Abstract
Background
Although stroke from large vessel athero-thromboembolism has a common pathogenesis, its topographic presentation is variable. Given the impact of cerebral infarct size and location on incident stroke magnitude and subsequent prognosis, we evaluated the determinants of cerebral infarct topography among patients with atherosclerotic stroke.
Methods
We analyzed data on 148 consecutive patients admitted over a 4-year period to a university medical center with acute ischemic stroke within the MCA distribution on DWI, presumed due to atherosclerosis. Based on the DWI data, we divided the patients into three stroke phenotypes: large cortical, small (< 1 cm in diameter) cortical, and deep pattern. Independent factors for each stroke phenotype were evaluated using logistic regression.
Results
After adjusting for covariates, premorbid statin use (OR, 3.05; 95 % CI, 1.40–6.65) and older age (OR, 1.05 per 1 year increase; 95 % CI, 1.02–1.08) were independently associated with the small cortical phenotypic pattern. In contrast, younger age (OR, 0.95 per 1 year increase; 95 % CI, 0.92–0.98), premorbid statin non-use (OR, 0.40; 95 % CI, 0.17–0.99), and higher levels of fasting s-glucose (OR, 1.01 per 1 mg/dl increase; 95 % CI, 1.00–1.02) and admission peripheral WBC counts (OR, 1.13 per 1 × 109 cells/L; 95 % CI, 1.00–1.27) were independently associated with the large cortical pattern. There was no relation between DWI patterns and LDL-cholesterol levels.
Conclusions
Age, premorbid statin use, s-glucose and WBC count predict atherosclerotic stroke phenotype. Further studies should examine whether modifying some of these factors may result in more favorable phenotypic patterns.
Keywords: stroke, ischemic; atherosclerosis; diffusion-weighted imaging; magnetic resonance imaging; risk factor; statin
Introduction
Given the limited options for acute stroke therapies and modest effects of current prevention strategies [29], there is a need to explore other avenues to reduce the devastating consequences of stroke. Specifically, efforts geared at identifying modifiable factors that may influence the phenotypic presentation of stroke could lead to strategies that mitigate stroke extent and improve clinical outcomes. For instance, it has been observed that although strokes of large vessel atherosclerotic origin have a common pathogenesis, their phenotypic expression can be quite varied. DWI studies reported variable lesion patterns in stroke patients with large artery atherosclerosis (LAA), even among patients with atherosclerosis in the same vascular system (extracranial [37] or intracranial [27]). However, there is a paucity of data regarding the frequencies, clinical determinants, and outcomes of topographic subtypes of ischemic strokes of LAA origin.
The objective of this study was to investigate the frequencies, clinical determinants, and outcomes of cerebral infarct topographic subtypes among patients with MCA infarction due to LAA.
Methods
Patient selection
We analyzed demographic, clinical, laboratory, and radiographic data collected prospectively on consecutive patients admitted for acute cerebral infarction to UCLA Stroke Center from December 2002 through December 2006. Inclusion criteria for this study were: (a) presentation within 5 days of symptom onset, (b) acute ischemic lesions within the MCA distribution on DWI, and (c) work-up disclosed modified TOAST algorithm diagnosis of LAA as the likely stroke mechanism [22]. Exclusion criteria were: (a) initial DWI performed only after active recanalization (fibrinolytic or mechanical) therapy administered (with some lesions potentially due to treatment complications rather than natural expression of stroke mechanism), (b) modified TOAST algorithm competing diagnosis of cardioembolic, small vessel, or other causes (such as arterial dissection, hypercoaguable state, vasculitis, and complicated migraine) [22].
Work-up
All patients underwent MRI (1.5 T Siemens Medical Systems, NJ). The MRI protocol also included DWI, gradient-recalled echo, fluid-attenuated inversion recovery, and T2*-perfusion-weighted imaging, and magnetic resonance angiography (MRA) imaging of the cervical and intracranial vessels, using MRI methods previously described [22]. Select patients also underwent additional vascular imaging including digital subtraction angiography at the attending physician’s discretion.
All the patients underwent routine blood tests, electrocardiography, cardiac telemetry for at least 24 hours, and echocardiography. Transesophageal echocardiography was performed in patients with infarcts unexplained after initial diagnostic evaluation. Aortic plaques on transesophageal echocardiogram were classified as moderate to severe if they were ≥4 mm in thickness, ulcerative, or contained mobile elements (most often thrombi) [1, 6]. Only plaques of the ascending and transverse aorta were considered to have the risk of brain embolus. Hemostatic markers of arterial prothrombotic tendency, including antiphospholipid antibodies (anticardiolipin antibody, dilute Russell Viper venom time, β2-glycoprotein-1 antibody), were measured in patients younger than 50 years-old or with stroke cause undefined after initial work-up. Hemostatic markers of venous prothrombotic tendency, including functional activity of protein C and protein S, factor V Leiden, prothrombin gene mutation, antithrombin-III functional activity, and factor VIII activity, were measured in patients with right-to-left shunts who were less than 50 years-old or had stroke cause undefined after initial work-up. Stroke mechanism categories were classified by the use of modified TOAST classification [22].
Conventional and non-conventional risk factors for atherosclerosis
Patients were evaluated according to the protocols that included demographic data, medical history, vascular risk factors, and the NIH Stroke Scale (NIHSS) score [34]. Type and dose of statin were recorded at the time of admission, and doses of statin were converted to an atorvastatin equivalent on the basis of estimates of their LDL-lowering potency [19]; as high-dose patients, who were taking ≥ 10 mg atorvastatin-equivalent dose, and low-dose patients, who were taking < 10 mg atorvastatin-equivalent dose.
All patients had fasting lipid panels drawn the day after hospital admission. In selected patients, C-reactive protein (CRP) levels at the time of admission (N = 94) and fasting homocysteine levels (N = 123) were evaluated, because levels of CRP and homocysteine have been associated with LAA [11, 13, 38]. To define the metabolic syndrome, we used the Adult Treatment Panel III guidelines [3] with some modification; we used body mass index (≥ 30 kg/m2) rather than waist circumference as the index of obesity, as used in the World Health Organization metabolic syndrome definition [5].
Subtypes of large vessel atherosclerotic stroke
Based on vessel imaging studies, we divided patients with LAA stroke within the MCA territory into three atherosclerosis lesion location groups according to previously published methods, namely those with (a) occlusive lesions (> 50 % stenosis or occlusion) of the extracranial portion of ICA [2], (b) those with occlusive lesions on the intracranial ICA or the proximal portion of the MCA (M1) [31], or (c) moderate-to-severe atherosclerotic changes on the ascending or transverse aorta [1, 6]. Patients with tandem stenoses in lesion-supplying extracranial and intracranial anterior circulation arteries were placed in the extracranial internal carotid disease category in group analyses. Patients with stenoses on the M2 segment were classified as intracranial atherosclerosis when there was coexistence of stenoses of non-relevant large intracranial vessels (intracranial portion of ICA or vertebral artery, basilar artery, or M1).
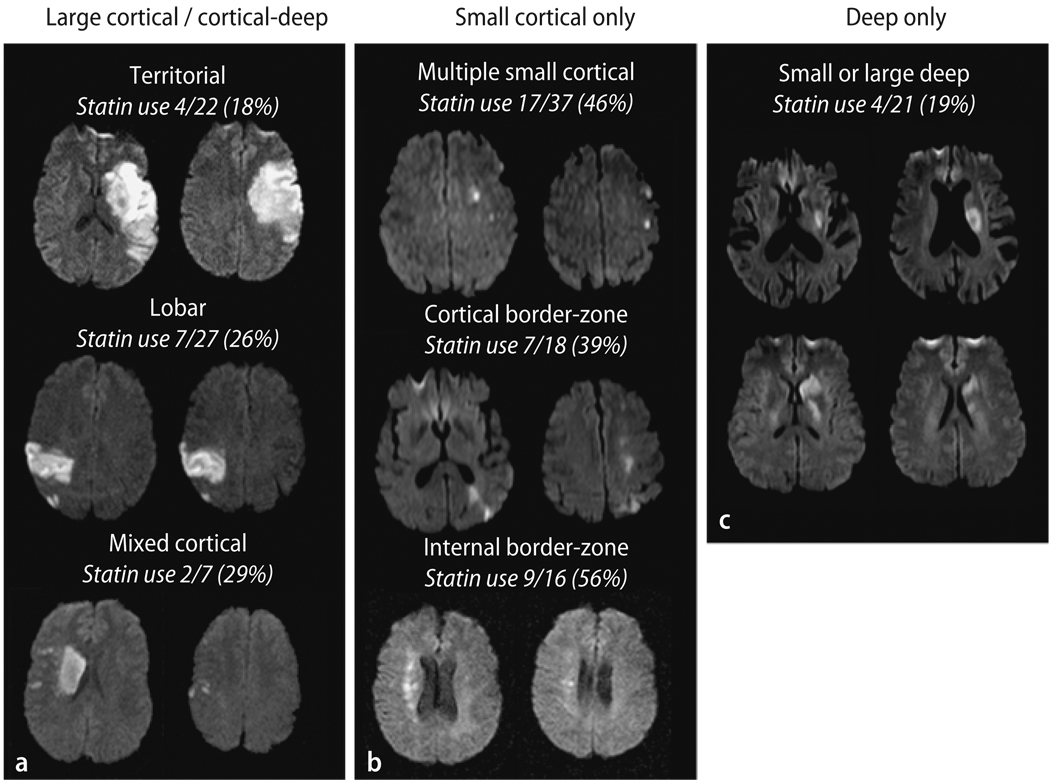
We divided the patients into three infarct topography pattern groups based on the observed DWI patterns (Fig. 1):
Large cortical/cortical-deep patterns included territorial infarcts involving two or three MCA subdivisions (superior, inferior, or deep) [17], cortical infarcts involving one subdivision (lobar type), and cortico-striatocapsular infarcts (mixed cortical-deep type), that is large striatocapsular lesions with concomitant small DWI lesions outside the striatocapsular area. These infarct patterns have been postulated to reflect artery to artery emboli caused by the rupture of unstable plaque.
Small cortical only pattern was infarcts with small single or multifocal ischemic lesions of < 1 cm in diameter on DWI. This category includes multiple small cortical infarcts suggesting microembolism [20] and multiple border-zone infarcts (either superficial or deep), suggesting hypoperfusion, microembolism, or both, as previously reported [40].
The deep only pattern was defined as deep infarcts restricted to the striatocapsular area without any cortical DWI lesions.
Fig. 1.
DWI lesion patterns
Two readers blinded to the clinical data analyzed the DWI data; inter-observer agreement was 97.3 % for the interpretation of DWI lesion pattern (large cortical/cortical-deep vs. small cortical only vs. deep only). A third reader’s opinion was obtained in cases of disagreement.
Statistical analysis
All potential predictors were entered into a logistic regression model with DWI pattern as the dependent variable and the potential risk factor as independent variable. Potential factors considered for inclusion in the model were (a) demographic and medical history variables: age, hypertension, diabetes, current smoking, previous stroke/TIA, coronary heart disease, and peripheral arterial disease, (b) blood work ups: fasting serum levels of glucose, lipid panels, and peripheral WBC count, (c) the site of atherosclerotic vascular lesions, and (d) taking statin and antithrombotic agents at the time of onset of index stroke.
Results
General characteristics
Among 915 patients admitted for acute ischemic stroke during the study period, 344 patients were cardioembolic stroke, 241 LAA, 152 small vessel disease, 96 other cause, and 82 undetermined (including 55 patients with cryptogenic stroke). Among the 241 (26.3 %) patients with LAA, 148 patients with acute infarcts within the MCA territory on DWI were included in this study. Reasons for exclusion of the remainder were acute ischemic lesions outside the MCA distribution – 79, DWI contraindicated or not done – 8, and DWI performed after active recanalization – 6.
The average age of the 148 study patients was 68.0 years (range 30 to 101) and 53.4 % were male. DWI was performed within 24 hours of last known well time in > 75 % of the LAA patients. MRAs and transthoracic echocardiography were performed in all LAA patients and transesophageal echocardiography in 52 (35 %).
Among the LAA patients, identified sites of atherosclerosis were: within the intracranial arterial system in 65 (43.9 %), extracranial carotid artery in 63 (42.6 %), and ascending or transverse aorta in 20 (13.5 %). Intracranial arterial system lesion sites included 13 intracranial ICA, 4 combined intracranial ICA and M1 or M2, 33 proximal M1, 11 distal M1, and 4 M2 occlusive lesions.
Large cortical/cortical-deep pattern DWI infarct patterns were observed in 56 patients (territorial – 22, lobar – 27, and mixed cortical-deep – 7). Small cortical only pattern were observed in 71 patients (multiple small cortical – 37, cortical border-zone – 18, and internal border-zone – 16), and deep infarct only pattern in 21 patients. Patients’ clinical characteristics according to the DWI pattern are shown in Table 1. Ethnicity and site of atherosclerosis were not different between the groups. The small cortical pattern group differed from the other groups in exhibiting older age, higher prevalence of hypertension and dyslipidemia, lower prevalence of the metabolic syndrome (P < 0.05 in all cases). The large cortical/cortical-deep group differed from the other groups in exhibiting higher WBC count (P = 0.011). Other risk factors and laboratory results were not different between the groups.
Table 1.
Demographic and clinical characteristics of 148 patients
| Large cortical/cortical-deep (N = 56) |
Small cortical only (N = 71) |
Deep only (N = 21) |
|
|---|---|---|---|
| Ethnicity | |||
| Caucasian | 37 (66.1 %) | 45 (63.7 %) | 14 (66.7 %) |
| African-American | 7 (12.5 %) | 9 (12.7 %) | 3 (14.3 %) |
| Asian-American | 5 (8.9 %) | 12 (16.9 %) | 3 (14.3 %) |
| Hispanics | 7 (12.5 %) | 5 (7.0 %) | 1 (4.8 %) |
| Site of atherosclerosis | |||
| Ascending/transverse aorta | 7 (12.5 %) | 12 (16.9 %) | 1 (4.8 %) |
| Carotid bifurcation | 27 (48.2 %) | 30 (42.3 %) | 6 (28.6 %) |
| Intracranial | 22 (39.3 %) | 29 (40.8 %) | 14 (66.7 %) |
| Vascular risk factors | |||
| Age | 63.3 ± 14.3 | 72.2 ± 11.51 | 66.4 ± 16.2 |
| Hypertension | 37 (66.1 %) | 58 (81.7 %)2 | 11 (52.4 %) |
| Diabetes | 12 (21.4 %) | 17 (23.9 %) | 5 (23.8 %) |
| Dyslipidemia | 19 (35.2 %) | 40 (56.3 %)2 | 6 (28.6 %) |
| Cigarette smoking | 13 (23.2 %) | 11 (15.5 %) | 5 (23.8 %) |
| Metabolic syndrome | 16 (51.6 %) | 11 (25.0 %)1 | 7 (50.0 %) |
| Previous stroke/TIA | 12 (21.4 %) | 25 (35.2 %) | 6 (28.6 %) |
| Coronary heart disease | 12 (21.4 %) | 17 (23.9 %) | 2 (9.5 %) |
| Peripheral arterial disease | 4 (7.4 %) | 1 (1.4 %) | 1 (5.0 %) |
| Laboratory results | |||
| Glucose | 136.8 ± 52.5 | 123.1 ± 42.8 | 132.8 ± 93.0 |
| Total cholesterol | 182.2 ± 49.7 | 164.0 ± 39.81 | 189.9 ± 54.4 |
| Triglyceride | 135.9 ± 88.3 | 129.7 ± 84.4 | 136.4 ± 85.8 |
| HDL-cholesterol | 47.1 ± 24.0 | 45.5 ± 12.3 | 47.1 ± 12.7 |
| LDL-cholesterol | 110.9 ± 39.9 | 93.8 ± 33.32 | 116.7 ± 44.2 |
| WBC, 109 cells/L | 9.6 ± 2.8 | 9.0 ± 3.5 | 7.2 ± 2.73 |
| C-reactive protein | |||
| Q1–2 (< 0.6 mg/dl) | 13 (39.4 %) | 24 (55.8 %) | 9 (50.0 %) |
| Q3 (0.6–1.84 mg/dl) | 7 (21.2 %) | 11 (25.6 %) | 5 (27.8 %) |
| Q4 (> 1.84 mg/dl) | 13 (39.4 %) | 8 (18.6 %) | 4 (22.2 %) |
| Homocysteine, µM/L | 8.4 ± 3.6 | 9.6 ± 3.6 | 8.4 ± 2.8 |
| NIH stroke score on admission | 14.5 ± 8.44 | 4.5 ± 5.7 | 6.0 ± 4.1 |
| Medications prior to onset | |||
| Statin use | |||
| Non-user | 43 (76.8 %) | 38 (54.5 %)2 | 16 (81.0 %) |
| Low-dose user | 3 (5.4 %) | 9 (12.9 %) | 2 (9.5 %) |
| High-dose user | 10 (17.9 %) | 23 (32.9 %)2 | 2 (9.5 %) |
| Antithrombotic medication | |||
| Antiplatelet agents | 16 (28.6 %) | 29 (40.8 %) | 5 (23.8 %) |
| Anticoagulants | 1 (1.8 %) | 4 (5.6 %) | 2 (9.5 %) |
Small cortical only pattern vs. large cortical/cortical-deep pattern, P < 0.05
Small cortical only pattern vs. other patterns, P < 0.05
Deep only pattern vs. other patterns, P < 0.05
Large cortical/cortical-deep pattern vs. other patterns, P < 0.001
A total 50 patients were taking statins; 26 – atorvastatin (10∼80 mg), 15 – simvastatin (10∼80 mg), 10 – lovastatin (10∼80 mg), 1 – rosuvastatin (20 mg), and 1 – pravastatin (20 mg). In one patient, information on type and dose of statin was unavailable. The prevalence of prior statin use was higher (P = 0.006) and total and LDL-cholesterol levels were lower in patients with small cortical pattern than in other groups (P = 0.027 and 0.013, respectively). Among statin users, 71 % (35 of 49 patients) were taking high-dose statins (≥ 10 mg atorvastatin-equivalent dose). Prior use of high-dose statins was more frequently observed in patients with small cortical pattern (32.9 %) than those with large cortical (17.9 %) or deep pattern (9.5 %).
Multivariable analysis
Table 2 shows the results of the multiple logistic regression model and the OR for each stroke phenotype. Significant factors in the model differed for the three stroke phenotypes. Age of patients and the premorbid use of statin were independently associated with DWI lesion pattern. Older age and premorbid statin use were strongly related to small cortical pattern. On the contrary, younger age and statin non-user were independently associated with large cortical/cortical-deep pattern, and when the large cortical/cortical-deep subdivided into three types, the premorbid statin use was lowest in patients with territorial type than those with lobar and mixed cortical type (Fig. 1 and Supplementary Table 1). In addition, elevated levels of fasting glucose and WBC on admission were independently associated with large cortical/cortical-deep pattern. Conversely, lower WBC counts were strongly related to the deep pattern (P = 0.15), and there were trends that patients with deep only pattern had intracranial atherosclerosis (vs. carotid bifurcation or aortic atheroma), and less likely to have hypertension (P = 0.096 and 0.091, respectively).
Table 2.
Results of logistic regression analysis
| Variables | Odds ratio (95% confidence interval) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Small cortical alone | Large cortical/cortical-deep | Deep | |||||||
| Univariate | Multivariate | P | Univariate | Multivariate | P | Univariate | Multivariate | P | |
| Site of atherosclerosis | |||||||||
| Ascending/transverse aorta | Ref | Ref | Ref | ||||||
| Carotid bifurcation | 0.74 | 0.87 | 0.92 (0.29–2.91) | 0.880 | 4.24 | 2.82 (0.28–28.05) | 0.376 | ||
| Intracranial | 0.91 | 0.35 | 0.37 (0.11–1.26) | 0.113 | 8.48 | 6.31 (0.72–55.17) | 0.096 | ||
| Vascular risk factor | |||||||||
| Age | 1.04 | 1.05 (1.02–1.08) | 0.004 | 0.95 | 0.95 (0.92–0.98) | 0.002 | 1.02 | ||
| Hypertension | 2.44 | 0.65 | 0.28 | 0.40 (0.14–1.16) | 0.091 | ||||
| Diabetes | 1.49 | 0.41 | 2.79 | ||||||
| Cigarette smoking | 0.44 | 2.29 | 1.03 | ||||||
| Previous stroke/TIA | 1.19 | 0.91 | 1.07 | ||||||
| Coronary heart disease | 0.44 | 3.02 | 1.16 | ||||||
| Peripheral arterial disease | 0.17 | 3.73 | 3.30 | ||||||
| Laboratory results | |||||||||
| Glucose | 0.99 | 1.02 | 1.01 (1.00–1.02) | 0.035 | 0.98 | ||||
| Total cholesterol | 1.01 | 0.99 | 1.01 | ||||||
| Triglyceride | 1.00 | 1.00 | 1.00 | ||||||
| HDL-cholesterol | 0.99 | 1.01 | 0.99 | ||||||
| LDL-cholesterol | 0.98 | 1.02 | 1.00 | ||||||
| WBC | 1.01 | 1.12 | 1.13 (1.00–1.27) | 0.056 | 0.76 | 0.76 (0.61–0.95) | 0.015 | ||
| Medications prior to onset | |||||||||
| Statin | 2.18 | 3.05 (1.40–6.65) | 0.005 | 0.47 | 0.40 (0.17–0.99) | 0.047 | 0.42 | ||
| Antithrombotic agents | |||||||||
| Antiplatelet agents | 1.11 | 1.14 | 0.41 | ||||||
| Anticoagulants | 1.26 | 0.26 | 1.51 | ||||||
The metabolic syndrome and CRP levels could not be included in this multivariate testing because of a significant numbers of missing data (36.4% for CRP and 39.8% for the metabolic syndrome).
Risk factor and metabolic profile depending on statin use
Patients with statins more frequently had a history of conventional atherosclerotic risk factors and of previous atherosclerotic events affecting brain or heart. However, no significant differences in laboratory findings at admission were found between premorbid statin users and non-users, including the distribution of CRP levels and metabolic syndrome abnormalities (Supplementary Table 2).
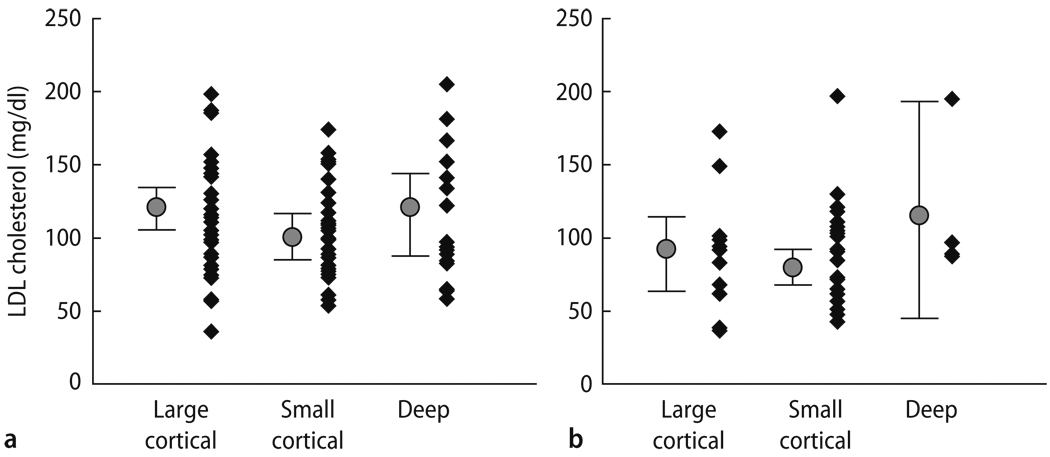
Total and LDL-cholesterol levels were significantly lower in statin users than in non-users (P < 0.001). However, no significant correlation between lipoprotein levels and DWI patterns were determined; the range of LDL-cholesterol levels was not significantly different between the large and small cortical groups in both (a) statin non-user and (b) statin user (Fig. 2).
Fig. 2.
Correlation between LDL-cholesterol levels and DWI topographic patterns, in (a) statin non-user and (b) statin user
Discussion
In this study, we found that several factors were independently associated with distinct stroke phenotypes among patients whose index ischemic stroke was due to LAA. In multivariable analysis, younger age and premorbid statin non-use were associated with large cortical pattern, whereas older age and premorbid statin use were strong independent factor for small cortical pattern. The association of older age with small cortical only pattern, accords with results from another study [32], and suggests that older patients are more vulnerable to border-zone infarctions potentially due to reduced collateral vigor. The association of statin non-use with large, multisectorial infarctions accords with several studies that found an association between premorbid statin use and better clinical stroke outcome [16, 21, 32]. However, previous studies encompassed all major stroke etiologic subtypes including cardioembolic and lacunar stroke, so it remains unclear whether these improved clinical outcomes with prior statin use may be due to facilitated recanalization, collateral perfusion enhancement, or a direct neuroprotective effect [32]. Statins have also been shown to have plaque stabilizing effects; with the occurrence of atherosclerotic plaque regression and reverse remodeling after statin therapy [24]. Our study indicates that statins may modify the pattern of infarct expression even within the subgroup of patients with large artery aortocervicocerebral atherosclerosis. Since we only included patients with LAA stroke in this study, it is conceivable that prestroke statin use may have stabilized symptomatic plaques to a certain extent, so that individuals on statins prior to the stroke were predisposed to smaller emboli (resulting in small cortical patterns) rather than large macroemboli (resulting in large cortical/cortical-deep patterns). Given the fact that statin-users were more likely to have advanced atherosclerosis than non-user (risk factors for atherosclerosis and previous vascular events on brain or heart were more frequently observed in statin users), it is interesting that non-disabling pattern was more prevalent in the former than the latter. Reflecting the fact that patients with deep pattern had lower prevalence of history of dyslipidemia and coronary arterial disease, statin use was low in these patients compared to other groups.
To further explore the potential mechanism behind the effect of statin use and LAA phenotype, we evaluated the difference in vascular risk factor and metabolic profiles between statin users and non-users, since among patients with coronary heart disease, it has been shown that those with severe metabolic and inflammatory abnormalities obtained greater incremental benefit with higher doses of statins [14]. However, the distributions of metabolic abnormalities and CRP levels were not different between statin users and non-users in our study. In addition, the presence of diffusion-perfusion mismatch was evaluated in 89 patients, but it was not different between statin user and non-user (data not shown). Nonetheless, given the limited number of patients checked CRP levels and lack of plaque image, it will be important to consider the impact of statin on the plaque and infarct images in future clinical investigations. It was recently reported that regression of atherosclerotic plaques is strongly associated with LDL-cholesterol reduction [24]. Interestingly, in the present study, although statin user had lower serum LDL levels compared to non-user, no significant correlation was observed between LDL-cholesterol levels and DWI topographic patterns. Statin has cholesterol-independent plaque stabilizing effect as well as LDL-lowering effects [23]. Statin has been shown to inhibit production of matrix metal-loproteinase (MMP) that promotes plaque instability, independent of lipoprotein changes [35]; the inhibitory effect of statin was reversed by the addition of mevalonate (a precursor of isoprenoids), providing non-lipid mechanism-directed antiatherosclerotic potential [10]. Our findings, therefore, support previous reports of cholesterol-independent plaque stabilizing effects in preclinical studies [23], and the use of statin regardless of LDL-cholesterol levels in patients with advanced large vessel artherosclerosis [30].
Our study also found that the probability of having large cortical infarcts increased, whereas the probability of having no cortical infarct (deep only pattern) decreased, with the increase of WBC counts on admission. Several mechanisms could contribute to the association between WBC count and stroke pattern. Leukocytosis has been associated with subclinical atherosclerosis and plaque instability [15, 25]. Greater plaque instability may generate larger artery-to-artery emboli, producing large infarcts, superficial or deep, rather than small cortical infarcts [36]. Conversely, although blood sampling was performed within several hours after onset in most of our cases, it is possible that WBC counts increased after onset of stroke as a result of inflammatory process triggered by the presence of large ischemic lesions [9]. A brain SPECT study using 99mTc-hexamethylpropyle-neamine oxime (HMPAO)-labeled leukocyte revealed that accumulation of leukocyte correlated with the severity of the brain tissue damage [4].
We found an association of elevated glucose levels with large multisectorial MCA infarcts. Hyperglycemia has previously been reported to predict increased stroke mortality independently of age, stroke type, and severity [39]. MR perfusion and spectroscopy studies have also shown that acute hyperglycemia increased brain lactate production and facilitates conversion of hypoperfused at-risk tissue into infarction [28].
We investigated DWI lesion patterns in aortocervicocephalic atherosclerotic stroke because several studies have suggested that infarct patterns are better delineated by DWI than other imaging modalities, correlate with the underlying stroke pathogenic mechanism [7], and are associated with stroke outcome [8]. In addition, infarct pattern on DWI may differ depending on the characteristics (type and nature) of clots [12, 26]. A clinicopathologic analysis of autopsy cases with atheromatous embolism showed that cholesterol crystal emboli lodged in the terminal leptomeningeal arteries, compatible with border-zone infarcts [26]. On the other hand, larger arteries were occluded by atheromatous emboli composed of thromboemboli containing fibrin and platelets as well as cholesterol crystals, which caused arterial territorial infarcts [26]. Patients with non-bacterial thrombotic endocarditis were found to uniformly demonstrate multiple, widely distributed, small and large strokes on DWI, consistent with source vegetations that fragment more easily and cause more widespread infarcts because they lack inflammatory reaction and have little cellular organization [33]. In the present study, we did not use DWI volumetric analysis. The role of DWI volumetric analysis in clinical practice is being challenged [18], and decision-making based on DWI lesion patterns has many advantages, particularly in the acute phase of ischemic stroke, in that it is a simpler and less time-consuming procedure than volumetric analysis.
Strengths of our study included the consecutive, prospective recruitment of a well-phenotyped group of patients. All subjects underwent comprehensive work-ups, including vascular, laboratory and cardiologic studies. However, the results of this study should be interpreted with caution because of the limited sample size and retrospective nature. Larger, multicenter studies will be required to prove or disprove our results. Our study demonstrates that LAA infarcts are a complex entity, not a unitary stroke subtype. LAA patients differ in predisposing risk factors, infarct patterns, and plaque location. Determinants of infarct pattern subtypes may also exist in cardioembolic (such as platelet activation in patients with atrial fibrillation) and small vessel disease strokes and merit further study. Given the diversity of stroke phenotypes and various determinant factors even within the same stroke subtype, not only occurrence of stroke but also the stroke phenotype should be considered as a measurable endpoint in future clinical trials. Statin therapy appears not only to avert the development of atherosclerosis, but also to favorably modify the infarct expression of established large artery disease, promoting small distal rather than large proximal or distal infarcts.
Abbreviations
- MCA
middle cerebral artery
- ICA
internal carotid artery
- DWI
diffusion-weighted imaging
- WBC
white blood cell
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
Footnotes
Electronic supplementary material The online version of this article (DOI 10.1007/s00415-009-0125-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare no conflict of interest.
Contributor Information
Oh Young Bang, Dept. of Neurology, Samsung Medical Center, Seoul, South Korea.
Bruce Ovbiagele, Dept. of Neurology, UCLA Medical Center, Los Angeles, CA, USA.
David S. Liebeskind, Dept. of Neurology, UCLA Medical Center, Los Angeles, CA, USA.
Lucas Restrepo, Dept. of Neurology, UCLA Medical Center, Los Angeles, CA, USA.
Sa Rah Yoon, Dept. of Radiology, UCLA Medical Center, Los Angeles, CA, USA.
Jeffrey L. Saver, UCLA Stroke Center, 710 Westwood Plaza, Los Angeles, CA 90095, SA; Dept. of Neurology, UCLA Medical Center, Los Angeles, CA, USA.
References
- 1.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The French Study of Aortic Plaques in Stroke Group. N Engl J Med. 1996;334:1216–1221. doi: 10.1056/NEJM199605093341902. [DOI] [PubMed] [Google Scholar]
- 2.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 3.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 4.Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorpho-nuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27:1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Amarenco P, Duyckaerts C, Tzourio C, Henin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–225. doi: 10.1056/NEJM199201233260402. [DOI] [PubMed] [Google Scholar]
- 7.Baird AE, Lovblad KO, Schlaug G, Edelman RR, Warach S. Multiple acute stroke syndrome: marker of embolic disease? Neurology. 2000;54:674–678. doi: 10.1212/wnl.54.3.674. [DOI] [PubMed] [Google Scholar]
- 8.Bang OY, Lee PH, Heo KG, Joo US, Yoon SR, Kim SY. Specific DWI lesion patterns predict prognosis after acute ischaemic stroke within the MCA territory. J Neurol Neurosurg Psychiatry. 2005;76:1222–1228. doi: 10.1136/jnnp.2004.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker KJ. Targeting the central nervous system inflammatory response in ischemic stroke. Curr Opin Neurol. 2001;14:349–353. doi: 10.1097/00019052-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Bellosta S, Via D, Canavesi M, Pfister P, Fumagalli R, Paoletti R, Bernini F. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol. 1998;18:1671–1678. doi: 10.1161/01.atv.18.11.1671. [DOI] [PubMed] [Google Scholar]
- 11.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. Jama. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 12.Caplan LR. Brain embolism, revisited. Neurology. 1993;43:1281–1287. doi: 10.1212/wnl.43.7.1281. [DOI] [PubMed] [Google Scholar]
- 13.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 14.Deedwania P, Barter P, Carmena R, Fruchart JC, Grundy SM, Haffner S, Kastelein JJ, LaRosa JC, Schachner H, Shepherd J, Waters DD. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919–928. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 15.Elkind MS, Cheng J, Boden-Albala B, Paik MC, Sacco RL. Elevated white blood cell count and carotid plaque thickness : the northern manhattan stroke study. Stroke. 2001;32:842–849. doi: 10.1161/01.str.32.4.842. [DOI] [PubMed] [Google Scholar]
- 16.Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253–258. doi: 10.1212/01.wnl.0000171746.63844.6a. [DOI] [PubMed] [Google Scholar]
- 17.Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory. Etiology and outcome patterns. Neurology. 1998;50:341–350. doi: 10.1212/wnl.50.2.341. [DOI] [PubMed] [Google Scholar]
- 18.Johnston KC, Wagner DP, Wang XQ, Newman GC, Thijs V, Sen S, Warach S. Validation of an acute ischemic stroke model: does diffusion-weighted imaging lesion volume offer a clinically significant improvement in prediction of outcome? Stroke. 2007;38:1820–1825. doi: 10.1161/STROKEAHA.106.479154. [DOI] [PubMed] [Google Scholar]
- 19.Jones P, Kafonek S, Laurora I, Hun-ninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hyper-cholesterolemia (the CURVES study) Am J Cardiol. 1998;81:582–587. doi: 10.1016/s0002-9149(97)00965-x. [DOI] [PubMed] [Google Scholar]
- 20.Kimura K, Minematsu K, Koga M, Arakawa R, Yasaka M, Yamagami H, Nagatsuka K, Naritomi H, Yamaguchi T. Microembolic signals and diffusion-weighted MR imaging abnormalities in acute ischemic stroke. AJNR Am J Neuroradiol. 2001;22:1037–1042. [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Savitz S, Schlaug G, Caplan L, Selim M. Antiplatelets, ACE inhibitors, and statins combination reduces stroke severity and tissue at risk. Neurology. 2006;66:1153–1158. doi: 10.1212/01.wnl.0000208406.45440.84. discussion 1135. [DOI] [PubMed] [Google Scholar]
- 22.Lee LJ, Kidwell CS, Alger J, Starkman S, Saver JL. Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke. 2000;31:1081–1089. doi: 10.1161/01.str.31.5.1081. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003;91:4B–8B. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- 24.Lima JA, Desai MY, Steen H, Warren WP, Gautam S, Lai S. Statin-induced cholesterol lowering and plaque regression after 6 months of magnetic resonance imaging-monitored therapy. Circulation. 2004;110:2336–2341. doi: 10.1161/01.CIR.0000145170.22652.51. [DOI] [PubMed] [Google Scholar]
- 25.Loimaala A, Rontu R, Vuori I, Mercuri M, Lehtimaki T, Nenonen A, Bond MG. Blood leukocyte count is a risk factor for intima-media thickening and subclinical carotid atherosclerosis in middle-aged men. Atherosclerosis. 2006;188:363–369. doi: 10.1016/j.atherosclerosis.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Masuda J, Yutani C, Ogata J, Kuriyama Y, Yamaguchi T. Atheromatous embolism in the brain: a clinicopathologic analysis of 15 autopsy cases. Neurology. 1994;44:1231–1237. doi: 10.1212/wnl.44.7.1231. [DOI] [PubMed] [Google Scholar]
- 27.Min WK, Park KK, Kim YS, Park HC, Kim JY, Park SP, Suh CK. Athero-thrombotic middle cerebral artery territory infarction: topographic diversity with common occurrence of concomitant small cortical and subcortical infarcts. Stroke. 2000;31:2055–2061. doi: 10.1161/01.str.31.9.2055. [DOI] [PubMed] [Google Scholar]
- 28.Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hypergly-cemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 29.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–e449. [PubMed] [Google Scholar]
- 30.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 31.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 32.Shook SJ, Gupta R, Vora NA, Tievsky AL, Katzan I, Krieger DW. Statin use is independently associated with smaller infarct volume in nonlacunar MCA territory stroke. J Neuroimaging. 2006;16:341–346. doi: 10.1111/j.1552-6569.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- 33.Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke. 2002;33:1267–1273. doi: 10.1161/01.str.0000015029.91577.36. [DOI] [PubMed] [Google Scholar]
- 34.Smaha LA. The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148:S46–S48. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Son JW, Koh KK, Ahn JY, Jin DK, Park GS, Kim DS, Shin EK. Effects of statin on plaque stability and thrombogenicity in hypercholesterolemic patients with coronary artery disease. Int J Cardiol. 2003;88:77–82. doi: 10.1016/s0167-5273(02)00368-6. [DOI] [PubMed] [Google Scholar]
- 36.Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- 37.Szabo K, Kern R, Gass A, Hirsch J, Hennerici M. Acute stroke patterns in patients with internal carotid artery disease: a diffusion-weighted magnetic resonance imaging study. Stroke. 2001;32:1323–1329. doi: 10.1161/01.str.32.6.1323. [DOI] [PubMed] [Google Scholar]
- 38.Van Der Meer IM, De Maat MP, Hak AE, Kiliaan AJ, Del Sol AI, Van Der Kuip DA, Nijhuis RL, Hofman A, Witteman JC. C-reactive protein predicts progression of atherosclerosis measured at various sites in the arterial tree: the Rotterdam Study. Stroke. 2002;33:2750–2755. doi: 10.1161/01.str.0000044168.00485.02. [DOI] [PubMed] [Google Scholar]
- 39.Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. 1997;314:1303–1306. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong SW, Bang OY, Lee PH, Li WY. Internal and cortical borderzone infarction: clinical and diffusion-weighted imaging features. Stroke. 2006;37:841–846. doi: 10.1161/01.STR.0000202590.75972.39. [DOI] [PubMed] [Google Scholar]