Abstract
BACKGROUND
The posterior approach for placing continuous interscalene catheters has not been studied in a controlled investigation. In this randomized, triple-masked, placebo-controlled study, we tested the hypothesis that an ultrasound-guided continuous posterior interscalene block provides superior postoperative analgesia compared to a single-injection ropivacaine interscalene block after moderately painful shoulder surgery.
METHODS
Preoperatively, subjects received a stimulating interscalene catheter using an ultrasound-guided, in-plane posterior approach. All subjects received an initial bolus of ropivacaine. Postoperatively, subjects were discharged with oral analgesics and a portable infusion device containing either ropivacaine 0.2% or normal saline programmed to deliver a perineural infusion over 2 days. The primary outcome was average pain on postoperative day (POD) 1 (scale: 0–10). Secondary outcomes included least and worst pain scores, oral opioid requirements, sleep disturbances, patient satisfaction, and incidence of complications.
RESULTS
Of the 32 subjects enrolled, 30 perineural catheters were placed per protocol. Continuous ropivacaine perineural infusion (n = 15) produced a statistically and clinically significant reduction in average pain (median [10th–90th percentile]) on POD 1 compared with saline infusion (n = 15) after initial ropivacaine bolus (0.0 [0.0–5.0] versus 3.0 [0.0–6.0], respectively; P < 0.001). Median oral opioid consumption (oxycodone) was lower in the ropivacaine group than in the placebo group on POD 1 (P = 0.002) and POD 2 (P = 0.002). Subjects who received a ropivacaine infusion suffered fewer sleep disturbances than those in the placebo group (P = 0.005 on POD 0 and 1 nights) and rated their satisfaction with analgesia higher than subjects who received normal saline (P < 0.001).
CONCLUSIONS
Compared to a single-injection interscalene block, a 2-day continuous posterior interscalene block provides greater pain relief, minimizes supplemental opioid requirements, greatly improves sleep quality, and increases patient satisfaction after moderate-to-severe painful outpatient shoulder surgery.
Interscalene perineural catheters placed using the anterolateral approach provide a number of benefits after painful shoulder surgery.1–3 However, using this approach, nonstimulating catheters may have a failure rate of up to 20%,2,4 and stimulating catheters may take too long to insert (sometimes >30 min).3,5–7 Other challenges related to the anterolateral approach include avoiding the external jugular vein, superficial placement potentially leading to catheter dislodgement and inclusion of the catheter site in the sterile surgical field.
A posterior approach to the brachial plexus may avoid these problems.8,9 The continuous cervical paravertebral block introduces the needle between the levator scapulae and trapezius muscles and directs the catheter anteriorly to lay along the brachial plexus.10–12 The needle is often advanced in an anterior direction until it contacts cervical vertebra, then further anterior and laterally until the brachial plexus is located using electrical stimulation.9,11,12 Recently, a case report described a new ultrasound-guided posterior approach using real-time imaging allowing needle/catheter insertion without requiring cervical vertebra contact.13
Unlike the anterolateral approach, the posterior approach has not been examined in a controlled investigation; and thus its benefits and risks remain unknown. Therefore, the objective of this randomized, triple-masked (patients, investigators, and statisticians), placebo-controlled study was to test the hypothesis that, compared to a single-injection ropivacaine interscalene block, an ultrasound-guided continuous posterior interscalene block provides superior postoperative analgesia after moderately painful shoulder surgery. The primary end point was average shoulder pain the day after surgery.
METHODS
The IRB (University of California, San Diego, CA) approved the protocol and oversaw the study through data analysis. Patients offered enrollment included adults (≥18 yr) scheduled for orthopedic surgery of the shoulder with anticipated moderate-to-severe postoperative pain who desired a regional anesthetic for postoperative analgesia. Exclusion criteria included known neuropathy of any etiology in the surgical extremity, history of chronic opioid use or substance abuse, severe pulmonary disease, morbid obesity (Body Mass Index >40 kg/m2), pregnancy, incarceration, and inability to communicate with the investigators and hospital staff.
Catheter Insertion Protocol
After receiving written, informed patient consent, an ultrasound-guided posterior interscalene catheter was placed by an attending anesthesiologist or a regional anesthesia fellow under the direct supervision of an attending physician using a standardized technique.13 All subjects had a peripheral IV catheter inserted and were placed in the lateral decubitus position with the operative shoulder nondependent. Standard noninvasive monitors were applied, and oxygen was administered via facemask. IV midazolam and fentanyl were titrated for patient comfort, while maintaining responsiveness to verbal cues. The area to be subsequently covered by the catheter dressing was shaved when necessary. Cutaneous landmarks were drawn, including the interscalene groove, as well as the intersection of the levator scapulae and trapezius muscles; the area was cleansed with chlorhexidine gluconate and isopropyl alcohol (ChloraPrep One-Step, Medi-Flex Hospital Products, Overland Park, KS); and a clear, sterile, fenestrated drape was applied.
Using a 6–13 MHz linear ultrasound probe (HFL38 MicroMaxx, SonoSite, Bothell, WA) in a sterile probe cover, the brachial plexus was identified between the anterior and middle scalene muscles in transverse cross-sectional view. At the approximate junction of the levator scapulae and trapezius muscles, 1% lidocaine was injected to anesthetize skin and the track into the middle scalene muscle under ultrasound guidance. With the bevel directed caudad and lateral, an 8.89 cm, 17 g, insulated Tuohy-tip needle (Stimucath, Arrow International, Reading, PA) was inserted through the lidocaine skin wheal. The needle was connected to a nerve stimulator (Stimuplex-DIG; B. Braun Medical, Bethlehem, PA) initially set at 1.2 mA, 0.1 ms, and 2 Hz.
Under continuous in-plane ultrasound guidance, the needle was directed anteriorly toward the brachial plexus, passing through the middle scalene muscle. Generalized shoulder contractions were sought at a current <0.5 mA via the needle. A 19 g stimulating catheter was then placed through the length of the needle and the nerve stimulator lead transferred from the needle to the catheter. The catheter was advanced 5 cm beyond the needle tip while maintaining a similar motor response at <0.8 mA of stimulating current via the catheter tip. The needle was withdrawn over the catheter and the catheter stylet removed. Using the 17 g Tuohy needle, the catheter was tunneled subcutaneously in a posterior and medial trajectory below the hairline to avoid the surgical field, and affixed to the contralateral shoulder using liquid adhesive, clear occlusive dressings, and an anchoring device. An initial 40 mL bolus of ropivacaine (0.5%) with epinephrine (2.5 µg/mL to act as an intravascular marker) was administered incrementally under ultrasound visualization to confirm local anesthetic spread around the brachial plexus.
Block Assessment
Within 30 min, the brachial plexus block was evaluated and considered successful when subjects demonstrated decreased ability to abduct the shoulder compared with preinjection abilities and a decrease in perceived sensation to cold of the skin over the deltoid muscle. Subjects with a successful block were retained in the study. Subjects with an unsuccessful block were offered a replacement perineural catheter.
Randomization
Treatment group allocation occurred after confirmation of a successful initial surgical block preoperatively. Subjects were randomized to 1 of 2 postoperative perineural infusions, ropivacaine 0.2% or normal saline (placebo), using a computer-generated table by Investigational Drug Service pharmacists. These same pharmacists prepared the study solution and filled and programmed the portable infusion pumps. Investigators, patients, and all clinical staff were unaware of treatment group assignments.
Intraoperative Management
During the surgical procedure, all subjects received a general anesthetic using inhaled anesthetic and oxygen. Ketorolac 30 mg IM was administered if not contraindicated. IV fentanyl was administered for cardiovascular responsiveness to noxious stimuli at the discretion of the anesthesia provider (and recorded).
Postoperative Management
In the recovery room, a portable infusion pump (Pain Pump II Blockaid, Stryker Instruments, Kalamazoo, MI) filled with 400 mL of study solution (either ropivacaine 0.2% or normal saline) was attached to the perineural catheter and the infusion initiated (8 mL/h basal, 4 mL bolus, 30 min lockout). IV opioids (fentanyl) were administered in the postanesthesia care unit for breakthrough pain as needed, and subjects were discharged home with an oral opioid prescription (oxycodone 5 mg tablets). In the event of breakthrough pain at home, patients were instructed to first use the bolus function of the infusion pump then to take oxycodone, as needed, if the pain had not resolved to a satisfactory level within 20 min. The subject and caretaker were given standard postoperative outpatient instructions as well as verbal and written instructions on the use of the infusion pump and catheter. Telephone and pager numbers for an Acute Pain Service physician available at all times were provided. Subjects were instructed to keep the operative limb well protected in a sling during the infusion period, unless instructed otherwise by their surgeon or physical therapist.
Follow-Up and Outcomes
Subjects were telephoned beginning the afternoon/evening of surgery, and each afternoon thereafter through the third postoperative day (POD) (day after catheter removal). Data collected included least, average, and worst pain scores using a 0–10 Numeric Rating Scale (0 = no pain), oral opioid consumption, sleep quality, and catheter-site discomfort (rated on the Numeric Rating Scale). Gross sensory and motor function were reviewed. If any part of their ipsilateral hand and/or fingers was completely insensate beginning the morning after surgery, the infusion was paused until feeling returned and restarted at half the prior basal rate. “Completely insensate” was defined as being unable to determine with eyes closed that another individual was touching various parts of the hand/fingers. Subjects were also questioned about symptoms of local anesthetic toxicity, dyspnea and the appearance of the catheter site. On POD 1, subjects were asked to rate their satisfaction with their postoperative analgesia on a scale of 0–10 (0 = not satisfied to 10 = very satisfied). In the afternoon of POD 2, or whenever the local anesthetic reservoir was exhausted (whichever came first), subjects' caretakers were instructed to remove the catheter, with a physician in telephone-contact throughout.
Statistical Analysis
Sample size calculations were based upon the hypothesis that a continuous posterior interscalene block decreases postoperative pain. To this end, we selected the average pain on POD 1 as our primary outcome to estimate a probable sample size. We considered a 50% reduction in average pain score to be clinically relevant (e.g., pain score decrease from 4 to 2 on the scale of 0–10). Based on a standard deviation of 1 for the ropivacaine group and 2.5 for the placebo group, and assuming a two-sided type I error protection of 0.05 and a power of 0.80, approximately 15 patients in each group were required (StatMate 2.0, GraphPad Software, San Diego, CA).
Continuous, normally distributed data are reported as mean ± sd. Nonparametric continuous or categorical data are reported as median [10th–90th percentiles] or percents, as appropriate. Comparisons of independent samples were performed using Student's t-test for parametric continuous variables or Mann-Whitney U-test for nonparametric or categorical variables. The χ2 test and Fisher's Exact test were used for differences in proportions, as appropriate. P < 0.05 was considered statistically significant for the primary outcome. Results of comparisons in secondary outcomes should be interpreted as suggestive, requiring confirmation in a future trial before considering them as definitive.
RESULTS
Of 32 patients enrolled, 2 (6%) did not have a catheter placed per protocol. In these subjects, no evoked motor response could be elicited via the insulated needle. Both patients subsequently had the catheter placed using ultrasound guidance alone, and both experienced a dense interscalene block before surgery and profound postoperative analgesia with pain scores of 0–1 on the Numeric Rating Scale of 0–10. However, since the study protocol required an evoked motor response via the placement needle at a current <0.5 mA, these two individuals were not randomized (per protocol).
The remaining 30 subjects were randomized to receive either ropivacaine (n = 15) or normal saline (n = 15) infusion. The demographic, morphometric, and surgical characteristics were similar in each group (Table 1 and Table 2). All patients were pain-free with a dense sensory block (determined grossly) upon discharge from the surgical facility.
Table 1.
Population Data and Procedural Information
| Ropivacaine (n = 15) |
Placebo (n = 15) |
|
|---|---|---|
| Age (yr) | 55 (28–65) | 42 (20–58) |
| Sex (female/male) | 4/11 | 6/9 |
| Height (cm) | 180 (165–185) | 175 (161–183) |
| Weight (kg) | 86 (80–100) | 78 (54–104) |
| Minimum current via needle (mA) |
0.47 (0.38–0.65) | 0.42 (0.30–0.56) |
| Minimum current via catheter (mA) |
0.28 (0.20–0.60) | 0.30 (0.23–0.55) |
| Total fentanyl (µg) | 250 (120–500) | 200 (120–330) |
| Surgical duration (min) |
156 (60–242) | 107 (59–168) |
Values are reported as median (10th–90th percentiles) or number of subjects, as indicated.
There were no statistically significant differences between the two treatment groups.
Table 2.
Primary Surgical Procedures
| Ropivacaine (n = 15) |
Placebo (n = 15) |
|
|---|---|---|
| Open rotator cuff repair | 1 | 1 |
| Open rotator cuff repair and humerus ORIF |
1 | 0 |
| Open rotator cuff repair and SAD |
1 | 0 |
| Open labral repair and debridement |
0 | 2 |
| Arthroscopic labral repair | 3 | 4 |
| Arthroscopic labral repair and SAD |
1 | 1 |
| Arthroscopic rotator cuff repair and SAD |
6 | 4 |
| Arthroscopic SAD and biceps tenodesis |
1 | 0 |
| Arthroscopic SAD | 1 | 3 |
There were no statistically significant differences between the two treatment groups.
ORIF = open reduction, Internal fixation; SAD = subacromial decompression.
Primary Outcome
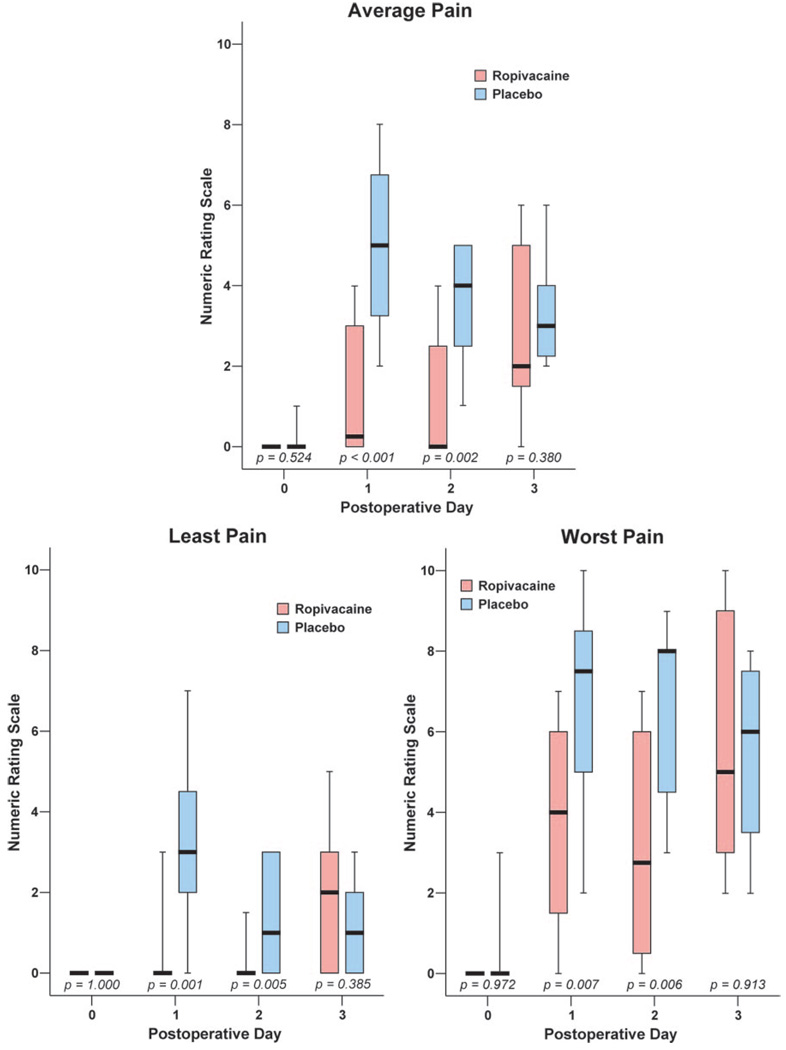
Subjects who received a ropivacaine perineural infusion reported a statistically and clinically significant reduction in average pain (median [10th–90th percentile]) on POD 1 compared to subjects who received a saline infusion after their ropivacaine bolus (0.0 [0.0–5.0] vs 3.0 [0.0–6.0], respectively, P < 0.001; Fig. 1).
Figure 1.
Effects of interscalene perineural infusion (ropivacaine, 0.2%, vs placebo) on average, least, and worst pain after outpatient shoulder surgery (Numeric Rating Scale: 0–10). Perineural infusions were discontinued on postoperative day 2. Postoperative day 2 pain scores represent the period of time from the previous phone contact until discontinuation of the infusion. Data are expressed as median (horizontal line), 25th–75th percentiles (box), and 10th–90th percentiles (whiskers). For tightly clustered data, the median horizontal line also represents 10th, 25th, 75th, and/or and 90th percentile(s), as appropriate.
Secondary Outcomes
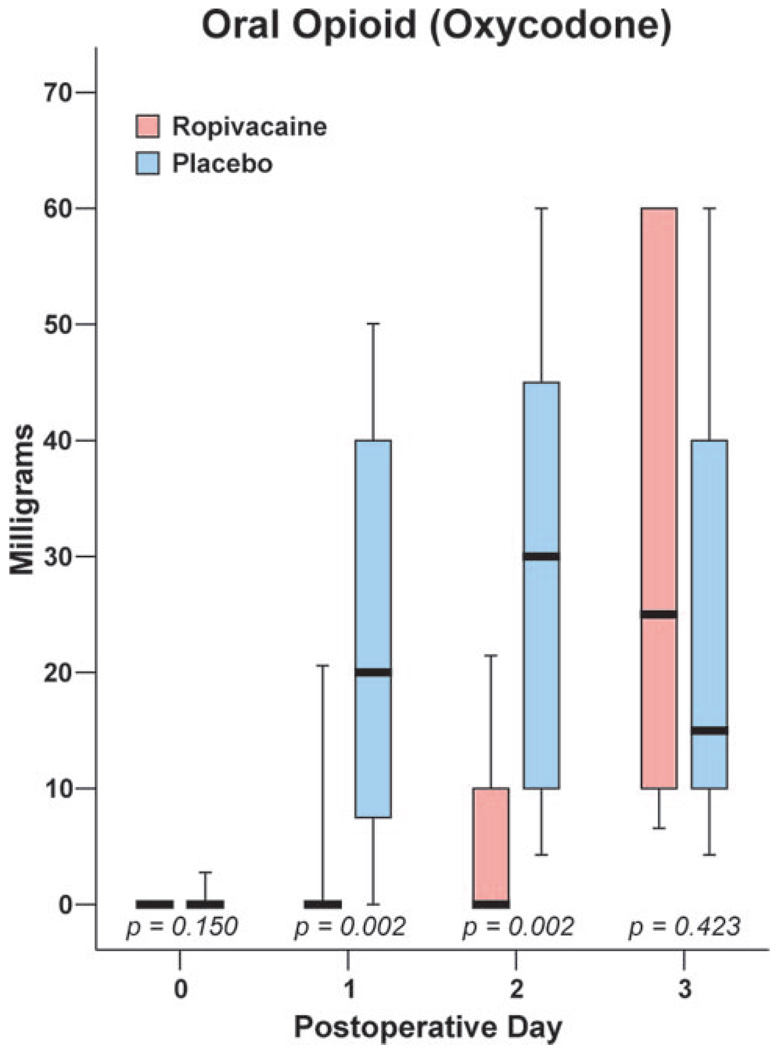
Beginning on POD 1 and for the duration of infusion, subjects in the ropivacaine group reported lower average, least and worst pain scores compared to placebo (Fig. 1). In addition, subjects in the ropivacaine group consumed less oxycodone (Fig. 2) and suffered fewer sleep disturbances (Table 3) compared with the placebo group. During the infusion for the first 2 days after surgery, 67% of subjects receiving ropivacaine required no supplemental opioid compared with 13% of subjects in the placebo group (P = 0.012). While the perineural catheter was in place, subjects in both groups rated catheter-associated neck pain as low (0.0 [0.0–2.6] for ropivacaine, 0.0 [0.0–1.8] for placebo; P = 0.254). One subject receiving a ropivacaine infusion experienced a completely insensate hand on POD 1, which resolved after a decrease in the basal infusion rate from 8 to 4 mL/h. Subjects who received a perineural infusion of ropivacaine also rated their satisfaction with analgesia higher (10.0 [7.0–10.0]) than subjects who received placebo (7.0 [3.2–9.4]; P < 0.001).
Figure 2.
Effects of interscalene perineural infusion (ropivacaine, 0.2%, vs placebo) on oral opioid consumption (oxycodone) after outpatient shoulder surgery. Perineural infusions were discontinued on postoperative day 2. Data are expressed as median (horizontal line), 25th–75th percentiles (box), and 10th–90th percentiles (whiskers). For tightly clustered data, the median horizontal line also represents 10th, 25th, 75th, and/or and 90th percentile(s), as appropriate.
Table 3.
Number of Awakenings Per Night
| Ropivacaine (n = 15) |
Placebo (n = 15) |
P | |
|---|---|---|---|
| POD 0 (with infusion) |
0 (0–0) | 0(0–8) | 0.005 |
| POD 1 (with infusion) |
0 (0–0) | 0 (0–2) | 0.005 |
| POD 2 (without infusion) |
0 (0–2) | 0 (0–2) | 0.669 |
Values are reported as median (10th–90th percentiles).
POD = postoperative day.
Protocol Deviations and Adverse Events
Two subjects receiving a ropivacaine infusion reported mild shortness of breath on POD 1 which resolved with decreasing the basal infusion from 8 to 4 mL/h. One catheter which had not been tunneled per protocol dislodged the afternoon of POD 0 in a patient receiving perineural ropivacaine, and the subject requested study withdrawal at that time. One subject who had received perineural normal saline requested catheter removal on POD 1 with 6 (of 10) catheter-site pain which resolved upon catheter removal. An additional patient receiving perineural normal saline reported mild superficial discomfort at the catheter site with gentle traction during attempted catheter removal. Upon returning to the surgical center, ultrasound imaging suggested traction at the skin insertion site. After sterile preparation and 1 mL of lidocaine 1% infiltration at the site, the catheter was withdrawn without difficulty. The patient was contacted later that day and he had no residual effects from the perineural infusion or catheter extraction.
DISCUSSION
This study provides evidence that a continuous interscalene ropivacaine perineural infusion delivered via a catheter placed by an ultrasound-guided posterior approach provides profound postoperative analgesia, minimizes supplemental opioid requirements, greatly improves sleep quality and increases patient satisfaction after shoulder surgery.
Anterior Versus Posterior Approach
Given the well described anterolateral interscalene approach, with its demonstrated efficacy and relatively high safety profile,14–16 some may question the need for an additional catheter placement technique. While published success rates for anterolateral interscalene nonstimulating catheter techniques approach 100%,1,14,17 others report failure rates as high as 20%.2,4 The ultrasound-guided posterior interscalene catheter technique we describe is, in our experience, easily mastered and has a high success rate (94% using the rigid study protocol of the current study; 100% ultimate successful catheter placement for the subjects of the current study). Other potential advantages of the posterior approach include avoidance of the external jugular vein and displacement of the catheter site further away from the planned sterile surgical field.
Side effects of respiratory distress, presumably from hemidiaphragmatic paralysis,17,18 and excessive numbness of the operative limb2,11 associated with other interscalene block techniques still occur after the ultrasound-guided posterior approach we describe. For the two subjects in our study who complained of shortness of breath and the one subject with an insensate extremity, decreasing the basal rate infusion resolved the symptoms.
Ultrasound Guidance
Using ultrasound-guided needle insertion may provide benefits over conventional techniques. Unlike traditional “blind” paravertebral approaches that describe contacting the cervical vertebra to gauge depth,9,11,12 the ultrasound-guided technique uses sonography to quickly locate the brachial plexus, maintain a needle trajectory consistently lateral to the cervical vertebra, theoretically minimize the number of needle passes (and possibly subsequent neck pain),19–21 and possibly decrease the risk of potential neuraxial complications.22–24 However, these are theoretical advantages of real-time image guidance, and only a large, randomized trial of these two techniques can definitively resolve these safety issues.
Although an ultrasound-guided technique has potential advantages, there are limitations as well. An ultrasound machine is required with its associated training and cost.25–27 In addition, ultrasound guidance is dependent on the practitioner's ability to correctly identify anatomic structures and visualize needle advancement, although the combined use of neurostimulation most likely decreases misidentification errors.
Study Limitations
Due to the limited sample size of this study, the true incidence of complications, especially rare complications, cannot be assessed. Furthermore, since we did not directly compare this new ultrasound-guided technique with the anterolateral or “blind” posterior approach, the relative benefits and risks of each remain unknown and deserve further study.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the invaluable assistance of the entire operating and recovery room staff at the University of California San Diego Hillcrest Hospital (San Diego, CA).
Supported by NIH grant GM077026 (P.I.: Dr. Ilfeld) from the National Institute of General Medical Sciences (Bethesda, MD, USA); and the Department of Anesthesiology, University of CA, San Diego Medical Center (San Diego, CA, USA).
Drs. Mariano, Loland, and Ilfeld as well as Ms. Maldonado have received funding for other research investigations from Arrow International (Reading, PA, USA) and Stryker Instruments (Kalamazoo, MI, USA). These companies did not provide funding for the current study, and had absolutely no input into any aspect of study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation. Drs. Mariano and Loland conduct continuous peripheral nerve block workshops for Stryker Instruments (Kalamazoo, MI, USA). None of the other authors has any personal financial interest in this research.
REFERENCES
- 1.Klein SM, Grant SA, Greengrass RA, Nielsen KC, Speer KP, White W, Warner DS, Steele SM. Interscalene brachial plexus block with a continuous catheter insertion system and a disposable infusion pump. Anesth Analg. 2000;91:1473–1478. doi: 10.1097/00000539-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Ilfeld BM, Morey TE, Wright TW, Chidgey LK, Enneking FK. Continuous interscalene brachial plexus block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 2003;96:1089–1095. doi: 10.1213/01.ANE.0000049824.51036.EF. [DOI] [PubMed] [Google Scholar]
- 3.Ilfeld BM, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Chmielewski TL, Spadoni EH, Wright TW. Ambulatory continuous interscalene nerve blocks decrease the time to discharge readiness after total shoulder arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology. 2006;105:999–1007. doi: 10.1097/00000542-200611000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Grant SA, Nielsen KC, Greengrass RA, Steele SM, Klein SM. Continuous peripheral nerve block for ambulatory surgery. Reg Anesth Pain Med. 2001;26:209–214. doi: 10.1053/rapm.2001.22256. [DOI] [PubMed] [Google Scholar]
- 5.Stevens MF, Werdehausen R, Golla E, Braun S, Hermanns H, Ilg A, Willers R, Lipfert P. Does interscalene catheter placement with stimulating catheters improve postoperative pain or functional outcome after shoulder surgery? A prospective, randomized and double-blinded trial. Anesth Analg. 2007;104:442–447. doi: 10.1213/01.ane.0000253513.15336.25. [DOI] [PubMed] [Google Scholar]
- 6.Ilfeld BM, Morey TE, Wright TW, Chidgey LK, Enneking FK. Interscalene perineural ropivacaine infusion: a comparison of two dosing regimens for postoperative analgesia. Reg Anesth Pain Med. 2004;29:9–16. doi: 10.1016/j.rapm.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Ilfeld BM, Morey TE, Thannikary LJ, Wright TW, Enneking FK. Clonidine added to a continuous interscalene ropivacaine perineural infusion to improve postoperative analgesia: a randomized, double-blind, controlled study. Anesth Analg. 2005;100:1172–1178. doi: 10.1097/01.ASN.0000145571.41015.D5. [DOI] [PubMed] [Google Scholar]
- 8.Vranken JH, van der Vegt MH, Zuurmond WW, Pijl AJ, Dzoljic M. Continuous brachial plexus block at the cervical level using a posterior approach in the management of neuropathic cancer pain. Reg Anesth Pain Med. 2001;26:572–575. doi: 10.1053/rapm.2001.26488. [DOI] [PubMed] [Google Scholar]
- 9.Borene SC, Rosenquist RW, Koorn R, Haider N, Boezaart AP. An indication for continuous cervical paravertebral block (posterior approach to the interscalene space) Anesth Analg. 2003;97:898–900. doi: 10.1213/01.ANE.0000072702.79692.17. [DOI] [PubMed] [Google Scholar]
- 10.Pippa P, Cominelli E, Marinelli C, Aito S. Brachial plexus block using the posterior approach. Eur J Anaesthesiol. 1990;7:411–420. [PubMed] [Google Scholar]
- 11.Boezaart AP, De Beer JF, Nell ML. Early experience with continuous cervical paravertebral block using a stimulating catheter. Reg Anesth Pain Med. 2003;28:406–413. doi: 10.1016/s1098-7339(03)00221-9. [DOI] [PubMed] [Google Scholar]
- 12.Boezaart AP, Koorn R, Rosenquist RW. Paravertebral approach to the brachial plexus: an anatomic improvement in technique. Reg Anesth Pain Med. 2003;28:241–244. doi: 10.1053/rapm.2003.50049. [DOI] [PubMed] [Google Scholar]
- 13.Mariano ER, Loland VJ, Ilfeld BM. Interscalene perineural catheter placement using an ultrasound-guided posterior approach. Reg Anesth Pain Med. 2009;34:60–63. doi: 10.1097/AAP.0b013e3181933af7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capdevila X, Dadure C, Bringuier S, Bernard N, Biboulet P, Gaertner E, Macaire P. Effect of patient-controlled perineural analgesia on rehabilitation and pain after ambulatory orthopedic surgery: a multicenter randomized trial. Anesthesiology. 2006;105:566–573. doi: 10.1097/00000542-200609000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Borgeat A, Dullenkopf A, Ekatodramis G, Nagy L. Evaluation of the lateral modified approach for continuous interscalene block after shoulder surgery. Anesthesiology. 2003;99:436–442. doi: 10.1097/00000542-200308000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Borgeat A. All roads do not lead to Rome. Anesthesiology. 2006;105:1–2. doi: 10.1097/00000542-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Borgeat A, Ekatodramis G, Kalberer F, Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology. 2001;95:875–880. doi: 10.1097/00000542-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Pere P, Pitkanen M, Rosenberg PH, Bjorkenheim JM, Linden H, Salorinne Y, Tuominen M. Effect of continuous interscalene brachial plexus block on diaphragm motion and on ventilatory function. Acta Anaesthesiol Scand. 1992;36:53–57. doi: 10.1111/j.1399-6576.1992.tb03421.x. [DOI] [PubMed] [Google Scholar]
- 19.Frohm RM, Raw RM, Haider N, Boezaart AP. Epidural spread after continuous cervical paravertebral block: a case report. Reg Anesth Pain Med. 2006;31:279–281. doi: 10.1016/j.rapm.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Voermans NC, Crul BJ, de Bondt B, Zwarts MJ, van Englelen BG. Permanent loss of cervical spinal cord function associated with the posterior approach. Anesth Analg. 2006;102:330–331. doi: 10.1213/01.ANE.0000190722.55703.61. [DOI] [PubMed] [Google Scholar]
- 21.Aramideh M, van den Oever HL, Walstra GJ, Dzoljic M. Spinal anesthesia as a complication of brachial plexus block using the posterior approach. Anesth Analg. 2002;94:1338–1339. doi: 10.1097/00000539-200205000-00054. [DOI] [PubMed] [Google Scholar]
- 22.Benumof JL. Permanent loss of cervical spinal cord function associated with interscalene block performed under general anesthesia. Anesthesiology. 2000;93:1541–1544. doi: 10.1097/00000542-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 23.Baker R, Dreyfuss P, Mercer S, Bogduk N. Cervical transforaminal injection of corticosteroids into a radicular artery: a possible mechanism for spinal cord injury. Pain. 2003;103:211–215. doi: 10.1016/s0304-3959(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 24.Brouwers PJ, Kottink EJ, Simon MA, Prevo RL. A cervical anterior spinal artery syndrome after diagnostic blockade of the right C6-nerve root. Pain. 2001;91:397–399. doi: 10.1016/S0304-3959(00)00437-1. [DOI] [PubMed] [Google Scholar]
- 25.Sandhu NS, Sidhu DS, Capan LM. The cost comparison of infraclavicular brachial plexus block by nerve stimulator and ultrasound guidance. Anesth Analg. 2004;98:267–268. doi: 10.1213/01.ANE.0000077685.55641.7C. [DOI] [PubMed] [Google Scholar]
- 26.Tsui B. Ultrasound-guidance and nerve stimulation: implications for the future practice of regional anesthesia. Can J Anaesth. 2007;54:165–170. doi: 10.1007/BF03022635. [DOI] [PubMed] [Google Scholar]
- 27.Sites BD, Gallagher JD, Cravero J, Lundberg J, Blike G. The learning curve associated with a simulated ultrasound-guided interventional task by inexperienced anesthesia residents. Reg Anesth Pain Med. 2004;29:544–548. doi: 10.1016/j.rapm.2004.08.014. [DOI] [PubMed] [Google Scholar]