Abstract
Primary open angle glaucoma (POAG) is a late onset disease usually accompanied by elevated intraocular pressure (IOP) that results from the failure of the trabecular meshwork (TM) to maintain normal levels of aqueous humor outflow resistance. Cells in the TM are subjected to chronic oxidative stress through reactive oxygen species (ROS) present in the aqueous humor (AH) and generated by normal metabolism. Exposure to ROS is thought to contribute to the morphological and physiological alterations of the outflow pathway in aging and POAG. Our results indicate that chronic exposure of TM cells to oxidative stress causes the accumulation of nondegradable material within the lysosomal compartment leading to diminished lysosomal activity and increased SA-β-Gal expression. Because the lysosomal compartment is responsible for maintaining general cellular turnover, such impaired activity may lead to a progressive cellular decline in the TM cell function and thus contribute to the progression of POAG.
Keywords: glaucoma, trabecular meshwork, oxidative stress, aging, lysosomes
The glaucomas are a group of eye diseases that are characterized by progressive degeneration of the optic nerve and consequent irreversible vision loss. With more than 70 million people affected worldwide, glaucoma constitutes the second leading cause of permanent blindness. Although the precise molecular mechanisms leading to glaucoma are far from being understood, aging and elevated intraocular pressure (IOP) are known to be the main causative factors for developing Primary Open Angle Glaucoma (POAG), the most prevalent form of the disease.1
Physiological levels of IOP are maintained by the trabecular meshwork (TM), a specialized tissue located at the chamber angle of the eye between the cornea and the sclera. It is composed of layers of collagen and elastin trabecular beams lined with endothelial-like cells. Functional failure of the TM tissue impedes aqueous humor outflow leading to elevated IOP. Why the TM tissue fails with aging and in glaucoma still remains unknown.2
Our laboratory has long hypothesized that aging of the TM cells, or more specifically, that the generation of free radicals associated with the normal process of aging can be, at least partially, responsible for the loss in TM tissue functionality in POAG.3-5 This hypothesis is supported by a growing body of evidence that shows increased presence of oxidative markers and decreased antioxidant defense in the glaucomatous TM.6,7 Also supporting a potential role of aging in the pathogenesis of POAG is our own study in which we report an increased number of cells positively stained for senescence-associated β galactosidase activity (SA-β-gal) in the glaucomatous TM tissue compared to age-matched control tissue.8 SA-β-Gal is an activity of the lysosomal β-galactosidase at pH 6 described in senescent cells.9 It is proposed to be a manifestation of residual β-galactosidase activity at suboptimal pH that becomes detectable with the increase in lysosomal content in aging cells.10,11
Cells in the TM are postmitotic differentiated cells characterized by a low renewal rate. A well-known effect of aging in other postmitotic cells, like neurons, cardiomyocytes or retinal pigmented epithelial cells, is the accumulation of intralysosomal oxidized cross-linked proteins and lipids that cannot be degraded by the proteolytic cellular machinery. A number of studies indicate that the presence of such waste material, known as lipofuscin, compromises the overall lysosomal pathway, thereby contributing to the progressive cellular dysfunction associated with aging.12,13
In our most recent publication, we investigated whether the occurrence of SA-β-gal might be somehow correlated with the presence of lipofuscin in aging TM cells.14 To this end, we mimicked in vitro the conditions to which aging TM cells are thought to be exposed in vivo, using the hyperoxic model. This experimental model, previously used by other investigators in similar studies with other cell types, consists of generating chronic oxidative stress by incubating confluent cell monolayers, in this case of TM cells, under a high oxygen concentration atmosphere (40% O2), while keeping the control cultures under physiological oxygen concentration (5% O2). Stressed cultures demonstrate the presence of several oxidative markers, including increased production of intracellular reactive oxygen species (ROS), increased DNA damage and protein carbonyl content, as well as decreased mitochondrial membrane potential. Morphologically, TM cells grown at 40% O2 were larger in size and were characterized by the presence of autofluorescent granules in the perinuclear region. Similarly, the levels of cellular autofluorescence, which are considered a measurement of lipofuscin content, were significantly higher in the stressed cultures.
Accompanying these changes, ultrastructural analysis showed a remarkable accumulation of intracytoplasmic membrane-bound organelles filled with an amorphous electron-dense material in the cells grown at 40% O2. Although we did not confirm the exact nature of these organelles, they shared morphological features resembling lipofuscin-loaded secondary lysosomes or autolysosomes found in other aging tissues. This is in accordance with the also observed dramatic increase in lysosomal mass (3-fold increase), and in autophagic vacuole content in the oxidatively stressed TM cultures. The question was if, as suggested by other investigators, this increase in lysosomal content correlated with detectable SA-β-Gal activity. Our results clearly suggested that both events were interconnected, and that the occurrence of SA-β-Gal activity was not necessarily mediated by the transcriptional upregulation of the β-galactosidase gene. It remains to be determined whether the increased SA-β-gal in the glaucomatous outflow pathway correlates with increased lysosomal mass in vivo. Also, following the same line of thinking, it would be very interesting to investigate whether other lysosomal hydrolases show abnormal activity at higher pH in aging cells.
A striking finding in our study was that oxidatively stressed TM cells did not display increased lysosomal proteolytic activity, as quantified using the fluorogenic substrate z-FR-AMC. This result was surprising since we indeed detected elevated cathepsin protein levels in the cells grown at 40% O2. Altogether, these data indicate that TM cells quickly respond to the accumulation of oxidized cross-linked material by activating the autophagy/lysosomal degradative pathway, with the consequent synthesis of new lysosomes and lysosomal enzymes. However, by reasons that are still unknown, the lysosomal proteolytic activity is compromised under oxidative stress conditions. One limitation in our study was the use of an omnicathepsin substrate and a pan-cathepsin antibody that recognizes the N terminus, which did not allow us to monitor either the activity or the processing of the different cathepsins. Ongoing experiments using more specific substrates for different cathepsins seem to indicate that not all lysosomal proteases are equally influenced by oxidative stress. Moreover, preliminary data suggest that 40% O2 might affect the proteolytic processing of some of the cathepsins in TM cells, which could lead to a defective activation of the lysosomal enzymes. We are in the process of confirming and investigating the causes of these exciting results.
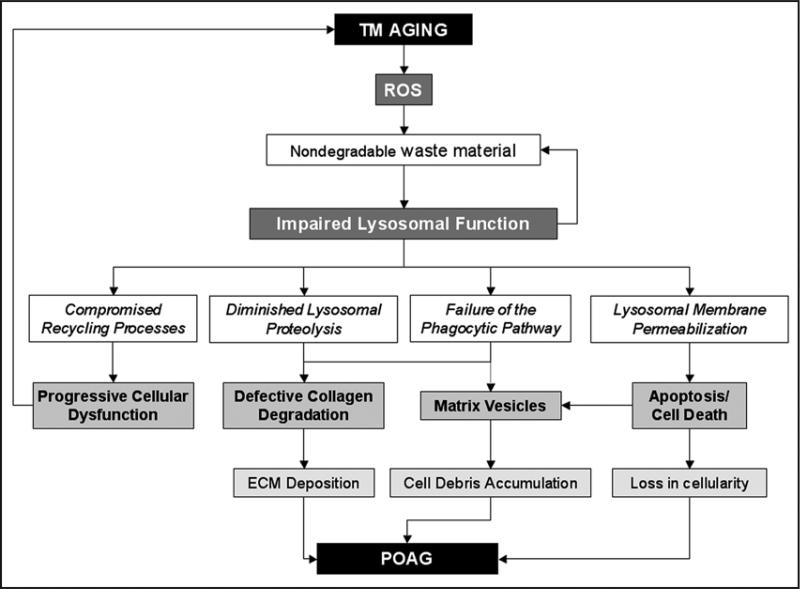
Our model for how this diminished degradative capacity within lipofuscin-loaded lysosomes in aging TM cells might affect TM tissue functionality and contribute to the pathogenesis of glaucoma is summarized in Figure 1. As proposed in the “garbage catastrophe theory of aging,”15,16 the first obvious consequence of lysosomal dysfunction is the failure of the recycling cellular processes with the consequent accumulation of biological garbage, including worn-out organelles, which further compromise the overall cell and TM tissue homeostasis.
Figure 1.
Proposed Model of the Potential Implications of Lysosomal Dysfunction in the Pathogenesis of POAG. The accumulation of lipofuscin-loaded lysosomes with diminished degradative capacity within aging TM cells might: (1) cause the accumulation of worn-out organelles and thus contribute to the progressive cellular dysfunction and tissue homeostasis associated with the aging process; (2) participate in collagen deposition due to inefficient intracellular and extracellular collagen degradation by lysosomal proteases; (3) impair the phagocytic TM cellular capacity and promote the accumulation of cellular debris and components of the ECM, in particular, collagen; and (4) induce lysosomal permeabilization with consequent TM cell death, which could explain the decreased cellularity, as well as the presence of extracellular lysosomal-related organelles in the glaucomatous TM.
A second less obvious effect of the accumulation of nondegradable material within the lysosomal acidic compartment might be its interference with other endolysosomal functions. Trabecular meshwork cells are known to be actively phagocytic; they are capable of ingesting endogenous and exogenous material, thus keeping the trabecular outflow channels free of potentially obstructive debris.17 Although not extensively investigated, a number of studies suggest a potential negative effect of oxidative stress in phagocytosis in retinal pigmented epithelial cells, macrophages, and kupffer cells.18-20 Also, oxidative stress and cytoplasmic saturation with indigestible material impairs phagocytosis of apoptotic cells in atherosclerotic plaques.21
Reduced phagocytic capacity together with impaired lysosomal proteolytic activity can additionally have an impact on physiological collagen turnover in the outflow pathway. Collagens of most connective tissues are subject to continuous remodeling and turnover, a phenomenon that occurs under both physiological and pathological conditions. There are two important pathways for collagen degradation: the extracellular pathway, which involves the action of both matrix metalloproteinases and secreted lysosomal enzymes (in particular, cathepsin K), and the intracellular route, mediated by phagocytosis, which involves lysosomal degradation by cysteine proteinases.22 Defects in these routes might explain the collagen lattice deposition observed in the basement membrane of the trabecular lamellae of the outflow pathway with age and in POAG.23
Also related to this subject is the study published by Rohen that demonstrated the presence of extracellular matrix vesicles (MV) in the glaucomatous outflow pathway.24 Two types of MV were observed: type I MV are small membrane-bound vesicles that contain electron dense material and are positively stained for acid phosphatase, suggesting a lysosomal origin. They may also appear as empty vesicles, sometimes still containing electron dense material at the inner aspect of their membranes. Type II MV vary largely in size and form. They are relatively large and round or oval, with content structurally similar to the cytoplasm of TM cells. The nature, function, and physiological significance of the MV in the outflow pathway remain completely unknown. Lipofuscin-loaded lysosomes are more sensitive to oxidative stress, jeopardizing lysosomal stability and causing cell death due to the release of lysosomal contents. In this scenario, it is very tempting to speculate that such matrix vesicles could derive from degeneration of lipofuscin-loaded cells due to lysosomal permeabilization, and possibly, defective phagocytic capacity in aging TM cells.
Speculations aside, several lines of evidence, in addition to increased SA-β-Gal activity, support a potential lysosomal dysfunction in the pathogenesis of glaucoma, including the cytoplasmic accumulation of pigment granules, lipid droplets and autophagic vacuoles, as well as the presence of membrane-limited vesicles filled with granular material in glaucomatous TM cells.25,26 It is also important to mention that the development of glaucoma is associated with several mucopolysaccharidoses, a group of congenital lysosomal storage diseases.27-30 Although this type of glaucoma belongs to the so-called secondary open angle glaucoma, it highlights the crucial role of the lysosomal system in proper outflow pathway functionality.
Acknowledgements
NEI EY01894, NEI EY016228, NEI EY05722, R21 EY019137, and Research to Prevent Blindness.
Footnotes
Addendum to: Liton PB, Lin Y, Luna C, Li G, Gonzalez P, Epstein DL. Cultured porcine trabecular meshwork cells display impaired lysosomal function when subjected to chronic oxidative stress. Invest Ophthalmol Vis Sci 2008; 49:3961−9; PMID: 18469195; DOI: 10.1167/iovs.08-1915.
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24:612–37. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Caballero M, Liton PB, Challa P, Epstein DL, Gonzalez P. Effects of donor age on protea-some activity and senescence in trabecular meshwork cells. Biochem Biophys Res Commun. 2004 doi: 10.1016/j.bbrc.2004.08.195. In press. [DOI] [PubMed] [Google Scholar]
- 4.Caballero M, Liton PB, Epstein DL, Gonzalez P. Proteasome inhibition by chronic oxidative stress in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;308:346–52. doi: 10.1016/s0006-291x(03)01385-8. [DOI] [PubMed] [Google Scholar]
- 5.De La Paz MA, Epstein DL. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalmol Vis Sci. 1996;37:1849–53. [PubMed] [Google Scholar]
- 6.Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84:389–99. doi: 10.1016/j.exer.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Zanon-Moreno V, Marco-Ventura P, Lleo-Perez A, Pons-Vazquez S, Garcia-Medina JJ, Vinuesa-Silva I, Moreno-Nadal MA, Pinazo-Duran MD. Oxidative stress in primary open-angle glaucoma. J Glaucoma. 2008;17:263–8. doi: 10.1097/IJG.0b013e31815c3a7f. [DOI] [PubMed] [Google Scholar]
- 8.Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745–8. doi: 10.1016/j.exger.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113:3613–22. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 11.Gerland L-M, Peyrol S, Lallemand C, Branche R, Magaud J-P, Ffrench M. Association of increased autophagic inclusions labeled for b-galactosidase with fibroblastic aging. Exp Gerontol. 2003;38:887–95. doi: 10.1016/s0531-5565(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 12.Terman A, Gustafsson B, Brunk UT. Autophagy, organelles and ageing. J Pathol. 2007;211:134–43. doi: 10.1002/path.2094. [DOI] [PubMed] [Google Scholar]
- 13.Terman A, Brunk UT. Oxidative stress, accumulation of biological ‘garbage’, and aging. Antioxid Redox Signal. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- 14.Liton PB, Lin Y, Luna C, Li G, Gonzalez P, Epstein DL. Cultured porcine trabecular mesh-work cells display altered lysosomal function when subjected to chronic oxidative stress. Invest Ophthalmol Vis Sci. 2008;49:3961–9. doi: 10.1167/iovs.08-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheldrake AR. The ageing, growth and death of cells. Nature. 1974;250:381–5. [Google Scholar]
- 16.Terman A. Garbage catastrophe theory of aging: imperfect removal of oxidative damage? Redox Rep. 2001;6:15–26. doi: 10.1179/135100001101535996. [DOI] [PubMed] [Google Scholar]
- 17.Chisholm IA, Grierson I. Particulate phagocytosis by trabecular meshwork endothelium. Can J Ophthalmol. 1977;12:293–9. [PubMed] [Google Scholar]
- 18.Izgut-Uysal VN, Tan R, Bulbul M, Derin N. Effect of stress-induced lipid peroxidation on functions of rat peritoneal macrophages. Cell Biol Int. 2004;28:517–21. doi: 10.1016/j.cellbi.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. Faseb J. 2004;18:562–4. doi: 10.1096/fj.03-0289fje. [DOI] [PubMed] [Google Scholar]
- 20.Videla LA, Tapia G, Fernandez V. Influence of aging on Kupffer cell respiratory activity in relation to particle phagocytosis and oxidative stress parameters in mouse liver. Redox Rep. 2001;6:155–9. doi: 10.1179/135100001101536265. [DOI] [PubMed] [Google Scholar]
- 21.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–61. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 22.Abraham LC, Dice JF, Lee K, Kaplan DL. Phagocytosis and remodeling of collagen matrices. Experimental Cell Res. 2007;313:1045–55. doi: 10.1016/j.yexcr.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutjen-Drecoll E, Rittig M, Rauterberg J, Jander R, Mollenhauer J. Immunomicroscopical study of type VI collagen in the trabecular meshwork of normal and glaucomatous eyes. Exp Eye Res. 1989;48:139–47. doi: 10.1016/0014-4835(89)90027-4. [DOI] [PubMed] [Google Scholar]
- 24.Rohen JW. Presence of matrix vesicles in the trabecular meshwork of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 1982;218:171–6. doi: 10.1007/BF02150090. [DOI] [PubMed] [Google Scholar]
- 25.Lutjen-Drecoll E, Rohen JW. Morphology of aqueous outflow pathways in normal and glaucomatous eyes. In: Ritch R, editor. The Glaucomas. Mosby Publishing; St. Louis, MO: 1996. pp. 89–123. [Google Scholar]
- 26.Tripathi R, Tripathi B. Functional anatomy of the anterior chamber angle. In: Jakobiec FJ, editor. Ocular Anatomy, Embryology and Teratology. Harper and Row Publishing; Philadelphia, PA: 1982. pp. 197–248. [Google Scholar]
- 27.Cahane M, Treister G, Abraham FA, Melamed S. Glaucoma in siblings with Morquio syndrome. Br J Ophthalmol. 1990;74:382–3. doi: 10.1136/bjo.74.6.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantor LB, Disseler JA, Wilson FM., 2nd Glaucoma in the Maroteaux-Lamy syndrome. Am J Ophthalmol. 1989;108:426–30. doi: 10.1016/s0002-9394(14)73311-2. [DOI] [PubMed] [Google Scholar]
- 29.Mullaney P, Awad AH, Millar L. Glaucoma in mucopolysaccharidosis 1-H/S. J Pediatr Ophthalmol Strabismus. 1996;33:127–31. doi: 10.3928/0191-3913-19960301-13. [DOI] [PubMed] [Google Scholar]
- 30.Nowaczyk MJ, Clarke JT, Morin JD. Glaucoma as an early complication of Hurler's disease. Arch Dis Child. 1988;63:1091–3. doi: 10.1136/adc.63.9.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]