Abstract
Echinacea purpurea (L.) Moench, a top selling botanical medicine, is currently of considerable interest due to immunomodulatory, anti-inflammatory, antiviral and cannabinoid receptor 2 (CB2) binding activities of its alkylamide constituents. The purpose of these studies was to comprehensively profile the alkylamide (alkamide) content of E. purpurea root, and to compare yields of alkylamide constituents resulting from various ethanolic extraction procedures commonly employed by the dietary supplements industry. To accomplish this goal, a high performance liquid chromatography- electrospray ionization mass spectrometry (HPLC-ESI-MS) method was validated for quantitative analysis of several E. purpurea alkylamides. Using this method, at least 15 alkylamides were identified and it was shown that fresh and dry E. purpurea extracts prepared from equivalent amounts (dry weight) of roots, with exceptions, exhibited similar yield of specific alkylamides. However, the amount of total dissolved solids in the dry extract was higher (by 38%) than the fresh extract. Two extracts prepared from dried roots at different ratios of root:solvent (1:5 w:v and 1:11 w:v) were similar in yield of total dissolved solids, but, there were differences in quantities of specific alkylamides extracted using these two root:solvent ratios. In addition, the important bioactive dodecatetraenoic acid isobutylamides are fully extracted from dry E. purpurea root in 2 days, suggesting that the manufacturing practice of macerating Echinacea extracts for weeks may be unnecessary for optimal alkylamide extraction. Finally, the identification of a new alkylamide has been proposed. These results demonstrate the differences of the described extractions and utility of the analytical methods used to determine the wide-ranging individual alkylamide content of commonly consumed Echinacea extracts.
Keywords: HPLC-ESI-MS, electrospray, Echinacea, alkylamide, isobutylamide, 2-methylbutylamide, quality assessment, ethanolic extraction, tincture
Introduction
The investigations described herein employ high performance liquid chromatography coupled to electrospray ionization mass spectrometry (HPLC-ESI-MS) for the comprehensive characterization of several extracts from the medicinal plant Echinacea purpurea (L.) Moench. Echinacea is widely used for the treatment of upper respiratory infections, and is a global top seller. Three main species of echinacea are used clinically and available to consumers, Echinacea pallida, E. purpurea and E. angustifolia. Of these, E. purpurea represents 80% of commercial production [1]. E. purpurea products range from the injectables prepared to rigorous European pharmaceutical manufacturing standards, to the low tech ethanolic extractions or “tinctures” that follow general manufacturing practices (GMPs) of the United States dietary supplements industry. Although in Germany the aerial parts are preferred, ethanolic extracts of echinacea root make up a large source of sales and clinical use in the United States. Manufacturing practices generally dictate whether the starting plant material should be fresh or dry, but in the case of echinacea species, both fresh and dry root extracts are commercially available. To further complicate matters, these extracts are prepared with varying ratios of plant:solvent depending on the manufacturer. Currently, there are few investigations comparing the efficiency of extracting active constituents under these various extraction conditions. The few studies investigating the extraction of alkylamides have generally utilized dried root, while alkylamide extraction of fresh roots has scarcely been studied [2]. There is currently a lack of information regarding differences in chemical composition among extracts prepared using fresh versus dried Echinacea. One of the goals of the studies conducted herein was to provide such information.
Four constituent groups are currently believed to be the source of activity in the echinacea genus; alkylamides (alkamides), phenylpropanoids (caffeic acid derivatives), polysaccharides, and glycoproteins [3]. However, in extractions with ethanol concentrations above 40%, only very low levels of polysaccharides are left in suspension, and denaturing of proteins is expected [4, 5]. Thus, the major constituents of ethanolic echinacea extracts are phenylpropanoids and alkylamides. To date, human pharmacokinetic studies of Echinacea spp. suggest that the alkylamides are the major constituent group circulated in plasma [6].
Alkylamides have been of pharmacological interest since the tingling paresthesia from chewing plants rich in these compounds were noted [7]. This anesthetic property was utilized by native Americans [8] and eventually by physicians in the early 20th century for a variety of purposes including as a sialogogue, antitussive and for toothache. Alkylamides were later recognized as insecticidal [9] and oncolytic [10]. Recent investigations have demonstrated immunomodulatory activity of alkylamides in vitro [11] and in vivo [12], as well as direct antiviral activity [13]. Most recently, these compounds have become a subject of interest due to their elucidation as agonists of the cannabinoid receptor 2 (CB2) receptor[14].
Given that the alkylamides appear to be one of the key constituent groups responsible for pharmacological activity of E. purpurea, the studies described herein focused on this class of constituents. A number of analytical studies, the bulk by Bauer et al, have relied on liquid chromatography with UV detection (LC-UV) to analyze echinacea alkylamides [15–18]. However, comprehensive profiling of echinacea alkylamide content using LC-UV alone has been challenging. These compounds are present at widely different concentrations, and many of them are isomeric. Consequently, co-elution of structurally similar alkylamides is common, and UV detectors may not detect minor alkylamide constituents because of low concentrations and/or co-elution with other compounds. Mass spectrometry (MS) provides a distinct advantage over UV detectors due to its sensitivity and the ability to select by mass the ions corresponding to the compounds of interest [19]. As the data presented here will demonstrate, this advantage makes HPLC-ESI-MS an ideal technique for the comprehensive analysis of the isomeric alkylamide content in Echinacea purpurea. Although several investigators have previously employed HPLC-ESI-MS to the analysis of alkylamides in Echinacea [20–22], none of the previous methods have been validated for quantitative purposes. Furthermore, because of the abundance of isomeric alkylamides in E. purpurea, even with the use of MS detectors, mis-identification or incomplete identification of alkylamides has been common [22–24]. With this study, we present the first validated HPLC-ESI-MS method for the analysis of alkylamides in E. purpurea. This method enables quantitative comparison of alkylamide content in various E. purpurea extracts. In addition, by relying on MS-MS fragmentation patterns to distinguish isomeric alkylamides, we report a more comprehensive profile of alkylamide content in E. purpurea than has previously been published.
2. Experimental
2.1 Reagents
The following chemicals and reagents were used: Acetonitrile (high-performance liquid chromatography (HPLC) grade (Honeywell Burdick and Jackson, Muskegon, MI), acetic acid (Fisher Chemical, Fairlawn, NJ), alkylamide standards (Chromadex Inc., Santa Anna, CA) ethanol (AAPER, Shelbyville, KY), nanopure water (Nanopure Diamond D11931, Barnstead International, Thermolyne, Dubuque, IA).
2.2 Plant Material
Cultivation of E. purpurea took place in Grants Pass, OR at Pacific Botanicals. Fresh, dormant roots of E. purpurea were harvested in March 2007. Species was verified by Richard Cech (Horizon Herbs, Williams, OR) and voucher specimens were submitted to the University of North Carolina Herbarium in Chapel Hill, NC (accession numbers 583416 and 583417). The roots were two-years-old at time of harvest.
2.3 Plant Extractions
A typical protocol [25] for the manufacture of ethanolic extracts was followed in all extractions, except that post washing, the roots were briefly soaked (5 min) in 70% ethanol as a disinfectant, and blown partially dry with compressed air. A loss of the isomeric dodeca-2,4,8,10-tetraenoic acid isobutylamides (tetraenes) of 1.4% (against final fresh root concentrations) was calculated from the initial rinse. The roots were then cut into small pieces (≤ 1 cm wide) and extracted using three different extraction techniques, fresh root extraction (1:2 w:v) and dry root extraction at two different root to solvent ratios, 1:11 and 1:5 (w:v). All ratios are expressed as mass raw plant material (E. purpurea roots) in weight (g) per volume (mL) of extraction solvent.
To prepare fresh root extracts, samples of the cut roots (65 g) were blended using a Waring Blender (Tarrington, CT) in a solvent of 95% ethanol (AAPER, Shelbyville, KY) at a ratio of 1 g roots:2 mL solvent. Samples of root from the same batch were dried in an oven at 50 °C and water content was determined to be 74.5%. Dry root extractions were carried out with the same method as the fresh except that the solvent consisted of 74.5% ethanol and 25.5% water (to account for the plant water removed upon drying). To make these dry root extracts, 16.6 g of dried root was added to 179 mL of solvent (74.5% ethanol) for a ratio of 1:11 and 16.6 g of dried root was added to 83 mL of solvent (74.5% ethanol) for a ratio of 1:5. Four replicate extracts were prepared at each extraction ratio (fresh 1:2, dry 1:11 and dry 1:5).
Aliquots (dry root 1:11, 200 μL) for the extraction as a function of time study were taken on a daily basis (days 2–33) during the process of maceration and stored at −70° C until the time of analysis for alkylamide content. After maceration for one month, the solvent was removed from all of the extracts using a hydraulic press. The extracts were then aliquoted into 1 mL portions in polypropylene microcentrifuge tubes and kept in the dark at room temperature until needed for analysis. Previous investigations have established stability of alkylamides under these conditions [26]. All extractions were macerated at 24 °C.
2.4 Preparation of Samples and Standards
Prior to analysis, samples were removed from storage and allowed to reach room temperature. Aliquots (500 μL) from all extractions were centrifuged at 14,000 rpm (Savant Speedvac Sc110, Farmingdale, NY) for 5 minutes. Supernatant was then diluted in the same solvent used for extraction (70% ethanol), and pipetted (300 μL) into autosampler vials (Agilent Technologies, Santa Clara, CA) for LC-MS analysis. Several dilutions were prepared from each extract to adjust alkylamide content to within the linear dynamic range of the method. Neat samples were used for determination of dodeca-2E-ene-8,10-diynoic acid and1000-fold dilutions were used for analysis of the isomers of dodeda-2,4,8,10-tetraenoic acid isobutylamide.
Alkylamide primary standards were purchased from Chromadex (Santa Anna, CA) with certificates of analysis verifying identity by NMR and HPLC, and purity of ≥99% by HPLC. Concentrated stock solutions of dodeca-2E-ene-8,10-diynoic acid isobutylamide (molecular weight 245.37, lot # 04950-601) and dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide (molecular weight 247.38, lot # 04953-102) were prepared at 5 mg/mL in ethanol and stored at 4 °C. The stock solutions were diluted in ethanol to produce final concentrations of 0.1, 10, 50, 100 and 500 μM.
2.5 HPLC-ESI-MS Analysis
An ion trap mass spectrometer with electrospray ionization source (LCQ Advantage, ThermoFisher, San Jose, CA) was employed. The solvent gradient, which was a minor variation on that previously published [21], was as follows, where solvent A is aqueous acetic acid (17mM, original pH 2.74) and solvent B is neat HPLC grade acetonitrile. For t = 0 to 4 min, a constant composition of A–B (90:10 v/v); for t = 4 to 15 min, a linear gradient from A–B (90:10, v/v) to A–B (60:40, v/v); for t = 15 to 30 min, a linear gradient from A–B (60:40, v/v) to A–B (40:60, v/v); for t = 30.1 to 35 min, a constant composition of A–B (0:100, v/v); for t = 35.1 to 43 min, a constant composition of A–B (90:10, v/v). The mass spectrometer was operated in the positive ion mode with a scan range of 50.00–2000.00. Spray, capillary, and tube lens offset voltages were 4.5 kV, 3 V and −60V, respectively.
2.6 Quantitative and Qualitative Analysis of Alkylamides
Constituents in the extracts were identified according to their molecular weights, HPLC retention times, and previously established MS–MS fragmentation patterns [27]. For quantitative determination of alkylamide content, calibration curves were plotted as the log of the area of the selected ion chromatogram for the protonated alkylamide of interest versus the log of concentration. Extract samples were analyzed neat and at 1000 fold dilution within the same run as the calibration standards. The concentrations of dodeca-2E-ene-8,10-diynoic acid isobutlyamide and of the isomers of dodeda-2,4,8,10-tetraenoic acid isobutylamide were determined by plugging the relevant peak area into the linear regression equation for the corresponding calibration curve. All samples for which quantitative comparisons were made were analyzed within a single run.
2.7 Method Validation
Method validation was conducted according to International Committee of Harmonization (ICH) guideline [28]. Alkylamide standards prepared as described in Section 2.4 were analyzed in triplicate on three separate days (for a total of 9 analyses of each standard). To assess accuracy, a “measured concentration” for each standard was back-calculated from the corresponding calibration curve. The measured concentration reported was an average of the measured concentrations calculated on three separated days. This measured concentration was then compared to the theoretical concentration of each standard, and the % relative difference was reported as the “residual”. Repeatability was determined as the relative standard deviation among the back-calculated concentrations for the triplicate analyses of each standard within a single run. Intermediate precision was calculated as the relative standard deviation among the back-calculated concentrations for three runs conducted on three separate days. The limit of detection (a measure of the sensitivity of the method) was determined based on the concentration required to give a signal to noise ratio (S:N) of 3:1 in the relevant selected ion chromatogram. Limit of quantitation was based on the signal necessary to achieve a S:N of 10:1.
2.8 Statistical analysis
The standard error of the mean (SEM) was determined for each set of concentrations or peak areas. Data are expressed as the mean ± SEM and comparison of means was conducted using a two tailed t-test for paired data when differences were observed. The mean values were considered significantly different if p < 0.05. Where appropriate, outlying data points were rejected on the basis of the Q-test. Statistical analyses were performed with Microsoft Excel (2003).
2.9 Determination of yield of dissolved solids
Polypropylene microcentrifuge tubes (1.5 mL) were weighed before addition of 500 μL aliquots of centrifuged extracts. After dehydration in the speedvac for 39 hours at 24 °C, the mass of dissolved solids for each sample was determined. The ratio of the mass of dissolved solids to the amount of dry root used in the equivalent volume of extract was then calculated, providing a measure of the quantity of dissolved solids extracted per mass of Echinacea root (extract yield). For the fresh extract, the dry weight of the root used for the extract was calculated by subtracting the mass of the water contained in the roots from the total mass of the fresh roots.
3. Results and Discussion
In this section, a comprehensive profile of alkylamide constituents in E. purpurea is listed, with a description of how these compounds can be identified using HPLC-ESI-MS. In addition, quantities of dissolved solids and specific alkylamides present in various E. purpurea extracts are compared. The extracts analyzed here were prepared using several different procedures commonly employed in the dietary supplement industry. Analysis of these extracts provides insight into the similarities and differences in extract composition that result from these variations in extraction technique.
3.1 Identification of Alkylamides
Table 1 lists alkylamides identified from Echinacea purpurea, with references that refer to publications in which these identifications were made. This is the most comprehensive listing of alkylamides of E. purpurea root to date. Most reports and reviews list some, but not all, of the alkylamides present in this species [16, 24, 29, 30]. Past estimates suggest the presence of eleven alkylamides in the roots of E. purpurea [31, 32]. Table 1, lists a total of 17 compounds, although, as described later on, some of these identifications are only tentative and one compound (C) was not detected in our samples of E. purpurea.
Table 1.
Alkylamides from Echinacea purpurea.
| Designation | Alkylamide | MW | Ref |
|---|---|---|---|
| A | Undeca-2E,4Z-diene-8,10-diynoic acid isobutylamide | 229.32 | [32] |
| B | Undeca-2Z,4E-diene-8,10-diynoic acid isobutylamide | 229.32 | [69] |
| C | Undeca-2E-ene-8,10-diynoic acid isobutylamide | 231.34 | [55] |
| D | Undeca-2E,4Z-diene-8,10-diynoic acid 2-methylbutylamide | 243.35 | [32] |
| E | Undeca-2Z,4E-diene-8,10-diynoic acid 2-methylbutylamide1 | 243.35 | |
| F | Dodeca-2Z,4E-diene-8,10-diynoic acid isobutylamide | 243.35 | [69] |
| G | Dodeca-2E,4Z-diene-8,10-diynoic acid isobutylamide | 243.35 | [32] |
| H | Dodeca-2E,4E,10E-triene-8-ynoic acid isobutylamide | 245.37 | [32] |
| J | Dodeca-2E-ene-8,10-diynoic acid isobutylamide2 | 245.37 | [55] |
| K | Dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide | 247.38 | [32] |
| L | Dodeca-2E,4E, 8Z,10Z-tetraenoic acid isobutylamide2 | 247.38 | [32] |
| M | Dodeca-2E,4E, 8E,10Z-tetraenoic acid isobutylamide | 247.38 | [38] |
| N | Dodeca-2E,4E,8Z-trienoic acid isobutylamide | 249.40 | [32] |
| O | Dodeca-2E,4E-dienoic acid isobutylamide | 251.41 | [31] |
| P | Trideca-2E,7Z-diene-8,10-diynoic acid isobutylamide | 257.38 | [31] |
| Q | Dodeca-2E,4Z-diene-8,10-diynoic acid 2-methylbutylamide | 257.38 | [40] |
| R | Dodeca-2,4,8,10-tetraenoic acid 2-methylbutylamide | 261.41 | [36] |
Proposed structure of newly identified alkylamide
Compounds J and L were utilized as standards.
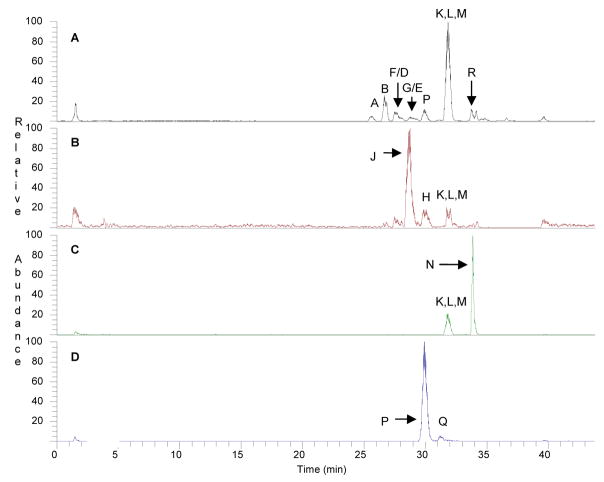
Many of these compounds were present in the E. purpurea extracts investigated here, as demonstrated in Figure 1. This figure shows a base peak chromatogram (Figure 1A) obtained from analysis of the 1:5 dry root E. purpurea extract. Peak labels correspond to alkylamide designations in Table 1, and were confirmed based on comparison of retention time with previous investigations [21] and MS-MS spectra (Table 2, Figure 2). A few of the minor alkylamides in the extract were obscured by coelution with major alkylamides, but can be visualized through the use of selected ion chromatograms (Figure 1B, C and D).
Figure 1. Characteristic chromatograms obtained by liquid chromatography-mass spectrometry analysis of an E. purpurea root extract (1:5).
Panel A shows a base peak chromatogram (plot of the most abundant ion in the mass spectrum versus time), with peak labels that correspond to the designations in Table 1. Several minor alkylamides that coelute with the compounds shown in the base peak chromatogram can be distinguished with selected ion chromatograms, as shown in panels B (mass range 245.5–246.5), C (mass range 249.5–250.5) and D (mass range 257.5–258.5).
Table 2.
Fragments formed by collisionally induced dissociation (MS-MS) of the MH+ ion of various E. purpurea alkylamides.
| Designation, name, m/z | group i4 | group ii5 | group iii6 | group iv7 | group v8 | |
|---|---|---|---|---|---|---|
| RI3 30–90% | RI 30–90% | RI 60–100% | RI 26–100% | RI < 3–20% | ||
| D 2441 |
Undeca-2E,4Z-diene-8,10- diynoic acid 2-metylbutyl amide | 157 | 129 | 131 | 174 | 188,202, 216 |
| E 244 |
Undeca-2Z,4E-diene-8,10- diynoic acid 2- methylbutylamide2 | 157 | 129 | 131 | 174 | 188,202, 216 |
| F 244 |
Dodeca-2Z,4E-diene-8,10- diynoic acid isobutylamide | 171 | 143 | 145 | 188 | 202, 216 |
| G 244 |
Dodeca-2E,4Z-diene-8,10- diynoic acid isobutylamide | 171 | 143 | 145 | 188 | 202, 216 |
| P 258 |
Trideca-2Z,7E-diene-8,10- diynoic acid isobutylamide | 185 | 157 | 159 | 202 | 216,230 |
| Q 258 |
Dodeca-2E,4Z-diene-8,10- diynoic acid 2- methylbutylamide | 171 | 143 | 145 | 188 | 202, 216 |
The number beneath the letter designation indicates the m/z value for the MH+ ion.
Proposed structure for compound E based on retention time and MS/MS fragmentation.
RI corresponds to relative Intensity.
The group i fragments correspond to acyllium ions as shown in Figure 2
The group ii fragments are carbocations that correspond to the alkyl chain of the alkylamide and are formed by loss of the amide portion (isobutylamide or 2-methylbutylamide).
The group iii fragments correspond to the alkyl chain of the alkylamide and are formed by the loss of the amide portion of the molecule and saturation of one of the double bonds on the akyl chain.
The group iv fragments correspond to the protonated alkylamide minus the N-alky group
The group v fragments correspond to the protonated alkylamide minus various portions of the N-alkyl group (see Figure 2).
Figure 2. MS/MS Spectra of Isomeric Alkylamides.
This figure compares fragments that result from collisionally induced dissociation precursor ions that represent the protonated molecular ion (MH+) of a number of isomeric alkylmaides. Even ions with the same MH+ ion have different fragmentation patterns, facilitating structural assignment.
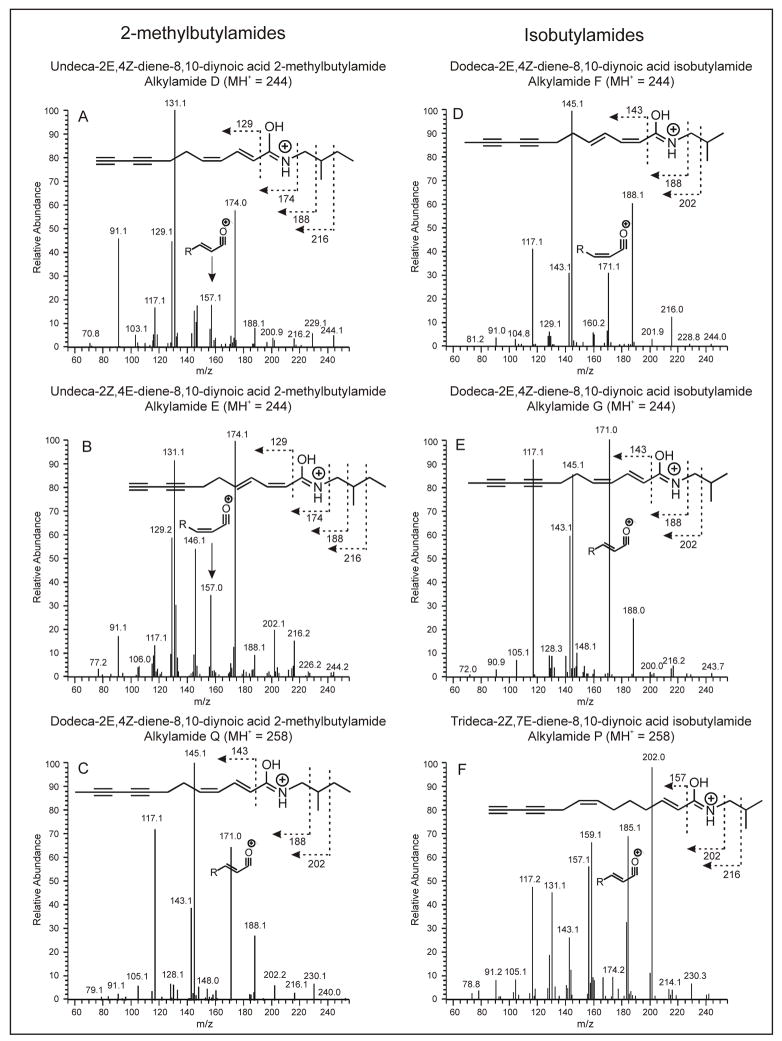
The isomeric compounds D, E, F and G all demonstrate MH+ ions at m/z 244 and compounds P and Q both have MH+ ions with m/z 258. Nonetheless, these alkylamides are distinguishable based on an MS-MS spectra. Table 2 illustrates the primary fragments that result from collisionally induced dissociation of several isomeric alkylamides. Structurally similar fragments have been grouped with designations of i, ii, iii, iv and v for ease of reference.
One of the major groups of fragments formed by collisionally induced dissociation is the acyllium ion (fragment group i in Table 2), as previously reported by Hiserodt et al [27]. These ions form due to a charge-remote hemolytic cleavage that yields a resonant distonic radical cation, which subsequently undergoes hydrogen rearrangement. Alkylamides F and G (Figures 2D and E) show an acyllium ion at m/z 171, while alkylamides D and E (Figures 2A and B) result in the acyllium ion at m/z 157.
Two additional fragments useful for elucidation of alkylamide structure are the group ii and group iii fragments (Table 2). The group ii fragments result from the loss of the amide portion alkylamide, and correspond to the remaining alkyl chain. The group iii fragments are observed at an m/z value 2 amu above the group ii fragments. Recent work utilizing deuterated alkylamides suggests that in the diene alkylamides, group iii fragments are formed when an unsaturated bond is lost and the remaining double bond shifts to the 3 position (in 2,4-dienes), with a subsequent gain of two hydrogens [27].
In combination, the group ii and iii ions can be used to determine 1) whether the alkylamide is a diene; 2) how many carbons are present in the akyl chain; and 3) the identity of the amide moiety (isobutyl versus 2-methylbutyl). Isobutylamides will have two fragments corresponding to a loss of 101 (group ii) and 99 (group iii) from the MH+ precursor ion (Figures 2D, E, and F). For 2-methylbutylamides, the fragments will reflect the additional carbon in the amide moiety, and fragment ions corresponding to a loss of 115 (group ii) and 113 (group iii) from the MH+ precursor ion will be observed (Figure 2A, B and C).
The group iv fragments (Table 2) correspond to the MH+ ion of the protonated alkylamide that remains after loss of the N-alkyl group. Loss of the N-isobutyl group results in a significant fragment (relative intensity 26–60%) at m/z 188 for compounds F and G and 185 for compound Q, while loss of the N-(2-methylbutyl) group results in a fragment with m/z 174 for compounds D and E (relative intensity 56–100%) and a fragment with m/z 202 for compound P. The mass that is lost to form the group iv fragment serves as additional confirmation to distinguish isobutylamides from 2-methylbutylamides.
The final fragments that result from collisionally induced dissociation of alkylamides are group v in Table 2. They are formed by cleavage of various C-C bonds on the N-alkyl substituent (Figure 2). The group v fragments are useful for verifying whether the N-alkyl substituent is a 2-methylbutylamide or isobutylamide.
An example of the utility of MS-MS for structural elucidation of alkylamides can be demonstrated for the specific case of alkylamide P, trideca-2E,7Z-diene-8,10-diynoic acid isobutylamide. The MS-MS spectrum of the precursor ion at m/z 258 for this compound is shown in Figure 2F. An acyllium ion (group i) is present at m/z 185. This suggests a thirteen carbon alkyl chain. This chain length is further confirmed by the group ii fragment at m/z 157. The presence of the group iii fragment (m/z 159) indicates that the compound is indeed a diene alkylamide, and, the characteristic loss of 99 to form this fragment (258–159 = 99) suggests that the compound is an isobutylamide rather than a 2-methylbutylamide. The high intensity group iv fragment at m/z 202 further confirms that the N-alkyl group is an isobutylamide. The low intensity group v fragments also confirm an N-isobutyl group rather than an N-2-methylbutyl group; additional group v fragments would be observed for a 2-methylbutylamide. Thus, the identity of the compound is proposed to be trideca-2E,7Z-diene-8,10-diynoic acid isobutylamide, which has previously been shown to occur in E. pallida, but rarely is reported as occurring in E. purpurea [33]. The E/Z assignments for trideca-2E,7Z-diene-8,10-diynoic acid isobutylamide have been made based on comparison of the relative retention times observed in this study with those reported previously [33], and are only tentative without NMR confirmation.
With HPLC-ESI-MS and MS-MS data such as those shown in Figure 1 and Figure 2, all of the previously identified alkylamides from E. purpurea in Table 1 except undeca-2E-ene-8,10-diynoic acid isobutylamide (compound C) were identified in the extracts prepared in this study. Although past work cites the presence of undeca-2E-ene-8,10-diynoic acid isobutylamide in E. purpurea root [34, 35], this compound was not detected in the echinacea extracts used for these studies. A commercially available standard of this compound was readily detectable with limit of detection of 0.15 μM, therefore, it can be concluded that undeca-2E-ene-8,10-diynoic acid isobutylamide was not present in the extracts at concentrations above 0.15 μM. Binns et al [36], using solely UV spectra and retention time, previously identified undeca-2E-ene-8,10-diynoic acid isobutylamide compound in E. purpurea roots of wild plants but not cultivated germlings. Hence, it is possible that some genetic strains of E. purpurea contain undeca-2E-ene-8,10-diynoic acid isobutylamide while others do not. However, our laboratory has investigated over 20 different US sources of E. purpurea (data not included) and thus far not detected this compound. Another possibility is that previous reports of the presence of undeca-2E-ene-8,10-diynoic acid isobutylamide in E. purpurea were due to improperly identified plant material. Another Echinacea species, E. angustifolia, does produce significant levels of undeca-2E-ene-8,10-diynoic acid isobutylamide, and the misidentification of echinacea species has often been documented [15, 37, 38].
In addition to aiding in structural elucidation of known alkylamides, with HPLC-ESI-MS it was possible to tentatively identify a new alkylamide, the structure of which has not been previously published. This compound is undeca-2Z,4E-diene-8,10-diynoic acid 2-methylbutylamide (compound E in Table 1). Identification of this compound was based on retention time and the correlation between MS-MS fragmentation pattern and alkylamide structure. The mass and fragmentation pattern for compound E (Figure 2) confirms that it is a 2-methylbutylamide, and indicates the level of saturation and length of the carbon chain. The mass spectral data do not indicate stereochemistry or bond position; however, relative retention time does suggest that this compound is the 2Z/4E isomer of compound D. For the previously identified alkylamide isomers that vary by the 2E/4Z and 2Z/4E stereochemistry, such as compounds A/B and F/G, we have demonstrated (Figure 1) that the 2E/4Z isomer elutes before the 2Z/4E isomer. Thus, it is logical to assume a similar relationship in stereochemistry between compounds D and E. However, as noted earlier, without NMR confirmation, the reported stereochemistry of this new alkylamide is only tentative.
Bauer et al previously demonstrated that with reversed phase HPLC, alkylamides with terminal alkynes elute early in the separation followed by tetraene alkylamides [33]. For the purposes of this discussion, compounds A–G, J, P and Q are designated as polyacetylene amides, while the term “tetraenes” refers to isobutylamides with four double bonds in the alkyl chain. Consistent with the Bauer study, our results (Figure 1) indicate that the polyacetylene amides (A, B, C, D/E, F/G, H, J, P, Q) elute early in the separation, between 25 and 31 minutes, followed by the tetraenes and dienes (K, L M, N and O). These findings are significant in that alkynes, specifically 8,10 terminal alkynes, have been shown to modulate CYP 450 function [34], while tetraene isomer L and alkylamides N and O are ligands of the CB2 receptor [14]. The results in Figure 1 suggest preparatory scale HPLC could be used to separate groups of alkylamides with differing physiological and pharmacological activity.
3.2 Calibration Results and Method Validation
Table 3 illustrates the linear regression equations and statistical data for the alkylamide standards. The linear range of the calibration curves was from 1 to 500 μM for Dodeca-2E-ene-8.10-diynoic acid isobutylamide (J), and from 1 to 100 μM for Dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide (L). Correlation coefficients (R2) of the alkylamide standards were 0.999 (J) & 0.993 (L). The limit of detection (concentration required to give a signal to noise ratio, S:N, of 3:1) for isobutylamides dodeca-2E-ene-8,10-diynoic acid and dodeca-2,4,8,10-tetraenoic acid were 0.051 and 0.99, respectively. Limits of quantitation (based on S:N of 10:1) were 1.7 and 3.3, respectively.
Table 3.
Calibration parameters, limits of quantification (LOQ) and limits of detection (LOD) for E. purpurea alkylamides.
| Slope (±SD1) | Intercept (±SD1) | R2 | Linearity (μM) | LOD (μM) | LOQ (μM) | |
|---|---|---|---|---|---|---|
| Dodeca-2E-ene- 8.10-diynoic acid isobutylamide (J) | 0.757 (±0.013) | 8.00 (±0.023) | 0.999 | 1.0 – 500 | 0.051 | 1.7 |
| Dodeca- 2E,4E,8Z,10Z- tetraenoic acid isobutylamide (L) | 0.813 (±0.040) | 6.95 (±0.070) | 0.993 | 1.0 – 100 | 0.99 | 3.3 |
SD – Standard Deviation
Table 4 shows the results of method validation for the quantitative analysis of alkylamide content, which was accomplished using International Committee of Harmonization (ICH) guidelines [28], as described under methods (Section 2.7). The values for the residuals, repeatability, and intermediate precision conform to the parameters for method validation according to the ICH.
Table 4.
Validation parameters for quantitative analysis of E. purpurea alkylamides
| Dodeca-2E-ene-8.10-diynoic acid isobutylamide (J) | ||||
|---|---|---|---|---|
| Theoretical concentration (μM)1 |
Measured concentration (μM)2 |
Residules (%)3 |
Repeatability (%)4 |
Intermediate precision (%)5 |
| 1.0 | 1.1 | 14 | 3 | 2 |
| 10 | 8.8 | −12 | 2 | 2 |
| 50 | 44 | −11 | 1 | 3 |
| 100 | 99.6 | −0.3 | 1 | 4 |
| 500 | 568 | 13 | 0.5 | 0.7 |
| Dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide (L)6 | ||||
| 1.0 | 0.99 | −.5 | 3 | 6 |
| 10 | 9.7 | −3 | 3 | 2 |
| 50 | 49 | −3 | 0.5 | 4 |
| 100 | 105 | 5 | 3 | 0.6 |
Theoretical concentration based on the mass of standard per volume of solution.
Measured concentration for a given standard is the average of the back-calculated concentration (from the calibration curve) for three analyses conducted on three separate days.
The residuals (Res) are calculated by first determining the difference between the measured concentration (CM) and the theoretical concentration (CT) and then dividing this value by the measured concentration: Res = (CM-CT)/CM × 100.
Repeatability corresponds to the relative standard deviation of the back-calculated concentration for triplicate analyses of the same standard on the same day.
Intermediate precision corresponds to the relative standard deviation of the back-calculated concentration for triplicate analyses of the same standard on three different days.
Validation results are reported for Dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide up to 100 μM, the upper limit of the linear range.
3.3 Comparison of alkylamide yield in various E. purpurea extracts
Three extracts were chosen for comparison of alkylamide content. One of these (fresh 1:2) was prepared from fresh E. purpurea roots using 1 g roots for every 2 mL of solvent. The other two were prepared from dry E. purpurea roots, one using 1 g dried roots per 11 mL solvent (1:11) and the other 1g dried roots per 5 mL solvent (1:5). All of three extracts contain the same percentage of ethanol (69%). The extracts differ only in the nature of starting material (fresh or dry root) and the ratio of root:solvent. Once the mass of the fresh roots is adjusted to account for water content, the fresh 1:2 and dry 1:11 extract have equivalent ratios of dry weight plant material:mL solvent. Therefore, by comparing the composition of the fresh 1:2 and dry 1:11 extracts, it should be possible to determine how extract composition differs depending on whether fresh or dried roots are used for extraction. The two extracts prepared from dried E. purpurea differ only in the ratio of g root:mL solvent (1:11 versus 1:5), therefore, by comparing the composition of the dry 1:11 and dry 1:5 extracts, it should be possible to determine whether changing the root:solvent ratio has an effect on extract composition.
Utilizing available alkylamide standards, the quantities of alkylamides in the fresh and dry E. purpurea root extracts were determined. Table 5 displays these results in terms of concentrations of the isomeric tetraenes (compounds K, L and M) and dodeca-2E-ene-8,10-diynoic acid isobutylamide (compound J) per mL of solvent. The three different Echinacea extracts, fresh 1:2, dry 1:11 and dry 1:5, all contained these alkylamides. However, as shown in Table 5, the dry 1:5 extract contained the greatest amount of these compounds. This is to be expected given the lower ratio of g roots:mL solvent used in the preparation of the 1:5 extract.
Table 5.
Quantities of the isomeric alkylamides K, L and M and alkylamide J in ethanolic extracts of E. purpurea root.
| Alkylamides K, L & M | Alkylamide J | |||||
|---|---|---|---|---|---|---|
| Type of Extract | Fresh 1:2 | Dry 1:11 | Dry 1:5 | Fresh 1:2 | Dry 1:11 | Dry 1:5 |
| Mean Concentration (mg/mL)1 | 1.4 | 1.6 | 7.2 | 0.0047 | .0072 | 0.013 |
| Mean Concentration (mM)1 | 5.7 | 6.4 | 29 | 0.019 | 0.029 | 0.054 |
| SE2 | 0.5 | 0.2 | 3 | 0.001 | 0.0004 | 0.002 |
The mean concentration was calculated for four replicate extractions (n = 4)
SE represents the standard error of the concentrations in mM
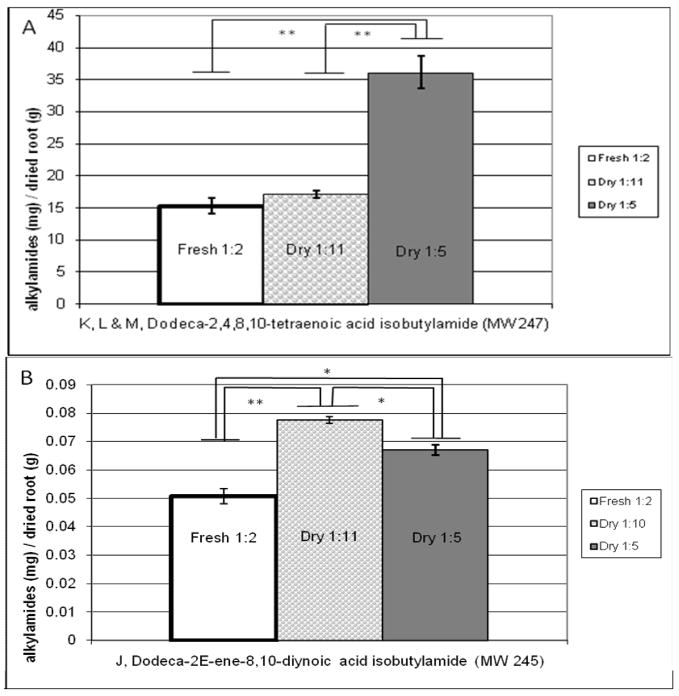
In order to easily compare how efficiently alkylamides were extracted in the three different E. purpurea extracts, the quantity of each alkylamide (mg) was divided by the dry weight of E. purpurea root (g) used to prepare an equivalent volume of extract. The resulting value is referred to as “alkylamide yield” (Figure 3). The only difference in the fresh 1:2 versus the dry 1:11 extracts is whether fresh or dry root was used in their preparation. Therefore, assuming no loss of alkylamide during the drying process, alkylamide yield would be expected to be very similar for these two extracts. Indeed, the alkylamide yield for the fresh 1:2 versus the 1:11 were comparable for the tetraenes K, L, M (Figure 3A), 15.3 ±2.5 vs. 17.1 ±1.3 mg/g, respectively. For alkylamide J, the yield was lower in the 1:2 extract as compared to the 1:11 extract (0.0506 ± 00.0053 vs. 0.0775 ± 0.0024 mg/g, respectively). This difference could be due to differences in particle size between the extracts, which may have given rise to more efficient extraction in the dry as compared to the fresh extract. Importantly, comparison of alkylamide yield for the 1:2 and 1:11 extracts does not indicate any significant degradation of alkylamides due to drying. The extracts in this study were prepared from roots immediately after completion of oven drying. Kabganian et al [39] came to the same conclusion finding no degradation of alkylamides in roots that were oven dried. Whether or not there is a loss in alkylamide content in E. purpurea roots stored for long periods of time would be a worthy subject of a future investigation.
Figure 3. Comparison of alkylamide yield in fresh 1:2, dry 1:11 and dry 1:5 E. purpurea root ethanolic extracts.
All concentrations represent the mean from four replicate extractions at room temperature. Error bars denote standard error of the mean (SEM). Comparisons were made between Fresh 1:2, Dry 1:11 and 1:5 extracts, with * indicating p<0.005; ** indicating p<0.001. Alkylamide yield is similar in the fresh 1:2 and dry 1:11 extracts, indicating no loss of alkylamides in the drying process. Better yield of alkylamide J was obtained for the fresh 1:2 versus dry 1:11 extract.
In comparing the extraction yield of the alkylamides between the 1:11 versus 1:5 dry root extracts, a logical prediction, provided a 1:5 extract is not saturated, would be that the alkylamide yield would be the same in the two extracts. As can be seen in Figure 3, however, this is not the case. While alkylamide yield is similar in the two extracts, there are statistically significant differences. For alkylamides K, L, M (Figure 3A), extraction is more efficient in the 1:5 as compared to the 1:11 extract. This is the opposite of what would be expected if the solution were saturated in the case of the 1:5 extract; therefore, saturation is not the cause of the differences. Conversely, for alkylamide J, extraction is more efficient in the 1:11 extract than the 1:5 extract (Figure 3B). Previous studies have established that solvent interactions of N-alkylamides differ depending on molecular structure and the surrounding phytochemical matrix [40]. Therefore, it is plausible that extraction of certain alkylamides is favored in more dilute extracts, while concentrated extracts favor the extraction of structurally different species.
3.4 Comparison of yield of dissolved solids in various E. purpurea extracts
The amount of total dissolved solids in the three extracts described in Section 3.3 was compared. Total dissolved solids are a measure of how much material overall (alkylamides as well as other compounds) was dissolved in the original extract. When the mass of dissolved solids is divided by the dry weight of the plant material used to produce an equivalent volume of extract, the resulting value is referred to as “extract yield,” which is a measure of how much of the initial starting material was converted into extract.
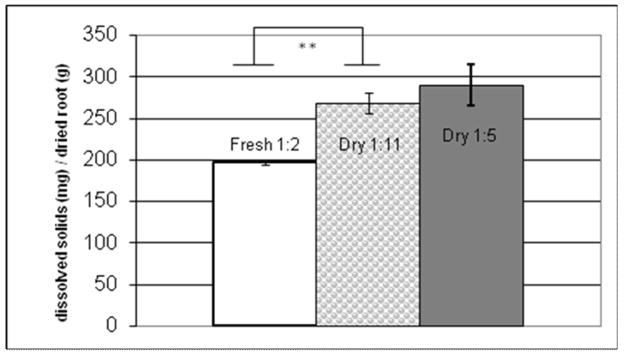
Figure 4 displays the extract yield (mg dissolved solids/g dry root) for the three E. purpurea extracts under investigation. Overall, the extract yield was similar for all three extracts. However, the yield for the fresh root extract (196.6 ± 2.7 mg/g.) was slightly lower than the two dry root extracts (269 ± 12 and 290 ± 24 mg/g for the 1:11 and 1:5 extracts, respectively, p < 0.001). As mentioned previously, a similar effect was observed for alkylamide J. This difference could possibly be attributed to differences in particle size in fresh versus dry extractions. Between the two dried extracts, there was no statistically significant difference in extract yield. This similarity between 1:11 and 1:5 extracts indicates that it is possible, by doubling the quantity of root used for the extraction, to double the amount of material dissolved in the solvent, at least up to a ratio of 1:5. It should be pointed out that because of the greater amount of root used to prepare the 1:5 extract, this extract does, overall, contain a greater concentration of dissolved solids then the 1:11 extract. However, there is no significant difference between the two extracts when the amount of dissolved solids is expressed relative to the mass of root used in the extract.
Figure 4. Yield of dissolved solids in ethanolic extracts of Echinacea purpurea roots.
Mass of dissolved solids was determined by evaporation of the ethanol/water solvent from aliquots of extracts, and this value was ratioed to the quantity of root (dry weight) used to prepare an equivalent volume of extract to calculate dissolved solids yield. Yield of dissolved solids in the 1:11 extraction does not statistically differ from 1:5 extraction. The fresh root extraction (1:2) differs from the 1:11 and the 1:5 extraction by 26.8% and 32.4% respectively.
*indicates p<0.005;** indicates p<0.001.
3.4 Extraction of the tetraene isomers as a function of maceration time
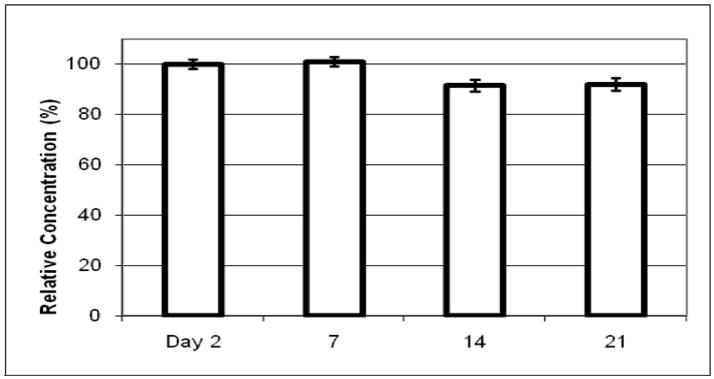
Lastly, the quantity of the isomeric dodeca-2,4,8,10-tetraenoic acid isobutylamides present in a macerating E. purpurea extract against time was measured (Figure 5). The results are all displayed relative to that achieved on the first day that concentration was measured (day 2). These tetraene isobutylamides are significant because they typically compose from 30 – 70% of the total alkylamides in echinacea products [41]. The results in Figure 5 demonstrate that the extraction of the dodeca-2,4,8,10-tetraenoic acid isobutylamides is complete by day 2. Thus, in terms of the extraction of specifically these compounds, maceration beyond day 2 should not be necessary. This finding is particularly interesting given that many dietary supplements manufacturers currently suggest that the appropriate time for a maceration is 2 weeks or longer, depending on the part of the plant used [25]. While commonly used long maceration times seem to have little effect on alkylamide content, long maceration times could actually be detrimental if some compounds (for example caffeic acid derivatives) degrade during maceration. Future investigations of the optimal maceration time for producing an extract with maximal concentrations of all desirable constituents are warranted.
Figure 5. Relative concentration of dodecatetraenoic acid isobutylamides (alkylamides K, L and M) in an E. purpurea root extraction over time.
Relative concentrations (CR) were calculated by dividing the concentration of each sample (CS) by the concentration at day 2 (Cday2) and converting to percent: CR = CS/Cday2 × 100. Samples were taken daily over 28 days from dry root (1:11) ethanolic maceration of E. purpurea. Results show that maximal extraction of dodecatetraenoic acid isobutylamide is achieved by day 2.
4. Conclusion
With these investigations, it has been demonstrated that HPLC-ESI-MS is an excellent technique for comprehensive analysis of the alkylamide content of Echinacea purpurea extracts. Using HPLC-ESI-MS, a more comprehensive alkylamide profile was obtained than is typically possible with other analytical approaches. By relying on collisionally induced dissociation, it was possible to distinguish between isomeric alkylamides, and to tentatively identify a new E. purpurea alkylamide, undeca-2Z,4E-diene-8,10-diynoic acid 2-methylbutylamide. In addition, the validated method facilitated quantitative comparison of alkylamide content among extracts prepared with different extraction procedures. All three extraction techniques investigated here (fresh 1:2, dry 1:5 and dry 1:11) resulted in very similar alkylamide profile, and gave similar yields of alkylamides and of total dissolved solids. The similarity in alkylamide content in fresh 1:2 and dry 1:11 extracts indicates that drying of root material at 50 °C does not result in a loss of alkylamides. It appears that either fresh or dried roots can be used to prepare extracts with high alkylamide content, although the overall yield was slightly lower for fresh extracts. Lastly, the analysis of a 1:2 fresh root ethanolic extract suggest that the maximum concentration of the tetraenes (dodecatetraenoic acid isobutylamides) is achieved by day 2 in an ethanolic extraction.
Although alkylamide yields were, overall, similar with the three extraction techniques, there were some statistically significant differences in quantities of alkylamides extracted. Notably the isomeric tetraenes were extracted more efficiently with a root to solvent ratio of 1:5 (w:v) as compared to a ratio of 1:11, while dodeca-2E-ene-8,10-diynoic acid isobutylamide was extracted more efficiently with a ratio of 1:11. Given that the biological activity of alkylamides differs depending on structure, these suggest that pharmacological activity of Echinacea purpurea extracts could differ depending on the ratio of root:solvent used in extraction. Ultimately, in vitro and in vivo studies are needed to elucidate the differences in pharmacokinetic and pharmacodynamic activity of extracts of various Echinacea spp. The results presented in this paper do, however, suggest that it would be erroneous to assume that all ethanolic extracts of Echinacea purpurea result in equivalent phytochemical profiles.
Acknowledgments
These studies were made possible by financial support of Research Corporation (Cottrell College Science Award # CC6595), the National Institutes of Health National Center for Complementary and Alternative Medicine (R15 AT001466-01), the National Science Foundation (MRI grant # 0420292) and the UNC Research Competitiveness Fund. We thank James Snow of Tai Sophia Institute for advice on technical aspects of medicinal plant extractions, Jason Reddick for technical advice, Richard Cech of Horizon Herbs for providing the voucher specimen for this study, and Tai Sophia Institute for educational support of KS.
Abbreviations Used
- CB2
cannabinoid 2 receptor
- HPLC-MS
high performance liquid chromatography-mass spectrometry
- ESI-MS
electrospray ionization mass spectrometry
- MW
molecular weight
- Tetraenes
isomers of dodeca-2,4,8,10-tetraenoic acid isobutylamide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li TSC. HortTechnology. 1998;8:122–129. [Google Scholar]
- 2.Sun L, Rezaei KA, Temelli F, Ooraikul B. J Agric Food Chem. 2002;50:3947–3953. doi: 10.1021/jf0200265. [DOI] [PubMed] [Google Scholar]
- 3.Bauer R. Z Arztl Fortbild. 1996;90:111–115. [PubMed] [Google Scholar]
- 4.Dalby-Brown L, Barsett H, Landbo AKR, Meyer AS, Molgaard P. J Agric Food Chem. 2005;53:9413–9423. doi: 10.1021/jf0502395. [DOI] [PubMed] [Google Scholar]
- 5.Brinker FHG. J Am Bot Coun. 1999;46:36–50. [Google Scholar]
- 6.Woelkart K, Marth E, Suter A, Schoop R, Raggam RB, Koidl C, Kleinhappl B, Bauer R. Int J Clin Pharm Ther. 2006;44:401–408. doi: 10.5414/cpp44401. [DOI] [PubMed] [Google Scholar]
- 7.Greger H. Planta Med. 1984;50:366–375. doi: 10.1055/s-2007-969741. [DOI] [PubMed] [Google Scholar]
- 8.Moerman DE. Native American ethnobotany. Timber Press; Portland, Or: 1998. [Google Scholar]
- 9.Jacobson M. J Am Chem Soc. 1948;70:4234. doi: 10.1021/ja01192a075. [DOI] [PubMed] [Google Scholar]
- 10.Voaden DJ, Jacobson M. J Med Chem. 1972;15:619–623. doi: 10.1021/jm00276a013. [DOI] [PubMed] [Google Scholar]
- 11.Sasagawa M, Cech NB, Gray DE, Elmer GW, Wenner CA. Int Immunopharmacol. 2006;6:1214–1221. doi: 10.1016/j.intimp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Goel V, Chang C, Slama JV, Barton R, Bauer R, Gahler R, Basu TK. Int Immunopharmacol. 2002;2:381–387. doi: 10.1016/s1567-5769(01)00163-1. [DOI] [PubMed] [Google Scholar]
- 13.Hudson J, Vimalanathan S, Kang L, Amiguet VT, Livesey J, Arnason JT. Pharm Biol. 2005;43:790–796. [Google Scholar]
- 14.Raduner S, Majewska A, Chen JZ, Xie XQ, Hamon J, Faller B, Altmann KH, Gertsch J. J Biol Chem. 2006;281:14192–14206. doi: 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- 15.Bauer R, Khan IA, Wagner H. Planta Med. 1988;54:426–430. doi: 10.1055/s-2006-962489. [DOI] [PubMed] [Google Scholar]
- 16.Bauer R, Remiger P, Wagner H. Phytochemistry. 1988;27:2339–2342. [Google Scholar]
- 17.Bauer R, Remiger P, Wagner H. Phytochemistry. 1989;28:505–508. [Google Scholar]
- 18.Bauer R, Remiger P, Wagner H. Planta Med. 1988;54:563–564. doi: 10.1055/s-2006-962563. [DOI] [PubMed] [Google Scholar]
- 19.Cech NB, Enke CG. Mass Spectrom Rev. 2001;20:362–387. doi: 10.1002/mas.10008. [DOI] [PubMed] [Google Scholar]
- 20.Luo XB, Chen B, Yao SZ, Zeng JG. J Chromatogr A. 2003;986:73–81. doi: 10.1016/s0021-9673(02)01922-2. [DOI] [PubMed] [Google Scholar]
- 21.Cech NB, Eleazer MS, Shoffner LT, Crosswhite MR, Davis AC, Mortenson AM. J Chromatogr A. 2006;1103:219–228. doi: 10.1016/j.chroma.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 22.He XG, Lin LZ, Bernart MW, Lian LZ. J Chromatogr A. 1998;815:205–211. [Google Scholar]
- 23.Bauer R, Khan IA, Wray V, Wagner H. Phytochemistry. 1987;26:1199–1200. [Google Scholar]
- 24.Wills RBH, Stuart DL. Food Chemistry. 1999;67:385. [Google Scholar]
- 25.Adams J, Tan E. Herbal Manufacturing, How to make Medicines from Plants. TAFE Printers; Preston, Australia: 1999. [Google Scholar]
- 26.Livesey J, Awang DV, Arnason JT, Letchamo W, Barrett M, Pennyroyal G. Phytomedicine. 1999;6:347–349. doi: 10.1016/S0944-7113(99)80057-9. [DOI] [PubMed] [Google Scholar]
- 27.Hiserodt RD, Pope BM, Cossette M, Dewis ML. J Am Soc Mass Spectrom. 2004;15:1462–1470. doi: 10.1016/j.jasms.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 28.ICH Expert Working Group. International Conference on International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 1995:13. [Google Scholar]
- 29.Gotti R, Pomponio R, Bertucci C, Cavrini V. J Sep Sci. 2002;25:1079–1086. [Google Scholar]
- 30.Gray DE, Pallardy SG, Garrett HE, Rottinghaus GE. Planta Med. 2003;69:50–55. doi: 10.1055/s-2003-37026. [DOI] [PubMed] [Google Scholar]
- 31.Bauer R. In: Phytomedicines of Europe: Chemistry and Biological Activity. Lawson LD, Bauer R, editors. American Chemical Society; Washington, DC: 1998. pp. 140–157. [Google Scholar]
- 32.Pietta P, Mauri P, Fuzzati N. In: Echinacea: the genus Echinacea. Miller SC, Yu HC, editors. CRC Press; Boca Raton: 2004. pp. 93–109. [Google Scholar]
- 33.Bauer R, Remiger P. Planta Med. 1989;55:367–371. doi: 10.1055/s-2006-962030. [DOI] [PubMed] [Google Scholar]
- 34.Matthias A, Gillam EM, Penman KG, Matovic NJ, Bone KM, De Voss JJ, Lehmann RP. Chem Biol Interact. 2005;155:62–70. doi: 10.1016/j.cbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Binns SE, Livesey JF, Arnason JT, Baum BR. J Agric Food Chem. 2002;50:3673–3687. doi: 10.1021/jf011439t. [DOI] [PubMed] [Google Scholar]
- 36.Binns SE, Arnason JT, Baum BR. Planta Med. 2002;30:837–854. [Google Scholar]
- 37.Rogers KL, Grice ID, Mitchell CJ, Griffiths LR. Aust J Exper Agric. 1998;38:403–408. [Google Scholar]
- 38.Foster S. HerbalGram J Am Bot Coun. 1985 Fall;:3. [Google Scholar]
- 39.Kabganian R, Carrier DJ, Sokansanj S, Herbs J. Spices Med Plants. 2002;10:11–18. [Google Scholar]
- 40.Liu Y, Murphy PA. J Agric Food Chem. 2007;55:120–126. doi: 10.1021/jf0619481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modarai M, Gertsch J, Suter A, Heinrich M, Kortenkamp A. J Pharm Pharmacol. 2007;59:567–573. doi: 10.1211/jpp.59.4.0012. [DOI] [PubMed] [Google Scholar]