of childbearing age had a urine pregnancy test. The characteristics of 161 participants who qualified are further described in Table 1. We asked participants not to exercise strenuously or take any medication for 24h prior to the study and not to consume any caffeine, nicotine or alcohol for 12h prior to testing.
Table 1.
Characteristics of the study population (mean±SEM)
| [0,3-5]Age group | [0,6-9]P | |||||||
|---|---|---|---|---|---|---|---|---|
| BP status |
A | B | C | Age effect |
||||
| 18–29 years | 30–49 years | 50–70 years | A vs. B |
A vs. C |
B vs. C |
|||
| N | Normal | 25 | 43 | 25 | ||||
| HTN | 5 | 23 | 34 | |||||
| Age | Normal | 23±0.7 | 41±0.8 | 56±1.01 | ||||
| HTN | 25±1.6 | 44±0.8 | 60±1.22 | |||||
| BMI | Normal | 24.57±0.86 | 26.34±0.87 | 26.54±1.04 | NS | NS | NS | NS |
| HTN | 26.93±2.20 | 29.48±1.67 | 29.01±0.93 | NS | NS | NS | NS | |
| HR | Normal | 70±2 | 71±2 | 73±2 | NS | NS | NS | NS |
| HTN | 74±4 | 74±2 | 73±2 | NS | NS | NS | NS | |
| SBP | Normal | 115±2 | 121±2 | 122±2 | NS | NS | NS | NS |
| HTN | 146±3 | 140±2 | 140±3 | NS | NS | NS | NS | |
| DBP | Normal | 68±1 | 75±1 | 76±2 | ‡ | † | † | NS |
| HTN | 83±1 | 86±2 | 81±2 | NS | NS | NS | NS | |
SBPs, DBPs and HRs are the average screening visit values obtained in seated position.
HTN, hypertension group; N , number of patients; NS, not significant.
P<0.01.
P<0.001, ANOVA with Holm—Sidak correction.
Data acquisition and calculations
The studies were carried out in two consecutive days between 10 AM and 2 PM in a dimmed, quiet, temperature-controlled room (22–25°C). Beat-to-beat BP and R—R interval data were collected with a Continuous Noninvasive Blood Pressure Monitor (Colin PILOT Model 9200; Colin Medical Instruments, San Antonio, Texas, USA). BP was recorded with the tonometric transducer placed over the left radial artery at the wrist level, and R—R interval data were collected from the ECG Standard Lead I. The invasive BRS, BRS in spectral domain and spectral domain HRV were calculated with a commercial software program (Colin TDA Program, Rev. 9-98). The Valsalva maneuver was performed, in the sitting position, on the first day of testing. The spontaneous BRS, phenylephrine BRS and amyl nitrite BRS tests were performed in that order, and in the supine position, on the second day of testing.
Spontaneous baroreflex
The spontaneous BRS in the frequency domain (BRSLFα) [5] was calculated with Colin TDA program Rev. 9-98 (Colin Medical Instruments). R—R and SBP power spectral densities (PSDs) were calculated over the 5min time period. Over the same time period, coherence (Coh) between the R—R and SBP was calculated with the following formula:
where SSBP*R-R denotes cross-spectrum of SBP and R—R, SSBP*SBP denotes power-spectrum of SBP and SR-R*R-R denotes power-spectrum of R—R. The mean BRSLFα was calculated as an average of the entire 5-min period and accepted only when the Coh between the SBP and R—R signals exceeded 0.5 [1]. Data for the BRSLFα estimate were collected during spontaneous breathing.
The spontaneous BRS in the time domain (BRS(+) and BRS(-)) [6] was calculated using custom-made software created in our laboratory with LabVIEW icon-based programming language (LabVIEW8.5; National Instruments, Austin, Texas, USA). BRS was determined over the 5min long period of R—R/SBP values. For each sequence of three consecutive beats in which R—R interval and SBP either increase at the same time, up-sequences (R—R(+)/SBP(+)), or decrease at the same time, down-sequences (R—R(-)/SBP(-)), a linear regression slope was calculated following (+1) R—R phase shift [9]. The average BRS over the 5-min time period was determined as a mean slope of all individual regression up-slopes [BRS(+)] and all individual regression down-slopes [BRS(-)].
Phenylephrine baroreflex
Phenylephrine (150μg) was injected as an intravenous bolus followed rapidly by a 5-ml flush of isotonic saline. BRSphe was calculated as the slope of the regression line of best fit according to the ‘Oxford Method’ [4]. Only regressions with correlation coefficient R more than 0.85 were accepted.
Amyl nitrite inhalation baroreflex
Amyl nitrite was inhaled after HR and BP changes from the phenylephrine test returned to control values. An ampule of amyl nitrite was broken and held under the nose for three normal inhalations [8,10]. The baroreflex was the slope of the regression line relating R—R interval changes to SBP changes. Only regressions with correlation coefficient R more than 0.85 were accepted.
Valsalva maneuver baroreflex
The Valsalva maneuver was performed in duplicate. The largest response was analyzed. All participants were first familiarized with the duration and intensity of strain of the Valsalva maneuver. At the end of a normal inspiration [11], each participant started forced expiration into the tubing connected to an aneroid manometer to quickly reach an expiratory pressure of 40mmHg for 15s. A small air leak in the system prevented closing of the glottis during the strain. After completion of the maneuver, the participants resumed normal respirations without deep breathing. The BRS was calculated as the slope of regression of R—R interval over systolic blood pressure during the part of the phase IV of the Valsalva maneuver with simultaneous and linear increase in BP and prolongation of the R—R interval [12]. Only regressions with a correlation coefficient R of more than 0.85 were accepted.
Assessment of heart rate variability
HRV during spontaneous breathing was determined in frequency domain over the same 5-min time period as spontaneous BRS. Power spectral indices of HRV [low frequency (LF), high frequency (HF) and total frequency (TF)] were determined as a mean value over the entire 5-min time period with commercial software (Colin PILOT Model 9200, TDA Program, Rev. 9-98) and as recommended by Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [1]. HRV in the LF and HF band was also expressed in normalized units (n.u.) as a percent of total frequency power (TF): LFn.u.=[LF (ms2)]/[LF(ms2)+HF(ms2)], HFn.u.=HF(ms2)/[LF(ms2)+HF(ms2)] [1].
Statistics
Ln transformation was used for nonnormally distributed data, such as BRS, HF and LF power, before using parametric measures. Correlations between BRS by the two methods and the LF, HF and LF/HF ratio of HRV were analyzed by Pearson’s correlation coefficient. P value of less than 0.05 was considered statistically significant. Effects of age and hypertension were determined by two-way analysis of variation (ANOVA) with Holm procedure for multiple comparisons (Sigma Plot 11; Systat Software, Inc., San Jose, California, USA). Agreement between different methods of measurement was determined by using the Bland—Altman method [13]. Since the distribution of the BRS values was skewed, we ln transformed original values in order to calculate the 95% confidence interval (CI) of differences between two different methods of measurement.
Results
Comparison of methods of baroreflex sensitivity measurement
Correlations ranged from R value of 0.54 for BRSLFα to R value of 0.29 for BRSamyl when compared with the phenylephrine method. Further comparison of differences between baroreflex methods by Bland—Altman plots [13] revealed that only BRSLFα had a weakly significant proportional bias vs. the phenylephrine method (Table 2, Fig. 1).
Table 2.
Regression analysis of differences between the two methods (ln-transformed data)
| Regression | R | Intercept | Slope | P | Proportional bias |
|---|---|---|---|---|---|
| (BRSLFα—BRSphe) vs. (mean difference) |
0.18 | 0.077 | -0.198 | 0.040 | Yes |
| (BRS(+)—BRSphe) vs. (mean difference) |
0.13 | -0.056 | -0.138 | 0.149 | No |
| (BRS(-)—BRSphe) vs. (mean difference) |
0.17 | 0.116 | -0.181 | 0.059 | No |
| (BRSamyl—BRSphe) vs. (mean difference) |
0.12 | -1.556 | 0.180 | 0.181 | No |
| (BRSVals—BRSphe) vs. (mean difference) |
0.00 | -0.519 | -0.002 | 0.989 | No |
| (BRS(+)—BRSLFα) vs. (mean difference) |
0.09 | 0.05 | -0.097 | 0.330 | No |
BRS, baroreflex sensitivity.
Fig. 1.
Comparison of differences between baroreflex methods and the phenylephrine method. (a—d) Relationship between the phenylephrine method and four other BRS measurement methods [spectral method (a), ramp method (b, c) and amyl nitrite method (d)]. All values are ln-transformed data. The dashed line is the line of identity, and the solid line is the best-fit regression between the two measures. (a1—d1) Bland—Altman plots of the difference between BRS measurements obtained with the one of four methods [spectral method (a1), ramp method (b1), Valsalva maneuver (c1), amyl nitrite method (d1)] versus the phenylephrine method. In these plots, there is proportionate bias if the slope of the regression is significantly different from zero. This is seen only in (a) revealing a proportionate bias between the phenylephrine method and BRSLFα. There is a fixed bias if the mean of the values differs from zero, and this is seen for four out of five measures. The dashed line shows the mean difference between the two methods, and the solid lines show the 95% limits of agreement between the two methods. gr1
However, four of five methods had a highly significant fixed bias vs. the phenylephrine technique (Fig. 1, Table 3). All methods underestimate BRSphe from 33% (BRS(-), with 95% CI from 26 to 39%) up to 67% (BRSamyl, with 95% CI from 63 to 71%) (Table 4). Consequently, even though all methods correlated with the phenylephrine technique, none of them could be used interchangeably with that technique. Although all of the techniques had some overlap with phenylephrine baroreflex measures (Fig. 1) the phenylephrine technique gave higher values than any other method.
Table 3.
Analysis of differences between the two methods by one-sample t-test (ln-transformed data)
| Difference | Average difference (±SEM) |
95% CI for average difference |
t | P | Fixed bias |
|---|---|---|---|---|---|
| BRSLFα—BRSphe | -0.476±0.0503 | -0.377, -0.576 |
-9.475 | <0.001 | Yes |
| BRS(+)—BRSphe | -0.444±0.0508 | -0.344, -0.545 |
-8.746 | <0.001 | Yes |
| BRS(-)—BRSphe | -0401±0.0501 | -0.302, -0.500 |
-7.994 | <0.001 | Yes |
| BRSamyl—BRSphe | -1.112±0.0641 | -0.970, -1.255 |
-14.990 | <0.001 | Yes |
| BRSVals—BRSphe | -0.524±0.0736 | -0.379, -0.651 |
-8.173 | <0.001 | Yes |
| BRS(+)—BRSLFα | -0.032±0.0270 | -0.085, 0.022 | 1.174 | 0.2427 | No |
BRS, baroreflex sensitivity; CI, confidence interval.
Table 4.
Relative bias
| Test | Bias relative to BRSphe [mean (95% CI)] |
|---|---|
| BRSLFα | 0.62 (0.56–0.69) |
| BRS(+) | 0.64 (0.58–0.71) |
| BRS(-) | 0.67 (0.61–0.74) |
| BRSamyl | 0.33 (0.29–0.37) |
| BRSVals | 0.59 (0.51–0.69) |
BRS, baroreflex sensitivity.
Effects of age and hypertension on baroreflex sensitivity
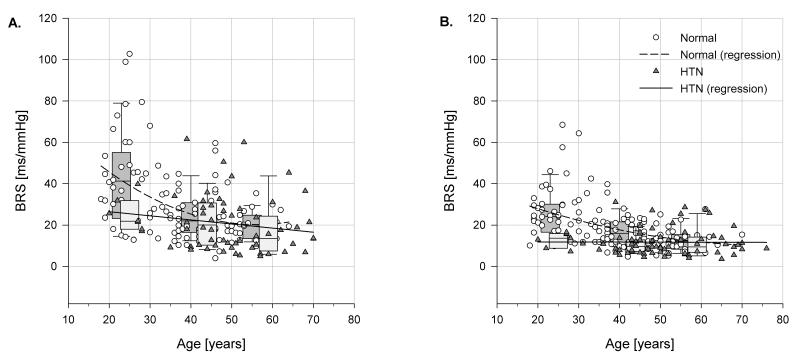
The BRS determined by phenylephrine bolus injection, BRSphe, significantly decreased with age only in the normotensive group, while the hypertensive patients had similar BRS across all age groups (Table 5). However, there was not a significant difference between hypertensive and normotensive BRS in any age group detected by the phenylephrine technique because of extensive overlap between groups (Table 5, Fig. 2). Spontaneous baroreflex measures, particularly BRSLFα, more clearly demonstrated a difference between hypertensive and normotensive patients, as BRSLFα values were lower in hypertensive patients in both the young and middle-age group (Fig. 2). The baroreflex in response to decreasing BP after inhalation of amyl nitrite did not show any significant change with age or BP status. Amyl nitrite-induced BP changes were similar in magnitude to those induced by phenylephrine in normotensive and hypertensive patients (-32 ± 1.65mmHg vs. -31 ± 1.86mmHg, respectively).
Table 5.
Baroreflex sensitivityin relation to age and hypertension
| [0,3-5]Age group | [0,6-10]P | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BP status |
A | B | C | Age effect |
Age × BP | ||||
| 18–29 years |
30–49 years |
50–70 years | A vs. B |
B vs. C |
A vs. C |
interaction | |||
| BRSphe | Normal | 43.16±2.70 (34) |
25.10 ± 1.99 (63) |
19.90 ± 3.29 (23) |
‡ | ‡ | NS | ‡ | |
| HTN | 25.09±4.46 (7) |
22.32 ± 2.36 (25) |
15.67 ± 2.31 (26) |
* | NS | NS | * | 0.07 | |
| BRSLFα | Normal | 24.26±1.41 (40) |
16.54 ± 1.06 (71) |
12.66 ± 1.71 (27) |
‡ | ‡ | NS | ‡ | |
| HTN | 12.22±2.32 (7)* |
10.77 ± 1.10 (31)* |
11.87 ± 1.04 (35) |
NS | NS | NS | NS | 0.02 | |
| BRS(+) | Normal | 26.63±1.72 (40) |
17.98 ± 1.29 (71) |
13.40 ± 2.10 (27) |
‡ | ‡ | NS | ‡ | |
| HTN | 16.84±2.69 (7) |
12.65 ± 1.28 (31)* |
13.08 ± 1.20 (35) |
NS | NS | NS | NS | 0.1 | |
| BRS(-) | Normal | 25.92±1.66 (40) |
18.53 ± 1.24 (71) |
14.59 ± 2.02 (27) |
‡ | ‡ | NS | ‡ | |
| HTN | 14.45±3.23 (7)* |
12.86 ± 1.54 (31)* |
14.12 ± 1.45 (35) |
NS | NS | NS | NS | 0.04 | |
| BRSamyl | Normal | 9.47±1.62 (18) |
10.54 ± 1.19 (33) |
8.26 ± 1.50 (21) |
NS | NS | NS | NS | |
| HTN | 7.07±2.99 (5) |
8.59 ± 1.42 (22) |
7.94 ± 1.26 (28) |
NS | NS | NS | NS | 0.82 | |
Values are means±SE.
BRS, baroreflex sensitivity; BP status, blood pressure at screening visit; BRS(-), BRS calculated from R—R/BP down-ramps; BRS(+), BRS calculated from R—R/BP up-ramps; BRS, baroreflex sensitivity; HTN, hypertension group (SBP/DBP>140/90mmHg); LFα low-frequency α coefficient; Phe, phenylephrine.
Data were analyzed by two-way ANOVA with Holm—Sidak test.
P<0.05.
P < 0.01.
P < 0.001.
Significant differences between normal volunteers and HTN are indicated in the HTN data box.
Fig. 2.
Baroreflex sensitivity in relation to age and blood pressure status. (a) BRS determined by phenylephrine bolus method; (b) spontaneous BRS determined in spectral domain by low-frequency α coefficient (BRSLFα). BRS, baroreflex sensitivity; HTN, hypertension group. gr2
Relationship between baroreflex sensitivity and heart rate variability
It is easier to measure HRV than baroreflex, and many baroreflex measurement techniques partly depend on spontaneous changes in HR. We compared spontaneous BRS determined in the time domain by the ramp method as BRS(+) and BRS(-) and in the spectral domain as the LF α-coefficient, BRSLFα. These measures strongly correlated with HRV in both normotensive and hypertensive groups, accounting for over 50% of variance in all cases (Table 6). However, BRS determined by artificially induced BP change rather than by spontaneous BP fluctuations correlated less well, whether participants had BP increased by phenylephrine, decreased by amyl nitrite or altered by the Valsalva maneuver (Table 6).
Table 6.
The correlation between heart rate variability over the entire frequency range and six measures of baroreflex sensitivity among normal volunteers and among hypertensive patients
| [0,2-5]Normotensive patients | [0,6-9]Hypertensive patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Test | Regression | R | P | n | Regression | R | P | n |
| BRS(+) | ln(BRS(+))=0.667 +0.396*ln(R—RTF) |
0.73 | ‡ | 135 | ln(BRS(+))=0.948 +0.304*ln(R—RTF) |
0.73 | ‡ | 71 |
| BRS(-) | ln(BRS(-))=0.897 +0.359*ln(R—RTF) |
0.71 | ‡ | 135 | ln(BRS(-))=0.833 +0.326*ln(R—RTF) |
0.73 | ‡ | 71 |
| BRSLFα | ln(BRSLFα)=0.795 +0.358*ln(R—RTF) |
0.71 | ‡ | 135 | ln(BRSLFα)=0.789 + 0.305*ln(R—RTF) |
0.72 | ‡ | 71 |
| BRSphe | ln(BRSphe)=2.412 +0.149*ln(R—RTF) |
0.26 | † | 118 | ln(BRSphe)=1.416 +0.287*ln(R—RTF) |
0.50 | ‡ | 60 |
| BRSamyl | ln(BRSamyl)=0.986 +0.204*ln(R—RTF) |
0.28 | * | 78 | ln(BRSamyl)=1.457 +0.074*ln(R—RTF) |
0.12 | * | 55 |
| BRSVals | ln(BRSVals)=2.234 +0.065*ln(R—RTF) |
0.11 | * | 73 | ln(BRSVals)=0.828 +0.307*ln(R—RTF) |
0.50 | ‡ | 56 |
BRS, baroreflex sensitivity.
P<0.05.
P<0.01.
P<0.001.
Effects of age and hypertension on heart rate variability
The LF, HF and TF components of HRV changed with age among normotensive patients whether expressed in absolute units (ms2) or as a fraction of TF in n.u. (Table 7). This age relationship was much weaker among hypertensive patients, as the two youngest groups of hypertensive patients had HRV similar to older hypertensive patients. Although younger hypertensive patients generally displayed less overall HRV than normotensive patients, hypertensive and normotensive patients older than 50 had remarkably similar HRV (Table 7). The ratio LF/HF is often employed as a measure of sympatho-vagal balance. The LF/HF ratio more than doubled with age among normotensive patients but failed to change with age among hypertensive patients. Although many measures of HRV differed between age groups, the hypertensive group differed from normotensive group only when comparing normalized values in the youngest age group. Thus, HRV correlated strongly with BRS but did not detect the cardiovascular effect of hypertension as well as some measures of BRS.
Table 7.
Heart rate variability in relation to age and hypertension
| [0,3-5]Age group | [0,6-9]P | |||||||
|---|---|---|---|---|---|---|---|---|
| HRV | BP status |
A | B | C | Age effect |
|||
| 18–29 years |
30–49 years |
>50 years | A vs. B |
A vs. C |
B vs. C |
|||
| LF (ms2) |
Normal | 231±26 (39) |
154±14 (69) |
150±44 (25) |
* | * | NS | NS |
| HTN | 287±73 (6) | 118±33 (29) |
149±31 (34) |
* | * | NS | NS | |
| HF (ms2) |
Normal | 257±32 (39) |
128±18 (69) |
101±38 (25) |
** | † | † | NS |
| HTN | 129±55 (6) | 81±25 (29) |
101±23(34) | NS | NS | NS | NS | |
| TF (ms2) |
Normal | 488±53 (39) |
282±29 (69) |
251±78 (25) |
‡ | † | * | NS |
| HTN | 416±120 (6) |
199±54 (29) |
250±50 (34) |
NS | NS | NS | NS | |
| LF (n.u.) |
Normal | 49±2.4 (39) | 58±1.8 (69) |
67±3.0 (25) | ‡ | * | ‡ | * |
| HTN | 73±6.0 (6)* | 58±2.7 (29) |
66±2.5 (34) | NS | NS | NS | NS | |
| HF (n.u.) |
Normal | 50±2.4 (39) | 41±1.8 (69) |
32±3.0 (25) | ‡ | † | ‡ | NS |
| HTN | 26±6.0 (6)* | 41±2.7 (29) |
33±2.5 (34) | * | * | NS | NS | |
| LF/HF | Normal | 1.17±0.21 (39) |
1.83±0.16 (69) |
2.82±0.26 (25) |
‡ | * | ‡ | † |
| HTN | 3.51±0.74 (6)† |
1.83±0.34 (29) |
2.90±0.31 (34) |
NS | NS | NS | NS | |
Values are means±SE. LF and HF are also displayed as normalized units (n.u.) corrected for total HRV . BP status: blood pressure at screening visit. Hypertension (HTN): SBP/DBP>140/90mmHg. Significant differences between normal volunteers and HTN are indicated in the HTN data box. HF, high frequency component of the heart rate; HRV, heart rate variability; HTN, hypertension group, LF, TF,low frequency and total frequency components of the heart rate.
P<0.05.
P<0.01.
P<0.001.
Discussion
This study compares common measures of the human baroreflex and shows which of these measures best uncover the baroreflex deficit of hypertensive patients and the elderly. The phenylephrine baroreflex has been considered the gold standard of baroreflex measures both because of its long use and because it has been used to document deficits among diabetic patients and patients with autonomic disorders [14]. Many studies [15-21] have reported correlation coefficients between the phenylephrine method and other nonpharmacological techniques but did not determine whether the methods gave interchangeable baroreflex measurements. We found significant correlations between the phenylephrine method and five other methods (Fig. 1). However, none of those methods may be used interchangeably with the phenylephrine method because of either fixed or proportionate bias. The phenylephrine method gives larger baroreflex numbers than the other methods, usually because young normal volunteers have a steeper baroreflex slope by the phenylephrine technique (Fig. 2). The BRSphe values may be larger compared with spontaneous BRS methods, because these two methods describe different aspects of baroreflex gain. By injecting phenylephrine, the baroreflex loop is virtually open and pressure change is large. In contrast, spontaneous BP oscillations in a closed baroreflex loop are much smaller, close to the set point of the baroreflex. Thus drug-induced BRS and spontaneous BRS are complimentary but are not interchangeable. There are several possible explanations for the poorer sensitivity of the phenylephrine method to detect age and hypertension-induced cardiovascular changes. Phenylephrine-induced peripheral vasoconstriction may increase afterload and cause the activation of other receptors such as cardiopulmonary mechanoreceptors. Phenylephrine may act directly on the vessel walls of carotid and aortic arteries, which might in turn modify the firing rate of afferent neurons [15]. These pharmacological actions of phenylephrine might cause cardiac slowing in addition to that caused by peripheral vasoconstriction.
We examined three active interventions that gave wide swings in BP (phenylephrine, amyl nitrite and Valsalva maneuver) and three measures that evaluated the baroreflex in response to small, spontaneous, changes in blood pressure around the baroreflex set point (BRS(+), BRS(-) and BRSLFα). It is surprising that the phenylephrine method correlated better with the spontaneous measures than with the active interventions that gave BP swings as large as those caused by a phenylephrine injection. Although the phenylephrine method correlated well with the spontaneous measures, Bland—Altman analysis revealed that BRSVals and BRSamyl significantly underestimated BRSphe as did BRSLFα and BRS(+). This suggests that differences in baroreflex measures between methods are not attributable to the size of the BP swings. The amyl nitrite method and the spontaneous down-ramps, BRS(-) , measured baroreflex responses to decreasing blood pressure. The phenylephrine method correlated quite well with the BRS(-) and only weakly with the amyl nitrite method. Thus, different baroreflex measures between the techniques are not due to the direction of BP change.
The baroreflex slows HR when BP increases, and it reflexively diminishes large swings in blood pressure. Patients with damage to the baroreceptors, hypertensive patients and the elderly all have wide swings in BP and diminished BRS [17]. As episodes of hypertension might cause vascular damage, it is of interest to document which baroreflex measure best documents the defect seen among hypertensive patients and the elderly. In our study, there were clear differences among the various baroreflex measures’ ability to distinguish the elderly and hypertensive patients from young normal volunteers. The decline in BRS is graphically displayed in Fig. 2. BRS of young hypertensive patients is no better than that of the elderly because BRS falls by half both with age and high blood pressure. The baroreflex response to amyl nitrite did not differ between the elderly and hypertensive patients. Thus, all groups retained a similar ability to protect themselves from a large drop in BP suggesting an intact sympathetic response, even though hypertensive patients and older patients had diminished responses to the small spontaneous decreases in blood pressure measured by BRS(-).
Although the magnitude of the BP changes induced by amyl nitrite in our study is similar to BP changes induced by phenylephrine, the BRS is smaller when arterial pressure is falling than when rising. Assessment of BRS with BP lowering drugs may be confounded by engagement of centrally and peripherally mediated mechanisms and by hysteresis of the baroreflex loop [18-20].
In general, baroreflex measures derived from spontaneous changes in blood pressure more clearly reflected the deficit of the elderly or the hypertensive patients. The baroreflex deficits caused by age and by hypertension were most clearly evident using BRSLFα. This provides a practical advantage: the baroreflex deficit of hypertensive patients is most clearly shown by noninvasive techniques that do not involve drugs or any appreciable risk.
All the baroreflex measures in our study were calculated from a comparison of changes in BP and R—R interval. It comes as no surprise then that there was a strong correlation between the baroreflex and HRV. HRV reflected over 50% of the variance in spontaneous baroreflexes. HRV diminished with age, and some normalized measures of HRV were smaller in young hypertensive patients. Nevertheless, none of the measures of HRV distinguished between young and old or between normotensive and hypertensive patients as well as BRSLFα, BRS(+) or BRS(-).
The baroreflex is initiated by stretch receptors in the large arteries. The decrease in BRS and HRV that occurs with age and hypertension is often attributed to rigidity of those arteries, preventing stretch of the baroreceptors in response to increasing blood pressure. If that were the only cause, then the baroreflex deficit seen with a hypertensive response to phenylephrine should have been mirrored by a similar deficit in response to hypotension from amyl nitrite. However, the reflex response to amyl nitrite hypotension was not impaired in either the old or hypertensive patient. This suggests that there are other causes for baroreflex deficits such as the genetic predisposition described by Parmer et al. [10] and neural resetting [19,21]. From a teleological viewpoint, an inability to respond to hypotension with advancing age would quickly impair survival and performance whereas an inability to respond appropriately to hypertension would give only a long-term survival disadvantage.
The inability of hypertensive patients to respond appropriately to hypotension would make antihypertensive drug therapy problematic. Thus, our finding that hypertensive patients and the elderly continue to mount a normal response to amyl nitrite hypotension mirrors real world observations about how they respond to antihypertensive drugs and the environment.
This comparison of BRS and HRV measures provides the practical information that studies of the cardiovascular deficits induced by age and hypertension are most clearly demonstrated by techniques that do not require a drug or physiologic challenge. The baroreflex indices BRSLFα, BRS(+) and BRS(-) were collected from patients at rest, and all differed according to age and blood pressure; BRSLFα gave the clearest distinctions. Our finding that old age and hypertension generate a defective response to small swings in BP, a less abnormal response to larger BP elevations and a normal response to a large BP decrease indicate that baroreflex deficits in cardiovascular disorders are not simply an overall loss of baroreflex function, but a more selective loss of fine regulation of BP control.
Acknowledgements
This research is supported in part by grants HL36005, HL44915, HL58120 and M01RR00827. There were no conflicts of interest.
Abbreviations
- BP
arterial blood pressure
- BRS
baroreflex sensitivity
- BRS(+)
spontaneous baroreflex sensitivity with blood pressure/R—R-interval up-ramps
- BRS(+)
spontaneous baroreflex sensitivity with blood pressure/R—R-interval down-ramps
- BRSamyl
baroreflex sensitivity with amyl nitrite inhalation
- BRSLFα
spontaneous baroreflex sensitivity in low frequency band
- BRSphe
baroreflex sensitivity with phenylephrine bolus
- BRSVals
baroreflex sensitivity with Valsalva maneuver
- Coh
coherence
- HF
high frequency
- HR
heart rate
- HRV
heart rate variability
- LF
low frequency
- n.u.
normalized units
- PSD
power spectral density
- SR—R*R—R
power-spectrum of R—R interval
- SSBP*R—R
cross-spectrum of systolic blood pressure and R—R interval
- SSBP*SBP
power-spectrum of systolic blood pressure
- TF
total frequency
References
- 1.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 2.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- 3.La Rovere MT, Bigger JTJ, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 4.Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: a quantitative method of assessing baroreflex sensitivity. Circ Res. 1969;24:109–21. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- 5.Pagani M, Somers V, Furlan R, Dell’orto S, Conway J, Basselli G, et al. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension. 1988;12:600–610. doi: 10.1161/01.hyp.12.6.600. [DOI] [PubMed] [Google Scholar]
- 6.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol Heart Circ Physiol. 1988;254:H377–H383. doi: 10.1152/ajpheart.1988.254.2.H377. [DOI] [PubMed] [Google Scholar]
- 7.Pitzalis MV, Mastropasqua F, Passantino A, Massari F, Ligurgo L, Forleo C, et al. Comparison between noninvasive indices of baroreceptor sensitivity and the phenylephrine method in post—myocardial infarction patients. Circulation. 1998;97:1362–1367. doi: 10.1161/01.cir.97.14.1362. [DOI] [PubMed] [Google Scholar]
- 8.Pickering TG, Gribbin B, Sleight P. Comparison of the reflex heart rate response to rising and falling arterial pressure in man. Cardiovasc Res. 1972;6:277–283. doi: 10.1093/cvr/6.3.277. [DOI] [PubMed] [Google Scholar]
- 9.Pellizer AM, Kamen PW, Jackman G, Brazzale D, Krum H. Non-invasive assessment of baroreflex sensitivity and relation to measures of heart rate variability in man. Clin Exp Pharmacol Physiol. 1996;23:621–624. doi: 10.1111/j.1440-1681.1996.tb02800.x. [DOI] [PubMed] [Google Scholar]
- 10.Parmer RJ, Cervenka JH, Stone RA. Baroreflex sensitivity and heredity in essential hypertension. Circulation. 1992;85:497–503. doi: 10.1161/01.cir.85.2.497. [DOI] [PubMed] [Google Scholar]
- 11.Mateika JH, DeMeersman RE, Kim J. Effects of lung volume and chemoreceptor activity on blood pressure and R-R interval during the Valsalva maneuver. Clin Auton Res. 2002;12:24–34. doi: 10.1007/s102860200007. [DOI] [PubMed] [Google Scholar]
- 12.Palmero HA, Caeiro TF, Iosa DJ, Bas J. Baroreceptor reflex sensitivity derived from phase 4 of the Valsalva maneuver. Hypertension. 1981;3(Suppl II):II-134–II-137. doi: 10.1161/01.hyp.3.6_pt_2.ii-134. [DOI] [PubMed] [Google Scholar]
- 13.Bland MJ, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 1986;8:307–10. [PubMed] [Google Scholar]
- 14.Robertson D, Hollister AS, Biaggioni I, Netterville JL, Mosqueda-Garcia R, Robertson M. The diagnosis and treatment of baroreflex failure. N Engl J Med. 1993;329:1449–1455. doi: 10.1056/NEJM199311113292003. [DOI] [PubMed] [Google Scholar]
- 15.Maestri R, Pinna GD, Mortara A, La Rovere MT, Tavazzi L. Assessing baroreflex sensitivity in post-myocardial infarction patients: comparison of spectral and phenylephrine techniques. J Am Coll Cardiol. 1998;31:344–51. doi: 10.1016/s0735-1097(97)00499-3. [DOI] [PubMed] [Google Scholar]
- 16.Watkins LL, Fainman C, Dimsdale J, Ziegler MG. Assesment of baroreflex control from beat-to-beat blood pressure and heart rate changes: a validation study. Psychophysiology. 1995;32:411–414. doi: 10.1111/j.1469-8986.1995.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 17.Watkins LL, Grossman P, Sherwood A. Noninvasive assessment of baroreflex control in borderline hypertension: comparison with the phenylephrine method. Hypertension. 1996;28:238–243. doi: 10.1161/01.hyp.28.2.238. [DOI] [PubMed] [Google Scholar]
- 18.Ma SX, Long JP. Central noradrenergic activity and the cardiovascular effects of nitroglycerin and amyl nitrite. J Cardiovasc Pharmacol. 1992;20:826–836. 7. [PubMed] [Google Scholar]
- 19.Studinger P, Goldstein R, Taylor JA. Mechanical and neural contributions to hysteresis in the cardiac vagal limb of the arterial baroreflex. J Physiol. 2007;583:1041–1048. doi: 10.1113/jphysiol.2007.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan N, Casadei B, Paterson DJ. Nitric oxide donors can increase heart rate independent of autonomic activation. J Appl Physiol. 1999;87:97–103. doi: 10.1152/jappl.1999.87.1.97. [DOI] [PubMed] [Google Scholar]
- 21.Parlow J, Viale JP, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans: comparison with drug-induced responses. Hypertension. 1995;25:1058–1068. doi: 10.1161/01.hyp.25.5.1058. [DOI] [PubMed] [Google Scholar]