Abstract
Introduction
Little is known about cross-national comparisons of the persistence of antihypertensive medication treatment, trends in persistence, and factors associated with persistence. The aim of this study was to describe and compare patterns of use of antihypertensive drugs in a population of elderly patients in the United States (Pennsylvania), Canada (British Columbia) and the Netherlands.
Methods
A retrospective cohort study of Medicare enrollees in a state pharmacy assistance programme in Pennsylvania (USA), residents from British Columbia (Canada) and residents from the Netherlands registered in the PHARMO database was conducted. Each population included patients 65 years and older who were initiated on blood pressure-lowering treatment between 1 January 1998 and 31 December 2003 and who had continuous follow-up for at least 365 days. In these populations, the proportion of patients with at least 180 consecutive days without medication available (non-persistence) were identified as were predictors of non-persistence using Cox proportional hazards.
Results
A total of 9664 Medicare enrollees (USA), 25 377 residents from British Columbia and 24 603 residents from the Netherlands were evaluated. During the first year after the initiation of treatment, the percentage of patients with at least 180 days without medication was 23.3% in Pennsylvania, 23.4% in British Columbia and 24.0% in the Netherlands. After 6 years, these percentages increased to 41.1, 36.3 and 38.2%, respectively. Factors associated with non-persistence were different between the three countries.
Conclusion
Despite differences in factors associated with persistence, non-persistence patterns are strikingly similar in all three populations. This suggests that the problem of non-persistence transcends international boundaries.
Keywords: adherence, antihypertensive drugs, discontinuation, hypertension, persistence
Introduction
Hypertension is the most common risk factor for cardiovascular morbidity and mortality [1] and is an important cause of disability-adjusted life-years [1]. To reduce this burden, the elucidation of effective treatment and the early identification of patients with hypertension has been stressed [2,3]. Nevertheless, only 30% of patients with hypertension reach their target blood pressure, whereas the remainder is uncontrolled [4]. Among patients who do not reach the target blood pressure, half of these failures can be attributed to suboptimal use or non-use [5,6]. Patients may fail to adhere to prescribed antihypertensive therapy for numerous reasons, such as the absence of symptoms associated with the condition, medication side effects, complexity of the dosing schedule or medication cost [7].
Efforts to quantify antihypertensive medication non-persistence are needed, especially when considering the current and future burden of this chronic disease [8]. A better understanding of factors associated with non-adherence may provide targets to intervene and improve medication persistence. Population-based studies may offer details about persistence rates and predictors to help identify patients with an increased risk of non-persistence. These studies can be used to assess the breadth of the non-persistence problem, and to explore whether residents of some countries are more adherent to antihypertensive medications. It seems plausible that different healthcare systems, different cultural attitudes about healthcare and different drug coverage policies could influence medication-taking behavior. Although cross-national multicenter randomized trials have been used in the field of hypertension to test effectiveness, [9–12] cross-national comparisons of drug utilization are scarce [13]. The aim of this study was to perform a cross-national population-based study on rates and predictors of non-persistence with antihypertensive drugs in the United States, Canada and the Netherlands.
Methods
Sources of data
United States
The US population was drawn from the Pharmaceutical Assistance Contract for the Elderly (PACE) programme in Pennsylvania. The PACE programme is the largest state prescription benefits programme for the elderly in the United States, and offers generous coverage to low-income elderly residents of the state. Full pharmacy and healthcare claims information was evaluated for this study, which included demographic characteristics and data for all filled prescriptions (including type of medication, quantity dispensed, and days supply). Drug names are coded according to the National Drug Code classification system [4]. Claims for services in hospitals and offices are coded according to International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and Diagnostic Related Groupings [14]. Medicare data have been demonstrated to be a reliable source of drug exposure [1,15,16]. On the basis of unique patient identifiers, data on the drug use of PACE enrollees were linked to Medicare data on hospitalizations and outpatient professional services and procedures.
The PACE programme has no deductibles and no maximum annual benefit [17]. There is a modest co-payment of US$6 for each generic prescription and US$9 for each branded prescription. The income ceiling for PACE eligibility is US$14 000 if single and US$17 200 for a couple. These benefits and eligibility requirements for enrollment result in essentially no out-of-pocket (i.e. out-of-system) medication costs. The maximum amount of days reimbursed without exemptions was 30 days.
Canada
The Canadian population was drawn from administrative files from the British Columbia Pharmacare Program [18–20]. The Ministry of Health collects data on all healthcare utilization claims of all registered residents of British Columbia. All residents of British Columbia who are 65 years or older are eligible for publicly funded healthcare, including pharmaceutical benefits. Information on drug use (including type of drug, quantity dispensed, days supplied), was entered and collected by pharmacies through a province-wide network called Pharmanet [18,20]. Drug use was linked to healthcare claims based on unique patient identifiers. This data source has been demonstrated to be a reliable and complete source of healthcare use [21]. Drug names are coded according to the Drug Information Number/Product Identification Number classification system [20]. Claims for services in hospitals and offices are coded according to ICD-9-CM and the Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedures [22].
From 1 January 1997 reference pricing was introduced in British Columbia [23,24]. Reference priced drugs include angiotensin-converting enzyme (ACE) inhibitors and calcium antagonists. Within each therapeutic class (e.g. ACE inhibitors) only one (often the least expensive) agent was fully covered. Patients who filled prescriptions for more expensive agents paid the difference in price out of pocket. From 1 January 2002, patients with an annual income of less than Can$16 000 were charged a Can$10 co-payment for each dispensing whereas patients with a greater annual income were charged Can$25 co-payment. In May 2003, the co-payment was replaced with a 25% co-insurance plus an income-based deductible policy. Linking deductible cost-sharing levels to income was intended to prevent low-income patients from underutilizing essential drugs [25]. The maximum amount of days reimbursed without exemptions was 90 or 100 days.
The Netherlands
Data for the Dutch population were obtained from the PHARMO record linkage system [26,27]. The PHARMO record linkage system includes drug dispensing records from community pharmacies and hospital discharge records of all 2 000 000 community dwelling inhabitants of 50 medium-sized areas in the Netherlands. Clustering of all pharmacies within each city results in drug dispensing histories that contain virtually all prescriptions dispensed to a particular patient. Data include demographic characteristics and data for all filled prescriptions (including type of medication, quantity dispensed, and days supplied). Drug names are coded according to the Anatomical Therapeutical Chemical classification system [3]. The hospital records include detailed information concerning the primary and secondary diagnoses, procedures and dates of hospital admission and discharge, coded according to ICD-9-CM. Outpatient procedures and diagnoses were not available for the Netherlands. Co-morbidities in the Dutch population were therefore based on proxy medications.
In the Netherlands, all prescription drugs of interest for this study were fully covered by insurance companies (funded by monthly premiums and income tax-derived government funding) during the study period and no other forms of co-payment or dispensing fees were charged at pharmacies [28]. Most privately insured patients had a small non-income-based deductible for all healthcare costs (approximately €50) throughout the study period. The maximum amount of days reimbursed without exemptions was 90 days.
Patients
For each of the three countries, we selected a new cohort of users of antihypertensive drugs and who had continuous follow-up for at least 365 days. For the Netherlands 2.0% of the patients were excluded because of limited follow-up, for British Columbia and Pennsylvania, these patients were not present in the initial database. New use was defined as having no antihypertensive drug fills in the 365 days before the first prescription of any antihypertensive drug. The date of the first prescription was defined as the index date. At the index date, all patients were 65 years or older. For the vast majority of patients enrolled in the PACE programme, this means that they were at least 66 years of age when eligible for our analysis. Antihypertensive drugs were classified according to their mechanism of action [3]: thiazide diuretics and chlorothalidone, selective beta-blockers, dihydropyridine calcium antagonists, ACE inhibitors, angiotensin receptor blockers (ARB) or miscellaneous antihypertensive drugs (including alpha-blockers and centrally acting antihypertensives, such as methyldopa). The simultaneous initiation of more than one antihypertensive drug, in a single pill combination or as two separate tablets, was defined as combination therapy. For the United States and Canada, an additional physician visit with a hypertension-related diagnosis in the 6 months up to and including the index date was required. Although important, information on the type and severity of the blood pressure elevation was not available. In addition, a claim or dispensing for a non-antihypertensive drug in at least three consecutive 6-month intervals before the index date was required to ascertain eligibility and new use. To ascertain ongoing eligibility and to avoid differential misclassification with regard to antihypertensive exposure status, the last dispensing of a non-antihypertensive drug was defined as the censor date. Patients were required to have at least one year of follow-up available. Patients who died were censored at 180 days before their date of death. Nursing home patients were excluded because of concerns that nursing home data are less reliable. To protect the confidentiality of all patients, all personal identifiers were removed before analysis.
Definition of persistence
Persistence was assessed by using the information on the dispensing date and prescribed duration (days supplied) of each filled prescription. If a prescription of the same antihypertensive drug was filled before the previous one expired, the amount left over was added to the dispensing date of the next prescription. For patients who used two or more antihypertensive drugs simultaneously, the overlapping amount was not included. Patients who had a consecutive 180-day period after the end date of a given prescription during which they filled no prescriptions for any antihypertensive medication were identified as non-persistent. This arbitrary threshold was chosen for two reasons. First, 180 days without medication between the end date of a prescription and the start of the consecutive prescription would lead to an adherence of at maximum 14% in the United States [30/(30+180)], based on a 30-day prescription) and 33% in the Netherlands and Canada [90/(90+180)], based on a 90-day prescription, which seems specific enough to detect actual discontinuation rather than continuous use with suboptimal adherence. In a previous study we found that extending this a 180-day gap did not categorize substantially more patients as persistent [29]. Second, 180 days without medication leads to significant blood pressure differences in placebo-controlled randomized controlled trials [30–33], although there is also an acute risk associated with discontinuation [34]. We performed sensitivity analyses evaluating non-persistence rates and factors associated with non-persistence while varying the treatment gap to 90 and 270 days. For the entire cohort, short-term (i.e. one year) and long-term (6 year) persistence were assessed.
Definition of predictors of persistence
Our selection of sociodemographic, clinical and treatment-related characteristics was based on previous literature [13,35]. The association of these items with persistence during the first year of treatment was studied. In addition to age and sex, which was available for all three countries, patient ethnicity was identified in the US population. Detailed information on annual income was also available for the US population and was dichotomized at a cut-off value of US$10 000. Categories of annual income for residents of British Columbia were estimated on the basis of the amount of income-dependent subsidy and subdivided into three classes: less than Can$16 000, Can$16 000–22 000, more than Cam$22 000 [36]. For residents of the Netherlands, income was categorized on the basis of the type of insurance (public versus private). The income limit for public insurance for those aged 65 years and older was €20 750 in 2004. Clinical characteristics present in the 12 months preceding the index date were assessed. These included: evidence of stroke, congestive heart failure, atrial fibrillation, peripheral vascular disease and coronary heart disease (CHD). CHD was further categorized into three groups based on previous literature: angina or coronary angiography (group 1), coronary artery bypass graft, percutaneous transluminal angioplasty or chronic CHD (group 2) or acute myocardial infarction (group 3). Patients who met the criteria for more than one group were assigned to the most severe group. Furthermore, evidence of hypercholesterolemia, diabetes, chronic obstructive pulmonary disease, depression, dementia and Parkinson’s disease, and for the US and Canadian population, the Charlson co-morbidity score was assessed [37]. In addition, treatment-related characteristics in the baseline year included the number of prescription medications used, number of physician visits (USA and Canada only) and type of antihypertensive treatment. To test for time trends, patients were categorized according to their year of initiating treatment, with 1998 as the reference year.
Statistical methods
Multivariable Cox regression was used to analyse the association of potential predictors and time to first 180-day episode without antihypertensive medication available. Predictors of suboptimal persistence were considered significant at the P<0.05 level. All statistical procedures were performed using SPSS 12.0 for Windows (SPSS Inc., Chicago, Illinois, USA).
Results
Study population
A total of 9664 patients from the United States, 25 377 patients from Canada and 24 603 patients from the Netherlands met the inclusion criteria of the study. Baseline characteristics of the study population are shown in Table 1. Although age was similarly distributed in the three populations, other demographic and clinical characteristics were not. Patients in Pennsylvania were predominantly women. In general, patients from Pennsylvania had the highest prevalence of cardiovascular co-morbidity, and patients from British Columbia the lowest. A similar pattern was observed for differences in other non-cardiovascular co-morbid conditions. Health services utilization was generally different between the three countries.
Table 1.
Baseline characteristics of the study population
| Pennsylvania (N=9664) |
British Columbia (N=25 377) |
The Netherlands (N=24 603) |
|
|---|---|---|---|
| Variable | Number (%) or mean (SD) | ||
| Demographics | |||
| Age (years) | 77.8 (±6.9) | 75.2 (±7.1) | 78.2 (±5.8) |
| 65–74 | 3270 (33.8) | 12 815 (50.5) | 7735 (31.4) |
| 75–84 | 4663 (48.3) | 9538 (37.6) | 13 123 (53.3) |
| ≥85 | 1731 (17.9) | 3024 (11.9) | 3735 (15.2) |
| Mena | 1564 (16.2) | 11 013 (47.1) | 10 345 (42.0) |
| Incomea | |||
| Low | 3651 (38.8) | 7180 (28.3) | 13 763 (55.9) |
| Medium | – | 2313 (9.1) | – |
| High | 6013 (62.2) | 14 647 (57.7) | 10 840 (44.1) |
| Race | |||
| White | 9203 (95.2) | – | – |
| Black | 340 (3.5) | – | – |
| Other, non-white | 121 (1.3) | – | – |
| Cardiovascular history in baseline year | |||
| CHD | |||
| Angina/angiography | 658 (6.8) | 386 (1.5) | 422 (1.7) |
| PTCA/CABG/chronic heart disease | 905 (9.4) | 665 (2.6) | – |
| Acute myocardial infarction | 467 (4.8) | 718 (2.8) | 2025 (8.2) |
| Stroke | 1798 (18.6) | 1351 (5.3) | 1092 (4.4) |
| Congestive heart failure | 2450 (25.4) | 2473 (9.7) | 989 (4.0) |
| Atrium fibrillation | 968 (10.0) | 390 (1.5) | 1164 (4.7) |
| Peripheral vascular disease | 895 (9.3) | 377 (1.5) | 397 (1.6) |
| Other co-morbid conditions | |||
| Hypercholesterolemia | 2197 (22.7) | 1089 (4.3) | 3285 (13.4) |
| Diabetes | 2966 (30.7) | 3523 (13.9) | 2713 (11.0) |
| COPD | 2966 (30.7) | 4576 (18.0) | 1085 (7.3) |
| Depression | 1198 (12.4) | 1551 (6.1) | 1453 (5.9) |
| Dementia | 748 (7.7) | 336 (1.3) | 58 (0.2) |
| Parkinson’s disease | 246 (2.5) | 201 (0.8) | 195 (0.8) |
| Charlson score | 1.94 (±1.9) | 0.27 (±0.71) | – |
| Health services used in baseline year | |||
| No. of hospitalizations | 0.50 (±0.9) | 0.26 (±0.64) | 0.47 (±1.0) |
| 0 | 6447 (66.7) | 20 602 (81.2) | 18 000 (73.2) |
| 1 | 2003 (20.7) | 3540 (13.9) | 3855 (15.7) |
| ≥2 | 1214 (12.6) | 1235 (4.9) | 2748 (11.2) |
| No. of prescription medications | 6.5 (±4.2) | 5.7 (±3.8) | 6.0 (±4.2) |
| 0–3 | 2607 (27.0) | 8328 (32.8) | 7177 (29.2) |
| 4–6 | 3124 (32.3) | 8623 (34.0) | 8173 (33.2) |
| 7–9 | 2013 (20.8) | 4772 (18.8) | 4933 (20.1) |
| ≥10 | 1920 (19.9) | 3654 (14.4) | 4320 (17.6) |
| No. of physician visits | 8.7 (±6.0) | 17.4 (±13.6) | – |
| 0–5 | 3132 (32.4) | 3149 (12.4) | – |
| 6–10 | 3680 (38.1) | 5732 (22.6) | – |
| 11–15 | 1742 (18.0) | 5089 (20.1) | – |
| ≥16 | 1110 (11.5) | 11 407 (45.0) | – |
| Year of initiation of treatment | |||
| 1998 | 1854 (19.2) | 4444 (17.5) | 3820 (15.5) |
| 1999 | 1711 (17.7) | 4635 (18.3) | 4420 (18.0) |
| 2000 | 1657 (17.1) | 4713 (18.6) | 4325 (17.6) |
| 2001 | 1532 (15.9) | 4455 (17.6) | 4118 (16.7) |
| 2002 | 1526 (15.8) | 3926 (15.5) | 4099 (16.7) |
| 2003 | 1384 (14.3) | 3204 (12.6) | 3819 (15.5) |
| First antihypertensive drug | |||
| Diuretic | 1003 (10.4) | 7439 (29.3) | 5726 (23.3) |
| Beta-blocker | 2713 (28.1) | 4102 (16.2) | 6201 (25.2) |
| Calcium antagonist | 1104 (11.4) | 1700 (6.7) | 1881 (7.6) |
| ACE inhibitor | 2921 (30.2) | 10 049 (39.6) | 6134 (24.9) |
| Angiotensin receptor blocker | 953 (9.9) | 418 (1.6) | 1738 (7.1) |
| Miscellaneous | 267 (2.8) | 383 (1.5) | 240 (1.0) |
| Combination therapy | 703 (7.3) | 1286 (5.1) | 2683 (10.9) |
ACE, Angiotensin-converting enzyme; CABG, coronary artery bypass graft; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; PTCA, percutaneous transluminal angioplasty.
Information on sex (4.9%) and income (4.9%) is missing for part of the British Columbia residents.
Long-term follow-up
In Table 2, the percentage of patients who had a period of at least 180 days without medication available during the course of the follow-up is shown. Of interest is the similarity between the proportions of non-persistent patients across all three countries. During the first year, almost 25% of the patients were non-persistent, with less than a one percentage difference in absolute non-persistence rates across countries. After 6 years, the difference between the population with the highest incidence of non-persistence, Pennsylvania, and the population with the lowest incidence, British Columbia, was less than 5%. The comparison does not materially change if 90 or 270 days without medication were used as a definition of non-persistence.
Table 2.
Percentage of patients who had at least one period of 180 days without blood pressure-lowering medication available
| 1 Year | 2 Years | 3 Years | 4 Years | 5 Years | 6 Years | |
|---|---|---|---|---|---|---|
| Pennsylvania | 23.3 (15.1–32.5) | 30.1 (23.3–41.0) | 34.6 (27.4–46.1) | 37.0 (30.0–49.4) | 39.3 (32.0–52.8) | 41.1 (33.6–55.1) |
| British Columbia | 23.4 (16.4–32.8) | 28.6 (23.0–39.8) | 31.6 (25.7–43.8) | 33.6 (27.6–46.8) | 35.0 (28.9–49.0) | 36.3 (29.1–50.5) |
| The Netherlands | 24.0 (17.0–33.9) | 31.6 (27.0–39.8) | 34.0 (29.8–43.4) | 35.8 (31.3–45.9) | 37.1 (32.4–47.7) | 38.2 (33.82–49.8) |
Percentages is brackets indicate a 270-day and 90-day period of non-use, respectively.
Predictors of non-persistence in the first year
In Table 3, the association between potential predictors and 180 days without medication in the first year after initiation are shown. Older age, male sex and frequent use of prescription medications in the baseline year were associated with non-persistence in the three populations. The previous occurrence of acute myocardial infarction was associated with higher persistence as was hypercholesterolemia. Angina pectoris was not associated with non-persistence in Pennsylvania [hazard ratio (HR) 1.02, 95% confidence interval (CI) 0.89–1.18], was associated with higher persistence in the Netherlands (HR 0.85, 95% CI 0.79–0.91) and with lower persistence in British Columbia (HR 1.20, 95% CI 1.01–1.44). Other demographic and clinical parameters demonstrated different associations between the three countries. In both the Netherlands and British Columbia, a time trend is visible, with patients who were initiated more recently achieving greater medication persistence. A corresponding improvement in persistence rates over time was not seen in the United States. Similarities were seen between the type of initial antihypertensive drug selected and persistence in all three countries. In general, patients treated with newer antihypertensive drugs (ACE inhibitors and ARB) were less likely to have a 180-day medication gap compared with patients treated with diuretics or beta-blockers (with the exception of patients initiated with beta-blockers in Pennsylvania). Using 90 and 270-day gaps as a definition of non-persistence did not qualitatively change the observed associations.
Table 3.
Association between potential predictors and non-persistence
| Pennsylvania (N=9664) | British Columbia (N=25 377) | The Netherlands (N=24 603) | ||||
|---|---|---|---|---|---|---|
| Variable | Adjusted HRa | P value | Adjusted HRa | P value | Adjusted HRa | P value |
| Demographics | ||||||
| Age (years) | ||||||
| 65–74 | 1.00 (reference) | NA | 1.00 (reference) | NA | 1.00 (reference) | NA |
| 75–84 | 1.03 (0.95–1.12) | 0.465 | 1.05 (1.00–1.10) | 0.052 | 1.01 (0.96–1.06) | 0.697 |
| ≥85 | 1.09 (0.98–1.21) | 0.129 | 1.23 (1.15–1.32) | <0.001 | 1.05 (1.00–1.15) | 0.037 |
| Sex | ||||||
| Women | 1.00 (reference) | NA | 1.00 (reference) | NA | 1.00 (reference) | NA |
| Men | 1.21 (1.10–1.33) | <0.001 | 1.13 (1.08–1.19) | <0.001 | 1.05 (1.01–1.09) | 0.025 |
| Income | ||||||
| Low | 1.00 (reference) | NA | 1.00 (reference) | NA | 1.00 (reference) | NA |
| Medium | – | 0.86 (0.79–0.93) | <0.001 | |||
| High | 1.06 (0.98–1.14) | 0.130 | 0.81 (0.77–0.85) | <0.001 | 0.95 (0.90–0.99) | 0.016 |
| Race | ||||||
| White | 1.00 (reference) | NA | – | – | ||
| Black | 1.42 (1.19–1.66) | <0.001 | – | – | ||
| Other, non-white | 1.29 (0.97–1.73) | 0.082 | – | – | ||
| Cardiovascular history in baseline year CHD | ||||||
| CHD | ||||||
| Angina/angiography | 1.02 (0.89–1.18) | 0.774 | 1.20 (1.01–1.44) | 0.038 | 0.85 (0.79–0.91) | <0.001 |
| PTCA/CABG/chronic heart disease | 1.04 (0.91–1.17) | 0.585 | 0.93 (0.80–1.08) | 0.323 | – | – |
| Acute MI | 0.85 (0.70–1.01) | 0.080 | 0.64 (0.54–0.76) | <0.001 | 0.59 (0.53–0.67) | <0.001 |
| Stroke | 0.93 (0.84–1.03) | 0.142 | 0.81 (0.73–0.91) | <0.001 | 1.01 (0.90–1.14) | 0.886 |
| Congestive heart failure | 1.10 (1.01–1.20) | 0.037 | 0.93 (0.86–1.02) | 0.111 | 0.95 (0.83–1.09) | 0.458 |
| Atrium fibrillation | 0.84 (0.74–0.96) | 0.008 | 1.40 (1.13–1.74) | 0.002 | 0.85 (0.76–0.97) | 0.016 |
| Peripheral vascular disease | 1.01 (0.89–1.14) | 0.906 | 1.01 (0.83–1.21) | 0.955 | 0.95 (0.79–1.14) | 0.553 |
| Other co-morbid conditions | ||||||
| Hypercholesterolemia | 0.87 (0.80–0.95) | 0.003 | 0.77 (0.68–0.88) | <0.001 | 0.77 (0.71–0.83) | <0.001 |
| Diabetes | 0.99 (0.91–1.08) | 0.702 | 0.93 (0.86–1.00) | 0.040 | 0.83 (0.76–0.90) | <0.001 |
| COPD | 1.09 (1.00–1.18) | 0.049 | 1.04 (0.98–1.10) | 0.256 | 1.04 (0.96–1.13) | 0.293 |
| Depression | 1.10 (0.99–1.14) | 0.075 | 1.14 (1.05–1.25) | 0.003 | 1.03 (0.94–1.12) | 0.560 |
| Dementia | 1.03 (0.90–1.18) | 0.657 | 0.92 (0.76–1.12) | 0.416 | 1.00 (0.65–1.54) | 0.987 |
| Parkinson’s disease | 1.14 (0.93–1.41) | 0.210 | 1.50 (1.21–1.85) | <0.001 | 0.95 (0.76–1.18) | 0.623 |
| Charlson score | 1.00 (0.97–1.02) | 0.651 | 1.00 (0.97–1.04) | 0.860 | – | |
| Health services used in baseline year | ||||||
| No. of hospitalizations | ||||||
| 0 | 1.00 (reference) | NA | 1.00 (reference) | NA | 1.00 (reference) | NA |
| 1 | 1.03 (0.94–1.14) | 0.532 | 0.95 (0.88–1.02) | 0.148 | 1.03 (0.96–1.12) | 0.383 |
| ≥2 | 1.14 (1.00–1.29) | 0.048 | 0.98 (0.87–1.10) | 0.725 | 1.06 (0.95–1.17) | 0.308 |
| No. of prescription medications | ||||||
| 0–3 | 1.00 (reference) | NA | 1.00 (reference) | NA | 1.00 (reference) | NA |
| 4–6 | 1.01 (0.92–1.11) | 0.857 | 1.01 (0.94–1.06) | 0.944 | 1.05 (0.99–1.11) | 0.091 |
| 7–9 | 1.03 (0.93–1.14) | 0.662 | 1.06 (0.99–1.14) | 0.112 | 1.20 (1.13–1.28) | <0.001 |
| ≥10 | 1.06 (0.94–1.20) | 0.335 | 1.25 (1.15–1.36) | <0.001 | 1.36 (1.27–1.46) | <0.001 |
| No. of outpatient physician visits | ||||||
| 0–5 | 1.00 (reference) | NA | 1.00 (reference) | NA | – | |
| 6–10 | 1.07 (0.98–1.17) | 0.154 | 0.88 (0.81–0.95) | 0.002 | – | |
| 11–15 | 1.15 (1.03–1.28) | 0.014 | 0.94 (0.86–1.02) | 0.136 | – | |
| ≥16 | 1.36 (1.20–1.55) | <0.001 | 0.94 (0.87–1.02) | 0.148 | – | |
| Year of initiation of treatment | ||||||
| 1998 | 1.00 (reference) | NA | 1.00 (reference) | NA | 1.00 (reference) | NA |
| 1999 | 1.05 (0.94–1.18) | 0.376 | 0.84 (0.78–0.90) | <0.001 | 0.94 (0.87–1.01) | 0.080 |
| 2000 | 0.96 (0.86–1.09) | 0.538 | 0.85 (0.79–0.91) | <0.001 | 0.87 (0.81–0.94) | <0.001 |
| 2001 | 1.05 (0.93–1.19) | 0.400 | 0.83 (0.77–0.89) | <0.001 | 0.86 (0.75–0.87) | <0.001 |
| 2002 | 1.02 (0.90–1.15) | 0.810 | 0.77 (0.71–0.82) | <0.001 | 0.75 (0.69–0.81) | <0.001 |
| 2003 | 0.96 (0.85–1.09) | 0.537 | 0.70 (0.65–0.76) | <0.001 | 0.72 (0.66–0.78) | <0.001 |
| First antihypertensive drug | ||||||
| Diuretic | 1.00 (reference) | NA | 1.00 (reference) | NA | 1.00 (reference) | NA |
| Beta-blocker | 0.57 (0.51–0.64) | <0.001 | 1.05 (0.98–1.12) | 0.151 | 0.92 (0.87–0.97) | 0.004 |
| Calcium antagonist | 0.54 (0.47–0.62) | <0.001 | 0.85 (0.78–0.94) | 0.001 | 0.77 (0.71–0.84) | <0.001 |
| ACE inhibitor | 0.48 (0.43–0.54) | <0.001 | 0.68 (0.64–0.72) | <0.001 | 0.62 (0.57–0.67) | <0.001 |
| Angiotensin receptor blocker | 0.50 (0.43–0.58) | <0.001 | 0.55 (0.44–0.69) | <0.001 | 0.48 (0.44–0.53) | <0.001 |
| Miscellaneous | 0.80 (0.65–0.98) | 0.032 | 2.18 (2.00–2.37) | <0.001 | 1.74 (1.49–2.04) | <0.001 |
| Combination therapy | 0.50 (0.42–0.59) | <0.001 | 0.78 (0.64–0.95) | 0.012 | 0.70 (0.56–0.85) | <0.001 |
ACE, Angiotensin-converting enzyme; CABG, coronary artery bypass graft; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; PTCA, percutaneous transluminal angioplasty.
Each variable was adjusted for the other variables in the table.
Choice of the initial antihypertensive drug
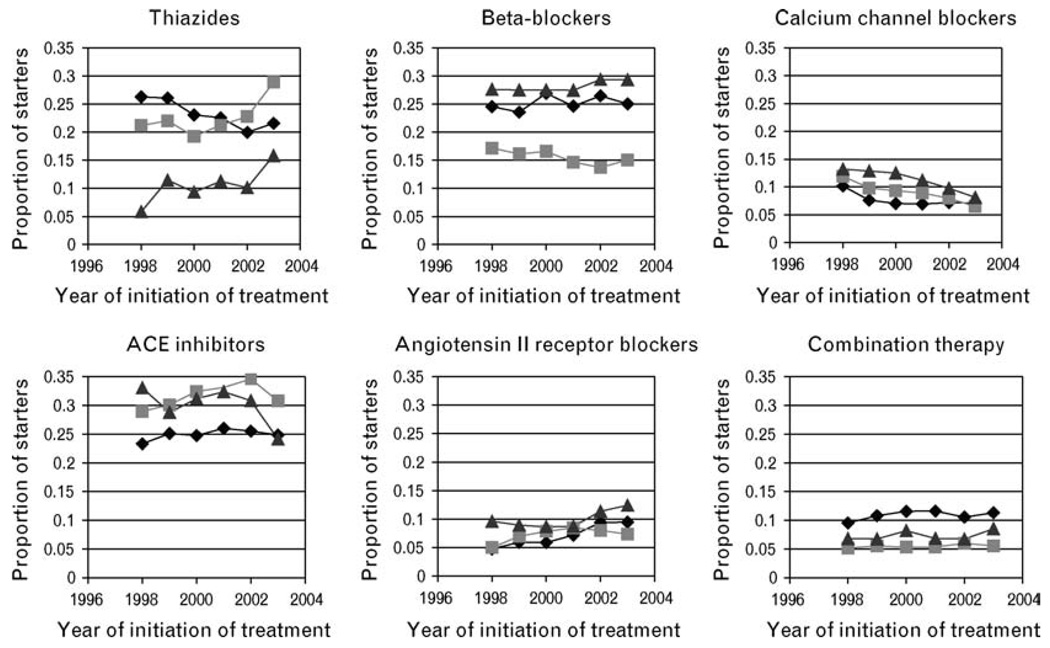
The choice of the initial antihypertensive drug differed remarkably between the three countries (Fig. 1). The initiation of treatment with thiazides was relatively low in Pennsylvania, beta-blockers were relatively less frequently prescribed in British Columbia, and ACE inhibitors were relatively less frequently prescribed in the Netherlands. All three countries demonstrate an increase in thiazide use after the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [38]. The use of calcium antagonists and ARB was generally similar.
Fig. 1.
Patterns of initial drug choice in Pennsylvania, British Columbia and the Netherlands. ACE, Angiotensin-converting enzyme. The Netherlands;
The Netherlands;  British Columbia;
British Columbia;  Pennsylvania.
Pennsylvania.
Discussion
In this study we aimed to assess and compare persistence patterns of new users of antihypertensive drugs in elderly populations from three different countries, the United States, Canada and the Netherlands. We found that, despite evidence of the effectiveness of antihypertensive treatment [3], almost a quarter of patients discontinue their medication for at least 180 consecutive days in the first year of treatment. In the subsequent 5 years, another 13–18% experienced at least one 180-day period without medication. The observed similarities in the patterns of non-persistence in all three countries were remarkable.
To the best of our knowledge, this is the first study comparing persistence with antihypertensive treatment in more than one country. Although many studies are available assessing long-term utilization patterns with antihypertensive drugs, [22,39,40] comparing persistence rates and their determinants is difficult because published studies often use different and incomparable methodologies [29]. In this study we used the same definition of persistence for the three populations. In addition, we used the same predictors of persistence and defined them similarly. Our 180-day gap is of clinical relevance and is conservative because we assumed that the preceding prescription was completely used. Furthermore, the populations were relatively similar with regard to drug coverage and co-payment, two factors that are known to influence adherence [41].
Nevertheless, these results should be interpreted in light of some limitations. First, treatment could be discontinued at the suggestion of the prescriber for clinically justifiable reasons such as intolerable side effects or lack of efficacy. We classified patients using any type of antihypertensive drug as continuous users, however, regardless of whether they switched to another agent (demonstrated to be a common occurrence) [22,42]. Because medication is often discontinued intentionally in the period preceding expected death, patients who died were censored 180-days before their date of death. Second, we could not measure the use of antihypertensive drugs obtained from pharmacies outside the state of Pennsylvania. This probably occurs infrequently because prescriptions required a minimal co-payment in Pennsylvania. All prescribing data in British Columbia and in the PHARMO area in the Netherlands were linked and were captured. We also could not capture the provision of free medication samples, which are offered in the United States and Canada, but not in the Netherlands. In addition, we required the ongoing filling of at least one non-antihypertensive prescription during each of the 180-day intervals after the initiation of treatment. Despite similarities in the methodologies used, there is still a potential for misclassifying patients with regard to the indication in the Netherlands as a result of differences in origins of the data (dispensing and hospital discharge data in the Netherlands compared with claims data including outpatient procedures in Pennsylvania and British Columbia). Although absolute persistence rates are probably marginally influenced by this, it is still a possible source of misclassification that we were unable to capture and quantify.
The implications of our findings are at least fourfold. First, considering that the proportion of patients with a prolonged period without medication available is large, and although the largest decline is observed shortly after the initiation of treatment, the need for adequate follow-up is ongoing. Unfortunately, few effective and feasible strategies to increase adherence for patients with hypertension are currently available [43], which argues for more well-performed research in this field. Second, there are remarkable similarities between the three countries with regard to both absolute persistence rates, after one year and even after 6 years of follow-up, as well as predictors of suboptimal persistence. Although certain predictors of suboptimal persistence appeared to be population specific in at least the direction of the association with inadequate use, other predictors were consistent across all countries studied, such as age and sex, previous myocardial infarction and the number of prescription medications. Third, the magnitude of the association of the vast majority of predictors of suboptimal persistence was small. Although the predictors we studied are similar to information available to clinicians who initiate and treat a patient with hypertension, there are probably other characteristics that are unavailable in prescription databases and therefore in our study, such as an interest in one’s own health and personal circumstances, which better predict the successful uptake of lifelong treatment.
Although this information is probably more difficult to assess considering the limited time available during consultations and the psychological skills required, knowledge about it could substantially increase the overall success of treatment in an individual patient. [7,44] Fourth, one of the strongest associations in magnitude was the type of antihypertensive treatment initiated. As reported by us and other authors in similar settings [22,39,40,45], patients starting with ACE inhibitors and ARB persisted better with treatment than patients starting with diuretics. This finding is in contrast to the evidence of efficacy from a thoughtful network meta-analysis [3] and the largest hypertension trial [38], which favor diuretics. In the ALLHAT trial, there were no material differences in discontinuation rates between patients initiated with diuretics, calcium antagonists and ACE inhibitors. As previously noted by other investigators, the artificial persistence-enhancing circumstances of the hypertension trials may not reflect the situation in daily practice [46], which is in line with patterns observed for lipid-lowering drugs [47]. Although more frequently reported, further research is needed to explore the observed associations between the type of antihypertensive drug and persistence in daily practice.
In the latest report of the World Health Organization on adherence to long-term therapy [44], access to affordable drugs is emphasized as one of the most important barriers to treatment adherence. With regard to hypertension, this is increasingly relevant as the prevalence of hypertension is expected to increase worldwide, with the highest incidence in the developing world where access can be a greater barrier [8]. As a result, antihypertensive drugs have been placed on the World Health Organization essential medicines list [48]. As the results of our study demonstrate, however, even if medication is affordable and available, there is still a substantial proportion of patients who, without proper guidance, will not adhere to therapy and will not optimally benefit from antihypertensive treatment. Further investigations about methods of improving persistence are critical to reducing adverse cardiovascular disease outcomes and improving public health.
Acknowledgments
The study was funded by the Dutch Organization for Scientific Research and by the Dutch Board of Health Insurance Organizations. W.H.S. is supported by a career development award from the National Heart, Lung and Blood Institute (K23 HL 090505-01).
B.L.G.vW. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- ACE-I
ACE-inhibitors
- ATC
Anatomical Therapeutical Chemical
- ARB
angiotensin receptor blockers
- CCP
Canadian Classification of Diagnostic Therapeutic, and Surgical Procedures
- CV
Cardiovascular
- CI
Confidence Interval
- CHF
congestive heart failure
- CABG
coronary artery bypass graft
- CHD
coronary heart disease
- DRGs
Diagnostic Related Groupings
- DHP-CCB
dihydropyridine calcium channel blockers
- DIN/PIN
Drug Information Number/Product Identification Number
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- MI
myocardial infarction
- NDC
National Drug Code
- OR
Odds Ratio
- PTCA
percutaneous transluminal angioplasty
- PVD
peripheral vascular disease
- PACE
Pharmaceutical Assistance Contract for the Elderly
- PHARMO-RLS
PHARMO record linkage system
Footnotes
There are no conflicts of interest.
References
- 1.Ezzati M, Vander Hoorn S, Lawes CMM, Leach R, James WPT, Lopez AD, et al. Rethinking the “diseases of affluence” paradigm: global patterns of nutritional risks in relation to economic development. PLoS Med. 2005;2:e133. doi: 10.1371/journal.pmed.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, He J, Muntner P. Prevalence, awareness, treatment and control of hypertension in North America, North Africa and Asia. J Hum Hypertens. 2004;18:545–551. doi: 10.1038/sj.jhh.1001701. [DOI] [PubMed] [Google Scholar]
- 3.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 4.Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampoli S, Joffres MR, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson J. Noncompliance may cause half of antihypertensive drug “failures”. JAMA. 1999;282:313–314. doi: 10.1001/jama.282.4.313. [DOI] [PubMed] [Google Scholar]
- 6.Burnier M, Schneider MP, Chiolero A, Stubi CL, Brunner HR. Electronic compliance monitoring in resistant hypertension: the basis for rational therapeutic decisions. J Hypertens. 2001;19:335–341. doi: 10.1097/00004872-200102000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 8.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 9.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, et al. for the INVEST Investigators. A calcium antagonist vs a noncalcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 10.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 11.Brown MJ, Palmer CR, Castaigne A, De Leeuw PW, Mancia G, Rosenthal T, Ruilope LM. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) Lancet. 2000;356:366–372. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- 12.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 13.Avorn J, Monette J, Lacour A, Bohn RL, Monane M, Mogun H, LeLorier J. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279:1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 14.Diagnosis Related Groups. Atlanta, GA: National Center for Health Statistics, Centers for Disease Controls and Prevention, Department of Health and Human Services; 2006
- 15.Avorn J, Soumerai SB. Improving drug-therapy decisions through educational outreach. A randomized controlled trial of academically based “detailing”. N Engl J Med. 1983;308:1457–1463. doi: 10.1056/NEJM198306163082406. [DOI] [PubMed] [Google Scholar]
- 16.Bright RA, Avorn J, Everitt DE. Medicaid data as a resource for epidemiologic studies: strengths and limitations. J Clin Epidemiol. 1989;42:937–945. doi: 10.1016/0895-4356(89)90158-3. [DOI] [PubMed] [Google Scholar]
- 17.Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Miller E, Blatman B, Einarson TR. A survey of population-based drug databases in Canada. Can Med Assoc J. 1996;154:1855–1864. [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson GM, Kerluke KJ, Pulcins IR, Hertzman C, Barer ML. Trends and determinants of prescription drug expenditures in the elderly: data from the British Columbia Pharmacare Program. Inquiry. 1993;30:199–207. [PubMed] [Google Scholar]
- 20.Bourgault C, Rainville B, Suissa S. Antihypertensive drug therapy in Saskatchewan: patterns of use and determinants in hypertension. Arch Intern Med. 2001;161:1873–1879. doi: 10.1001/archinte.161.15.1873. [DOI] [PubMed] [Google Scholar]
- 21.Williams J, Young W. Inventory of studies on the accuracy of Canadian health administrative databases: technical report. Toronto: Insitute for Clinical Evaluative Sciences (ICES); 1996. [Google Scholar]
- 22.Bourgault C, Senecal M, Brisson M, Marentette MA, Gregoire JP. Persistence and discontinuation patterns of antihypertensive therapy among newly treated patients: a population-based study. J Hum Hypertens. 2005;19:607–613. doi: 10.1038/sj.jhh.1001873. [DOI] [PubMed] [Google Scholar]
- 23.Schneeweiss S, Soumerai SB, Glynn RJ, Maclure M, Dormuth C, Walker AM. Impact of reference-based pricing for angiotensin-converting enzyme inhibitors on drug utilization. Can Med Assoc J. 2002;166:737–745. [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa B, Hudson R. Reference-based pricing. Can Med Assoc J. 2000;162:12. 14; author reply 14, 18. [PMC free article] [PubMed] [Google Scholar]
- 25.Deaton A. Policy implications of the gradient of health and wealth. Health Aff (Millwood) 2002;21:13–30. doi: 10.1377/hlthaff.21.2.13. [DOI] [PubMed] [Google Scholar]
- 26.Herings RMC. PHARMO: a record linkage system for postmarketing surveillance of prescription drugs in the Netherlands, dissertation. Utrecht, the Netherlands: Department of Pharmaco-Epidemiology, Utrecht University; 1993. [Google Scholar]
- 27.Herings RM, Bakker A, Stricker BH, Nap G. Pharmaco-morbidity linkage: a feasibility study comparing morbidity in two pharmacy based exposure cohorts. J Epidemiol Community Health. 1992;46:136–140. doi: 10.1136/jech.46.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoek JF, Penninx BW, Ligthart GJ, Ribbe MW. Healthcare for older persons, a country profile: the Netherlands. J Am Geriatr Soc. 2000;48:214–217. doi: 10.1111/j.1532-5415.2000.tb03915.x. [DOI] [PubMed] [Google Scholar]
- 29.Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Refill persistence with chronic medication assessed from a pharmacy database was influenced by method of calculation. J Clin Epidemiol. 2006;59:11–17. doi: 10.1016/j.jclinepi.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 31.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 32.Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 33.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 34.Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA. 1990;263:1653–1657. [PubMed] [Google Scholar]
- 35.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 36.Warburton R. Takeup of income-tested healthcare premium subsidies: evidence and remedies for British Columbia. Can Tax J. 2005;53:1–28. [Google Scholar]
- 37.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 38.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 39.Gerth WC. Compliance and persistence with newer antihypertensive agents. Curr Hypertens Rep. 2002;4:424–433. doi: 10.1007/s11906-002-0021-6. [DOI] [PubMed] [Google Scholar]
- 40.Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Rate and determinants of 10-year persistence with antihypertensive drugs. J Hypertens. 2005;23:2101–2107. doi: 10.1097/01.hjh.0000187261.40190.2e. [DOI] [PubMed] [Google Scholar]
- 41.Shrank WH, Hoang T, Ettner SL, Glassman PA, Nair K, DeLapp D, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006;166:332–337. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- 42.Van Wijk BL, Klungel OH, Heerdink ER, De Boer A. The association between compliance with antihypertensive drugs and modification of antihypertensive drug regimen. J Hypertens. 2004;22:1831–1837. doi: 10.1097/00004872-200409000-00029. [DOI] [PubMed] [Google Scholar]
- 43.Fahey T, Schroeder K, Ebrahim S. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD005182.pub2. CD005182. [DOI] [PubMed] [Google Scholar]
- 44.Sabate E. [Accessed: October 2007];Geneva: World Health Organization; Adherence to long-term therapies: evidence for action. 2003 Available at http://www.who.int/chp/knowledge/publications/adherence_report/en/
- 45.Burke TA, Sturkenboom MC, Lu SE, Wentworth CE, Lin Y, Rhoads GG. Discontinuation of antihypertensive drugs among newly diagnosed hypertensive patients in UK general practice. J Hypertens. 2006;24:1193–1200. doi: 10.1097/01.hjh.0000226211.95936.f5. [DOI] [PubMed] [Google Scholar]
- 46.Cardinal H, Monfared AA, Dorais M, LeLorier J. A comparison between persistence to therapy in ALLHAT and in everyday clinical practice: a generalizability issue. Can J Cardiol. 2004;20:417–421. [PubMed] [Google Scholar]
- 47.Andrade SE, Walker AM, Gottlieb LK, Hollenberg NK, Testa MA, Sapiera GM, Platt R. Discontinuation of antihyperlipidemic drugs - do rates reported in clinical trials reflect rates in primary care settings? N Engl J Med. 1995;332:1125–1131. doi: 10.1056/NEJM199504273321703. [DOI] [PubMed] [Google Scholar]
- 48. [Accessed: October 2007];Geneva: World Health Organization; List of Essential Medicines. 2005 Available at http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf.