Abstract
Enhanced stress responsiveness has been implicated as a potential mechanism contributing to the pathophysiology of irritable bowel syndrome (IBS), and should be reflected in altered function of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system. Both of these systems can modulate mucosal immune function. The aims of this study were: (i) to characterize the basal circadian rhythm of adrenocorticotropin hormone (ACTH) and cortisol in IBS vs healthy controls; (ii) to compare stimulated ACTH, cortisol and noradrenaline responses to a pelvic visceral stressor (sigmoidoscopy) in IBS and controls; and (iii) to correlate neuroendocrine responses with colonic mucosal cytokine expression and symptoms in IBS. Two separate studies were conducted in women. In Study 1, basal cortisol levels were analysed in 41 IBS and 25 controls using 24-h collections of plasma ACTH and cortisol (q10 min sampling). In Study 2, 10 IBS patients with diarrhoea (IBS-D) and 10 controls underwent sigmoidoscopy with measurements of stimulated neuroendocrine responses and cytokine mRNA expression in colonic tissue. Basal ACTH levels were significantly blunted (P < 0.05), while basal and stimulated plasma cortisol levels were higher in patients. Basal cortisol levels prior to an experimental visceral stressor positively correlated with anxiety symptoms (P < 0.004), but not IBS symptoms. Irritable bowel syndrome patients with diarrhoea had significantly decreased mRNA expression of mucosal cytokines [interleukin (IL)-2, IL-6] in the sigmoid colon vs controls (P < 0.05). Although dysregulations in stress-responsive systems such as the HPA axis and mucosal immune function are demonstrated in IBS, they do not appear to have a primary role in modulating IBS severity and abdominal pain.
Keywords: adrenocorticotropin hormone, corticotrophin-releasing factor, cortisol, cytokines, hypothalamic-pituitary-adrenal axis, irritable bowel syndrome
INTRODUCTION
In response to a variety of physical and psychological stressors, corticotropin-releasing factor (CRF) released from the paraventricular nucleus (PVN) of the hypothalamus plays a prominent role in orchestrating the behavioural, neuroendocrine and autonomic responses to stress.1,2 The hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) are the two major branches of this central stress response system. Alterations in these two output systems in the form of adrenocorticotropin hormone (ACTH) and cortisol, and catecholamine levels have been reported in several stress-sensitive disorders, including anxiety,3 depression4 and irritable bowel syndrome (IBS).5–8 For example, IBS patients have been shown to have increased basal levels of catecholamines3,7,9,10 or increased sympathetic/vagal tone.6,11,12
The topic of HPA axis alterations in IBS topic has recently received greater attention due to its possible implication in abnormal immune activation in the gut,13,14 and to the intense interest in the development of CRF receptor antagonists as possible IBS treatments.15 There are several ways in which alterations in the HPA axis could be related to recently reported alterations in mucosal immune activation in the colon.13,14,16 For example, increased mucosal immune activation and resulting increases in plasma cytokine levels have been suggested to play a possible role in HPA axis stimulation,5 resulting in a hyperresponsive HPA axis.
A limited number of studies in IBS patients have measured basal10,17 and stimulated HPA axis hormone levels in response to a meal,6 hormone challenge,5,8,17 or mental stress.9,18,19 Two studies reported basal free cortisol levels in IBS patients with conflicting results.10,17 Most, but not all, studies examining stimulated HPA axis hormone levels have demonstrated normal to increased HPA axis responses in IBS compared to controls.5,6,8,9,17–19 It has been suggested that elevated HPA axis responses may be more a reflection of a history of traumatic early life events, rather than a specific marker for a given disorder, e.g. depression, or post-traumatic stress disorder (PTSD).20 If this hypothesis is correct, different prevalence rates of early trauma history in the reported studies could in part explain the disparate results in some of these studies.
In this study, we wanted to test the general hypothesis that female IBS patients exhibit an abnormal profile of the HPA axis under baseline conditions over 24 h, and in response to a visceral stressor. We wanted to explore the following four questions: (i) do IBS patients have altered basal HPA axis regulation compared to healthy controls? (ii) do IBS patients have altered stimulated HPA axis and SNS responses to a visceral stressor? (iii) do gut-related immune markers such as mucosal cytokine expression correlate with plasma levels of cortisol and noradrenaline (NA) and (iv) do clinical factors including IBS symptoms correlate with HPA axis hormone levels?
METHODS
Study 1: Assessment of basal rhythm and pulsatility of ACTH and cortisol
Healthy controls
Twenty-five healthy women without evidence of acute or chronic illness were recruited by newspaper advertisement. None had a history of acute or chronic pain or abdominal symptoms.
IBS patients
Forty-one women with Rome II-positive IBS,21 were recruited from advertisement and the UCLA Functional Bowel Disease Clinic. Inflammatory or other structural intestinal disease was excluded based on clinical and endoscopic evidence. The bowel habit subtype was determined using Rome II subclassification criteria for IBS with diarrhoea (IBS-D) and IBS with constipation (IBS-C).21 Patients who did not meet diagnostic criteria for IBS-D or IBS-C and reported alternating loose/watery stools and hard/lumpy stools were classified as IBS with alternating bowel habits (IBS-A). Seventeen (41%) of the IBS patients met diagnostic criteria for fibromyalgia (FM)22 which is a chronic somatic pain disorder that frequently coexists with IBS.23,24
Other relevant history
Subjects completed a bowel symptom questionnaire25 and the Hospital Anxiety and Depression Scale (HAD)26 and underwent a structured clinical interview diagnostic for DSM-IV (SCID)27 conducted by a licensed therapist (MM). The subject was excluded if they met criteria for an Axis I psychiatric disorder or a positive HAD (anxiety or depression score > 11) profile. A history of childhood (≤13 years of age) and adult (>13 years of age) trauma, and early adverse life events was assessed using a 25-item Trauma Questionnaire28 and interview. Gynaecological history including menstrual cycle information and use of oral contraceptive agents (OCP) or hormone replacement therapy (HRT) was obtained. None of the study subjects were on centrally acting drugs (anxiolytics, narcotics, antidepressants), corticosteroid agents or other medications which could affect neuroendocrine function in the past 2 months. None of the patients had a history of smoking or alcohol abuse. Fifteen additional subjects were excluded at time of screening because they failed to meet inclusion and exclusion criteria.
Study protocol
Fasting subjects were admitted to the UCLA General Clinical Research Center (GCRC). Standard meals and only non-calorie and non-caffeinated drinks were given. Subjects remained supine in bed at a 30° angle except to use the bathroom. Lights were turned off at 22:00 and subjects were encouraged to go to sleep. This time corresponded to within 1 h of the usual sleep time for most participants. Beginning at 09:00, blood samples for ACTH and cortisol were collected every 10 min over a 24-h period. All studies were conducted in a room specially designed for serial blood collections and allowed subjects not to be disturbed during the studies. Specific activities of the patient (bathroom, meals, sleep, etc.) were recorded.
Using 20-cm validated verbal descriptor anchored visual analogue scales,29 IBS patients rated the intensity and unpleasantness of their abdominal pain for both the past 3 months (chronic) and the past 24 h (current). The patients were also asked to ‘rate the overall severity of your IBS symptoms during the past week on a scale from 0 (no symptoms) to 20 (the most intense symptoms imaginable)’. The Stress Symptom Scale (SSR) measured acute changes in mood (i.e. stress, anger, anxiety, fatigue, arousal and attentiveness) was administered.9
The UCLA GCRC Core Laboratory measured plasma ACTH levels using immunoassays, and plasma cortisol levels using liquid chromatography in tandem mass spectrometry assays (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA).
Data preprocessing and analysis of the circadian rhythm of HPA axis hormones
Time-series containing large sections of missing data-points were considered ‘unusable’ (control = 2, IBS = 8 for both ACTH and cortisol) and were not used in subsequent analyses. One of the control’s ACTH time-series was discarded as an outlier. The few missing measurements in the usable data were filled using either duplicate measurements when available or using a linear interpolation between the nearest available measures. Missing initial or terminal values of the time-series were replaced with the nearest available measure. The average of the duplicate measurements was used for analysis.
Summary statistics such as averages, minimums, maximums and area under the curve (AUC) using the trapezoidal rule were computed for each time-series. Area under the curve was also computed for six 4-h intervals of the hormonal circadian rhythms (10:00–14:00, 14:00–18:00, etc.) and for 12-h blocks (daytime: 10:00–22:00 and nighttime: 22:00–10:00).
Circadian rhythms of the time-series were analysed using the standard cosinor method which involves a non-linear least squares cosine curve fit to all time-points in the series.30 The mesor (mean), acrophase (time of peak) and amplitude were obtained from the fitted cosines for each of the time-series.
Measurement of pulsatility in the time-series of HPA axis hormones
Hormonal pulse activity was measured using an algorithm called Smoothing Bases Plus Pulses (SBPP). This method,31 which has been used in the analysis of ACTH and cortisol hormonal concentrations,32,33 models the pulsatile component of a series with a slowly changing baseline or circadian rhythm. The fitted values include the estimated baseline, average amplitude, half-life and number of pulses (i.e. secretory episodes with a probability greater than a user-specified threshold; it was set at 95%). Analysis was performed on square-root transformed pulse measures. We obtained a profile of the number of pulses coinciding with each of the recorded subject’s activities (within a window of two points on either side to account for duration of activities and variability in recording time).
Statistical analyses
Compared to controls, the 24-h plasma ACTH and cortisol variables were remarkably similar in IBS patients with and without FM. Therefore, we combined the two IBS patient groups to improve statistical power of the tests. An analysis of variance (ANOVA) with diagnosis as the between-subjects factor and time-period as the within-subjects repeated factor was used to assess the effects of group and time-period. Differences between hormonal levels for the six 4-h blocks were assessed using two-sided t-tests. Two-sided chi-squared or Fisher’s exact test (when counts were low) was used to study the association between diagnosis and the stress indicators (abuse, alcoholism, etc.). Pearson’s correlation test was used where indicated to assess correlations. Results that show statistical significance (P < 0.05) and marginal significance/trends (P ≤ 0.1) are reported. Stricter P-value thresholds were used to determine significance based on number of comparisons performed, i.e. when comparing hormone levels across the six 4-h time-blocks between groups and assessing correlations. Where indicated, values are reported as mean ± SEM. Differences in clinical features and stress indicators between diagnostic groups were analysed using two-sided t-tests.
STUDY 2: Measurement of stimulated HPA axis response to a visceral stressor and colonic mucosal cytokine expression
Subjects
Ten female healthy controls and 10 IBS patients were studied. The inclusion and exclusion criteria, recruitment, and gynaecological history and baseline psychological and bowel symptom assessments were similar to that in Study 1. Seven of the 10 female control subjects and seven of the 10 IBS patients in Study 2 completed Study 1 on a separate occasion.
Study protocol
Subjects were admitted to the Medical Procedures Unit. Irritable bowel syndrome patients rated the intensity and unpleasantness of their abdominal pain for both the past 1 month (chronic) and the past 24 h (current) using validated visual analogue scales.29 Serial blood samples were collected at four time-points: following the 30-min rest period (baseline), immediately after and at 10 and 20 min after the sigmoidoscopy.
Flexible sigmoidoscopy with colonic mucosal tissue biopsies was performed between the late afternoon hours of 16:00–17:00. Tap water enema bowel preparations were used. No macroscopic abnormalities (e.g. erythema, ulcerations) were endoscopically visualized in any of the subjects. Ten biopsies were collected in the rectum and sigmoid colon (10 and 30 cm from the anal verge respectively).
Blood sample measurements for ACTH, cortisol and catecholamine levels were conducted at an experienced commercial laboratory.9 Plasma cortisol, NA and adrenaline levels were measured using a standardized high-performance liquid chromatography (HPLC) methodology. For adrenaline values within normal range of ≤10 pg mL−1, a value of 10 pg mL−1 was designated. Adrenocorticotropin hormone levels were measured using a chemiluminescent immunoassay. RNA extraction and reverse transcription were accomplished using the techniques described previously.34
Statistical analysis
An ANOVA with diagnosis as the between-subjects factor and time-stage as the repeated factor was used to assess the effects of group and time. Differences in neuroendocrine levels were assessed using t-tests. Given the small sample sizes and distributions of the respected variables, non-parametric tests were used to analyse the study data and relationship to neuroendocrine measures and symptoms. Specifically, the Mann–Whitney test was employed to compare cytokine mRNA levels between IBS patients and controls. The Spearman rank correlation test was used to assess correlations. Results that show statistical significance (P < 0.05) and marginal significance/trends (P ≤ 0.1) are reported. Stricter P-value thresholds were used to determine significance based on number of comparisons performed, i.e. when comparing cytokine levels between groups and assessing correlations. Where indicated, values are reported as mean ± SEM.
In both Study 1 and 2, verbal and written consent was obtained from each subject. Compensation was provided to study participants. This study was approved by the UCLA Human Subjects Protection Committee and the UCLA GCRC Advisory Committee.
RESULTS
STUDY 1: Assessment of basal rhythm and pulsatility of ACTH and cortisol
Clinical characteristics of study subjects
IBS patients were significantly older and had a more prevalent history of abuse during adulthood than the control group (Table 1). However, age did not correlate significantly with HPA axis hormone levels and was not included in the statistical model.
Table 1.
Clinical characteristics of Study 1 subjects
| Description | Controls (n = 25) | IBS (n = 41) | P-value | |
|---|---|---|---|---|
| Age (years ± SEM) | 33.0 ± 2.1 | 39.9 ± 1.5 | = 0.01 | |
| BMI (±SEM) | 26.3 ± 1.3 | 27.2 ± 1.0 | ns | |
| Bowel habits [n (%)] | IBS-A | – | 17 (41) | |
| IBS-C | – | 9 (22) | ||
| IBS-D | – | 15 (37) | ||
| Symptom severity | IBS symptom severity (0–20 cm) | – | 10.5 ± 0.8 | |
| Abdominal pain | Chronic abdominal pain (unpleasantness) | – | 12.5 ± 0.4 | |
| Chronic abdominal pain (intensity) | – | 13.1 ± 0.4 | ||
| Current abdominal pain (unpleasantness) | – | 8.2 ± 0.7 | ||
| Current abdominal pain (intensity) | – | 9.6 ± 0.8 | ||
| Gynaecological history [n (%)] | ||||
| Premenopausal phase | Follicular | 8 (32) | 15 (37) | ns |
| Luteal | 12 (48) | 11 (27) | ||
| Menses | 1 (4) | 4 (10) | ||
| Provera | 1 (4) | 1 (2) | ||
| Postmenopausal | 3 (12) | 9 (22) | ns | |
| OCP | 5 (20) | 8 (20) | ns | |
| HRT | 1 (4) | 1 (2) | ns | |
| Hysterectomy | 1 (4) | 3 (7) | ns | |
| Chronic stress indicators [n (%)] | Childhood abuse | 4 (16) | 8 (20) | ns |
| Adult abuse | 1 (4) | 10 (25) | 0.029 | |
| Early adverse event | 1 (4) | 6 (15) | ns | |
| HAD scores | Anxiety | 3.8 ± 0.625 | 6.1 ± 0.4 | 0.002 |
| Depression | 2.0 ± 0.6 | 4.2 ± 0.4 | 0.003 |
BMI, body mass index; OCP, oral contraceptive agent; HRT, hormone replacement therapy. Chronic pain symptoms were rated over previous 3 months. Current pain was rated over previous 24 h. For premenopausal women not taking OCP, the menstrual cycle phase was determined by the count forward/backward method: menses, first 3 days of menses; follicular, days 4–14; luteal, day 14 onward and before menses.
Twenty-four hour circadian rhythm of plasma ACTH and cortisol levels
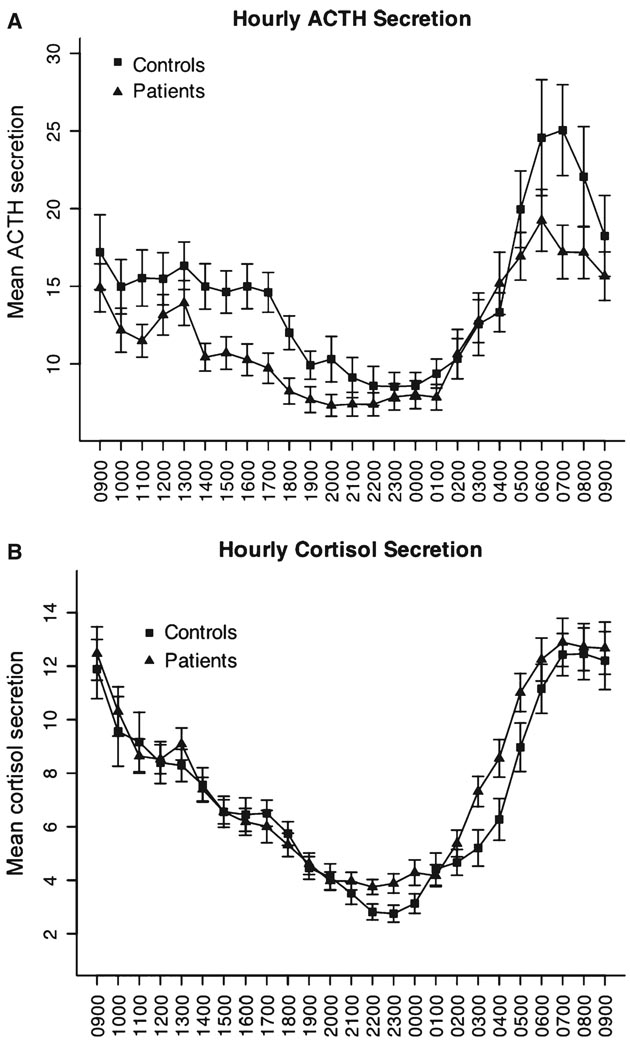
There was a significant effect of time (P < 0.05) on plasma ACTH and plasma cortisol levels with the usual circadian variation. As shown in Fig. 1 and Table 2, minimum ACTH levels were lower in IBS patients than controls (P < 0.05). Irritable bowel syndrome patients had especially blunted ACTH levels compared to controls, for the 4-h time-block 14:00–18:00 (P = 0.004). With regard to cortisol 12-h daytime and nighttime AUC, there was a marginal group × time-block interaction (P = 0.055). Cortisol levels were higher in patients than controls during the 02:00–06:00 time-period, but this was not significant when adjusted for multiple comparisons (time-periods). The patients reached their maximal ACTH and cortisol elevations earlier than controls, and the ACTH acrophase (time of maximal value) occurred significantly earlier in patients (P < 0.05). There was no significant group difference in the mean amplitude of the fitted cosines.
Figure 1.
Mean plasma ACTH (A) and cortisol (B) levels in controls and patient groups over a 24-h period. The mean ± SEM hourly concentrations obtained after averaging over six neighbouring measurements (each measurement taken at 10-min intervals). Minimum ACTH levels in IBS patients were significantly lower than controls (P < 0.05).
Table 2.
Summary statistics for ACTH (pg mL−1) and cortisol (µg dL−1)
| Mean | Maximum | Minimum | Total AUC | Amplitude | Acrophase | ||
|---|---|---|---|---|---|---|---|
| ACTH | Controls | 14.31 ± 1.4 | 50.74 ± 5.4 | 4.68 ± 0.6 | 20560 ± 1955 | 6.07 ± 0.8 | 09:03 ± 0:26 |
| IBS | 11.64 ± 0.9** | 39.97 ± 3.3** | 3.41 ± 0.3* | 16717 ± 1346** | 5.17 ± 0.5 | 07:53 ± 0:19* | |
| Cortisol | Controls | 6.97 ± 0.5 | 19.28 ± 1.0 | 1.55 ± 0.2 | 9983 ± 701 | 4.24 ± 0.4 | 09:17 ± 0:19 |
| IBS | 7.50 ± 0.4 | 20.40 ± 1.0 | 1.79 ± 0.2 | 10745 ± 529 | 4.47 ± 0.3 | 08:34 ± 0:17** |
Values are shown as mean ± SEM.
P < 0.05
P ≤ 0.1.
Pulsatility analysis
The number of cortisol secretory episodes (pulses) was marginally higher in IBS patients than controls (13.39 ± 1.2 vs 10.48 ± 1.3, P = 0.08). About 50% of the pulses corresponded to recorded subject activities, but they did not differ between groups.
Correlations of ACTH and cortisol measurements with clinical factors
Abdominal pain and IBS severity ratings did not correlate with measurements of ACTH or cortisol in the IBS patients. Although acute stress symptom ratings did not significantly differ between controls and IBS patients, the ratings of arousal and anxiety positively correlated with mean and maximal levels of plasma cortisol (all P < 0.05, but this was not significant after Bonferroni correction for multiple comparisons). No differences were found between ACTH and cortisol plasma time-series measurements in terms of menstrual status or phase, abuse history, or bowel habit subgroup. However, these relationships should be further explored in future studies with larger sample sizes.
STUDY 2: Association of HPA axis response with a visceral stressor and colonic mucosal cytokine expression
Clinical characteristics
Clinical characteristics were similar between groups although IBS patients had significantly higher HAD anxiety and depression symptom scores than controls (Table 3). However, both anxiety and depression scores were within the normal range and all patients with psychiatric comorbidity by SCID were excluded.
Table 3.
Clinical characteristics of Study 2 subjects with IBS-D
| Description | Controls | IBS (n = 10) | P-value (n = 10) | |
|---|---|---|---|---|
| Age (years ± SEM) | 33.9 ± 3.5 | 40 ± 2.3 | ns | |
| BMI (±SEM) | 25.5 ± 1.7 | 28.6 ± 2.7 | ns | |
| Symptom severity | IBS symptom severity (0–20 cm) | – | 13.5 ± 1.5 | |
| Abdominal pain | Chronic abdominal pain (unpleasantness) | – | 12.4 ± 0.8 | |
| Chronic abdominal pain (intensity) | – | 14.1 ± 0.6 | ||
| Current abdominal pain (unpleasantness) | – | 11.7 ± 1.2 | ||
| Current abdominal pain (intensity) | – | 13.1 ± 1.1 | ||
| Gynaecological history (n) | ||||
| Premenopausal phase | Follicular | 4 | 5 | ns |
| Luteal | 4 | 4 | ||
| Menses | 1 | 0 | ||
| Postmenopausal | 1 | 1 | ns | |
| Chronic stress indicators [n (%)] | Childhood abuse | 1 (10) | 2 (20) | ns |
| Adult abuse | 0 (0) | 1 (10) | ns | |
| Early adverse event | 0 (0) | 1 (10) | ns | |
| HAD scores | Anxiety | 2.22 ± 0.43 | 5.2 ± 0.92 | 0.01 |
| Depression | 0.33 ± 0.24 | 3.7 ± 0.97 | <0.01 |
BMI, body mass index. Chronic pain symptoms were rated over previous 1 month. Current pain was rated over previous 24 h. For premenopausal women not taking OCP, the menstrual cycle phase was determined by the count forward/backward method: menses, first 3 days of menses; follicular, days 4–14; luteal, day 14 onward and before menses.
Stimulated HPA axis hormones and catecholamines to a visceral stressor
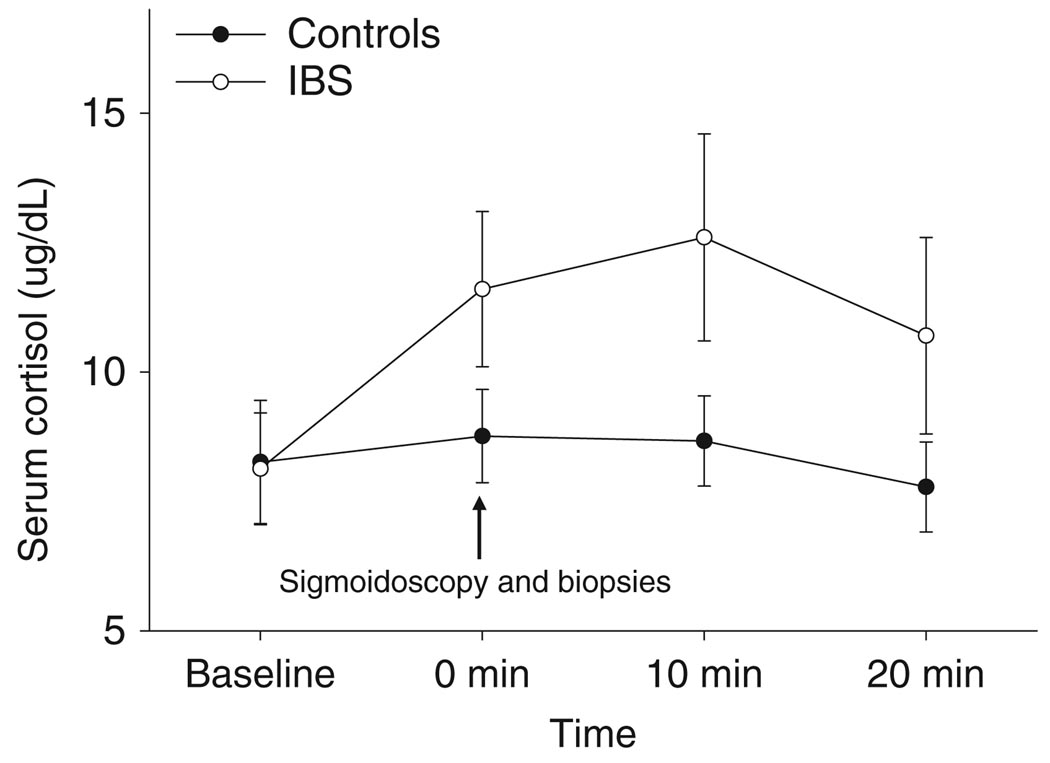
As shown in Fig. 2, there was a significant change in cortisol levels over time to the sigmoidoscopy (P = 0.019). There was a marginal group × time interaction (P = 0.067). Cortisol levels in IBS patients were higher compared to controls but these were not statistically significant. In patients, basal (i.e. preprocedure) cortisol levels positively correlated with HAD anxiety symptom scores (r = 0.81, P < 0.004). Positive correlations were also observed with 1-month chronic IBS symptom ratings of intensity (r = 0.70, P < 0.05) and unpleasantness (r = 0.68, P < 0.05), but these were not significant after Bonferroni correction. There was a marginal effect of time (P = 0.075) and group × time interaction (P = 0.097) for NA levels during the sigmoidoscopy, where it was observed that levels rose higher than baseline in IBS patients vs controls immediately after sigmoidoscopy and dropped back to baseline levels at 10 min. No group differences were observed with ACTH or adrenaline levels (Table 4).
Figure 2.
Serial plasma cortisol levels in response to a pelvic visceral stressor (sigmoidoscopy). Overall, there were significant changes of cortisol levels over time in response to the sigmoidoscopy (P = 0.019). There was a trend for a group × time interaction (P = 0.067) with increasing levels of plasma cortisol in the women with IBS following the sigmoidoscopy compared to a more blunted response in healthy women.
Table 4.
Plasma ACTH, NA and adrenaline levels at baseline and following sigmoidoscopy
| Plasma neuroendocrine measure |
Controls (n = 10) |
IBS-D (n = 10) |
|---|---|---|
| ACTH (pg mL−1) | ||
| Baseline | 19.3 ± 2.5 | 16.8 ± 2.3 |
| 0 min (immediately after sigmoidoscopy) | 20.7 ± 3.0 | 36.1 ± 13.0 |
| 10 min | 19.3 ± 2.3 | 26.8 ± 8.9 |
| 20 min | 18.4 ± 2.7 | 21.2 ± 6.7 |
| NA (pg mL−1) | ||
| Baseline | 239.9 ± 23.6 | 264 ± 35.6 |
| 0 min (immediately after sigmoidoscopy) | 250.4 ± 19.2 | 325.4 ± 39.9 |
| 10 min | 247.1 ± 22.8 | 241.8 ± 30.8 |
| 20 min | 258.9 ± 28.8 | 244.6 ± 32.7 |
| Adrenaline (pg mL−1) | ||
| Baseline | 31.1 ± 10.9 | 36.9 ± 14.4 |
| 0 min (immediately after sigmoidoscopy) | 34.7 ± 11.3 | 41.1 ± 15.5 |
| 10 min | 32.2 ± 8.9 | 32.2 ± 15.6 |
| 20 min | 39.9 ± 22.2 | 42.8 ± 18.3 |
Values are shown as mean ± SEM.
Colonic mucosal cytokine mRNA expression in IBS and controls
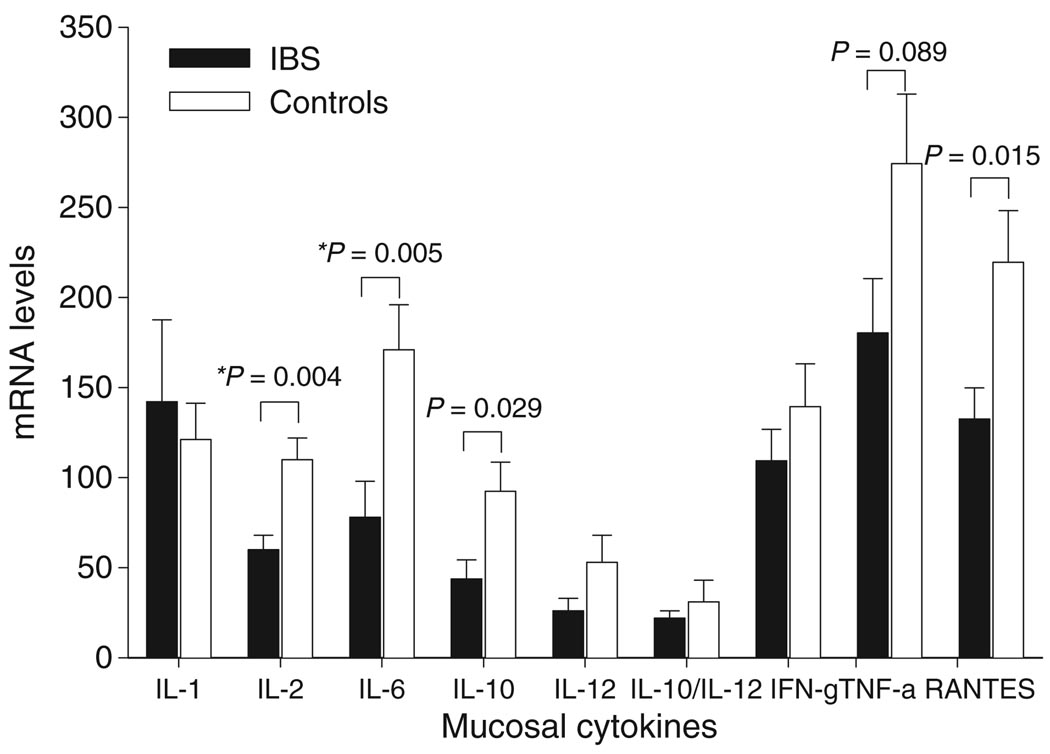
Irritable bowel syndrome patients had decreased expression of interleukin (IL)-2, IL-6, IL-10, and the chemokine RANTES compared to controls, but only the differences in IL-2 and IL-6 mRNA expression in the sigmoid colon maintained significance after correction for multiple comparisons (Fig. 3). Expression of the other cytokines in the sigmoid colon and rectum (data not shown) were similar in IBS and controls. Negative correlations were found between poststressor NA levels and interferon (IFN)-δ (r = −0.52, P = 0.029) and RANTES (r = −0.55, P = 0.015) mRNA expression in the sigmoid colon but were not significant after correction for multiple comparisons. There were no other significant correlations between neuroendocrine measures or expression of mucosal cytokines with current IBS symptom ratings.
Figure 3.
Expression of colonic and rectal mucosal cytokines in IBS patients and healthy controls. mRNA measurements (mean ± SEM) from mucosal biopsy tissue obtained in the sigmoid colon of IBS-D patients and healthy controls are shown. IL-2, IL-6, IL-10 and RANTES mRNA levels in the sigmoid colon were lower in IBS vs controls but only the differences between IL-2 and IL-6 (*) maintained significance after correction for multiple comparisons. The mRNA expression of the other cytokines was similar in IBS and controls. IL-1 and RANTES values are expressed as values × 10−2 and IL-10, IL-10/IL-12, IFN-δ and TNF-α are expressed as values × 10−1.
DISCUSSION
This study aimed to identify the differences in the basal and stimulated levels of HPA axis hormones, and stimulated plasma NA in women with IBS and healthy women. There were four main findings in IBS patients: (i) basal levels of plasma ACTH were significantly decreased, while both 24-h basal plasma cortisol levels and stress-induced cortisol levels tended to be increased; (ii) basal cortisol levels prior to a visceral stressor positively correlated with the presence of anxiety symptoms; and (iii) exploratory evaluation of mucosal cytokine expression demonstrates decreased or normal levels of cytokines in the sigmoid colon.
Evidence of a dysregulated HPA axis under basal conditions in IBS
Mildly elevated plasma cortisol, but significantly blunted ACTH levels in IBS suggests a dysregulation of the HPA axis. While no other studies have previously evaluated the diurnal rhythm and pulsatility of the HPA axis to this extent in IBS, there are two studies which support abnormal basal urinary and salivary cortisol levels (both representing free cortisol) in IBS.10,17 However, one study found a trend for higher urinary cortisol levels10 and the other found lower salivary cortisol levels17 in IBS. Observed differences may be due to the different patient populations, sex of study participants, type of cortisol measured (i.e. salivary vs plasma) and methodology (serial vs spot collections). Most importantly, as psychiatric comorbidity and a history of early life trauma have been shown to be associated with altered HPA axis responses,20 and as none of the previous publications reported these factors, such psychiatric factors may be an important differentiating factors.
In contrast, basal plasma ACTH levels were blunted. Decreased ACTH levels have been demonstrated in other chronic stress-related conditions such as PTSD35 and major depressive disorder, with or without history of childhood abuse.20 In patients with FM and/or chronic fatigue syndrome (CFS), a delay in the decline of cortisol in patients (i.e. increased cortisol levels) and a suggestion of fewer ACTH pulses (i.e. lower ACTH levels) were observed.33 Secretion of ACTH from the pituitary is stimulated by CRF (via CRF1 receptors) and arginine vasopressin (via V1B receptors), released from the PVN of the hypothalamus, while cortisol via mineralocorticoid and glucocorticoid receptors in the PVN, pituitary gland and hippocampus, exerts a negative feedback inhibition.2,36 Thus, decreased ACTH levels can result from a downregulation of the CRF/CRF1 receptor signalling system at the level of the pituitary and/or due to enhanced negative feedback of cortisol. The former is less likely given that two of three CRF stimulation studies reported increased ACTH responses,5,8 although the remaining study showed a blunted ACTH response.17 Enhanced negative feedback of cortisol would be supported with the demonstration of increased number glucorticoid receptors and increased suppression of ACTH secretion by glucocorticoids (dexamethasone suppression test), as has been shown in PTSD.35,37 Glucorticoid receptor number on peripheral immune cells or activity has not been studied in IBS, but two studies failed to find increased suppression of cortisol following dexamethasone in IBS, although ACTH levels were not measured in these studies.5,17 Further studies are needed to determine the mechanisms responsible for the observed ACTH and cortisol levels in IBS which differ from that of controls.
Our findings suggest that, at least under the controlled baseline conditions, lower levels of ACTH secretion do not necessarily translate into significant differences in basal cortisol levels. Normal-high cortisol levels in the presence of decreased ACTH levels would suggest enhanced adrenal cortical sensitivity to ACTH as this would result in higher than expected cortisol levels relative to ACTH levels. Cortisol response to ACTH stimulation, which has not been reported in IBS, would help to determine if this is present in IBS. Normal cortisol levels despite increased ACTH levels have also been demonstrated in IBS.8,18 Taken together with our findings, normal cortisol levels in the presence of either elevated8,18 or decreased38 ACTH levels suggest tightly regulated, homeostatic cortisol secretion. In summary, there appears to be good evidence for a dysregulated HPA axis under basal conditions in IBS consistent with an enhanced central stress response system.
HPA axis hormone and NA levels in response to a visceral stressor
We chose a pelvic visceral stressor because it is a clinically relevant stressor given the typically reported lower abdominal/pelvic pain and tenderness, and the commonly reported hypersensitivity to rectosigmoid balloon distension reported in IBS patients.39 Neuroendocrine responses to this type of stressor have not been previously reported in IBS, and sigmoidoscopy allowed the simultaneous collection of colonic tissue to measure mucosal cytokine mRNA expression. There was a trend for increased responses of stress markers in the plasma in terms of cortisol, but no significant differences in ACTH or NA responses in women with IBS-D compared to healthy women. The failure of stimulated cortisol levels to reach statistical significance between groups could be due to the small sample sizes. In another ongoing study where we have enrolled a greater number of subjects, salivary (free) cortisol levels were higher at baseline immediately prior to the same visceral stressor in IBS patients vs controls.40
Published reports on stimulated cortisol levels have been somewhat inconsistent in IBS. Out of seven studies which measured cortisol responses to hormone challenge or stress, four also measured plasma ACTH levels. Three of these four studies reported increased ACTH responses compared to controls,8,18,41 while the other demonstrated blunted ACTH (and cortisol) responses.17 Only two of seven published studies demonstrated elevated cortisol responses6,41 which suggest that, in general, stimulated cortisol responses in IBS patients are normal but they may be increased in a subset of IBS patients. This may be dependent on the type and relevance of the stimulus/stressor to the individual, to psychiatric comorbidity or to a presence or absence of early life trauma.
Interestingly, there was a significant positive correlation of basal cortisol levels just prior to the visceral stressor and anxiety symptom scores, while the correlation of basal cortisol levels in the absence of an experimental stressor did not. The observed correlation is not surprising given that CRF has anxiogenic effects15 and the relatively recent finding that a CRF receptor antagonist induced a reduction in anxiety ratings to electrical stimulation in the rectum in IBS-D patients.42 Thus, one may speculate that cortisol levels obtained immediately prior to expected abdominal discomfort a surrogate marker for anticipatory anxiety in IBS. In contrast, cortisol and ACTH levels did not correlate with other clinical symptoms. Overall, previous studies measuring HPA axis levels in IBS have not demonstrated a strong relationship between these measures and IBS symptoms. Due to the low prevalence of early adverse life events or trauma in our population, we were unable to assess the impact of these factors on the observed HPA axis activity.
Expression of mucosal cytokines in IBS and healthy controls
In this exploratory study, IBS patients had decreased expression of the pro-inflammatory cytokines, IL-2 and IL-6 and there were no correlations with plasma neuroendocrine measures or clinical symptoms. Although this is consistent with a recent preliminary report where the expression of certain mucosal chemokines was decreased and pro-inflammatory cytokines were similar in IBS and controls,43 it was unexpected as several reports suggest that that IBS patients may have enhanced mucosal immune activation in terms of intraepithelial lymphocytes13,16 and mast cells.44 Three of four published studies measuring peripheral cytokine levels in IBS reported plasma levels only.5,45,46 Elevated plasma levels of IL-6,5,46 IL-6R,5 IL-1β,46 and TNF-α46 and a lower IL-10/IL-12 ratio45 have been reported in IBS patients compared to controls, presumably suggesting that IBS may be associated with enhanced pro-inflammatory cytokine release. However, plasma cytokine levels do not necessarily reflect the levels or expression of various cytokines in the gut mucosa, but may arise from activated immunocytes in the spleen or liver.47 For example, in inflammatory bowel disease, changes in intestinal mucosal cytokine expression are not always accompanied by corresponding changes in plasma cytokine levels.48 Furthermore, elevated levels of pro-inflammatory plasma cytokines such as IL-6 have been reported during somatic stress, depression, and even with intense exercise.49,50 It is therefore conceivable that previously reported increased plasma cytokine levels IBS patients may be less related to mucosal immune activation, but rather reflect the effects of stress or comorbid psychiatric conditions. There is only one report which described increased IL-β mRNA levels in the rectal mucosa of postinfectious IBS patients (PI-IBS) after acute gastroenteritis compared to controls who suffered a gastroenteritis but did not develop IBS.51 In contrast to all previously reported studies, subjects with either psychiatric comorbidity or a history of prior gastroenteritis were excluded in our study.
Differences in mucosal cytokines were found in the sigmoid colon but not rectum. This may be due to qualitative and quantitative differences in the inflammatory infiltrate at the rectum and colon which have been described.52 The greater cell density in the rectum is thought to be due to its proximity to the external environment which may be associated with differences in antigenic stimulation.52 Our findings are intriguing but require further study in larger numbers of well-characterized IBS patients and healthy controls.
Strengths and limitations
In this study, we made efforts to eliminate potentially confounding psychiatric factors which have been shown to affect HPA axis regulation, by using structured psychiatric interviews, and by administering instruments to assess early life trauma and symptoms of anxiety and depression. As these factors are common in IBS patients, in particular those observed in tertiary referral clinics, our findings cannot be extrapolated to all IBS patients. We also used high-frequency sampling over a 24-h period in a large sample of control and IBS subjects to allow for in depth characterization of HPA axis kinetics. While the sample size for mucosal cytokine collection was relatively small, we did find that 50% of cytokines tested were expressed at levels in the completely opposite direction (lower rather than higher) than expected and were consistent with another study.43 However, given the small sample sizes, these latter results should be interpreted with caution but warrant the need for larger studies in well-characterized IBS patients to further investigate the expression of mucosal cytokines. An additional limitation is the lack of subjective pain measurements taken at the time of the visceral stressor, i.e. sigmoidoscopy, which would have been helpful to explore the relationship of pain sensitivity and the neuroendocrine and cytokine measures.
SUMMARY AND CONCLUSIONS
Our findings are consistent with a dysregulation of the HPA axis in women with IBS, without psychiatric comorbidity. The fact that plasma cortisol levels were not associated with current IBS symptoms argues against a primary role of HPA axis dysregulation in modulating gastrointestinal symptoms in IBS, but this should be explored further in larger samples. Elevated cortisol and blunted ACTH levels may be more indicative of a generalized upregulation of the central stress system with enhanced negative feedback, which is not specific to IBS, and may be related to overall state of well being in stress-related disorders. In the sample studied, our findings do not support increased immune activation at the level of the rectosigmoid colonic mucosa in women with IBS-D. Although in this study plasma cortisol levels by themselves were not related to mucosal cytokine expression, it is possible that the interactions of NA, cortisol and acetylcholine (released from vagal afferents)47,53 determine the state of mucosal immune activation. However, well-designed studies are needed to investigate this issue further in IBS.
Acknowledgments
GRANT SUPPORT
Supported by NIH grants AR41622 (LC), GCRC M01-RR00865, DK48351, P50 DK64539 and R24 AT002681 (EAM), the Mucosal Immunology Core, UCLA Center for AIDS Research (CFAR) National Institutes of Health grant AI28697, and GlaxoSmithKline.
Footnotes
CONFLICT OF INTEREST
Dr Lin Chang and Dr Emeran Mayer are consultants for GlaxoSmithKline. Dr Vanessa Ameen was formerly an employee of GlaxoSmithKline.
REFERENCES
- 1.Elenkov IJ, Wilder RL, Chrousos GP, et al. The sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:585–638. [PubMed] [Google Scholar]
- 2.Fish EW, Shahrokh D, Bagot R, et al. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 3.Esler MD, Goulston KJ. Levels of anxiety in colonic disorders. N Engl J Med. 1973;288:16–20. doi: 10.1056/NEJM197301042880104. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. J Am Med Assoc. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 5.Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalmic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential marker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Elsenbruch S, Orr WC. Diarrhea- and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses. Am J Gastroenterol. 2001;96:460–466. doi: 10.1111/j.1572-0241.2001.03526.x. [DOI] [PubMed] [Google Scholar]
- 7.Elsenbruch S, Holtmann G, Oezcan D, et al. Are there alterations of neuroendocrine and cellular immune responses to nutrients in women with irritable bowel syndrome? Am J Gastroenterol. 2004;99:703–710. doi: 10.1111/j.1572-0241.2004.04138.x. [DOI] [PubMed] [Google Scholar]
- 8.Fukudo S, Nomura T, Hongo M. Impact of corticotrophin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1999;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickhaus B, Mayer EA, Firooz N, et al. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol. 2003;98:135–143. doi: 10.1111/j.1572-0241.2003.07156.x. [DOI] [PubMed] [Google Scholar]
- 10.Heitkemper M, Jarrett M, Cain K, et al. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol. 1996;91:906–913. [PubMed] [Google Scholar]
- 11.Heitkemper M, Jarrett M, Cain KC, et al. Autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. 2001;46:1276–1284. doi: 10.1023/a:1010671514618. [DOI] [PubMed] [Google Scholar]
- 12.Cain KC, Jarrett ME, Burr RL, et al. Heart rate variability is related to pain severity and predominant bowel pattern in women with irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:110–118. doi: 10.1111/j.1365-2982.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 14.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 15.Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- 16.Dunlop SP, Jenkins D, Neal KR, et al. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Bohmelt AH, Nater UM, Franke S, et al. Basal and stimulated hypothalamic-pituitary-adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosom Med. 2005;67:288–294. doi: 10.1097/01.psy.0000157064.72831.ba. [DOI] [PubMed] [Google Scholar]
- 18.Posserud I, Agerforz P, Ekman R, et al. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsenbruch S, Lovallo WR, Orr WC. Psychological and physiological responses to postprandial mental stress in women with the irritable bowel syndrome. Psychosom Med. 2001;63:805–810. doi: 10.1097/00006842-200109000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Heim C, Newport DJ, Bonsall R, et al. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatr. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 21.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia, a report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 23.Sperber AD, Atzmon Y, Neumann L, et al. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol. 1999;94:3541–3546. doi: 10.1111/j.1572-0241.1999.01643.x. [DOI] [PubMed] [Google Scholar]
- 24.Whitehead WE, Palsson O, Jones KR. Systemic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 25.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 27.Spitzer RL, Williams JBW, Gibbon M, et al. Structured Clinical Interview for DSM-III-R. Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- 28.Green BL. The Trauma History Questionnaire (THQ) In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Lutherville: Sidran Press; 1996. pp. 366–369. [Google Scholar]
- 29.Gracely RH, McGrath P, Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain. 1976;5:19–29. doi: 10.1016/0304-3959(78)90021-0. [DOI] [PubMed] [Google Scholar]
- 30.Posener JA, Charles D, Veldhuis JD, et al. Process irregularity of cortisol and adrenocorticotropin secretion in men with major depressive disorder. Psychoneuroendocrinology. 2004;29:1129–1137. doi: 10.1016/j.psyneuen.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Guo W, Wang Y, Brown MB. A signal extraction approach to modeling hormone time series with pulses and a changing baseline. J Am Stat Assoc. 1999;94:746–756. [Google Scholar]
- 32.Young EA, Carlson NE, Brown MB. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25:267–276. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 33.Crofford LJ, Young EA, Engleberg NC, et al. Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav Immun. 2004;18:314–325. doi: 10.1016/j.bbi.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 34.McGowan I, Elliott J, Fuerst M, et al. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr. 2004;37:1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 35.Yehuda R, Yang RK, Buchsbaum MS, et al. Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology. 2006;31:447–451. doi: 10.1016/j.psyneuen.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Chrousos GP. Regulation and dysregulation of the hypothalamic-pituitary-adrenal axis. The corticotropin-releasing hormone perspective. Endocrinol Metab Clin North Am. 1992;21:833–858. [PubMed] [Google Scholar]
- 37.Yehuda R, Giller EL, Jr, Levengood RA, et al. Hypothalamic-Pituitary-Adrenal Functioning in Post-Traumatic Stress Disorder: Expanding the Concept of the Stress Response Spectrum. Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to Post-Traumatic Stress Disorder. Philadelphia: Lippincott-Raven Publishers; 1995. pp. 351–366. [Google Scholar]
- 38.Klerman EB, Goldenberg DL, Brown EN, et al. Circadian rhythms of women with fibromyalgia. J Clin Endocrinol Metab. 2001;86:1034–1039. doi: 10.1210/jcem.86.3.7293. [DOI] [PubMed] [Google Scholar]
- 39.Munakata J, Naliboff B, Harraf F, et al. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 40.Videlock EJ, Yuan PQ, Taché Y, et al. Stress-induced cortisol response and colonic mucosal CRF-1 mRNA receptor expression correlate with symptoms of chronic stress and quality of life in irritable bowel syndrome (IBS) Gastroenterology. 2007;132:A-72. [Google Scholar]
- 41.Dinan TG, O’Keane V, O’Boyle C, et al. A comparison of the mental status, personality profiles and life events of patients with irritable bowel syndrome and peptic ulcer disease. Acta Psychiatr Scand. 1991;84:26–28. doi: 10.1111/j.1600-0447.1991.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 42.Sagami Y, Shimada Y, Tayama J, et al. Effect of a corticotrophin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quigley EM, McSharry J, O’Mahoney L, et al. Mucosal cytokine imbalance in irritable bowel syndrome (IBS) Am J Gastroenterol. 2007;102 Suppl.:632. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- 44.Barbara G, Wang B, Grundy D, et al. Mast cells are increased in the colonic mucosa of patients with irritable bowel syndrome and excite visceral sensory pathways. Gastroenterology. 2005;128:A-626. [Google Scholar]
- 45.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 46.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 47.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen OH, Vainer B, Madsen SM, et al. Established and emerging biological activity markers of inflammatory bowel disease. Am J Gastroenterol. 2000;95:359–367. doi: 10.1111/j.1572-0241.2000.t01-1-01790.x. [DOI] [PubMed] [Google Scholar]
- 49.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 50.Mastorakos G, Ilias I. Interleukin-6: a cytokine and/or a major modulator of the response to somatic stress. Ann N Y Acad Sci. 2006;1088:373–381. doi: 10.1196/annals.1366.021. [DOI] [PubMed] [Google Scholar]
- 51.Gwee KA, Collins SM, Read NW, et al. Increased rectal mucosal expression of interleukin 1á in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–526. doi: 10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caballero T, Nogueras F, Medina MT, et al. Intraepithelial and lamina propria leucocyte subsets in inflammatory bowel disease: an immunohistochemical study of colon and rectal biopsy specimens. J Clin Pathol. 1995;48:743–748. doi: 10.1136/jcp.48.8.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]