Abstract
Androgens have been used in the treatment of bone marrow failure syndromes without a clear understanding of their mechanism of action. Blood counts of patients with dyskeratosis congenita or aplastic anemia with mutations in telomerase genes can improve with androgen therapy. Here we observed that exposure in vitro of normal peripheral blood lymphocytes and human bone marrow–derived CD34+ cells to androgens increased telomerase activity, coincident with higher TERT mRNA levels. Cells from patients who were heterozygous for telomerase mutations had low baseline telomerase activity, which was restored to normal levels by exposure to androgens. Estradiol had an effect similar to androgens on TERT gene expression and telomerase enzymatic activity. Tamoxifen abolished the effects of both estradiol and androgens on telomerase function, and letrozole, an aromatase inhibitor, blocked androgen effects on telomerase activity. Conversely, flutamide, an androgen receptor antagonist, did not affect androgen stimulation of telomerase. Down-regulation by siRNA of estrogen receptor-α (ERα), but not ERβ, inhibited estrogen-stimulated telomerase function. Our results provide a mechanism for androgen therapy in bone marrow failure: androgens appear to regulate telomerase expression and activity mainly by aromatization and through ERα. These findings have potential implications for the choice of current androgenic compounds and the development of future agents for clinical use.
Introduction
Telomere attrition has been associated with the process of normal aging and as etiologic of aneuploid malignancies (in mouse “knockout” models) and of a variety of human diseases (due to mutations in relevant genes).1 Telomeres consist of T2AG3 repeats and proximate proteins located at the end of chromosomes that serve to prevent recombination, end-to-end fusion, and activation of DNA damage responses.2 As DNA polymerase is unable to fully duplicate telomeres during cell division—the “end replication problem”3—telomeres are eroded until reaching critically short lengths, signaling the cell to cease proliferation (cellular senescence) and apoptosis.2 To maintain telomeres, some highly proliferative cells, including hematopoietic progenitor and stem cells, express telomerase (TERT), a specialized reverse transcriptase capable of adding DNA repeats to the 3′ end of telomeric leading strand using an RNA molecule (TERC) as a template. Telomerase also is expressed in the majority of malignant cells of many tissues.4
Abnormal telomere maintenance is a feature of a variety of human diseases. Dyskeratosis congenita, a constitutional type of aplastic anemia, is caused by mutations in genes involved in telomere maintenance (DKC1 is mutated in X-linked dyskeratosis congenita5,6; TERC, TERT, and TINF2 are mutated in autosomal dominant dyskeratosis congenita7–9; and TERT, NOP10, and NHP2 are mutated in autosomal recessive dyskeratosis congenita10,11). Mutations in TERT and TERC also are genetic risk factors for acquired aplastic anemia.12,13 Although most acquired aplastic anemia is the result of an immune process destroying hematopoietic stem and progenitor cells,14 predisposition to the development of marrow failure appears to be conferred by inherited TERT or TERC mutations. These genetic alterations result in low telomerase activity by haploinsufficiency, short telomeres in leukocytes, and reduced hematopoietic function. Of clinical relevance, aplastic anemia patients with telomerase mutations tend to respond poorly to therapy with immunosuppressive drugs.15,16
Androgens have been employed as therapy for marrow failure syndromes since the 1960s, but their mechanism(s) of action on hematopoiesis is not understood. Dyskeratosis congenita17 and acquired aplastic anemia with telomerase complex mutations often respond to treatment with androgens.1 Other bone marrow failure syndromes, such as Fanconi anemia, also may improve with hormonal therapy. There is evidence that androgens control telomerase expression in prostate cancer cells18 and normal reproductive tissues.19 For these reasons, we hypothesized that androgens might act similarly on hematopoietic cells. In the present study, we investigated the effects of sex steroids on telomerase activity and expression in primary blood and marrow cells from healthy persons and telomerase-mutant individuals.
Methods
Peripheral blood mononuclear cell separation and culture
Peripheral blood and bone marrow samples were collected after informed consent was obtained in accordance with the Declaration of Helsinki and research protocol approved by the NHLBI Institutional Review Board. Twenty milliliters of peripheral blood were collected from 2 healthy carriers of TERT codon Ala202Thr mutation and 1 healthy carrier of the codon Val1090Met mutation, and healthy volunteers. Mononuclear cells were separated by density gradient centrifugation at 500g for 35 minutes at room temperature using LSM lymphocyte separation medium (MP Biomedicals LLC). After 2 washes in phosphate-buffered saline (PBS; Mediatech Inc), cells were resuspended in phenol-free RPMI 1640 (Mediatech Inc) with l-glutamine supplemented with charcoal-treated 10% fetal bovine serum (HyClone), penicillin G sodium (100 μg/mL), streptomycin sulfate (292 μg/mL; Gibco), phytohemagglutinin (5 μg/mL; Sigma-Aldrich), and interleukin-2 (IL-2; 40 IU per milliliter; PeproTech Inc) at 37°C with 5% CO2 in the presence or absence of androgen (methyltrienolone [R1881; Perkin Elmer], 6β-hydroxy-testosterone [6β-HT], 19-nortestosterone-17 decanoate [19-NT; Sigma-Aldrich]), estradiol (Sigma-Aldrich) and/or 4-hydroxy-tamoxifen (Sigma-Aldrich), and/or letrozole (provided by Novartis, Basel, Switzerland under material transfer agreement number 25304), flutamide (Sigma-Aldrich), hydrocortisone 21-succinate (Sigma-Aldrich), and cyclosporine (Novartis) at various concentrations. Cells were cultured from 1 to 8 days.
Bone marrow and peripheral blood CD34+ cell separation and culture
Bone marrow samples were collected from the iliac crest of normal volunteers, diluted 10-fold in RPMI 1640 with 100 U/mL DNAse II-S (Sigma-Aldrich), shaken gently at room temperature for 45 minutes, filtered through 30-μm nylon mesh, and mononuclear cells separated by density gradient centrifugation at 400g for 35 minutes at room temperature using LSM. Peripheral blood samples were collected by leukocytapheresis from healthy volunteers. Cells obtained from either source were washed twice in PBS containing 2 mM ethylenediaminetetraacetic acid, and labeled directly with magnetic anti-CD34 monoclonal antibody (Direct CD34 Progenitor Cell Isolation Kit; Miltenyi Biotec) according to the manufacturer's instructions. Magnetic-labeled cells were positively selected by dual column separation. A cell aliquot was incubated with anti–CD34-PE (clone 8G12), anti–CD45-APC, and/or anti–CD71-PE monoclonal antibodies (BD Biosciences), and the purity of CD34+ cells was analyzed in a LSRII flow cytometer (Becton Dickinson). Purified cells were cultured in StemSpan H3000 media (StemCell Technologies) supplemented with recombinant human–Flt-3 ligand (100 ng/mL), recombinant human-stem cell factor (100 ng/mL), and IL-3 (20 ng/mL) and IL-6 (20 ng/mL; StemSpan CC100 Cytokine Cocktail), with or without methyltrienolone at 0.5 and 5 μM, testosterone at 1 μM, or estradiol at 1 μM, at 37°C with 5% CO2.
Quantification of telomerase activity
Telomerase activity was measured by the fluorescent telomeric-repeat amplification protocol (TRAP assay) with the TRAPeze XL Telomerase Detection kit (Chemicon), according to the manufacturer's instructions. Briefly, cultured cells were washed twice in PBS, lysed in CHAPS buffer 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate) at a concentration of 106 cells/200 μL for 30 minutes on ice, and centrifuged at 12 000g for 20 minutes at 4°C. Protein concentration was determined using the bicinchoninic acid protein assay kit (Pierce). Telomerase activity was assayed with 2 μg protein per reaction, and experiments were performed in triplicate. Fluorescence was measured in an optically clear 96-well plate read in the Victor3 spectrofluorometer (Perkin Elmer). Telomerase activity was calculated as total product generated units relative to the TSR8 standard curve, according to the manufacture's recommendations. As absolute telomerase activity may vary in total product generated units from experiment to experiment, telomerase activity was normalized as “relative telomerase activity,” considering telomerase activity of stimulated lymphocytes or CD34+ cells in the absence of any sex hormone as 100%.
TERT mRNA expression
Total RNA was extracted from lymphocytes using the PicoPure RNA Isolation kit (Arcturus). TERT mRNA expression was first determined by reverse transcriptase polymerase chain reaction (RT-PCR), as previously described.20 Briefly, 1 μg RNA template was amplified using the following primers: forward, 5′-CGGAAGAGTGTCTGGAGCAA-3′ and reverse, 5′-GGATGAAGCGGAGTCTGGA -3′, and the one-step RT-PCR kit (QIAGEN) in the following conditions: 50°C for 30 minutes; 95°C for 15 minutes; 94°C for 45 seconds; 60°C for 45 seconds; 72°C for 90 seconds, 31 cycles; and 72°C for 10 minutes. GAPDH gene was amplified as RNA loading control, with the primers (forward, 5′-CTCAGACACCATGGGGAAGGTGA-3′; reverse, 5′-ATGATCTTGAGGCTGTTGTCATA-3′) in the following conditions: 50°C for 30 minutes; 95°C for 15 minutes; 94°C for 45 seconds; 55°C for 45 seconds; 72°C for 90 seconds, 16 cycles; and 72°C for 10 minutes. To confirm RT-PCR results, TERT mRNA levels were determined by real-time RT-PCR using the TaqMan One-Step RT-PCR Kit (Applied Biosystems) and fluorescein phosphoramidite-labeled probe for TERT and Vic-Tamra–labeled probe for β-microglobulin; 250 μg total RNA were used per reaction, and RT-PCR was performed in a PTC-200 thermal cycler equipped with Chromo4 detector (MJ Research), according to manufacturer's instruction. A plasmid containing TERT was used to build the standard curve in serial dilutions (106-101 copies per reaction), and β2-microglobulin was used as control.
Expression of androgen receptor, estrogen receptors, and aromatase
For the androgen receptor (AR) and estrogen receptor α (ERα) expression was measured by immunoblotting. Peripheral blood leukocytes were separated as above and breast cancer cell line MCF7 (HTB-22, lot no. 0706420; ATCC) and prostate cancer cell line LNCaP (CRL-, lot no. 766845; ATCC) were cultured as recommended by ATCC. Cells were lysed in mPER and cell lysates electrophoresed in 4% to 20% Tris-glycine polyacrylamide gels (Invitrogen). Protein was transferred to nitrocellulose membranes and incubated with primary antibodies (for AR, rabbit polyclonal N-20, lot no. K0907; for ERα, mouse monoclonal D-12, lot no. G1607; for α-tubulin, mouse monoclonal AA12, lot no. I2508, all from Santa Cruz Biotechnology; and for actin, monoclonal antibody ACTN05, Abcam Inc). For the detection of ERβ (ESR2) and aromatase (CYP19) expression, we measured mRNA by RT-PCR. Fifty micrograms of total RNA template were amplified using the following primers: forward, 5′-TGA AAA GGA AGG TTA GTG GGA ACC-3′, and reverse, 5′-TGG TCA GGG ACA TCA TCA TGG-3′ by RT-PCR using conditions similar to those for TERT, except for a annealing temperature of 53°C and a total of 35 cycles. For CYP19, 50 μg RNA template was amplified using the following primers: forward, 5′-GAA TAT TGG AAG GAT GCA CAG ACT-3′ and reverse, 5′-GGG TAA AGA TCA TTT CCA GCA TGT-3′ and using the same RT-PCR conditions as above, except for an annealing temperature of 57°C (total of 35 cycles).
Estrogen receptor knockdown
Five million lymphocytes obtained from peripheral blood of healthy volunteers were electroporated with small interference siRNA using the Amaxa Nucleofector II program U-014 and the Human T Cell Nucleofector kit (lot no. FAC-04 760, all from Amaxa Biosystems/Lonza, Walkersville Inc). siRNAs were obtained from Dharmacon RNAi Technologies: nontargeting pool (scramble; lot no. 869521), ESR1 (ERα; lot no. 071120), and ESR2 (ERβ; lot no. 071120).
Statistical analysis
Differences in telomerase activity at various drug concentrations were assessed by the nonparametric Kruskal-Wallis test, followed by the Dunn multiple comparison test. When only 2 groups were analyzed, the Mann-Whitney test was performed. A P value less than .05 was considered statistically significant. Prism 4 software (GraphPad Software) was used to perform statistical tests.
Results
Androgens stimulate telomerase activity and TERT gene expression in normal stimulated lymphocytes
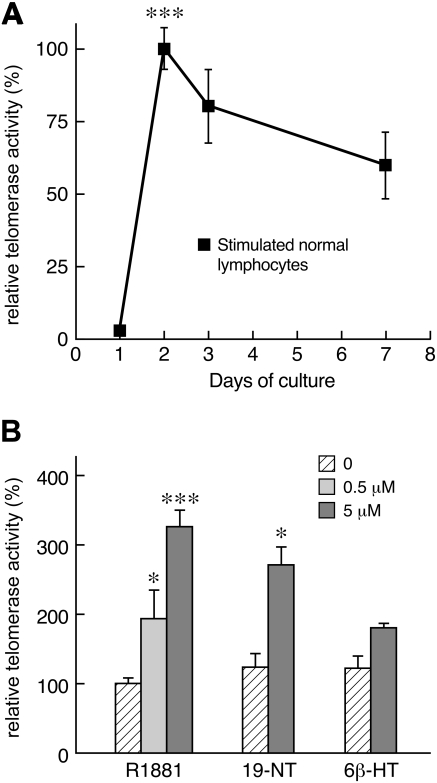
To determine the kinetics of telomerase activation in hematopoietic cells, normal peripheral blood lymphocytes were cultured with IL-2 and phytohemagglutinin (PHA): in vitro, telomerase activity was initially very low, significantly increased at day 2 (P < .001) and then slightly declined over time (Figure 1A). To test whether androgens modulated telomerase function in primary lymphocytes, we chose 3 androgenic compounds; selection was dictated in part by the availability of these heavily regulated compounds, but also to test drugs used in clinical settings or reported in previous publications, and based on their known ability to undergo aromatization. Methyltrienolone (R1881), a readily available synthetic androgen, was added to the cell media at concentrations of 500 nM and 5 μM; R1881 significantly augmented telomerase activity at day 3 of culture in a dose-dependent fashion (P < .001; Figure 1B). Cells were harvested on day 3 because telomerase activity was still very high compared with day 2 and the larger number of cells allowed for protein and RNA extraction. Likewise, 19-nortestosterone-17-decanoate (19-NT), widely used in the clinic, at 5 μM increased telomerase activity of cultured lymphocytes (P < .05; Figure 1B); 6β-hydroxy-testosterone (6β-HT), a metabolic product of 6β-hydroxylase, caused more modest increase in telomerase function, not reaching statistical significance.
Figure 1.
Telomerase activity in cultured normal peripheral blood lymphocytes. (A) The kinetics of telomerase activity in normal cultured peripheral blood lymphocytes was determined in 2 separate experiments by measuring time-dependent changes in telomerase activity from 3 healthy donors. Each time point was measured in triplicate for each individual. Telomerase activity was absent upon collection, very low during the first day in vitro, significantly increased at day 2, and then slightly decreased over time until day 7. (B) Androgens activate telomerase activity of cultured lymphocytes. Methyltrienolone (R1881) induced telomerase function in a dose-dependent fashion (n = 10, 5 men and 5 women; each measurement done in triplicate; data are combined results from 3 different experiments). 19-nortestosterone (19-NT) also induced telomerase function but not 6β-hydroxy-testosterone (n = 2, in 2 experiments; *P < .05; ***P < .001).
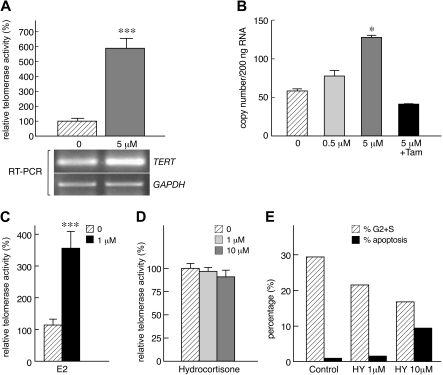
We next addressed whether androgens affected TERT gene expression in lymphocytes. Using RT-PCR, we found that R1881 induced TERT mRNA expression, in correlation with higher telomerase activity (Figure 2A). These results were confirmed by real-time PCR: TERT mRNA levels were significantly higher when cells were cultured with R1881 (P < .05; Figure 2B). Taken together, our data indicated that both androgens stimulated TERT gene transcription and increased telomerase activity.
Figure 2.
Androgens stimulate TERT gene expression. (A) Exposure of lymphocyte to androgen (R1881) was associated with higher TERT mRNA levels by RT-PCR (GADPH was used and loading control; results from one representative experiment). (B) To confirm these results, TERT expression was evaluated by real-time PCR in 2 separate experiments (n = 3): in lymphocytes, R1881 up-regulated TERT mRNA expression, an effect that was inhibited by tamoxifen (Tam). (C) Estradiol activates telomerase enzymatic activity in cultured lymphocytes (E2; n = 3, in 2 different experiments). (D) In a single separate experiment, lymphocytes were also cultured with hydrocortisone (n = 2). (E) In the same experiment, hydrocortisone (HY) inhibited cell cycle and at higher doses (10 μM) induced cell death by apoptosis (*P < .05; ***P < .001).
Estradiol also stimulates telomerase activity
Normal peripheral blood lymphocytes were cultured with IL-2 and PHA with or without addition of β-estradiol (E2) (1,3,5-Estratriene-3,17β-diol). Similar to androgens, E2 also increased telomerase function in stimulated lymphocytes compared with controls (P < .05; Figure 2C). In addition, 2 CD34+ cell samples of healthy controls were treated in the presence of E2, which significantly stimulated telomerase activity in comparison to control condition (P < .05).
Hydrocortisone does not modulate telomerase function
As sex hormones are steroids, we also addressed whether other steroids, specifically glucocorticoids, might modulate telomerase function. Peripheral blood lymphocytes were cultured for 3 days in the presence of hydrocortisone at concentrations of 1 μM and 10 μM. Hydrocortisone did not influence telomerase activity of lymphocytes (Figure 2D), although some apoptosis was induced, as demonstrated by the sub-G0 DNA population in cell-cycle analysis (Figure 2E); corticosteroids also inhibited cell-cycle transit, as indicated by the reduction in G2 plus S population (Figure 2E).
Inhibition of sex steroids effects on telomerase activity by tamoxifen
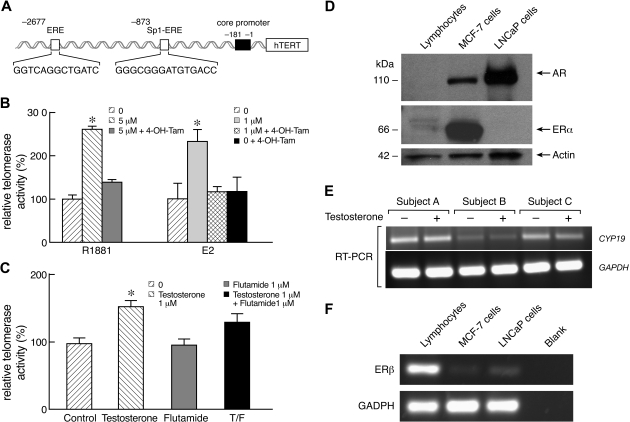
Because telomerase was activated by both androgens and estradiol in human primary lymphocytes, we investigated the cellular pathways involved and specifically the role of the ERs, which bind through estrogen receptor elements (EREs) in the TERT promoter (Figure 3A).21,22 To address this mechanism in hematopoietic cells, lymphocytes were cultured with either R1881 (5 μM) or E2 (1 μM) in the absence or presence of 4-hydroxy-tamoxifen, an estrogen antagonist, at 1 μM. The increase in telomerase activity induced by both R1881 and E2 was abrogated by tamoxifen (Figure 3B), without altering the rate of cell death (data not shown). As a control, tamoxifen alone at the same concentration did not modulate telomerase function in lymphocytes, while higher doses of tamoxifen (10 μM and 100 μM) induced cell death (data not shown).
Figure 3.
Involvement of the ER pathway in androgen-mediated activation of telomerase. (A) Schematic representation of the TERT gene promoter region, emphasizing ERE at position −2677 and Sp1-ERE at position −873. The first base of TERT promoter is −1. (B) In 2 independent experiments, 4-hydroxy-tamoxifen (4-OH-Tam) inhibited both methyltrienolone (R1881)– and estradiol (E2)–dependent activation of telomerase activity (n = 4; each individual was measured in triplicate). Tamoxifen alone at 1 μM did not influence telomerase activity. (C) One microgram of testosterone and/or 1 μM flutamide, an androgen receptor inhibitor, was added to lymphocyte culture for 3 days and telomerase activity measured. Addition of testosterone alone increased telomerase activity but not flutamide alone. However, flutamide did not inhibit testosterone effects on telomerase activity. (D) Western blot of cell lysates of lymphocytes, MCF-7, a breast cancer cell line, and LNCaP (a prostate cancer cells line). AR was not expressed by lymphocytes (representative, n = 3) but was present in MCF-7 and LNCaP cell lines. ERα had low expression in lymphocytes in comparison with MCF-7 lines but was absent in LNCaP. Actin was used as loading control. (E) Expression of ERβ was measured in the same cells using RT-PCR (GADPH was used as expression control). ERβ was more expressed in lymphocytes than in MCF-7 and LNCaP cell lines. Blanks represent the negative control for the RT-PCR reaction. (F) RT-PCR for CYP19 mRNA expression in peripheral blood lymphocytes of healthy male (subjects A and C) and female (B) subjects cultured in the presence or absence of testosterone 1 μM. CYP19 was to be expressed in lymphocytes in both sexes, but mainly in male subjects, but was not affected but addition of testosterone to cell culture. GADPH was used as expression control (*P < .05; **P < .01).
Inhibition of the androgen receptor pathway does not affect telomerase activity in lymphocytes
We next asked whether the AR pathway contributed to telomerase activation in peripheral blood lymphocytes after exposure to androgens. Cells were cultured with IL-2, PHA, and testosterone, which is a compound metabolized by both 5α-reductase (androgenic effects) and by aromatase (estrogenic effects), in the presence or absence of flutamide, a drug that competes with testosterone for binding to ARs. Although telomerase activity was slightly reduced in the presence of flutamide, this effect was not statistically different from that of testosterone alone (Figure 3C). AR also was not detected by immunoblot in cell lysates prepared from lymphocytes of healthy donors (Figure 3D).
Inhibition of androgen effects on telomerase activity by letrozole
To further elucidate the role of the ER pathway, we investigated whether intracellular conversion of androgens to estrogens in human lymphocytes was necessary for androgens to activate telomerase. Aromatase (CYP19), the enzyme that converts androgens into estrogen, was expressed in lymphocytes, as assessed by RT-PCR (Figure 3E), as previously observed.23–25 Lymphocytes were then cultured with R1881 in the presence or absence of letrozole, an aromatase inhibitor. When letrozole was added to cultures, androgen-dependent increases in telomerase activity were not observed (Table 1). We inferred that androgen conversion into estrogen was necessary to activate telomerase, further implicating the ER as the pathway of telomerase activation by sex steroids in lymphocytes.
Table 1.
Effects of letrozole, an aromatase inhibitor, on androgen-dependent telomerase activation
| Relative telomerase activity (n = 4) Letrozole concentration |
||
|---|---|---|
| 0 | 1 μM | |
| Methyltrienolone (R1881) concentration | ||
| 0 | 100% | 116 ± 9.5%† |
| 0.5 μM | 153 ± 3.8%* | 108 ± 9.1%† |
Telomerase activity of cultured lymphocytes is expressed as relative telomerase activity (considering telomerase activity of each donor in the absence of hormones as reference activity). Letrozole is an aromatase (CYP19) inhibitor, preventing androgens from being converted into estrogen. Normal peripheral blood lymphocytes from 4 healthy subjects were cultured for 3 days in two separate experiments each.
P < .05 in Kruskal-Wallis test; P < .05 when compared to condition without R1881 and letrozole.
Difference not statistically significant in comparison to control (no drugs added).
Estrogen receptor-α mediates telomerase activation
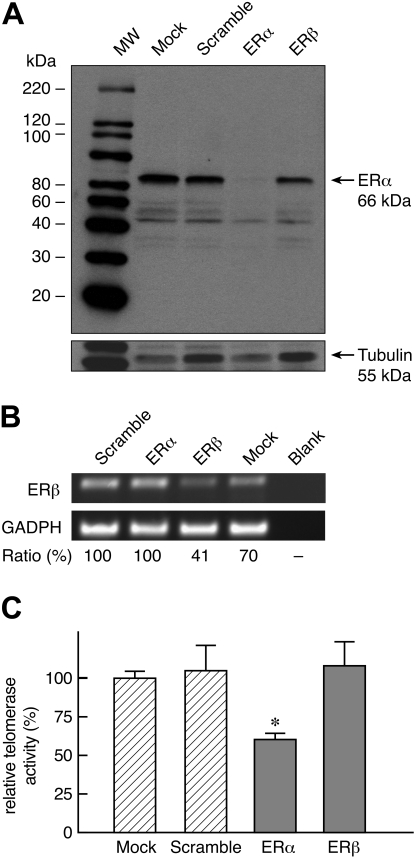
Because the ER appeared to be the main pathway of TERT activation for both androgens and E2, we investigated whether ERα or ERβ mediated sex steroid activation of telomerase. Both ERα and ERβ were detected in lymphocytes either by immunoblot (ERα; Figure 3D) or RT-PCR (ERβ; Figure 3F). We used siRNA to “knock down” ERα or ERβ in peripheral blood lymphocytes and then stimulated the cells with IL-2, PHA, and E2 (Figure 4). In 5 experiments, siRNA knocked down either ERα (85% reduction in expression in comparison to scramble control; Figure 4A) or ERβ (60% reduction; Figure 4B). When ERα expression was reduced, the E2-mediated increase in telomerase activity was abrogated, but this effect was not observed when ERβ was targeted (Figure 4C). Although the reduction in ERβ expression was within the range observed in siRNA experiments and usually capable of altering cell function, a nearly complete abrogation was not achieved, and this receptor cannot be rigorously excluded as a mediator between estrogens and telomerase expression.
Figure 4.
siRNA “knockdown” of ERα but not ERβ abrogates increased telomerase activity induced by estrogen. In 5 separate experiments, peripheral blood lymphocytes were transfected with either scramble siRNA, siRNA for ERα (ESR1), for ERβ (ESR2), or no siRNA at all (mock) and cultured for 3 days in the presence of PHA, IL-2, and E2 (1 μM). (A) Representative Western blot indicates that siRNA effectively knocked down ERα expression (> 85%). (B) RT-PCR of a representative experiment indicates that siRNA also knocked down ERβ (59% reduction). (C) Reduction in ERα expression correlated with significant decrease in telomerase activity (*P < .05).
Androgens stimulate telomerase activity of bone marrow CD34+ cells
Based on the positive effects of methyltrienolone on telomerase activity in peripheral blood lymphocytes, we investigated whether similar effects could also be produced by androgens in hematopoietic progenitors, the cells ultimately responsible for blood cell production. We collected bone marrow– and peripheral blood–derived CD34+ cells from normal subjects. Cells were separated immunomagnetically and cultured in vitro for 8 days, in the presence or absence of methyltrienolone or testosterone; the purity of CD34+ cells after separation in all our samples was greater than 94% (94%-97%; data not shown). Increased telomerase activity was observed in CD34+ cells exposed to androgen (Table 2).
Table 2.
Induction of telomerase activity in normal bone marrow CD34+ cells by methyltrienolone (R1881)
| Donor | Relative telomerase activity R1881 concentration |
||
|---|---|---|---|
| 0 | 0.5 μM | 5 μM | |
| 1 | 100% ± 5.9% | 142% ± 7.1% | 133% ± 11.8% |
| 2 | 100% ± 5.7% | 152% ± 24% | 139% ± 14% |
| 3 | 100% ± 5.6% | 142% ± 19% | 134% ± 13% |
| 4 | 100% ± 5.8% | 132% ± 6% | 146% ± 0.9% |
| 5 | 100% ± 5.5% | 116% ± 9.9% | 139% ± 2.1% |
| Average | 100% ± 2.3% | 137% ± 6.1%* | 138% ± .3%* |
Telomerase activity of cultured CD34+ cells is expressed as relative telomerase activity (considering telomerase activity of each donor in the absence of hormones as reference activity). CD34+ bone marrow cells from 3 healthy donors were purified and cultured for 8 days in liquid media in the presence of methyltrienolone.
P < .01, Kruskal Wallis test; P < .05 for each column in comparison to “0” (no androgen) using the Dunn multiple comparison test.
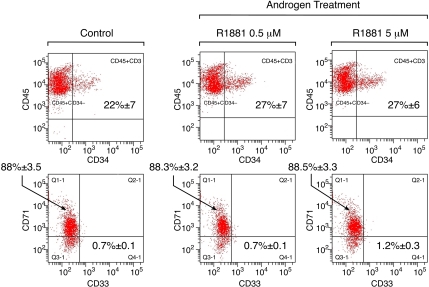
The increase in enzymatic activity did not correlate with changes in cell cycle (data not shown) or cell differentiation into erythroid (indicated by CD71 expression) or myeloid (evidenced by CD33 expression) lineages (Figure 5). Taken together, these results indicate that androgens stimulate telomerase activity of CD34+ hematopoietic cells in a similar fashion to that observed in peripheral blood lymphocytes.
Figure 5.
Influence of androgen on telomerase activity of hematopoietic stem and progenitor cells. CD34+ cells were separated on immunomagnetic columns and cultured in long-term liquid media in the presence of methyltrienolone (R1881) for 8 days (n = 3), when they were harvested for flow and cell-cycle analyses and protein extraction. No changes in CD71 (transferrin receptor, erythroid marker) or CD33 (myelomonocytic marker) expression patterns were observed upon male steroid treatment.
Androgen up-regulates telomerase activity of TERT mutant lymphocytes
As androgens were able to activate telomerase function in primary lymphocytes of healthy volunteers, we examined whether androgens also modulated telomerase activity in hematopoietic cells from TERT mutant individuals. Peripheral blood lymphocytes that were heterozygous for TERT mutations (codon Ala202Thr and codon Val1090Met)16 were cultured with R1881 for 3 days and telomerase activity measured. Both loss-of-function mutations are known to abolish telomerase activity of the mutant allele and to reduce overall cellular telomerase function to approximately 50% by haploinsufficiency.16 Telomerase was reduced in all 3 mutation-carriers in comparison to controls, but when R1881 was added to cell culture, telomerase function was restored to normal levels (P < .01; Table 3), indicating that androgens were able to stimulate telomerase activity in cells of telomerase-mutant patients.
Table 3.
Induction of telomerase activity by methyltrienolone (R1881) in lymphocytes of TERT-mutation carriers
| Relative telomerase activity R1881 concentration |
||
|---|---|---|
| 0 | 5 μM | |
| Carriers* (n = 3) | 53% ± 4.8% | 107% ± 3.7%† |
| Noncarriers (n = 3) | 100% ± 21%* | 222% ± 26%† |
Telomerase activity of cultured lymphocytes is expressed as relative telomerase activity (considering telomerase activity from noncarriers' lymphocytes in the absence of hormones as reference activity).
Two clinically healthy individuals with codon Ala202Thr and one with codon Val1090Met mutations were studied; all carriers were clinically healthy and were relatives of patients with aplastic anemia with TERT mutations.
P< .01.
Discussion
In the series of experiments reported here, we showed that androgens stimulated telomerase gene expression and enzymatic activity in normal and TERT-mutant human peripheral blood lymphocytes and in bone marrow CD34+ cells. That aromatization and estrogen receptors mediate this process was inferred from the inhibition of both androgen and estrogen effects on telomerase by 4-hydroxy-tamoxifen, which blocks the ER, and further by letrozole, which blocks the enzyme aromatase and prevents androgen conversion into estrogen. “Knockdown” experiments suggested that the ERα is implicated in this mechanism. In contrast, our inability to detect the androgen receptor in target cells and absence of an effect of the androgen antagonist flutamide argue against a major role for the androgen receptor in TERT stimulation.
Historically, androgens have been used to treat both constitutional and acquired aplastic anemias. In studies performed decades ago, male hormones appeared to increase the survival of patients and to induce partial and complete hematologic responses in acquired aplastic anemia.26–28 However, large randomized trials have failed to demonstrate significant benefits of androgens.29,30 Many hematologists have the experience of occasional aplastic patients who remain androgen-dependent after initial response.31 Androgens continue to be a mainstay of therapy of constitutional marrow failure syndromes. In dyskeratosis congenita, approximately 60% of patients show an increase in the 3 hematopoietic cell lineages with hormonal therapy.17 Patients who are known to have telomerase complex mutations may become transfusion-independent and have adequate neutrophil counts after treatment with androgens.1 Patients with Fanconi anemia, who also frequently present telomere shortening that correlates with the degree of marrow failure,32 may significantly benefit from androgen therapy.33 Androgen therapy usually is reserved to transfusion-dependent patients or to patients with clinically significant neutropenia or thrombocytopenia who are not eligible for hematopoietic stem cell transplantation. However, the contribution of telomere shortening to disease development in Fanconi anemia is uncertain.
One known mechanism of hematologic effects of androgens is to increase red cell mass by stimulating expansion of erythroid progenitors through increased erythropoietin production by the kidneys.26,34 How androgens used therapeutically would lead to increased myelopoiesis and megakaryocytopoiesis has been unclear. Our results provide a direct molecular mechanism of action for androgens on telomerase activity of hematopoietic cells. The TERT promoter region contains an imperfect palindromic ERE at position −2677 and an ERE adjacent to an Sp1 site (Sp1-ERE) at position −873 (Figure 3A).21,22 In ER-positive cell lines (MCF-7, derived from a patient with epithelial breast cancer), estradiol activates telomerase activity; ERα bound to ERE in the TERT promoter region in gel shift assays, and mutations in this element or tamoxifen exposure significantly reduce estrogen-induced TERT activation.21,22 In our experiments, knockdown of ERα but not of ERβ abrogated estrogen effects on telomerase activity. As ERβ expression was not completely abrogated by siRNA (it was reduced to approximately 40%), we cannot entirely exclude ERβ involvement as a mediator of estrogen effects on telomerase function. However, it has been previously shown that ERβ does not bind to ERE in the TERT promoter region.22 Taken together, these results implicate ERα in this pathway. Androgens also were found to modulate telomerase activity in normal prostate and malignant cells of this gland. 5α-dihydrotestosterone stimulated telomerase in LNCaP cells, an androgen-sensitive prostate cancer cell line, but not in normal prostate cells.35 R1881 also induces telomerase in LNCaP cells,18 but this effect was indirect, as TERT promoter constructs transfected into HeLa cells were not activated. Androgen seems to exert its effects on telomerase in prostate cancer cell lines through intracellular conversion to estradiol.36 Androgens may act on telomerase expression in hematopoietic tissue after conversion in peripheral tissues to metabolites, such as 17β-estradiol and 5α-dihydrotestosterone; leukocytes express aromatase (CYP19), the enzyme responsible for aromatization of the testosterone A ring by conversion to 17β-estradiol (Figure 3E).23,24,37
Androgens increased low baseline telomerase activity in individuals carrying a loss-of-function TERT mutation to normal levels (Table 3). These results have direct implications for patients with aplastic anemia carrying telomerase complex mutations and in dyskeratosis congenita, potentially explaining the effectiveness of male hormones in these syndromes, as well as guiding what type of androgenic compounds (readily aromatized) or even estrogens may be even more effective in the future. Although estrogens have not been used in the treatment of bone marrow failure syndromes, our results suggest that these hormones may be therapeutic for telomerase-deficient marrow failure states. Indeed, there are suggestive anecdotal experiences in bone marrow failure, such as remissions, especially of some constitutional syndromes, with the onset of puberty, and the onset or relapse of acquired aplastic anemia with pregnancy, when estrogen levels decrease.
That most of the experiments were performed in peripheral blood lymphocytes due to the paucity of bone marrow CD34+ cells for multiple assays is the major limitation in our study, and of course observations made in lymphocytes do not necessarily reflect the pathways in the hematopoietic stem cell, the etiologic cell in aplastic anemia. However, our major finding, androgen-mediated up-regulation of telomerase activity, was similar in both lymphocytes and bone marrow CD34+ cells; estradiol also stimulated telomerase activity in CD34+ cells. These results suggest that cells of the same tissue origin might share similar molecular pathways of telomerase activation. In addition, the androgen and estrogen doses used in our study were pharmacological, and the effects observed here do not necessarily reflect the physiologic effects of sex hormones on telomerase activity in hematopoietic cells.38
In this latter context, sex hormone effects of telomerase may be cautiously extrapolated, positively to the physiology of aging and negatively to the treatment of malignancies. Sex hormone differences might explain why women have slightly longer telomeres of leukocytes than do men.39 Further, hormone replacement therapy has been reported to decrease the rate of telomere shortening in postmenopausal women: women 55 to 69 years old receiving estrogens for at least 5 years had longer telomeres than did age-matched controls, suggesting that hormonal replacement attenuated the pace of telomere attrition.40 For both sexes, telomeres of peripheral blood leukocytes shorten very rapidly, at a rate of more than 1 kb/year, during the first decade of life, but telomere length reaches a plateau during much of adulthood, with later gradual attrition after the fifth to sixth decades of life.41 The plateau—more evident in women—roughly coincides with higher sex hormone levels, consistent with sex hormone mediated telomere length stabilization in normal adults.1
Telomere shortening has been linked etiologically to cancer in animals. Epithelial aneuploid tumors, unusual in the laboratory rodent, are prevalent in Terc knockout animals that also bear p53 gene mutations.42 The anticipated end-to-end chromosome fusions and breakage-fusion-bridge cycles are present in these animals. In humans, we have recently reported constitutional hypomorphic TERT mutations in acute myeloid leukemia.43 Sex hormone effects on telomerase regulation in tissues such as prostate and breast are well known. Our data indicate that, at high doses in vitro, sex hormones affect telomerase activity. In vivo, they might offer a simple method to modulate telomerase activity in genetically susceptible individuals. In this context, patients with aplastic anemia and short telomeres in the absence of telomerase mutations also would benefit from sex hormones by preventing evolution to clonal disorders, which is more common in this group of patients.
In conclusion, our results are consistent with a direct effect of androgens on TERT gene expression and telomerase activity, which may explain the hormone stimulation of hematopoiesis in dyskeratosis congenita and in acquired aplastic anemia patients, leading to hematopoietic response by activating telomerase in hematopoietic cells, especially stem cells. Higher telomerase activity in hematopoietic stem cells might increase hematopoietic cell proliferation and recovery of peripheral blood cell counts. These findings provide one biological basis for the treatment with sex hormones of patients with aplastic anemia carrying telomerase mutations, and they have potential physiologic implications for telomere stability during the human reproductive period and for a relationship between sex hormones and cancer susceptibility.
Acknowledgment
This work was supported by the National Institutes of Health Intramural Research Program.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.T.C. designed and performed research, analyzed data, and wrote the paper; W.T.Y., K.L.W., J.A.R., and S.K. performed research; and C.A.S. and N.S.Y designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rodrigo T. Calado, MD, PhD, Hematology Branch, NHLBI/NIH, 10 Center Dr, Bldg 10/CRC, Rm 3E-5140, Bethesda, MD 20892-1202; e-mail: calador@nhlbi.nih.gov.
References
- 1.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 3.Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 4.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 5.Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 6.Knight SW, Heiss NS, Vulliamy TJ, et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am J Hum Genet. 1999;65:50–58. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vulliamy T, Marrone A, Goldman F, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 8.Vulliamy T, Walne A, Baskaradas A, et al. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol Dis. 2005;34:257–263. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Savage SA, Giri N, Baerlocher GM, et al. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walne AJ, Vulliamy T, Marrone A, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrone A, Walne A, Tamary H, et al. Telomerase reverse transcriptase homozygous mutations in autosomal recessive dyskeratosis congenita and Hoyeraal-Hreidarsson syndrome. Blood. 2007;110:4198–4205. doi: 10.1182/blood-2006-12-062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogarty PF, Yamaguchi H, Wiestner A, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi H, Baerlocher GM, Lansdorp PM, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 14.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2511–2521. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ly H, Calado RT, Allard P, et al. Functional characterization of telomerase RNA variants found in patients with hematological disorders. Blood. 2005;105:2332–2339. doi: 10.1182/blood-2004-09-3659. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi H, Calado RT, Ly H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 17.Dokal I, Vulliamy T. Dyskeratosis congenita: its link to telomerase and aplastic anaemia. Blood Rev. 2003;17:217–225. doi: 10.1016/s0268-960x(03)00020-1. [DOI] [PubMed] [Google Scholar]
- 18.Guo C, Armbruster BN, Price DT, Counter CM. In vivo regulation of hTERT expression and telomerase activity by androgen. J Urol. 2003;170:615–618. doi: 10.1097/01.ju.0000074653.22766.c8. [DOI] [PubMed] [Google Scholar]
- 19.Bayne S, Liu JP. Hormones and growth factors regulate telomerase activity in ageing and cancer. Mol Cell Endocrinol. 2005;240:11–22. doi: 10.1016/j.mce.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura TM, Morin GB, Chapman KB, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 21.Kyo S, Takakura M, Kanaya T, et al. Estrogen activates telomerase. Cancer Res. 1999;59:5917–5921. [PubMed] [Google Scholar]
- 22.Misiti S, Nanni S, Fontemaggi G, et al. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol. 2000;20:3764–3771. doi: 10.1128/mcb.20.11.3764-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vottero A, Kirschner LS, Yue W, Brodie A, Stratakis CA. P450arom gene expression in peripheral blood lymphocytes: identification of a cryptic splice site for exon-1 after Epstein-Barr virus transformation. J Steroid Biochem Mol Biol. 1998;64:245–250. doi: 10.1016/s0960-0760(97)00193-3. [DOI] [PubMed] [Google Scholar]
- 24.Vottero A, Rochira V, Capelletti M, et al. Aromatase is differentially expressed in peripheral blood leukocytes from children, and adult female and male subjects. Eur J Endocrinol. 2006;154:425–431. doi: 10.1530/eje.1.02102. [DOI] [PubMed] [Google Scholar]
- 25.Larionov AA, Poroshina TE, Zimarina TS, Leenman EE, Berstein LM. Aromatase (estrogen-synthetase) gene expression in human leukocytes. Russ J Physiol. 2000;80:10–17. [PubMed] [Google Scholar]
- 26.Shahidi NT. Androgens and erythropoiesis. N Engl J Med. 1973;289:72–80. doi: 10.1056/NEJM197307122890205. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Medal L, Gomez-Leal A, Duarte L, Rico MG. Anabolic androgenic steroids in the treatment of acquired aplastic anemia. Blood. 1969;34:283–295. [PubMed] [Google Scholar]
- 28.Najean Y, Haguenauer O. Long-term (5 to 20 years) evolution of nongrafted aplastic anemia. Blood. 1990;76:2222–2228. [PubMed] [Google Scholar]
- 29.Champlin RE, Ho WG, Feig SA, et al. Do androgens enhance the response to antithymocyte globulin in patients with aplastic anemia? A prospective randomized trial. Blood. 1985;66:184–188. [PubMed] [Google Scholar]
- 30.Bacigalupo A, Chaple M, Hows J, et al. Treatment of aplastic anemia (AA) with antilymphocyte globulin (ALG) and methylprednisolone (MPred) with or without androgens: a randomized trial from the EBMT SAA working party. Br J Haematol. 1993;83:145–151. doi: 10.1111/j.1365-2141.1993.tb04645.x. [DOI] [PubMed] [Google Scholar]
- 31.Stebler C, Tichelli A, Gratwohl A, Hoffmann T, Nissen C, Speck B. Androgen dependence in patients with severe aplastic anemia after antilymphocyte globulin therapy. Bone Marrow Transplant. 1991;7(suppl 2):101. [PubMed] [Google Scholar]
- 32.Li X, Leteurtre F, Rocha V, et al. Abnormal telomere metabolism in Fanconi′s anaemia correlates with genomic instability and the probability of developing severe aplastic anaemia. Br J Haematol. 2003;120:836–845. doi: 10.1046/j.1365-2141.2003.04225.x. [DOI] [PubMed] [Google Scholar]
- 33.Bagby G, Alter B. Fanconi anemia. Semin Hematol. 2006;43:147–156. doi: 10.1053/j.seminhematol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Fried W, Morley C. Effects of androgenic steroids on erythropoiesis. Steroids. 1985;46:799–826. doi: 10.1016/0039-128x(85)90031-5. [DOI] [PubMed] [Google Scholar]
- 35.Soda H, Raymond E, Sharma S, et al. Effects of androgens on telomerase activity in normal and malignant prostate cells in vitro. Prostate. 2000;43:161–168. doi: 10.1002/(sici)1097-0045(20000515)43:3<161::aid-pros1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 36.Nanni S, Narducci M, Della Pietra L, et al. Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J Clin Invest. 2002;110:219–227. doi: 10.1172/JCI15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samy TSA, Zheng R, Matsutani T, et al. Mechanism for normal splenic T lymphocyte functions in proestrus females after trauma: enhanced local synthesis of 17beta-estradiol. Am J Physiol Cell Physiol. 2003;285:C139–C149. doi: 10.1152/ajpcell.00058.2003. [DOI] [PubMed] [Google Scholar]
- 38.Federman DD. The biology of human sex differences. N Engl J Med. 2006;354:1507–1514. doi: 10.1056/NEJMra052529. [DOI] [PubMed] [Google Scholar]
- 39.Vasa-Nicotera M, Brouilette S, Mangino M, et al. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76:174–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee DC, Imm JA, Kim JH, Lee HR, Shim JY. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J. 2005;46:471–479. doi: 10.3349/ymj.2005.46.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Artandi SE, Chang S, Lee SL, et al. Telomere dysfunction promotes nonreciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 43.Calado RT, Regal JA, Hills M, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Nat Acad Sci U S A. 2009;106:1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]