Abstract
Women who have outlived child-bearing have long been described as post-reproductive. But contributions they make to the survival or fertility of their descendants enhance the reproduction of their genes. Consequently natural selection affects this characteristic stage of human life history. Grandmother effects can be measured in data sets that include births and deaths over several generations, but unmeasured covariates complicate the task. Here we focus on two complications: cohort shifts in mortality and fertility, and maternal age at death. We use the Utah Population Database to show that longevity of grandmothers may be associated with fewer grandchildren, as reported by Madrigal and Melendez-Obando (2008) for a Costa Rican sample, even when grandmother effects are actually positive.
Keywords: Human life history, Historical demography, Human longevity
A grandmother hypothesis to explain the evolution of human life history proposes that distinctive human longevity evolved when ancestral females whose fertility was declining increased their fitness by provisioning grandchildren (Hawkes et al., 1998; O’Connell et al., 1999; Alvarez, 2000; Hawkes, 2003). That hypothesis was prompted in part by ethnographic observations of food gathering by Hadza hunter-gatherers and the weight changes of Hadza children (Hawkes et al., 1997). Children’s weight changes correlated with their mothers’ foraging effort unless their mothers had a nursing infant. Then the older youngsters’ weight gains depended on their grandmothers’ foraging instead. The tradeoffs suggested an evolutionary scenario about adaptive shifts in an ancestral population facing ecological changes that reduced the availability of food young juveniles could handle for themselves. The scenario involves a shift from an ancestral life history like that of chimpanzees and the other living great apes to the greater longevity, later maturity, and shorter birth intervals that characterize humans (Hawkes, 2003, Hawkes et al., 2003; Hawkes and Blurton Jones, 2005; Hawkes, 2006; Robson et al., 2006). The Hadza example suggests that grandmother effects may persist in modern populations, and many – though not all - analyses of ethnographic and historical data sets have found them (Sear and Mace, 2008 for review).
Ethnographic observations, like those of Hadza foragers, have the advantage of tracking the activities and conditions of individuals. But they have the disadvantage of small sample size and short time scales. Historical data sets, by contrast, rarely include direct information on individual time allocation and economic productivity, or individual growth, morbidity, and mortality correlates. But they have the substantial advantages of large sample size and time frames that encompass realized fertility and mortality. (Lahdenperä et al., 2004; Voland et al., 2005; Sear and Mace, 2008).
Recently Madrigal and Melendez-Obando (2008) used a historical Costa Rican data set to evaluate a version of the grandmother hypothesis. They concluded that instead of increased fitness for their descendants, “the maternal grandmother is exerting a negative influence” (208:228). This they interpreted as “strong grounds for questioning the universality of the grandmother hypothesis” (2008:223).
Madrigal and Melendez-Obando consider both a grandmother hypothesis and a mother hypothesis to explain why women usually outlive their fertility. The mother hypothesis proposes a fitness advantage for ending child-bearing early so that maternal effort can be invested in previously born children (Williams, 1957). Like the grandmother hypothesis, this assumes that survival past fertility is not necessarily post-reproductive. In this case the fitness benefit comes from investments that improve the survival of offspring. Madrigal and Melendez-Obando explicitly recognize this to be the predicted benefit to mothers for stopping early. Nevertheless, for both hypotheses their “proxy for fitness is the number of children produced by a woman, not the number who survived…” (p. 224), since they are “simply not in a position to test [effects on offspring survival], and must work with the” data at hand (p 228). They find that a woman’s longevity is positively correlated with her own fertility, but negatively correlated with the fertility of her daughter. They conclude that “the relatively large number of studies on the grandmother hypothesis have overshadowed a simpler explanation for the evolution of postmenopausal longevity: postcycling females achieve higher fertility by supporting their own offspring, not their grandchildren“ (p 228).
Madrigal and Melendez-Obando would not, as they note, be the first to find mothers having no positive effects on their daughters’ fertility. Mothers may even protect their daughters from higher fertility that could impose costs on the welfare of the daughter and her other children (Leonetti et al., 2007, Sear and Mace, 2008). Such tradeoffs between offspring numbers and their survival are a central assumption of the mother hypothesis, as recognized by Madrigal and Melendez-Obando. Those tradeoffs imply negative, not positive effects on fertility (e.g., Blurton Jones, 1986; Borgerhoff Mulder, 2001; Strassman and Gillespie, 2002). That makes positive correlations between a woman’s longevity and her fertility – here called mother effects – initially surprising, and more so combined with negative grandmother effects. The combination prompted us to consider whether the findings might be less a reflection of what Costa Rican mothers and grandmothers were doing than a consequence of the demographic transition in this data set and the death ages of the women sampled.
The Costa Rican “data consist of maternal genealogies started from 152 living subjects… Most genealogies are at least seven generations long…Considering all individuals across all generations and all lineages [there are] …1,172 individuals… Several families extend back to the 1500s” (Madrigal and Melendez-Obando 2008:224). That suggests an average of 7.7 individuals per lineage, but moving back through the generations the lineages intertwine. Since the same women occur in different lineages, Madrigal and Melendez-Obando measured mother effects by randomly selecting a woman from each lineage and correlating the longevity and the fertility of the selected set. They measured grandmother effects by correlating the longevity of each selected woman with the fertility of her linking daughter. Using bootstrap resampling techniques, they generated 2000 samples and reported the 90% confidence intervals from the 2000 correlations. Correlations for a woman’s longevity on her own fertility have a 90% confidence interval of +0.015 to +0.236 around a mean r of +0.1258. Correlations for the grandmother effects have a 90% confidence interval of −0.2 to +0.007 around a mean r of −0.106. As they note these confidence intervals do not overlap.
The Costa Rican data are not fully described, but they clearly extend over four centuries. That time period witnessed a demographic transition from high fertility and high mortality to low fertility and low mortality (Kirk, 1996). Pre-transition longevities would consequently be shorter and fertilities higher, while post-transition lifespans would be longer and fertilities lower. With lineages pooled over this transition, and a woman drawn at random from each pool, a negative relationship between women’s longevity and their daughters’ fertility might well be common. However, the same thing should be true of the relationship between a woman’s longevity and her own fertility. Pre-transition women have shorter lifespans and higher fertilities, post-transition women live longer and bear fewer children. But Madrigal and Melendez-Obando report a positive correlation between a woman’s longevity and her own fertility. One possible source for this contradiction could be the death ages of the women sampled. If women are included when their fertile span was cut short by death during their childbearing years, the very short lifespans and low parities of those women would make correlations between age at death and fertility especially strong - perhaps strong enough to override the demographic transition effects. We hypothesize that 1) negative grandmother effects could be artifacts of pooling over a demographic transition with 2) mother effects strong enough to overwhelm this pooling when samples include women whose fertility was curtailed by their own early death.
MATERIALS AND METHODS
Since the Cost Rican data set is not published, we turned to the Utah Population Data Base (UPDB, Bean et al., 1990) to test these hypotheses. If we found these patterns in the Utah data that also encompass the demographic transition, the plausibility that they characterize the Costa Rican data would be strengthened. For mother effects, correlations between women’s longevity and their own fertility, we used the 101,607 parous women born in 1900 or before with complete information on longevity and fertility. For grandmother effects we used the 51,012 women born before 1870 on whom there are complete fertility data for a randomly selected daughter. This design assumes an average 30 year generation time, with the births of the children and grandchildren distributed across the same years. The large samples make unnecessary the bootstrapping used by Madrigal and Melendez-Obando who were working with very small lineages. Otherwise we calculate correlation coefficients just as they did.
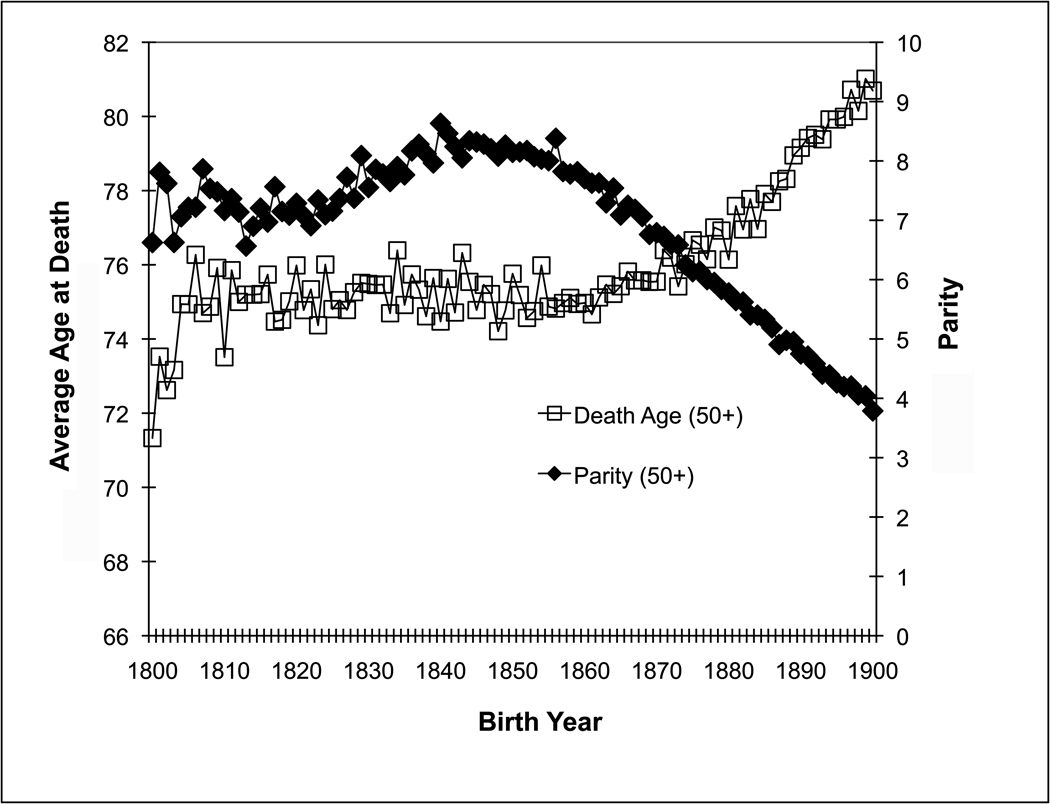
Figure 1 displays the average death age and average parity for Utah women who were born in the 1800s and lived at least to the age of 50 according to their birth year. The demographic transition began with women born in the middle of this century. Average death age for those who lived at least to 50 started to increase from about 75 years in those born in mid- 1800s to pass 80 for those born at the turn of the century. At the same time, average parities of those women decreased from about eight births to less than four.
Figure 1.
Average age at death and average parity for women in the Utah Population Database by birth year across the nineteenth century. Only women who lived at least to 50 years are included.
RESULTS
Table 1 reports the correlation between age at death and parity for the mother sample and age at death and a daughter’s fertility for the grandmothers. Results are given with all women pooled and then with birth year controlled to partial out demographic transition effects. We also distinguish between all parous women and those who survived past 50. The latter restriction excludes women whose early deaths curtailed their fertility and sharply limited any grandmothering.
Table 1.
Correlation coefficients between age at death and own fertility (mothers) and between age at death and a daughter’s fertility (grandmothers) for women in the Utah Population Database who were born in the nineteenth century. The mother sample includes all parous women born before 1900. Column 2 lists sample sizes. Column 3 lists correlations for samples pooled across the demographic transition (Figure 1), with significance levels in column 4. In column 5, the effect of pooling across the demographic transition is removed by controlling the woman’s birth year. Without this control, the shorter lifespans of the higher fertility pre-transition women combined with the longer lifespans of the lower fertility post-transition women have a negative effect on correlations between longevity and fertility. Line 2 removes women who died during the child-bearing years. Their inclusion has a strong effect on associations between death age and fertility because their early deaths impose low parities. The grandmother sample includes all women born before 1870 with complete information about the fertility of a randomly chosen daughter. We use the fertility of a daughter to parallel the Madrigal and Melendez-Obando (2008) measure of grandmother effects as the correlation between a woman’s longevity and the fertility of her linking daughter. Line four removes women who died before reaching their grandmothering years.
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Sample | N | Parity by death age |
p | Parity by death age controlling for birth year |
p | |
| 1 | Mothers | 101,607 | 0.08163 | <0.0001 | 0.12118 | <0.0001 |
| 2 | Mothers who lived >50 |
87,923 | −0.06371 | <0.0001 | −0.01356 | <0.0001 |
| Fertility of a Randomly selected daughter by death age |
Fertility of a Randomly selected daughter by death age controlling for birth year |
|||||
| 3 | Grandmothers | 51,012 | −0.02033 | <0.0001 | 0.00410 | 0.3547 |
| 4 | Grandmothers who lived >50 |
43,698 | −0.01777 | 0.0002 | 0.00825 | 0.0846 |
The first row shows that for the mother sample, there is a postive relationship between age at death and number of children (column 3) as reported by Madrigal and Melendez-Obando for Costa Rica. Continuing across the row, the association is even stronger (column 5) when the demographic transition effects are removed by controlling birth year. That control in column 5 takes account of the cohort differences between lower average lifespan, high average fertility, pre-transition women and longer average lifespan, low fertility post transition women. Line two removes the women whose fertility was interrupted by death during their fertile years. Considering only those who actually outlived their fertility, the association between parity and death age changes sign. Row two, column 3 pools these women across the demographic transition, giving a correlation coefficient that is strongly negative. Removing the demographic transition effects (column 5) leaves a correlation that is still negative but less so because the systematic cohort variation is now removed. Comparison between columns 3 and 5 indicates the strength of the effect of pooling across the demographic transition. Comparison between rows 1 and 2 indicates the large contribution that early adult mortality makes to correlations between longevity and fertility for the mother sample.
The third row (column 3) shows that when grandmother data are pooled across the demographic transition, the correlation between a woman’s longevity and the fertility of a randomly selected daughter is negative. Just as Madrigal and Melendez-Obando reported for their Costa Rican data set, grandmothers’ longevity appears to have a negative effect on the number of grandchildren they leave. But the pooling combines high mortality, high fertility conditions with low mortality low fertility conditions. When this systematic secular variation is removed by controlling grandmother’s birth year (column 5) the negative relationship disappears. Row four shows that for the women who actually reached their grandmothering years, the correlation is still negative, as long as the demographic transition is ignored (column 3). As above, the negative grandmother effect disappears when we control for the systematic variation in longevity and fertility across cohorts that span the demographic transition (column 5).
DISCUSSION
We found correlations in the UPDB like the ones Madrigal and Melendez-Obando report for their historical Costs Rican data set. In the pooled UPDB, women with longer lifespans had daughters with lower fertility, an apparently negative effect of grandmothers’ survival on their number of grandchildren. But this negative relationship resulted from pooling pre-transition lower survival, higher fertility women with post-transition higher survival, lower fertility women. When this secular variation is controlled the negative relationship disappears.
The same pooling across the demographic transition that results in this apparently negative grandmother effect should also affect associations between a woman’s longevity and her own fertility. It does. But those associations can still be positive if women whose fertility was curtailed by death during the childbearing years are included. Those necessarily low parities and young ages at death weight the correlation enough to override the counter effects of pooling pre- and post-transition women. The correlation changes from positive to negative when the mothers who died young are removed. If only women who outlived their fertility are included, then, like the grandmothers the relationship is negative. And like the grandmothers, controlling for birth year to remove the negative effect of pooling across the demographic transition moves both the full and the restricted sample in a positive direction.
Our analyses indicate that measuring grandmother effects in the Utah population requires attention to covariates (see Smith et al., submitted). The same is likely true for the Costa Rican case. Patterns there may be different of course. Madrigal and Melendez-Obando report very well-behaved results from bootstrapping samples of women drawn from sparse lineages extending over major demographic shifts. But the UPDB analyses demonstrate that similar results can be analytical artifacts. We found that 1) negative grandmother effects can result from pooling pre- and post-demographic transition conditions, while 2) associations between a woman’s longevity and her own fertility overwhelm the negative effects of this pooling when women who died during their childbearing years are included. Maybe, as Madrigal and Melendez-Obando conclude, a Costa Rican woman’s “postreproductive longevity is…detrimental to her inclusive fitness” (2008:229). But maybe not. To falsify our hypotheses that their findings arise from the same confounds that allow us to reproduce similar associations in the Utah data, we need to know more about the Costa Rican data set itself and more about the details of their analyses.
ACKNOWLEDGEMENTS
This study was supported in part by the National Institute of Aging by grant AG022095 (The Utah Study of Fertility, Longevity, and Aging). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. We thank the Pedigree and Population Resource (funded by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance and support of the Utah Population Database (UPDB). We also acknowledge Dr. Geraldine P. Mineau and Alison Fraser, MSPH, for their careful management of and assistance with the data used for this study.
Contributor Information
Kristen Hawkes, Department of Anthropology, University of Utah, 270 South 1400 East, Stewart 102, Salt Lake City UT 84112; USA, Ph: 801-581-6117, hawkes@anthro.utah.edu.
Ken R. Smith, Department of Family and Consumer Studies, University of Utah, 225 South 1400 East, Emery 228, and, Huntsman Cancer Institute, University of Utah, 2000 Circle of Hope, Salt Lake City UT 84112; USA
LITERATURE CITED
- Alvarez HP. Grandmother hypothesis and primate life histories. Am J Phys Anthropol. 2000;133:435–450. doi: 10.1002/1096-8644(200011)113:3<435::AID-AJPA11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bean LL, Mineau GP, Anderton DL. Fertility change on the American frontier: adaptation and innovation. Berkeley: University of California Press; 1990. [Google Scholar]
- Blurton Jones N. Bushman birth spacing: A test for optimal interbirth intervals. Ethol Sociobiol. 1986;7:91–105. [Google Scholar]
- Borgerhoff Mulder M. Optimizing offspring: the quantity-quality tradeoff in agropastoral Kipsigis. Evol Hum Behav. 2001;21:391–410. doi: 10.1016/s1090-5138(00)00054-4. [DOI] [PubMed] [Google Scholar]
- Kirk D. Demographic Transition Theory. Pop Stud. 1996;50:361–387. doi: 10.1080/0032472031000149536. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Grandmothers and the evolution of human longevity. Am J Hum Biol. 2003;15:380–400. doi: 10.1002/ajhb.10156. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Slow life histories and human evolution. In: Hawkes K, Paine R, editors. The Evolution of Human Life History. Santa Fe: School of American Research Press; 2006. pp. 95–126. [Google Scholar]
- Hawkes K, Blurton Jones NG. Human age structures, paleodemography, and the grandmother hypothesis. In: Voland E, Chasiotis A, Schiefenhovel W, editors. Grandmotherhood: the evolutionary significance of the second half of female life. New Brunswick: Rutgers University Press; 2005. pp. 118–140. [Google Scholar]
- Hawkes K, O'Connell JF, Blurton Jones NG. Hadza women's time allocation, offspring provisioning and the evolution of long postmenopausal life spans. Curr Anthropol. 1997;38:551–578. [Google Scholar]
- Hawkes K, O'Connell JF, Blurton Jones NG. Human life histories: primate tradeoffs, grandmothering socioecology, and the fossil record. In: Kappeler P, Pereira M, editors. Primate Life Histories & Socioecology. Chicago: University of Chicago Press; 2003. pp. 204–227. [Google Scholar]
- Hawkes K, O’onnell JF, Blurton Jones NG, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. Proc Nat Acad Sci USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D. Demographic transition theory. Pop Stud. 1996;50:361–387. doi: 10.1080/0032472031000149536. [DOI] [PubMed] [Google Scholar]
- Lahdenperä M, Lummaa V, Helle S, Tremblay M, Russell AF. Fitness benefits of prolonged post-reproductive lifespan in women. Nature. 2004;428:178–181. doi: 10.1038/nature02367. [DOI] [PubMed] [Google Scholar]
- Leonetti DL, Nath DC, Hemam NS. In-law conflict: Women's reproductive lives and the roles of their mothers and husbands among the matrilineal Khasi. Curr Anthropol. 2007;48(6):861–890. [Google Scholar]
- Madrigal L, Melendez-Obando M. Grandmothers’ longevity negatively affects daughters’ fertility. Am J Phys Anthropol. 2008;136(2):223–229. doi: 10.1002/ajpa.20798. [DOI] [PubMed] [Google Scholar]
- O'Connell JF, Hawkes K, Blurton Jones NG. Grandmothering and the evolution of Homo erectus. J Hum Evol. 1999;36:461–485. doi: 10.1006/jhev.1998.0285. [DOI] [PubMed] [Google Scholar]
- Robson SL, van Schaik CP, K Hawkes K. The derived features of human life history. In: Hawkes K, Paine R, editors. The Evolution of Human Life History. Santa Fe: School of American Research Press; 2006. pp. 17–44. [Google Scholar]
- Sear R, Mace R. Who keeps children alive? A review of the effects of kin on child survival. Evol Hum Behav. 2008;29:1–18. [Google Scholar]
- Smith KR, Christensen ER, Hawkes K, Rogers AR, Mineau GP. Do Grandmothers Influence the Fertility of their Daughters? Submitted. [Google Scholar]
- Strassmann BI, Gillespie B. Life-history theory, fertility and reproductive success in humans. Proc R. Lond B. 2002;269:553–562. doi: 10.1098/rspb.2001.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voland E, Chasiotis A, Schiefenhovel W. Grandmotherhood: The evolutionary significance of the second half of female life. New Brunswick: Rutgers University Press; 2005. [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]