Abstract
Dorsal root-evoked stimulation of sensory afferents in the hemisected in vitro rat spinal cord produces reflex output, recorded on the ventral roots. Transient spinal 5-HT2C receptor activation induces a long-lasting facilitation of these reflexes (LLFR) by largely unknown mechanisms. Two Sprague-Dawley substrains were used to characterize network properties involved in this serotonin (5-HT) receptor-mediated reflex plasticity. Serotonin more easily produced LLFR in one substrain and a long-lasting depression of reflexes (LLDR) in the other. Interestingly, LLFR and LLDR were bidirectionally interconvertible using 5-HT2A/2C and 5-HT1A receptor agonists, respectively, regardless of substrain. LLFR was predominantly Aβ afferent fiber mediated, consistent with prominent 5-HT2C receptor expression in the Aβ fiber projection territories (deeper spinal laminae). Reflex facilitation involved an unmasking of polysynaptic pathways and an increased receptive field size. LLFR emerged even when reflexes were evoked three to five times/h, indicating an activity independent induction. Both the NMDA and AMPA/kainate receptor-mediated components of the reflex could be facilitated, and facilitation was dependent on 5-HT receptor activation alone, not on coincident reflex activation in the presence of 5-HT. Selective blockade of GABAA and/or glycine receptors also did not prevent reflex amplification and so are not required for LLFR. Indeed, a more robust response was seen after blockade of spinal inhibition, indicating that inhibitory processes serve to limit reflex amplification. Overall we demonstrate that the serotonergic system has the capacity to induce long-lasting bidirectional changes in reflex strength in a manner that is nonassociative and independent of evoked activity or activation of ionotropic excitatory and inhibitory receptors.
INTRODUCTION
Bulbospinal serotonergic systems potently modulate spinal cord sensory and motor activity (Hochman et al. 2001; Millan 2002; Schmidt and Jordan 2000). One hypothesis is that activation of serotonergic systems reconfigures spinal neural networks into various functional “states” (Hochman et al. 2001). For example, serotonin (5-HT2) receptor activation facilitates flexion reflexes (Machacek et al. 2000) and a “state” of facilitated flexion withdrawal reflexes could serve to protect damaged tissue from further injury. Most studies have demon-strated that spinal 5-HT2 receptors mediate increases in spinal cord excitability. 5-HT2 receptors depolarize dorsal horn neurons in frog (Tan and Miletic 1992) and facilitate focal stimulation-induced synaptic activity in ventral horn cells (Yamazaki et al. 1992). 5-HT2 receptor activity also depolarizes (Holohean et al. 1990; Wang and Dun 1990) and increases glutamate-evoked firing in motoneurons (Jackson and White 1990) and facilitates extensor stretch reflex and tonic motor activity (Miller et al. 1996). In addition, 5-HT2 receptors facilitate an L-type calcium current and thus promote plateau potentials in turtle motoneurons (Perrier and Hounsgaard 2003).
Activation of monoaminergic metabotropic receptors by descending neuromodulatory commands can also produce long-lasting changes in spinal cord function (Hori et al. 1996; Lewis et al. 1993). For example, 5-HT2 receptors mediate a long-lasting facilitation of miniature excitatory postsynaptic currents in superficial dorsal horn neurons (Hori et al. 1996). Also in dorsal horn, low concentrations of serotonin and selective 5-HT2 agonists induce a lasting synaptic enhancement at least in part as a result of recruitment of previously silent synapses (Li and Zhuo 1998; Li et al. 1999). 5-HT2 receptors are also involved in long-term facilitation both of postsynaptic responses in neonatal rat hypoglossal motoneurons (Bocchiaro and Feldman 2004) and of adult respiratory motor output (Baker-Herman and Mitchell 2002).
Although 5-HT has an overall depressive effect on spinal reflexes (Crick and Wallis 1991; Hedo and Lopez-Garcia 2002; Hedo et al. 2002; Machacek et al. 2001), we previously demonstrated that the washout of 5-HT or 5-HT2C receptor agonists produces long-lasting facilitation of monosynaptic and longer-latency reflexes (Machacek et al. 2001). Although an increased monosynaptic reflex may in part be explained by altered activity in muscle spindle afferents, the afferents mediating longer-latency reflex facilitation remain unknown. Central sensitization, for example, is a long-lasting facilitation in spinal cord nocisponsive activity mediated by Aδ and C fiber nociceptive input (Coderre et al. 1993) and facilitated nociceptive afferent responses may be responsible for our previously observed amplification in longer-latency reflexes (Machacek et al. 2001).
The experiments described in the present study explore the spinal network properties and modulatory mechanisms that produce long-lasting alterations in spinal cord reflex excitability even after the washout of the receptor agonists. We examined whether the expression of LLFR was dependent on the coincident activation of reflex pathways during 5-HT receptor activation, on GABAergic and glycinergic inhibitory actions, and characterized the ionotropic glutamate receptors required for the expression of LLFR. In addition to understanding properties of 5-HT2 receptor-induced LLFR, we also identified a 5-HT1A receptor-induced long-lasting depression of reflexes (LLDR) and demonstrate that lasting facilitation and depression are interconvertible.
METHODS
Dissection
Neonatal-juvenile Sprague-Dawley rat pups of both sexes, age range P2-P17 (but primarily P8-P14) were anesthetized with 10% wt/vol urethane (1.5-2.0 g/kg injected intraperitoneally). The animals were then submerged in an ice slurry bath for 5 min to reduce body temperature. One of two Na+ substitution solutions were used during the surgical isolation of the spinal cord: 1) a high sucrose-containing artificial cerebral spinal fluid (aCSF) [(in mM): sucrose 250; KCl 2.5; NaHCO3 26; NaH2PO4 1.25; D-glucose 25; MgCl2 3; CaCl2 1; and kynurenate 1] or 2) a high choline-containing solution [(in mM): choline chloride 110; KCl 2.5; NaH2PO4 1.2; Na-pyruvate 2.4; L-ascorbic acid 1.3; dextrose 20; CaCl2 0.5; MgCl2 7] (Hoffman and Johnston 1998). The animals were decapitated and the cervical to sacral spinal cord was isolated through a ventral approach. Vertebral bodies were removed with special care to maintain both ventral and dorsal spinal roots. After the dura was removed the whole spinal cord was pinned ventral side up in a Sylgard-coated (Dow) petri dish. Sagittal hemisection of the cord from C7-S1 was performed using insect pins (1.0 mm, Fine Science Tools) and each hemisection was transferred to a separate dish in oxygenated aCSF containing (in mM): NaCl 128; KCl 1.9; D-glucose 10; MgSO4 1.3; CaCl2 2.4; KH2PO4 1.2; and NaHCO3 26. Ventral and dorsal lumbar roots were pinned down to the coated dish at the most peripheral cut ends. The preparation was then allowed to recover for about 1 h before any further manipulation.
Experimental setup
Bipolar glass suction electrodes were attached to dorsal roots L2 and L5 to allow for electrical stimulation as well as L2 and L5 ventral roots to record evoked reflexes (Fig. 1A). In some experiments, an additional suction electrode was attached to the L5 dorsal root to record afferent volleys. Constant current stimulators (Eide 1972) delivered single-shock stimuli of defined stimulation parameters to the dorsal roots. Although the present work sought to examine modulatory actions on reflexes evoked by low-threshold nonnociceptive afferents, initial experiments delivered stimuli of 50 μA and 50 μs, 500 μA and 50 μs, and 500 μA and 500 μs, which roughly correspond to progressive recruitment of Aβ,Aδ, and C afferent fibers, respectively (Thompson et al. 1990). In several experiments, L2 and L5 dorsal roots were stimulated separately with an 800-ms delay between stimulation of L2 and L5 while recording reflexes from L2 and L5 ventral roots. Raw data were collected with Clampex software (v. 7.0, Axon Instruments, Union City, CA) and stored in a PC for off-line analysis. Reflex responses were collected after dorsal root stimulation frequencies of 0.02-0.1 Hz. In the majority of experiments, after an initial stabilization period, no time-dependent changes in reflex amplitude were observed at these frequencies. We used a static bath preparation that was directly oxygenated with a gas mixture of 95% O2-5% CO2.
Fig. 1.
Serotonin (5-HT) is capable of inducing long-lasting facilitation of reflexes (LLFR) and long-lasting depression of reflexes (LLDR) but selective 5-HT2A/2C receptor activation with 1-(2,5)-dimethoxy-4-iodoamphetamine (DOI) induces only LLFR. A: experimental setup. Drawing of a midsagittally hemisected rat spinal cord. Glass suction electrodes are attached to dorsal roots for stimulation and ventral roots for recording of reflexes. In addition, in some experiments, a recording electrode was also attached to the L5 dorsal root to record afferent fiber volleys. B and C: scatterplots of changes in reflex strength in 2 different animals after application of 5-HT and its removal. In B, LLFR is induced whereas in C, 5-HT induces LLDR. D: effect of DOI on the sample population (n = 34). Values are presented as reflex strength normalized to the mean amplitude value of reflexes evoked in the 30-min period before DOI application. This period was always used to define baseline. Mean reflex values were first obtained for a given animal over 10-min intervals and were used to calculate the population mean ± SE.
Measurement of the reflex
To allow for quantitative analysis, reflex responses were rectified and integrated. The rectified/integrated reflex amplitude (area under the curve) was compared between baseline, drug, and wash conditions. Scatterplots indicate the area under the curve of the reflex as a whole (i.e., both early and later latency components) measured to 100 ms after the stimulus artifact beginning at the onset of the reflex response. Because of our interest in plasticity of Aβ afferent fiber-evoked reflexes this latency period was chosen to largely exclude a contribution from nociceptive afferents. Hedo et al. (1999) demonstrated in this preparation that C fiber-mediated reflexes begin at a minimal latency of 100 ms. In some cases we divided the early reflex into short- versus longer-latency responses. Short-latency, presumably monosynaptic, components were defined as from the onset of reflex activity to 15 ms after the stimulus artifact and longer-latency components were measured from 15 to 100 ms after the stimulus artifact (Crick and Wallis 1991). Unless otherwise stated all measured reflexes combine both short- and longer-latency components (≤100 ms after stimulus artifact).
Electrophysiology
We examined the stability of reflex amplitude over time under control conditions that included different stimulation frequencies (0.1 Hz, n = 5; 0.05 Hz, n = 4; and 0.033 Hz, n = 7). In several instances, reflex amplitude decayed in the first 10-30 min, but then stabilized and remained stable even after several solution exchanges or rest periods of 10 min (n = 12/16). Occasionally, the wash procedure produced slight but visible mechanical perturbations of the preparation and commonly caused a short period of facilitated reflex responses (about 5-15 min), perhaps as a result of transient activation of mechanosensitive events (Grillner et al. 1984). Alternatively, the wash procedure could enhance the diffusion of oxygen into the tissue and at the same time wash out lactate and other metabolites. This could transiently facilitate the reflex activity. However, control experiments where this procedure was repeated several times showed that the reflex returned to baseline amplitude in nine of ten experiments. In the tenth experiment the shorter-latency component continued to be facilitated after the bath solution exchange. Thus we waited until a steady reflex amplitude was obtained and define this amplitude as our control baseline (between 30 and 90 min).
Reflexes were monitored in the same way throughout the experiments. For the majority of experiments, dorsal root stimulation parameters were 500 μA, 500 μs, at 0.03 Hz unless otherwise indicated. Only preparations that expressed a steady control baseline were used for analysis.
Pharmacology
Stock solutions of drugs (10-100 mM) were made and stored at -20°C until needed. Bath application reflects final concentrations. Drug application involved applying stock solutions of test drugs, dissolved in the appropriate solvent (aCSF, water, dimethyl sulfoxide [DMSO], or ethanol) mixed directly into the bath. A static bath was used to minimize the overall volume of drugs used in experiments. Solution exchange was achieved by replacing the bath solution three times. The neurochemicals and concentrations used were as follows: serotonin (5-HT, 10 μM); 6-chloro-2-(1-piperazinyl) pyrazine (MK212, 1 μM); 1-(2,5)-dimethoxy-4-iodoamphetamine (DOI, 1-10 μM); 8-hydroxy-2-(dipropylamino)-tetralin (8-OH-DPAT, 1 μM); clozapine (0.5-2 μM); kynurenic acid (1 mM); bicuculline methiodide (10-20 μM); phenylbenzene-ω-phosphono-α-amino acid (PMBA, 50-100 μM); D-2-amino-5-phosphonovaleric acid (AP5, 50 μM), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 1-10 μM). All drugs were obtained from Sigma-RBI.
Immunohistochemistry
Six male Sprague-Dawley rats (Raleigh, NC) aged P14 were perfused with a solution containing 0.9% saline, 0.1% sodium nitrate, and 0.01% heparin followed by Lana's fixative [4% paraformaldehyde, 0.2% picric acid, 0.16 M phosphate (PO3) buffer (pH 6.9)]. Spinal cords were isolated, postfixed for 2 h in 4% paraformaldehyde, and cryoprotected in 10% sucrose with 0.1 M phosphate (PO3) then stored at 4°C. Lumbar spinal cords were frozen at -80°C, then sectioned in 10-μm-thick slices on a cryostat and collected on microscope slides. Slides were rehydrated for 4 h in 0.1 M PO3-buffered saline (PBS) at room temperature (RT). They were then washed overnight in PBS containing 0.3% Triton X-100 (PBS-t) at 4°C. Tissue was incubated in mouse anti 5-HT2A (diluted 1:500, Pharmingen) or mouse anti 5-HT2C (diluted 1:250, Pharmingen) for 48-72 h at 4°C, then washed three times in PBS-t for 30 min each time at RT. Tissue was then incubated in biotinylated donkey anti-mouse (diluted 1:250, Jackson Labs) for 1.5 h at RT and then washed three times for 20 min each in PBS-t. After this, tissue was incubated in extravidin-Cy3 (Sigma) diluted 1:1,000 for 1.5 h, washed once for 20 min in PBS-t, followed by washing two times for 20 min each in 50 mM Tris-HCl. Finally, slides were coverslipped with Vectashield (Vector Labs) and photographed with either a Nikon E-800 Microscope using a DXM-1200 Digital Camera or scanned using a BioRad MRC-600 confocal microscope on a Zeiss axiovert-135 inverted microscope.
Data analysis
In determining differences between control and drug conditions, the evoked responses from the last 10 min of each condition were compared to ensure that the full drug effect was measured. Population means were determined as a percentage change compared with the control condition. Figures typically show an example of the response as well as the population histograms. Values are given as means ± SE. LLFR and LLDR were considered to have occurred when the area under the curve of the rectified responses remained significantly different from the control baseline reflex response for ≥1 h after washout (SigmaPlot and SigmaStat; SPSS Science, Chicago, IL). Normality was tested (P ≤ 0.05) and paired t-test or ANOVAs were used unless normality failed, in which case nonparametric tests were used. All differences were considered significant if P < 0.05. There was no statistical difference in the degree of facilitation after washout of DOI when comparing hemisects obtained from the same animal to those obtained from different animals (P ≤ 0.05, n ≥ 12) and thus the data from all hemicords were pooled together. Unless otherwise stated, experiments were conducted on the Sprague-Dawley rat substrain obtained from North Carolina.
RESULTS
The purpose of these experiments was to characterize the actions of 5-HT receptor activation on long-lasting changes in the input-output properties of spinal segmental reflexes. The sample population consisted of 186 hemisected spinal cords obtained from 136 animals. Control experiments were run to define the general properties of afferent fiber recruitment as well as to test the variability of evoked spinal reflexes elicited by electrical stimulation of afferents in the dorsal roots. Two suction electrodes, one for stimulating and one for recording, were placed on the same dorsal root (usually L5) at a mean distance of approximately 4 mm apart. It was previously reported that electrical stimulation of peripheral nerves progressively recruited Aβ, Aδ, and C fiber volleys with stimulus parameters of 50 μA and 50 μs, 500 μA and 50 μs, and 500 μA and 500 μs, respectively (Thompson et al. 1990). Although this was also commonly observed in our sample with electrical stimulation of dorsal roots, in many animals C fibers could also be recruited at intensities lower than 500 μA and 500 μs but never at an intensity of 50 μA and 50 μs. Therefore although we did not determine stimulation as a multiple of threshold for each experiment we are confident that we were not recruiting C fibers at 50 μA and 50 μs. Afferent conduction velocity, estimated from onset of stimulus artifact to the first peak negativity of the triphasic volley, was 2.3 and 0.24 m/s, respectively, for the fastest arriving A fibers and C fiber components. The C fiber conduction velocity is consistent with that previously reported in vitro (Hedo et al. 1999; Lu and Perl 2003). The conduction velocity of Aβ fibers recorded here is similar to but lower than that observed in sciatic nerve recordings in similar aged rats (8 m/s) at RT and much slower than that observed in adult as reported at 36°C (Aβ = 22.4 m/s, Aδ = 8.0 m/s) (Hedo et al. 1999; Park et al. 1999). We assume these differences are temperature dependent.
5-HT receptor-mediated long-lasting changes in reflex strength: substrain comparison
Reflexes were recorded in ventral roots after dorsal root stimulation (typically L5; Fig. 1A). Previously, we observed that after washout of bath-applied 5-HT, long-lasting facilitation of reflexes (LLFR) was observed in 13/14 Sprague-Dawley rats (Machacek et al. 2001). The animals in that study were obtained from the Charles River colony in Montreal, Canada. The present series of experiments began with Sprague-Dawley rats obtained from the Charles River colony in Raleigh, NC. In this population, after 5-HT washout, LLFR was observed in only four of 13 cases (Fig. 1B), whereas a novel observation, a long-lasting depression of reflexes (LLDR), occurred in six of 13 animals (Fig. 1C). Whereas the present report focuses on aspects of 5-HT2 receptor-induced LLFR, the observation that LLDR as well as LLFR can be observed from this colony demonstrates that reflex gain can be modulated upward and downward, affording a bidirectional characterization of 5-HT receptor-induced plasticity in reflex strength (described later).
One explanation for the observed differences in reflex plasticity between the present and previous work is substrain differences. To confirm this, we retested the Sprague-Dawley rats from Montreal and observed a robust LLFR after 5-HT washout in four of five experiments (not illustrated). Additional differences in reflex excitability and plasticity in these substrains are reported below and summarized in Table 1.
Table 1.
Comparison of 5-HT and DOI actions on spinal reflexes between Montreal and Raleigh colonies of Charles River Sprague-Dawley rats
| Colony | 5-HT-Induced LLFR | 5-HT-Induced LLDR | DOI-Induced LLFR | Peak Reflex Strength |
|---|---|---|---|---|
| Montreal | 80% (4/5) | 0% (0/5) | 94% (16/17) | 8/15 below C strength |
| Raleigh | 27% (4/13) | 46% (6/13) | 88% (15/17) | 7/9 at C strength |
| Occurrence of Evoked Reflexes at 50 μA and 50 μs |
||||
|---|---|---|---|---|
| Short-Latency Control | Short Latency After DOI Wash | Longer-Latency Control | Longer Latency After DOI Wash | |
| Montreal | 47% (7/15) | 73% (11/15) | 60% (9/15) | 93% (14/15) |
| Raleigh | 0% (0/9) | 11% (1/9) | 44% (4/9) | 67% (6/9) |
DOI is a potent nonselective 5-HT2A/2C receptor agonist that we previously reported to induce LLFR in five of five animals (36). Figure 1D depicts the time course of DOI-induced LLFR for the present sample population (n = 34) where LLFR was observed in 31/34 hemicords. Unlike the observation with 5-HT, the ability of DOI to induce LLFR appeared to be equally effective for both substrains, producing LLFR in 15/17 from Raleigh and 16/17 from Montreal (Table 1; 3rd data column).
We next compared the effects of DOI on both substrains at two constant-current stimulus intensities that recruit a subpopulation of Aβ fibers or fully recruit all afferents (Fig. 2, A and B). In comparison to the Raleigh colony, spinal cord reflex excitability in the Montreal colony was significantly greater by several measures (Table 1). For example, at stimulus strength of 50 μA and 50 μs, which usually recruited only a small portion of the Aβ fiber volley, the Montreal colony has a greater incidence of evoked short- and long-latency reflexes both before and after DOI application. Because the longlatency reflexes were observed when only a small component of the fastest DR volley was recruited, these actions must be mediated by Aβ afferents. Interestingly, after DOI, in 27 and 33% of the Montreal animals, short- and long-latency reflexes were unmasked, respectively, at a stimulation intensity that recruits only a small portion of Aβ fibers (see Fig. 2B, left). Overall, comparing the two populations, in the Montreal colony, reflexes are more easily evoked and Aβ fiber-mediated reflexes are also more likely to be facilitated after 5-HT2 receptor activation (Table 1).
Fig. 2.
Comparison of facilitatory actions of DOI at 2 stimulation intensities. A and B: top waveforms in first 3 rows are recordings of L5 ventral root activity at the indicated intensities whereas last row is a recording of the dorsal root to identify recruited afferent volleys. Examples represent 5 raw traces superimposed. A: at left, short- and long-latency reflexes are evoked after selective recruitment of Aβ fibers (at 50 μA, 50 μs). Although after DOI washout LLFR is observed at all stimulus intensities, the greatest relative facilitation occurs at the low stimulation strength. B: in this example, reflexes are absent at a low stimulus intensity in control and DOI conditions but both short- and long-latency reflexes become unmasked after DOI washout. Note that in A and B recruitment of C fibers (right) does not enhance LLFR. C: under control conditions, stimulation of the L5 dorsal root (500 μA, 500 μs, 1/30 s) does not evoke reflexes in the L2 ventral root (top row, right column). However, after application of DOI and its wash, L5 dorsal root stimulation now evokes heterosegmental reflexes in the L2 ventral root (bottom row, right column). Gray bars indicate timing for the monosynaptic reflex. Note that there was no evidence of a heterosegmentally evoked monosynaptic reflex. Examples represent 20 superimposed raw traces.
Afferent fibers involved in LLFR
We next compared different stimulation intensities in relation to the DOI-induced LLFR in animals in which we recorded afferent fiber volleys. In 11/11 animals, recruitment of a small fraction of Aβ fibers alone (at 50 μA and 50 μs) produced a reflex that was susceptible to DOI-induced LLFR (Fig. 2, A and B, left). Indeed, even when much greater stimuli were delivered that maximally recruit Aβ fibers and also recruit Aδ and C fibers (Fig. 2, A and B, right) the early-latency-facilitated reflex amplitude was not substantially greater (examined in nine animals at 500 μA and 500 μs). Thus within the reflex period examined, the reflex facilitated by DOI is mediated predominantly by Aβ fibers. Therefore to ensure maximal recruitment of Aβ fibers, all of the following experiments were conducted at a stimulation intensity of 500 μA and 500 μs. In 31 animals with DOI-induced LLFR, long-latency components were always observed and were facilitated. Short-latency monosynaptic reflexes were observed in 27/31 of these animals, 25/27 of which were facilitated by DOI. In the four animals initially without monosynaptic reflexes, short-latency reflexes emerged in three animals after DOI. Thus the predominantly Aβ-mediated short- and longer-latency reflexes appear to be equally facilitated. Thus for the subsequent experiments where reflexes are quantified, monosynaptic and longer-latency actions are grouped together (the first 100 ms of the reflex after the stimulation artifact).
Heterosegmental actions between L2 and L5 spinal segments
In eight animals both L5 and L2 dorsal roots were stimulated at 500 μA and 500 μs (800-ms delay between stimuli) while simultaneously recording from L5 and L2 ventral roots. Before application of DOI, long-latency heterosegmental reflexes observed in the L5 ventral root after dorsal root stimulation of L2 were seen in one of eight animals. Similarly, heterosegmental actions in L2 were observed after stimulation of L5 in two of eight animals. Subsequent to DOI, heterosegmental actions were observed in eight of eight animals after stimulation of L2 and in five of eight cases after stimulation of L5. The average latency for evoked reflexes between these segments was 24.7 ± 4.1 ms. Because the heterosegmental actions did not include short-latency, presumably monosynaptic reflexes (measured as 10.5 ± 1.3 ms intrasegmentally), the facilitation is likely attributable to an increased excitability in interposed interneuronal pathways (Fig. 2C).
Facilitated reflexes are not the result of activity-dependent homosynaptic strengthening
Previously, reflexes were obtained using stimulation of dorsal root afferents at low frequency (0.02 Hz) (Machacek et al. 2001). At this frequency, it may be suggested that the 5-HT-induced changes are independent of stimulation-evoked intrinsic network synaptic activity. In the present study, to further minimize a contribution from electrically evoked reflex recruitment to the observed plasticity, only three to five stimuli were delivered per hour to capture reflex strength before, during, and after DOI application (Fig. 2, A and B). Under these conditions, marked (62% increase) and long-lasting increases in reflex strength were still observed (n = 18/19: 14/15 Montreal colony and 4/4 Raleigh colony). Thus the induction of DOI-induced alterations in reflex strength appears to occur by mechanisms dependent on 5-HT receptor activation, but independent of coincident activity of the reflex circuit.
Spinal distribution of 5-HT2C and 5-HT2A receptors
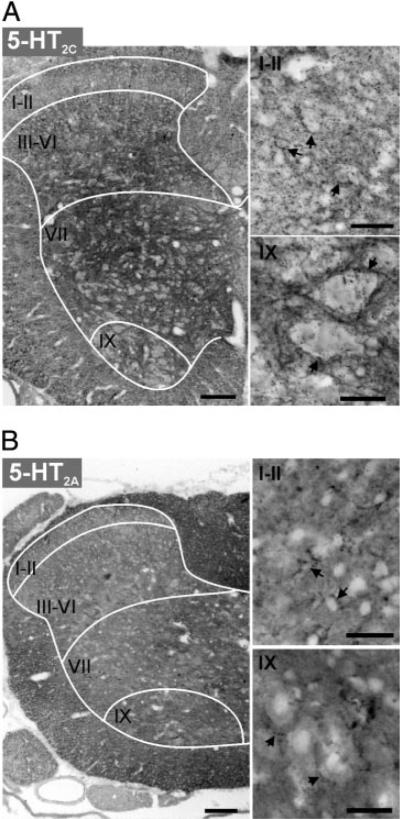
Although pharmacological studies clearly suggest that LLFR can be induced by 5-HT2C receptor activation for its induction (Machacek et al. 2001) a contribution from 5-HT2A receptors cannot be ruled out. For example, the most robust LLFR is evoked after washout of DOI, a potent 5-HT2A/2C agonist. Therefore using immunocytochemistry, we compared the distribution of 5-HT2C and 5-HT2A receptors in the lumbar spinal cord of six P14 rats. Figure 3 provides a representative example of the labeling pattern observed. Note that 5-HT2C receptor labeling appears to predominate in the gray matter, with greatest labeling density in the deep dorsal horn, intermediate gray matter, and motor nuclei. The labeling is punctate, intense, and includes perisomatic labeling (Fig. 3A). In com-parison, 5-HT2A receptor labeling is dominant in the white matter and is not clearly juxtaposed to neuronal cell somas (Fig. 3B), although labeling in some neurons cannot be ruled out. Thus the actions of DOI are likely to be by 5-HT2C receptor activation. The prominence of 5-HT2C receptor labeling in the intermediate gray matter and ventral horn coincides with the location of many interneurons and motoneurons associated with the coordination of motor activity (Baldissera et al. 1981; Goulding and Pfaff 2005; Jankowska 1992, 2001). In addition, Aβ fibers terminate in these regions (Willis Jr and Coggeshall 1991), consistent with our electrophysiologic observations of an effect predominantly mediated by low-threshold afferents.
Fig. 3.
5HT2C and 5-HT2A receptor labeling in lumbar spinal cord of a P14 rat. For both 5-HT2C (A) and 5-HT2A receptors (B), left panel presents low power photomicrograph of one side of a spinal cord transverse section (scale bar = 100 μm) and right panels are confocal images displaying labeling at higher magnification (scale bar = 25 μm) of superficial dorsal horn (laminae I-II) and ventral horn lamina IX (top and bottom, respectively). Images are presented as grayscale negatives. Drawn white lines approximate white matter/ gray matter border and major divisions of Rexed's laminae. A: 5-HT2C receptor labeling is strongly expressed in the deep dorsal horn (lamina III-VI) intermediate lamina VII and motor nuclei (lamina IX). Arrows in right panels point to cell somas with perisomatic punctate labeling in dorsal horn (top) and lamina IX neurons in ventral horn (bottom). B: 5-HT2A receptor labeling is strongest in the white matter. Note that labeling in the gray matter is uniformly weak. Arrows point to punctate labeling that is not clearly juxtaposed to cell somata in the superficial dorsal horn (laminae I-II) and in the motor nucleus (lamina IX).
Effects of GABAA and glycine receptor antagonists
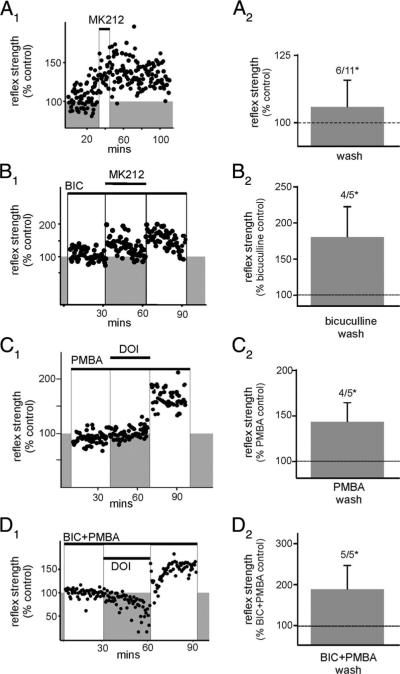
The observed increases in reflex strength could result from a reduction in the actions of inhibitory interneurons, either interposed in reflex pathways or producing a presynaptic inhibition at excitatory synapses. Thus we tested the contribution of inhibitory interneurons to the evoked plasticity. Instead of DOI we chose to examine the actions of the selective but partial 5-HT2C receptor agonist MK212 (Porter et al. 1999). MK212, a weak partial agonist, was chosen instead of DOI in these experiments because the occurrence and strength of the evoked LLFR is much smaller (see Table 2) and thus should provide a better model to examine the contributory effects of a disinhibited spinal cord. Spinal reflexes were examined in 11 experiments with the Raleigh colony of rats. MK212 produced LLFR in six of 11 cases (Fig. 4).
Table 2.
Summary of data for all conditions
| Condition | Percentage Change From Control | n |
|---|---|---|
| DOI | 162 ± 14 | 31/34 |
| MK212 + bicuculline | 181 ± 42 | 4/5 |
| DOI + AP5 | 148 ± 8 | 3/3 |
| DOI + CNQX | 141 ± 16 | 3/3 |
| DOI + kynurenate | 179 ± 24 | 4/4 |
| DOI + PMBA | 142 ± 21 | 4/5 |
| DOI + bicuculline + PMBA | 187 ± 58 | 5/5 |
| MK212 or DOI + bicuculline + AP5 | 140 ± 27 | 7/12 |
| MK212 | 106 ± 10 | 6/11 |
Values are means ± SE; n represents the fraction of total cords showing long-lasting facilitation of the reflex, P < 0.05.
Fig. 4.
Blocking inhibitory synaptic transmission is not essential for the induction of LLFR. Stimuli were delivered at 500 μA, 500 μs, 0.03 Hz. A: activation of 5-HT2C receptors with the partial agonist 6-chloro-2-(1-piperazinyl) pyrazine (MK212) induces a weak LLFR. A1: individual experiment demonstrating the most robust LLFR observed after MK212 application. y-axis represents the reflex strength normalized to 100%. A2: mean percentage change in reflex strength after MK212 washout (n = 11), expressed as a percentage of control. LLFR was observed in 6/11 cases, P < 0.05. B: after the block of GABAA receptors with bicuculline, a profound increase in reflex facilitation after washout of MK212 is observed. B1: individual experiment demonstrating MK212-induced LLFR while in the presence of bicuculline (compare episodes in white boxed regions). B2: mean percentage change in reflex strength after MK212 washout but still in the presence of bicuculline, expressed as a percentage of bicuculline control (LLFR in 4/5, P < 0.05). C: DOI is capable of inducing LLFR during block of glycine receptors with phenylbenzene-ωphosphono-α-amino acid (PMBA). C1: individual experiment demonstrating DOI-induced LLFR while in the presence of PMBA (compare white boxes). C2: mean percentage change in reflex strength after DOI washout but still in the presence of PMBA, expressed as a percentage of PMBA control (LLFR in 4/5, P < 0.05). D: DOI induces LLFR during simultaneous blockade of both GABAA and glycine receptors. D1: individual experiment demonstrating DOI-induced LLFR in the presence of both bicuculline and PMBA (compare white boxes). D2: mean percentage change in reflex strength after DOI washout but still in the presence of bicuculline + PMBA, expressed as a percentage of bicuculline + PMBA control (LLFR in 5/5, P < 0.05). LLFR in these and all other figures was determined by comparing control to washout with a paired t-test, stimulation was applied at 500 μA, 500 μs and the first 100 ms after stimulation artifact were quantified as described in METHODS.
To determine whether a decrease in activity from GABAergic inhibitory interneurons mediated LLFR, experiments were conducted with bicuculline already in the bath before 5-HT2C receptor activation. In the presence of bicuculline, application then removal of MK212 with bicuculline produced LLFR in four of five cases (Fig. 4B). The reflex facilitation produced by MK212 in the absence of γ-aminobutyric acid type A (GABAA) receptor activity was greater than that produced by MK212 alone (P < 0.05). This suggests that GABAergic mechanisms limit the strength of 5-HT2C receptor-mediated reflex enhancement. To study the possible contribution of glycinergic inhibition, we used PMBA as a glycine receptor antagonist (Hosie et al. 1999). The more commonly used glycine receptor antagonist strychnine was not used because it produced irreversible increases is spinal reflex excitability, probably arising from its membrane permeance and consequent block of various ion channels (Lee et al. 1975; Shapiro 1977). However, experiments with PMBA suggested that it might be blocking MK212 actions on the 5-HT2 receptor. To overcome this, we applied the full agonist DOI at 10 μM, and under these conditions, LLFR was observed in four of five instances in the presence of glycine receptor blockade (Fig. 4C). Further, when both GABAA and glycine receptors were blocked simultaneously, DOI induced LLFR in five of five cases (Fig. 4D). Thus neither GABAA nor glycine receptor activity is required for the induction of 5-HT2 receptor-mediated increases in reflex strength.
Effects of NMDA and non-NMDA receptor antagonists
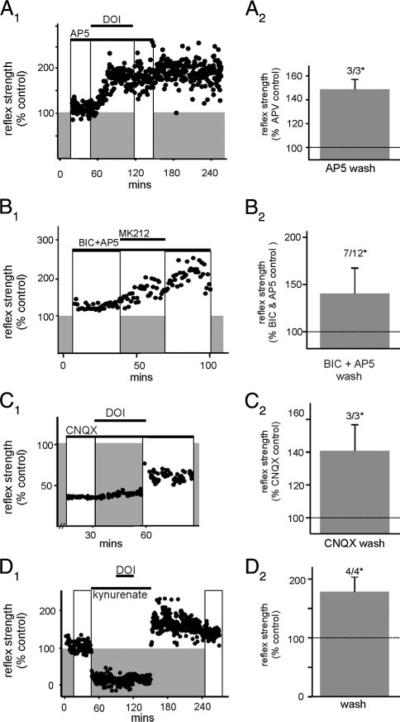
We next studied whether 5-HT2A/2C receptor-induced LLFR requires activation of N-methyl-D-aspartate (NMDA) and non-NMDA ionotropic glutamate receptors. Activation of 5-HT2A/2C receptors with DOI in the presence of the NMDA receptor antagonist AP5 induced LLFR in three of three cases (Fig. 5A). In other experiments we preincubated the spinal cord in bicuculline to better isolate reflex modulation resulting from alterations in glutamatergic synaptic activity (Fig. 5B). Subsequent addition of AP5 depressed longer-latency reflexes in 11/12 cases while leaving the short-latency reflex component largely unchanged (not shown). Comparison of reflex responses in the presence of AP5/bicuculline before and after activation of 5-HT2 receptors with MK212 or DOI revealed LLRF in seven of 12 cases. Thus 5-HT2 receptor activation can potentiate non-NMDA receptor reflex components independent of NMDA receptor activity and this facilitation cannot be based entirely on a reduction in tonic GABAA receptor activity (Fig. 5B). Experiments were performed where DOI was added in the presence of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonist CNQX. In five of five cases CNQX depressed both short- and reducedlatency reflex components. Addition of DOI did not alter the reflex blocking actions of CNQX and after removal of all drugs, reflexes reemerged slowly, generally returning to control values. Even when inhibitory input was blocked with bicuculline, addition of 1 μM DOI in the presence of CNQX (10 μM) failed to cause LLFR (n = 3). Interestingly, when a higher concentration of DOI (10 μM) was used, LLFR emerged (three of three; Fig. 5C). Because increased concentrations of DOI overcome the CNQX effect, it is possible that CNQX blocks 5-HT2 receptors as well as its commonly accepted block of AMPA/kainate receptors. In support of this, in the presence of NMDA and AMPA/kainate the receptor antagonist kynurenic acid (1 mM), which completely blocked reflex activity, 1 μM DOI caused a robust LLFR after its washout (four of four; Fig. 5D). In total, these results suggest that facilitated responses include contributions from both NMDA and AMPA/kainate receptors but that neither is required for the induction of LLFR.
Fig. 5.
Ionotropic glutamate receptors are not required for the induction of LLFR and N-methyl-D-aspartate (NMDA) or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor activity alone is sufficient for the expression of LLFR. A1: scatterplot of reflex strength demonstrating that, in the presence of D-2-amino-5-phosphonovaleric acid (AP5), DOI is still capable of inducing LLFR (compare boxed regions). A2: graph showing mean reflex strength after DOI washout but still in the presence of AP5 (n = 3) expressed as a percentage of AP5 control (LLFR in 3/3, P < 0.05). B1: scatterplot of MK212-induced LLFR in the presence of bicuculline and AP5. B2: effect of MK212 or DOI on mean reflex strength (n = 12) after agonist washout but still in the presence of bicuculline/AP5, expressed as a percentage of bicuculline/AP5 control (LLFR in 7/12, P < 0.05). C1: scatterplot of DOI-induced LLFR in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). C2: effect of DOI on mean reflex strength after DOI washout but still in the presence of CNQX (n = 3) normalized to that observed in CNQX before DOI application (LLFR in 3/3, P < 0.05). D1: scatterplot showing actions of DOI in the in the presence of kynurenate. Note that the DOI-induced LLFR is only observed once kynurenate is removed from the bath (compare white boxes). D2: effect of DOI on reflex strength normalized to control, after washout of both DOI and kynurenate (LLFR in 4/4, P < 0.05).
Interconvertibility of LLFR and LLDR by receptor-selective activation
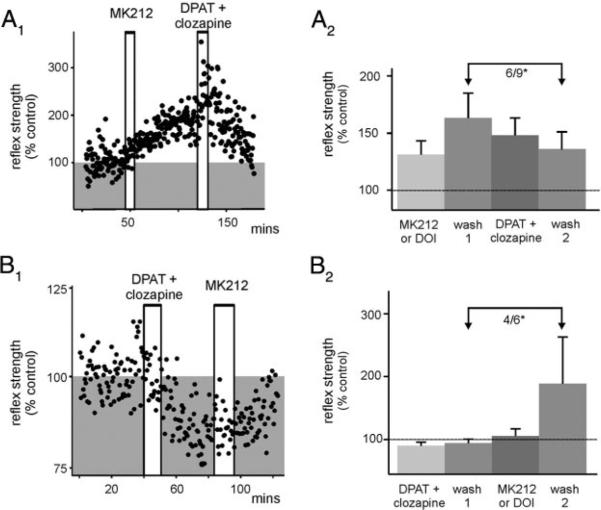
Although 5-HT2 receptors generally facilitate spinal neural responsiveness, 5-HT1 receptor activation usually depresses neural activity (see Hochman et al. 2001). Thus we tested whether 5-HT2-mediated LLFR could be reversed with activation of 5-HT1 receptors and vice versa. Interestingly, when 5-HT1A receptors were selectively activated with the 5-HT1A/7 receptor agonist DPAT (in the presence of clozapine to block DPAT's actions at 5-HT7 receptors) the 5-HT2A/2C recepto-rmediated LLFR was partly reversed in seven of nine cases (six of nine significantly; Fig. 6A). Conversely, 5-HT1A receptor activation induced a long-lasting reflex depression (LLDR; four of six) that was reversed with subsequent 5-HT2A/2C receptor activation (six of six), and even increased reflex strength to LLFR (wash 2 compared with wash 1) in four of six instances (Fig. 6B). Thus reflex strength can be modulated bidirectionally depending on the 5-HT receptor subtype activated.
FIG. 6.
5-HT1 receptor activation can induce a long-lasting reflex depression (LLDR) and can reverse LLFR. A1: Scatterplot showing that MK212-induced LLFR can be reversed after selective 5-HT1A receptor activation for 10-30 min (DPAT + clozapine). A2: effect of 5-HT2 receptor activation (MK212 or DOI) then 5-HT1 receptor activation (DPAT and clozapine) normalized to predrug control reflex amplitude. 5-HT1 receptor activation reversed the 5-HT2 receptor induced LLFR in 6/9 cases (P < 0.05). This is shown with arrows comparing reflex amplitude after MK212 or DOI washout to reflex amplitude after DPAT and clozapine washout. Stimuli were delivered at 500 μA, 500 μs, 0.03 Hz and in one case 500 μA, 100 μs, 0.03 Hz. B1: scatterplot example showing that after selective activation of 5-HT1A receptors [8-hydroxy-2-(dipropylamino)-tetralin (8-OH-DPAT; 5-HT1A/7 agonist) and clozapine (5-HT7 antagonist)], LLDR is observed which can be reversed after MK212 application. B2: effect of DPAT + clozapine on reflex normalized to control (LLDR in 4/6, P < 0.05), then after application of DOI (or MK212), normalized to wash 1 (6/6 reversed but LLFR in 4/6, P < 0.05). This is shown with arrows comparing reflex amplitude after DPAT and clozapine washout to reflex amplitude after MK212 or DOI washout. Stimuli were delivered at 500 μA, 500 μs, 0.03 Hz; and in one case 100 μA, 100 μs, 0.03 Hz.
DISCUSSION
We observed that transient 5-HT2 receptor activation in the juvenile isolated rat spinal cord can result in enhanced reflex strength that is maintained for ≥1 h. Although not explored here, LLFR could be observed even 12 h after its induction (data not shown). Reflex facilitation commonly required removal of the agonist for expression. After washout, 5-HT2A/2C receptor-mediated LLRF was produced regardless of whether there is concurrent antagonism of ionotropic excitatory glutamatergic and/or inhibitory GABAergic/glycinergic receptor activity. Therefore although the reflexes themselves appear to be mediated by glutamate, induction and maintenance of LLFR are independent of classic, fast transmission ionotropic receptor activity. 5-HT2A/2C receptor-induced LLFR also appeared to be independent of other mechanisms associated with afferent-evoked activity because it was observed when measured with as few as three stimuli per hour. The magnitude of the induced LLFR was limited by GABAergic inhibitory actions because block of GABAergic actions considerably increased the degree of reflex enhancement. These results are summarized in Table 2.
An important additional observation was that 5-HT could also induce a long-lasting depression of reflexes (LLDR), mimicked by activation of 5-HT1A receptors, and that LLFR and LLDR are interconvertible with 5-HT2A/2C and 5-HT1A receptor agonists, respectively. Therefore the serotonergic system has the capacity to induce long-lasting bidirectional changes in reflex strength.
Facilitatory effects of serotonin in spinal cord
Serotonergic neurons are not intrinsic to the spinal cord so modulatory actions normally arise from activity in several descending bulbospinal systems (e.g., raphe nuclei; see Hochman 2001 for review; however, see Newton and Hamill 1988). Both facilitatory and depressant actions have been reported with a heuristically attractive but oversimplified scheme, suggesting that 5-HT inhibits sensory and promotes motor activity (Hochman et al. 2001; Jacobs and Fornal 1993). Based on pharmacological evidence in an earlier study, we determined that 5-HT produced a lasting reflex amplification by activation of 5-HT2C receptors (Machacek et al. 2001). Immunohistochemical analysis of 5-HT2C receptor distribution here indicates a relative lack of receptors in the superficial dorsal horn (lamina I and II) with much greater labeling in deeper spinal laminae (Fig. 3). Because the majority of nociception-encoding C fibers terminate in laminae I and II (Willis Jr and Coggeshall 1991), one would expect that the 5-HT2 receptor-induced reflex facilitation does not primarily involve nociceptive afferent input. Indeed, the 5-HT2A/2C receptor-induced facilitation studied here was primarily mediated by Aβ, low-threshold afferent fibers, which project to 5-HT2C receptor-rich deeper spinal laminae. 5-HT2C receptors are thought to be postsynaptic on the somatodendritic membrane (Leysen 2004), consistent with the presently observed spinal perisomatic labeling.
Irrespective of the substrain differences observed, both the short- and longer-latency (presumably polysynaptic) reflex responses appear to be facilitated by 5-HT2A/2C receptor activation after agonist washout. Because in some instances only the longer-latency reflex component was facilitated, whereas the monosynaptic reflex was unchanged, the modulation of spinal cord reflex excitability is unlikely to simply reflect changes in motoneuron properties (Bennett et al. 1998; Carlin et al. 2000; Lee and Heckman 1998; Perrier and Hounsgaard 2003). Indeed, the emergence of long-latency heterosynaptic actions between L2 and L5 spinal segments after 5-HT2A/2C receptor activation support an increased excitability through interneuronal pathways. Although some heterosynaptic facilitatory actions could be caused simply by a lasting postsynaptic motoneuronal depolarization, previous recordings demonstrate a corresponding facilitated convergent input onto interneurons from distant spinal segments (Machacek et al. 2001). Moreover, unlike the long-lasting actions currently described, previous in vitro studies on motoneuron properties demonstrated that the actions of 5-HT or 5-HT2 receptor agonists recover after agonist removal (Berger and Takahashi 1990; Elliott and Wallis 1992; Elliott et al. 1999; Takahashi and Berger 1990; Wang and Dun 1990). The involvement of interneurons in increased reflex excitability was also suggested by observing long-lasting increases in excitatory postsynaptic potential (EPSP) amplitudes in a subpopulation of laminae IV-VII neurons (Machacek et al. 2001). Moreover, complementary studies in cat have demonstrated that polysynaptic flexion and crossed-extension reflexes are facilitated by DOI, and in a temporally distinct manner from facilitated stretch reflexes (Machacek et al. 2000). Thus the heterosegmental facilitation of reflexes observed here is consistent with spinal plasticity at the interneuronal level. Intracellular recordings from motoneurons and interneurons will be required to better clarify the relative contribution of pre- versus postsynaptic mechanisms to the production of LLFR.
Because the 5-HT2 receptor mechanisms inducing reflex plasticity were observed to be independent of activity in the afferent fibers reporting LLFR, plasticity occurs by nonassociative mechanisms. In a similar manner, 5-HT2 receptors have been reported to be involved in long-lasting facilitated postsynaptic responses in neonatal rat hypoglossal motoneurons by activity-independent mechanisms (Bocchiaro and Feldman 2004). 5-HT2 receptor activity may also be responsible for facilitation of other synaptic responses in spinal cord independent of synaptic activation. For example, 5-HT2 receptor activation has been found to be involved in activating protein kinase C (PKC) and phosphorylating NMDA receptors, thereby potentiating NMDA receptor-mediated ion currents (Blank et al. 1996) and activation of PKC, which has been implicated in persistent pain and hyperalgesia (Wajima et al. 2000; Yashpal et al. 1995).
Effects of GABAA and glycinergic disinhibition
LLFR was observed after blockade of GABAA and/or glycine receptors. This demonstrated that alterations in inhibitory mechanisms are not required to induce or maintain LLFR. Also, because the strength of the evoked LLFR was much greater when GABAA receptors were blocked, factors associated with GABAergic inhibition appear to be able to limit LLFR strength. Although not explored it is possible that 5-HT2 receptor activation also facilitates transmission through inhibitory pathways.
Because temporary increases in network excitability, such as with GABAA or glycine receptor block (bicuculline and PMBA, respectively), could predispose activated synapses toward long-lasting changes in excitability, it was critical to compare 5-HT2A/2C receptor LLFR in experiments where GABAA or glycine receptor blockade was present throughout the test period. Indeed, PMBA and bicuculline on their own were observed to be capable of producing long-lasting increases in reflex strength after washout (not shown). These effects are unlikely attributable to incomplete drug washout because, in a similar preparation, these drugs are reversible with washout when examining other spinal mechanisms (Cowley and Schmidt 1995; Kremer and Lev-Tov 1997; Sajovic and Levinthal 1983).
NMDA and non-NMDA receptor-mediated facilitation
Evoked reflexes include non-NMDA (AMPA/kainate) receptor-mediated monosynaptic and NMDA and non-NMDA receptor-mediated polysynaptic reflexes (Evans 1989; King et al. 1992). We had previously demonstrated that both components underwent a 5-HT receptordependent LLFR (Machacek et al. 2001). Here, initial experiments using the quinoxalinedione CNQX to block AMPA/kainate receptors prevented DOI-induced LLFR. However, radioligand binding assays suggest that various quinoxalinediones compete for binding at 5-HT receptors (Soskic and Joksimovic 1998). We thus increased the concentration of DOI to 10 μM to surmount a possible competitive antagonism and, under these conditions, LLFR was observed. Furthermore, kynurenic acid, a broad-spectrum ionotropic glutamate receptor antagonist that blocks both AMPA/ kainate and NMDA receptors, blocked reflex activity while in the bath, but did not prevent DOI-induced LLFR after washout. Together these results clearly demonstrate that 5-HT2A/2C receptor-induced facilitation occurred independently of NMDA and AMPA/kainate receptor activation.
Properties of 5-HT2 receptors and possible mechanisms producing enhanced facilitation after agonist removal
Once activated, the DOI-induced LLFR is maintained even after the addition of normethyl-clozapine (Machacek et al. 2001) to block 5HT2C receptors. Thus maintenance of LLFR is not dependent on agonist-based continued activation of 5HT2C receptors. Indeed, in the majority of experiments, reflex facilitation required removal of agonist for expression. Because 5-HT2A/2C receptor-induced LLFR occurred after activation with its endogenous ligand (5-HT), the long-lasting changes do not arise from unusual properties of an exogenous agonist. Interestingly, DOI induced LLFR much more robustly than MK212 and it is known that different 5-HT2 receptor agonists can bias activation toward different signal transduction pathways (Berg et al. 1998). In this regard the actions of DOI appear to better approximate the actions of the endogenous ligand 5-HT. The different results obtained between DOI and MK212 may also partly be explained by the partial agonist activity of MK212 at 5-HT2C receptors versus the full agonist activity of DOI (Jerman et al. 2001) and an involvement of 5-HT2A receptors to the DOI-induced reflex enhancement cannot be ruled out (Eide and Hole 1993; Hori et al. 1996; Lewis and Coote 1993).
Although the mechanism behind increased facilitation after removal of agonist is currently unknown, the complex signaling properties of 5-HT2 receptors are implicated (Leysen 2004; Van Oekelen et al. 2003). The 5-HT2C receptor is capable of coupling to several G proteins and consequently multiple signal transduction cascades. They are: 1) phospholipase C (PLC) activation by Gq, 2) phospholipase A2 (PLA2) by Gαi/o associated Gβ,γ-mediated activation of extracellular signal related kinases (ERKs), 3) p38 by Gα12,13, and 4) phospholipase D activation by Gα13 and free Gβ-γ (Leysen 2004). Further-more, 5-HT2C receptors can become constitutively active (Grotewiel and Sanders-Bush 1999). Although these complex properties may help explain the unusual properties of facilitation after agonist unbinding, further studies are necessary to identify the cellular mechanisms involved.
Modulation of reflex strength is bidirectional
To be behaviorally relevant the observed lasting changes in reflex strength should be bidirectional to maintain reflex pathway operation within a normal range of sensorimotor gain. Therefore an important observation was that 5-HT can also induce a long-lasting reflex depression (LLDR). Moreover, pharmacological studies demonstrated that LLFR and LLDR are interconvertible by 5-HT2A/2C and 5-HT1A receptor activation, respectively. Therefore the serotonergic system has the demonstrated capacity to induce long-lasting bidirectional changes in reflex strength. In these experiments, the facilitatory actions produced by 5-HT2A/2C receptor activation were stronger than the depressant actions produced by activation of 5-HT1A receptors. Even though the 5-HT receptor pharmacology responsible for LLFR has been clearly determined to be by 5-HT2C receptor activation (Machacek et al. 2001), we have yet to thoroughly investigate the 5-HT receptors responsible for LLDR. Therefore it is possible that other 5-HT receptors are capable of producing LLDR or acting in a complementary manner with 5-HT1A receptors to produce a more profound depression. It is also possible that LLDR is more strongly induced when spinal cord reflexes are already in a facilitated state. These observations warrant further study.
Overall, these experiments suggest that serotonergic systems can be used to produce lasting changes in reflex gain. These changes appear to be able to act like a slow switch to either increase or decrease reflex excitability. We have not attempted to determine the temporal limits of the 5-HT receptor activation required to induce reflex plasticity. However, a reflex plasticity that requires agonist activity for several minutes would be expected to contribute to long-lasting transitions in excitability as occurs in the sleep/wake cycle, but would not be suited to transient excitability shifts as observed in changing postural and locomotor activities.
Acknowledgments
GRANTS This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-40893-01.
REFERENCES
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Handbook of Physiology. The Nervous System. Motor Control. pt. 1 vol. II. Williams & Wilkins; Baltimore, MD: 1981. Integration in spinal neuronal systems; pp. 509–595. [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Berger AJ, Takahashi T. Serotonin enhances a low-voltage-activated calcium current in rat spinal motoneurons. J Neurosci. 1990;10:1922–1928. doi: 10.1523/JNEUROSCI.10-06-01922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Zwart R, Nijholt I, Spiess J. Serotonin 5-HT2 receptor activation potentiates N-methyl-D-aspartate receptor-mediated ion currents by a protein kinase C-dependent mechanism. J Neurosci Res. 1996;45:153–160. doi: 10.1002/(SICI)1097-4547(19960715)45:2<153::AID-JNR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Effects of inhibitory amino acid antagonists on reciprocal inhibitory interactions during rhythmic motor activity in the in vitro neonatal rat spinal cord. J Neurophysiol. 1995;74:1109–1117. doi: 10.1152/jn.1995.74.3.1109. [DOI] [PubMed] [Google Scholar]
- Crick H, Wallis DI. Inhibition of reflex responses of neonate rat lumbar spinal cord by5-hydroxytryptamine. Br J Pharmacol. 1991;103:1769–1775. doi: 10.1111/j.1476-5381.1991.tb09861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E. An electrically isolated stimulator for neurophysiological research. Acta Physiol Scand. 1972;84:3. (Abstract) [Google Scholar]
- Eide PK, Hole K. The role of 5-hydroxytryptamine (5-HT) receptor subtypes and plasticity in the 5-HT systems in the regulation of nociceptive sensitivity. Cephalalgia. 1993;13:75–85. doi: 10.1046/j.1468-2982.1993.1302075.x. [DOI] [PubMed] [Google Scholar]
- Elliott P, Wallis DI. Serotonin and L-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuro-science. 1992;47:533–544. doi: 10.1016/0306-4522(92)90163-v. [DOI] [PubMed] [Google Scholar]
- Elliott P, Wallis DI, Foster GA, Stringer BMJ. Ionic mechanisms underlying excitatory effects of serotonin on embryonic rat motoneurons in long-term culture. Neuroscience. 1999;90:1311–1323. doi: 10.1016/s0306-4522(98)00534-x. [DOI] [PubMed] [Google Scholar]
- Evans RH. The pharmacology of segmental transmission in the spinal cord. Prog Neurobiol. 1989;33:255–279. doi: 10.1016/0301-0082(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr Opin Neurobiol. 2005;15:14–20. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Grillner S, Williams T, Lagerback PA. The edge cell, a possible intraspinal mechanoreceptor. Science. 1984;223:500–503. doi: 10.1126/science.6691161. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Sanders-Bush E. Differences in agonist-independent activity of 5-Ht2A and 5-HT2c receptors revealed by heterologous expression. Naunyn-Schmiedebergs Arch Pharmacol. 1999;359:21–27. doi: 10.1007/pl00005318. [DOI] [PubMed] [Google Scholar]
- Hedo G, Ajubita M, Lopez-Garcia JA. Role of serotonin 1A receptors on the modulation of rat spinal mono-synaptic reflexes in vitro. Neurosci Lett. 2002;334:41–44. doi: 10.1016/s0304-3940(02)01064-9. [DOI] [PubMed] [Google Scholar]
- Hedo G, Laird JM, Lopez-Garcia JA. Time-course of spinal sensitization following carrageenan-induced inflammation in the young rat: a comparative electrophysiological and behavioural study in vitro and in vivo. Neuroscience. 1999;92:309–318. doi: 10.1016/s0306-4522(98)00734-9. [DOI] [PubMed] [Google Scholar]
- Hedo G, Lopez-Garcia JA. 5-HT(1B) but not 5-HT(6) or 5-HT(7) receptors mediate depression of spinal nociceptive reflexes in vitro. Br J Pharmacol. 2002;135:935–942. doi: 10.1038/sj.bjp.0704526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL. Motor Neurobiology of the Spinal Cord. CRC Press; Boca Raton, FL: 2001. 5-HT receptors and the neuromodulatory control of spinal cord function; pp. 47–87. [Google Scholar]
- Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holohean AM, Hackman JC, Davidoff RA. Changes in membrane potential of frog motoneurons induced by activation of serotonin receptor subtypes. Neuroscience. 1990;34:555–564. doi: 10.1016/0306-4522(90)90164-y. [DOI] [PubMed] [Google Scholar]
- Hori Y, Endo K, Takahashi T. Long-lasting synaptic facilitation induced by serotonin in superficial dorsal horn neurones of the rat spinal cord. J Physiol. 1996;492:867–876. doi: 10.1113/jphysiol.1996.sp021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Akagi H, Ishida M, Shinozaki H. Actions of 3-[2′-phosphonomethyl[1,1′-biphenyl]-3-yl]alanine (PMBA) on cloned glycine receptors. Br J Pharmacol. 1999;126:1230–1236. doi: 10.1038/sj.bjp.0702402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DA, White SR. Receptor subtypes mediating facilitation by serotonin of excitability of spinal motoneurons. Neuropharmacology. 1990;29:787–797. doi: 10.1016/0028-3908(90)90151-g. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol. 2001;533:31–40. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman JC, Brough SJ, Gager T, Wood M, Coldwell MC, Smart D, Middlemiss DN. Pharmacological characterisation of human 5-HT2 receptor subtypes. Eur J Pharmacol. 2001;414:23–30. doi: 10.1016/s0014-2999(01)00775-0. [DOI] [PubMed] [Google Scholar]
- King AE, Lopez-Garcia JA, Cumberbatch M. Antagonism of synaptic potentials in ventral horn neurones by 6-cyano-7-nitroquinoxaline-2,3-dione: a study in the rat spinal cord in vitro. Br J Pharmacol. 1992;107:375–381. doi: 10.1111/j.1476-5381.1992.tb12754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol. 1997;77:1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- Lee KC, Klee MR, Lee HJ. The effect of strychnine on membrane properties of spinal motoneurons in the cat. Yonsei Med J. 1975;16:1–28. doi: 10.3349/ymj.1975.16.2.1. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lewis DI, Coote JH. The actions of 5-hydroxytryptamine on the membrane of putative sympatho-excitatory neurones in the rostral ventrolateral medulla of the adult rat in vitro. Brain Res. 1993;609:103–109. doi: 10.1016/0006-8993(93)90861-g. [DOI] [PubMed] [Google Scholar]
- Lewis DI, Sermasi E, Coote JH. Excitatory and indirect inhibitory actions of 5-hydroxytryptamine on sympathetic preganglionic neurones in the neonate rat spinal cord in vitro. Brain Res. 1993;610:267–275. doi: 10.1016/0006-8993(93)91410-t. [DOI] [PubMed] [Google Scholar]
- Leysen JE. 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature. 1998;393:695–698. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek DW, Garraway SM, Shay BL, Hochman S. Serotonin 5-HT(2) receptor activation induces a long-lasting amplification of spinal reflex actions in the rat. J Physiol. 2001;537:201–207. doi: 10.1111/j.1469-7793.2001.0201k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek DW, Livingston BP, Hochman S, Nichols TR. Spinal 5-HT2 receptor activation produces long-lasting modulation of the flexor-with-drawal reflex and changes in stiffness regulation. Soc Neurosci Abst. 2000;26:152. [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ. Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol. 1996;75:620–628. doi: 10.1152/jn.1996.75.2.620. [DOI] [PubMed] [Google Scholar]
- Newton BW, Hamill RW. The morphology and distribution of rat serotoninergic intraspinal neurons: an immunohistochemical study. Brain Res Bull. 1988;20:349–360. doi: 10.1016/0361-9230(88)90064-0. [DOI] [PubMed] [Google Scholar]
- Park JS, Nakatsuka T, Nagata K, Higashi H, Yoshimura M. Reorganization of the primary afferent termination in the rat spinal dorsal horn during post-natal development. Dev Brain Res. 1999;113:29–36. doi: 10.1016/s0165-3806(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol. 2003;89:954–959. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajovic P, Levinthal C. Inhibitory mechanism in zebrafish optic tectum: visual response properties of tectal cells altered by picrotoxin and bicuculline. Brain Res. 1983;271:227–240. doi: 10.1016/0006-8993(83)90285-8. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Shapiro BI. Effects of strychnine on the sodium conductance of the frog node of Ranvier. J Gen Physiol. 1977;69:915–926. doi: 10.1085/jgp.69.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soskic V, Joksimovic J. Bioisosteric approach in the design of new dopaminergic/serotonergic ligands. Curr Med Chem. 1998;5:493–512. [PubMed] [Google Scholar]
- Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Miletic V. Diverse actions of 5-hydroxytryptamine on frog spinal dorsal horn neurons in vitro. Neuroscience. 1992;49:913–923. doi: 10.1016/0306-4522(92)90367-b. [DOI] [PubMed] [Google Scholar]
- Thompson SWN, King AE, Woolf CJ. Activity-dependent changes in rat ventral horn neurons in vitro; summation of prolonged afferent evoked postsynaptic depolarizations produce a D-2-amino-5-phosphonovaleric acid sensitive windup. Eur J Neurosci. 1990;2:638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Van Oekelen D, Luyten WH, Leysen JE. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003;72:2429–2449. doi: 10.1016/s0024-3205(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Wajima Z, Hua XY, Yaksh TL. Inhibition of spinal protein kinase C blocks substance P-mediated hyperalgesia. Brain Res. 2000;877:314–321. doi: 10.1016/s0006-8993(00)02714-1. [DOI] [PubMed] [Google Scholar]
- Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol. 1990;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Jr, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 2nd ed. Plenum Press; New York: 1991. [Google Scholar]
- Yamazaki J, Fukuda H, Nagao T, Ono H. 5-HT2/5-HT1C receptor-mediated facilitatory action on unit activity of ventral horn cells in rat spinal cord slices. Eur J Pharmacol. 1992;220:237–242. doi: 10.1016/0014-2999(92)90753-q. [DOI] [PubMed] [Google Scholar]
- Yashpal K, Pitcher GM, Parent A, Quirion R, Coderre TJ. Noxious thermal and chemical stimulation induce increases in 3H-phorbol 12,13-dibutyrate binding in spinal cord dorsal horn as well as persistent pain and hyperalgesia, which is reduced by inhibition of protein kinase C. J Neurosci. 1995;15:3263–3272. doi: 10.1523/JNEUROSCI.15-05-03263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]