Abstract
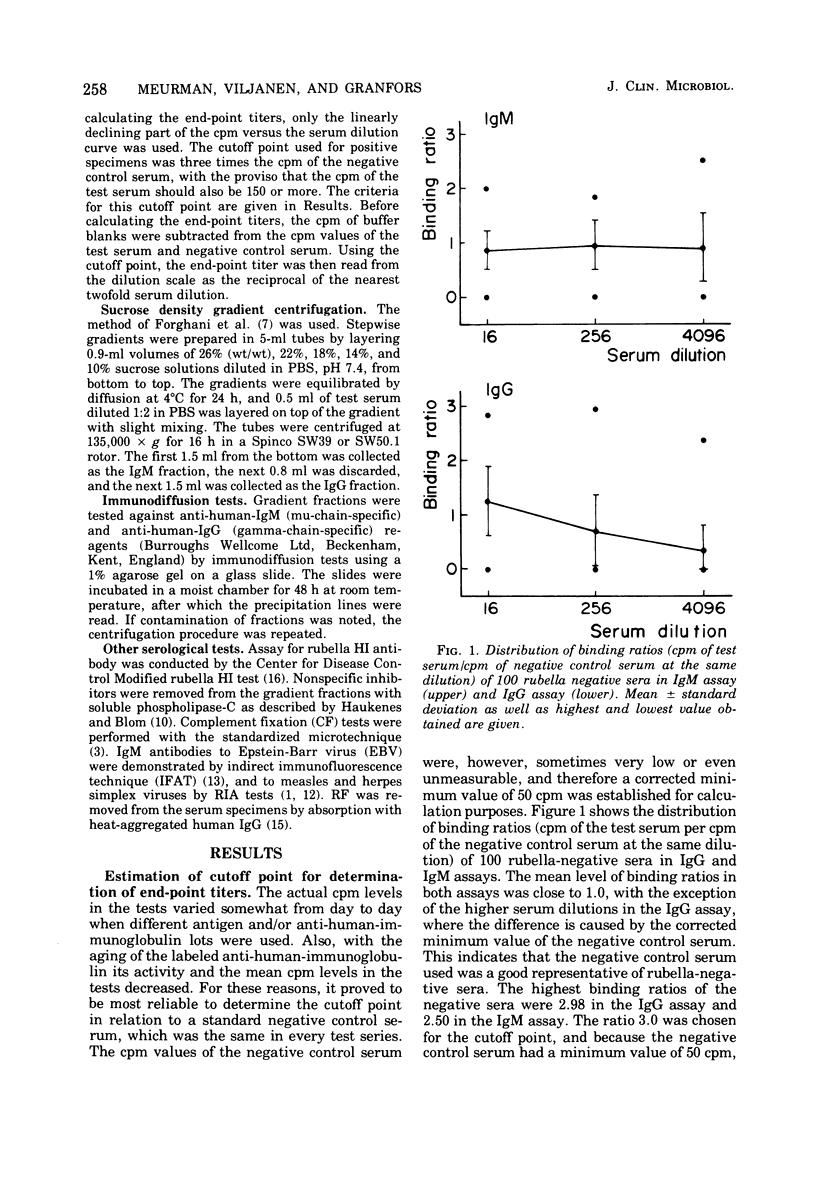
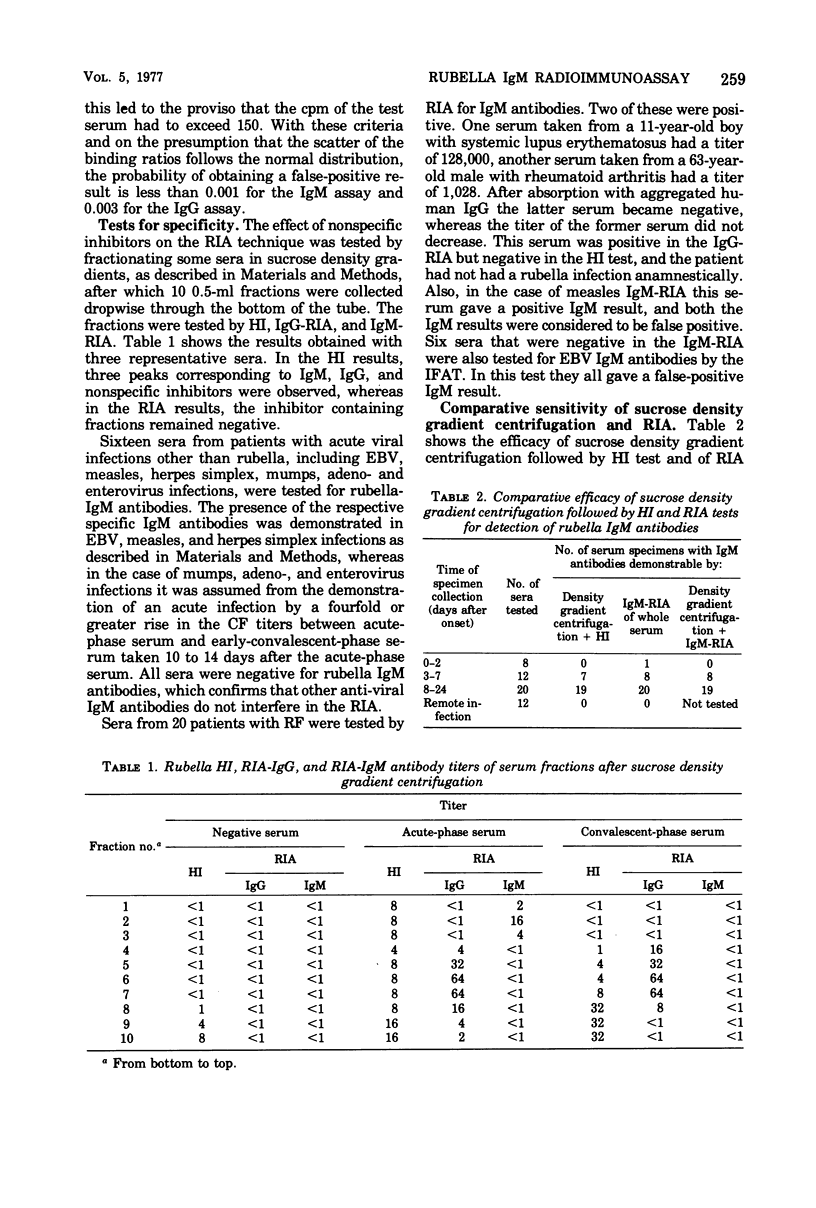
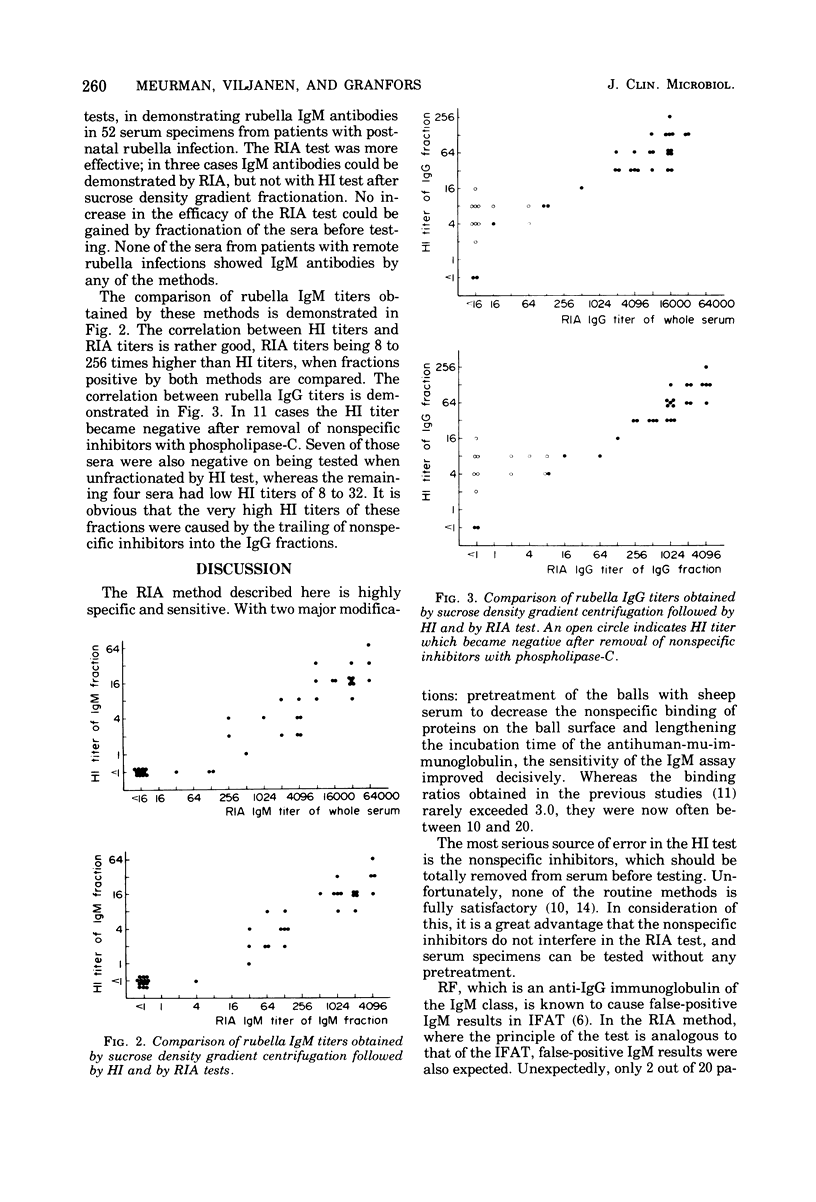
The solid-phase radioimmunoassay (RIA) method developed in our laboratory for demonstrating rubella virus-specific immunoglobulin G (IgG) antibodies (Kalimo et al., 1976) was further developed for demonstrating IgM antibodies. A total of 188 serum specimens were tested. The statistical probability of obtaining a false-positive IgM result, based on determinations of 100 rubella-negative sera, was below 0.001. Nonspecific inhibitors and IgM antibodies against other viruses tested did not interfere in the assay. In 2 out of 20 (10%) serum specimens with rheumatoid factor, a false-positive IgM result was obtained. The new RIA method was compared with sucrose density gradient centrifugation, followed by hemagglutination inhibition testing of the separated immunoglobulins with respect to demonstrating IgM antibodies. In patients with acute rubella infection, IgM antibodies were demonstrated by RIA in 9 out of 20 acute-phase sera and in all 20 early-convalescent-phase sera, compared with 7 out of 20 acute-phase sera and 19 out of 20 early-convalescent-phase sera by sucrose density gradient centrifugation. The results obtained indicate that the RIA method is reliable and sensitive and suitable for routine diagnostic use.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arstila P., Vuorimaa T., Kalimo K., Halonen P., Viljanen M., Granfors K., Toivanen P. A solid-phase radioimmunoassay for IgG and IgM antibodies against measles virus. J Gen Virol. 1977 Jan;34(1):167–176. doi: 10.1099/0022-1317-34-1-167. [DOI] [PubMed] [Google Scholar]
- Best J. M., Banatvala J. E., Watson D. Serum IgM and IgG responses in postnatally acquired rubella. Lancet. 1969 Jul 12;2(7611):65–68. doi: 10.1016/s0140-6736(69)92386-1. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. The specificity and polyvalency of binding of a monoclonal rheumatoid factor. Immunochemistry. 1976 Apr;13(4):355–359. doi: 10.1016/0019-2791(76)90347-5. [DOI] [PubMed] [Google Scholar]
- FRANKLIN E. C., HOLMAN H. R., MULLER-EBERHARD H. J., KUNKEL H. G. An unusual protein component of high molecular weight in the serum of certain patients with rheumatoid arthritis. J Exp Med. 1957 May 1;105(5):425–438. doi: 10.1084/jem.105.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Demonstration of rubella IgM antibody by indirect fluorescent antibody staining, sucrose density gradient centrifugation and mercaptoethanol reduction. Intervirology. 1973;1(1):48–59. doi: 10.1159/000148832. [DOI] [PubMed] [Google Scholar]
- Fraser K. B., Shirodaria P. V., Stanford C. F. Fluorescent staining and human IgM. Br Med J. 1971 Sep 18;3(5776):707–707. doi: 10.1136/bmj.3.5776.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen R., Nagel J., Brand-Saathof B., De Graaf S. Immunofluorescence test for IgM rubella antibodies in whole serum after absorption with anti-gammaFc. Clin Exp Immunol. 1975 Dec;22(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Haukenes G., Blom H. False positive rubella virus haemagglutination inhibition reactions: occurrence and disclosure. Med Microbiol Immunol. 1975;161(2):99–106. doi: 10.1007/BF02121750. [DOI] [PubMed] [Google Scholar]
- Haukenes G. Negative complement-fixation tests with rheumatoid factor positive sera. A simple method for selective removal of rheumatoid factor. Ann Rheum Dis. 1974 Sep;33(5):461–464. doi: 10.1136/ard.33.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimo K. O., Meurman O. H., Halonen P. E., Ziola B. R., Viljanen M. K., Granfors K., Toivanen P. Solid-phase radioimmunoassay of rubella virus immunoglobulin G and immunoglobulin M antibodies. J Clin Microbiol. 1976 Aug;4(2):117–123. doi: 10.1128/jcm.4.2.117-123.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoskelainen J., Hänninen P. Antibody response to Epstein-Barr virus in infectious mononucleosis. Infect Immun. 1975 Jan;11(1):42–51. doi: 10.1128/iai.11.1.42-51.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Variables of the rubella hemagglutination-inhibition test system and their effect on antigen and antibody titers. Appl Microbiol. 1970 Mar;19(3):491–504. doi: 10.1128/am.19.3.491-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodaria P. V., Fraser K. B., Stanford F. Secondary fluorescent staining of virus antigens by rheumatoid factor and fluorescein-conjugated anti-IgM. Ann Rheum Dis. 1973 Jan;32(1):53–57. doi: 10.1136/ard.32.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T., Vaheri A. Rubella: a method for rapid diagnosis of a recent infection by demonstration of the IgM antibodies. Br Med J. 1968 Jan 27;1(5586):221–223. doi: 10.1136/bmj.1.5586.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljanen M. K., Granfors K., Toivanen P. Radioimmunoassay of class-specific antibodies (RIACA): chicken antibodies to bovine serum albumin. Immunochemistry. 1975 Aug;12(8):699–705. doi: 10.1016/0019-2791(75)90218-9. [DOI] [PubMed] [Google Scholar]


