Abstract
When successful, human leukocyte antigen (HLA)–matched bone marrow transplantation with reduced-intensity conditioning is a cure for several nonmalignant hematologic disorders that require chronic transfusion, such as sickle cell disease and aplastic anemia. However, there are unusually high bone marrow transplant (BMT) rejection rates in these patients. Rejection correlates with the number of transfusions before bone marrow transplantation, and it has been hypothesized that preimmunization to antigens on transfused blood may prime BMT rejection. Using a novel mouse model of red blood cell (RBC) transfusion and major histocompatibility complex–matched bone marrow transplantation, we report that transfusion of RBC products induced BMT rejection across minor histocompatibility antigen (mHA) barriers. It has been proposed that contaminating leukocytes are responsible for transfusion-induced BMT rejection; however, filter leukoreduction did not prevent rejection in the current studies. Moreover, we generated a novel transgenic mouse with RBC-specific expression of a model mHA and demonstrated that transfusion of RBCs induced a CD8+ T-cell response. Together, these data suggest that mHAs on RBCs themselves are capable of inducing BMT rejection. Cellular immunization to mHAs is neither monitored nor managed by current transfusion medicine practice; however, the current data suggest that mHAs on RBCs may represent an unappreciated and significant consequence of RBC transfusion.
Introduction
When successful, bone marrow transplantation is a cure for nonmalignant hematologic disorders such as sickle cell disease, aplastic anemia, Diamond-Blackfan anemia, and Fanconi anemia.1–8 Currently, most transplantations are performed in the context of treating neoplasia, and stringent recipient conditioning is used to attack the malignancy. In this context, bone marrow transplant (BMT) rejection is now a rare event, as the conditioning regimens essentially eliminate recipient immunity. However, for disorders in which no malignancy is being treated (eg, sickle cell disease), it is difficult to justify the use of stringent myeloablative conditioning regimens, which can lead to substantial morbidity and mortality from toxic side effects. Accordingly, it is highly desirable to develop reduced-intensity BMT conditioning regimens that minimize toxic side effects.
Unfortunately, current reduced-intensity regimens result in significant rates of BMT rejection in patients with nonmalignant hematologic disorders.9–12 One factor that potentially contributes to rejection is the requirement for chronic transfusion therapy. There is a strong correlation between the number of transfusions a patient has received and the likelihood of BMT rejection.13–15 Moreover, canine studies have demonstrated that transfusion of peripheral blood from bone marrow donors before transplantation can induce BMT rejection in otherwise healthy animals.16–18 Together, these data are most consistent with the hypothesis that transfusion primes the recipient to reject bone marrow through immunization to donor antigens. Because the donors and recipients are major histocompatibility complex (MHC)–matched (in both human trials and animal studies), immunization against minor histocompatibility antigens (mHAs) is most probably responsible for the rejection.19,20
Leukocytes present in the transfused blood are traditionally thought to be the source of immunization against both human leukocyte antigen (HLA) antigens and mHAs. However, implementation of universal leukoreduction in multiple centers has failed to decrease the rates of BMT rejection in multiply transfused patients.21,22 Transfusion standards require that leukoreduced red blood cells (RBCs) contain fewer than 5 × 106 leukocytes per unit in the United States23 and fewer than 106 leukocytes per unit in Europe.24 Hence, leukoreduction decreases but does not eliminate leukocytes; thus, it remains possible that the residual leukocytes are responsible for BMT rejection. However, an alternate hypothesis is that exposure to mHAs on the RBCs themselves can lead to recipient immunization through processing and presentation of mHAs by recipient antigen-presenting cells (APCs), leading to BMT rejection. To test this hypothesis, we engineered a mouse model of leukoreduced blood transfusion and MHC-identical–mHA-mismatched bone marrow transplantation.
Here, we report that transfusion of leukoreduced RBC units between MHC-identical–mHA-mismatched mice induces subsequent BMT rejection. Although such rejection could be the result of direct presentation of mHAs on residual leukocytes, we demonstrate that leukoreduction is sufficient to prevent transfusion-induced BMT rejection resulting from antigens expressed on leukocytes but not RBCs. Using a novel transgenic mouse with RBC-specific expression of a model antigen, the HEL-OVA-Duffy mouse (HOD mouse), we report that mHAs on transfused RBCs undergo crosspresentation with subsequent expansion of recipient CD8+ T cells specific for the transfused mHA. Together, these findings demonstrate the potential of mHAs on RBCs to induce sufficient immunity to reject a subsequent BMT.
Methods
Mice
C57BL/6 (B6), BALB/c, B6.PL-Thy1a (B6.Thy1.1), C.B10-H2b (BALB.B), and B6.C-H2d (B6.H2d) mice were purchased from The Jackson Laboratory and housed in Emory University Department of Animal Resources facilities. Mice were 8 to 12 weeks of age, and all procedures were performed according to Institutional Animal Care and Use Committee-approved protocols. HOD mice were created as described. HOD and mHEL25 mice were bred by the Emory Division of Animal Resources Animal Husbandry service (mHEL mice are available from The Jackson Laboratory).
Generation and characterization of the HOD mouse
The whole open reading frame (ORF) for HEL (including the N terminal signal sequence) was amplified by PCR. Primers were designed to insert a BamHI site on the 5′ end, engineer a Kozak consensus sequence upstream of the start codon, remove the stop codon, and insert a SmaI site at the 3′ end (5′-TCG GAT CCG CCA CCA TGA GGT CTT TGC TAA TCT T-3′; 5′-GGC CCG GGC AGC CGG CAG CCT CTG ATC C-3′). The portion of the OVA ORF encoding amino acids 251 to 349 was amplified with primers that inserted a SmaI site on the 5′ end (in frame with the HEL ORF), removed the stop codon, and inserted an XhoI site on the 3′ end (5′-GGC CCG GGG GCC TTG AGC AGC TTG AGA G-3′; 5′-GGC TCG AGC ACT CCA GCC TCT GCT GAC CC-3′). Finally, the complete ORF for Duffy was amplified with primers designed to insert an XhoI site on the 5′ end in frame with the start codon and insert an XbaI site on the 3′ end after the stop codon (5′-TGA ACT CGA GAT GGC CTC CTC TGG GTA TGT CC-3; 5-CTC TAG ACT AGG ATT TGC TTC CAA GGG TGT-3′). Plasmids containing cDNA templates for amplification for HEL and OVA were generous gifts from Dr Judith Kapp (University of Alabama at Birmingham) and Dr Emil Unanue (Washington University, St Louis, MO). Human Duffy was amplified from a Quick-Clone cDNA library synthesized from human bone marrow (Clontech). PCR was carried out with high- fidelity pfu enzyme (Stratagene), the amplified fragments were ligated into pGEM-T Easy (Promega), and the inserts were sequenced in both directions. The HOD cDNA was assembled through sequential ligation in pGEM-T Easy. The complete ORF was reamplified with the 5′ HEL primer and a new 3′ Duffy primer designed to introduce a BglII site in the 3′ end, and complete sequencing was carried out. The completed HOD ORF was excised by digestion with BamHI and BglII and was ligated into (μ′LCR β pr delta Nco Bgl) that was digested with BglII. Recombinants were isolated and orientation was confirmed through sequencing. The final construct was linearized and bacterial elements were removed through digestion with ClaI and SacII. The linearized construct was purified and submitted to the Emory Core Transgenic Facility for pronuclear injection. Founder pups were screened by PCR and subsequently characterized by flow cytometry.
HOD expression was assessed by staining peripheral blood with polyclonal murine antisera to HEL or OVA generated through immunizing B6 mice with HEL or OVA protein. Expression of Duffy antigens was assessed using human antisera to Fya or Fyb, or monoclonal anti-Fy3 (MIMA-29, generous gift from Marion Reid and Greg Halverson at the New York Blood Center). APC-conjugated allospecific anti-IgG1b or anti–human Ig (BD Biosciences) was used as secondary antibodies. Expression on leukocytes or platelets was analyzed by also staining with anti-CD45, anti-CD41, and anti–TER-119 (BD Biosciences). Leukocytes were defined as CD45+TER-119− events and PLT as CD41+TER-119− events. Flow cytometry was carried out using a BD FACSort and data were analyzed using FlowJo software (TreeStar).
Leukoreduction of mouse blood
Mouse blood was obtained by retro-orbital bleeding into anticoagulant containing adenine, citrate, and dextrose (ACD Solution A; BD). RBCs were washed with phosphate-buffered saline (PBS) adjusted to 340 mOsm with NaCl (modified PBS or mPBS) and leukoreduced over a neonatal leukoreduction filter (Purecell Neo; Pall Medical) previously primed with 12 mL mPBS. The blood was then washed 3 times with mPBS to remove platelets. Each transfusion consisted of 100 μL of packed RBCs diluted with 400 μL PBS injected in the tail vein.
Enumeration of residual white blood cells
Residual white blood cells (rWBCs) in the leukoreduced blood were enumerated by flow cytometry using the method previously described.26 Briefly, a 25-μL aliquot of blood was stained using 150 ng/mL propidium iodide (PI; Invitrogen) in a 1-g/L sodium citrate dihydrate solution with 0.7% Zap-oglobin II reagent (Beckman Coulter), 0.7% RNAse cocktail (New England Biolabs), effectively staining all nucleated cells. Nuclei were enumerated on a BD FACSort flow cytometer. A known quantity of APC-labeled beads were added to each sample to allow acquisition of absolute counts (BD).
BMT
Whole bone marrow was prepared from the femur and tibia of donor mice. For BALB.B BMT, B6.Thy1.1 recipients were irradiated with a dose of 650 cGy, on the same day 5 × 106 whole bone marrow cells from BALB.B donor were injected intravenously. For HY mismatched BMT, female B6.Thy1.1 recipients were irradiated with a dose of 500 cGy and injected on the same day with 5 × 106 whole bone marrow cells from male B6 donors.
Bone marrow engraftment analysis
Bone marrow engraftment was monitored by flow cytometry. Peripheral WBCs were stained with fluorescein isothiocyanate-labeled anti-CD3, phycoerythrin-labeled anti-Thy1.2 (BD), and APC-labeled anti-Thy1.1 (eBioscience) antibodies. Percent donor engraftment was calculated using Thy1.1+ events/(Thy1.1+ events + Thy1.2+ events) × 100. Engraftment was defined as a sustained chimerism of greater than 5%, 6 weeks after transplantation. The use of CD45.1/CD45.2 markers was purposely avoided in our system, as CD45.1/CD45.2 has been reported to be an mHA capable of inducing rejection.27
In vivo killing assay
In vivo killing assays were performed according to a method adapted from Barber et al.28 Briefly, RBC lysed spleen cells from B6 and B6.H2d mice were labeled with a different concentration of carboxyfluorescein succinimidyl ester (CFSE): 3 μM (B6 control population) or 0.25 μM (B6.H2d cells). A total of 5 × 106 cells of each population were mixed and injected into experimental groups. Twelve to 15 hours after injection, spleens from recipient mice were harvested and remaining CFSE-labeled target cells were analyzed by flow cytometry. Percent specific removal of CFSE-labeled donor target cells was calculated as follows: [1 − (B6.H2d/B6)/(B6.H2d in a naive mouse/B6 in a naive mouse)] × 100.
In vivo cross-presentation to OT-I T cells
On day −1, 15 × 106 splenocytes from OT-I donors were labeled with 3 μM CFSE (Invitrogen) and adoptively transferred intravenously into 8- to 12-week-old B6.Thy1.1 recipients and left to circulate for 24 hours. On day 0, whole blood from FVB and HOD donor mice was collected and anticoagulated with ACD. Some of the HOD blood was leukoreduced as described in “Leukoreduction of mouse blood.” The blood was then washed 3 times in mPBS, and 100 μL of packed blood diluted with 400 μL PBS was injected intravenously into the recipient mice. On day 4, expansion of the OT-I T cells in the spleen of the recipient mice was measured by staining with an anti-CD8 antibody (BD) and OT-I specific MHC tetramer Kb/SIINFEKL. After gating on CD8+CFSE+ cells, the proliferation of the OT-I T cells was measured by CFSE dilution. Samples were analyzed on a BD FACSort flow cytometer.
Statistical analyses
For bone marrow transplantation studies, the significance of the rejection rate was analyzed with a Fisher exact test on the contingency table formed by the engraftment and rejection events in the groups of mice transfused with mismatched leukoreduced blood and transfused with syngeneic leukoreduced blood. For the crosspresentation to OT-I studies, the significance of the expansion of the OT-I T cells was analyzed with a one-way analysis of variance with Dunnett posttest comparing the HOD and HOD LR group with the FVB control group. Analysis was performed using GraphPad Prism, Version 5.0a for Macintosh (GraphPad Software; www.graphpad.com).
Results
Transfusing leukoreduced units of RBCs induces BMT rejection across mHA barriers
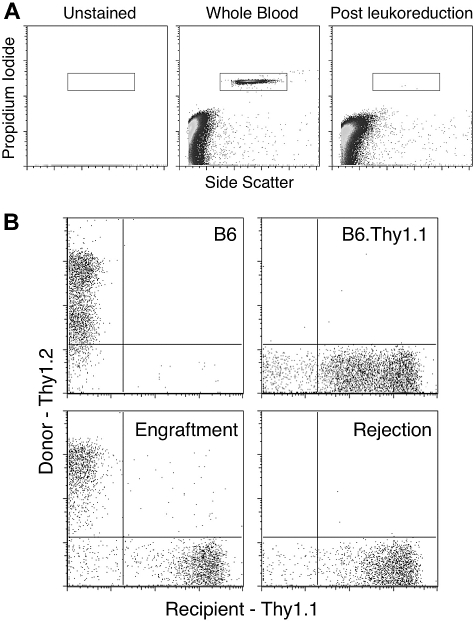
We engineered a murine system in which processing of RBC units models current methodologies of manufacturing human RBCs units, including collection solution and filter leukoreduction (as described in “Leukoreduction of mouse blood”). To assess the degree of leukoreduction, rWBCs were quantified in each RBC preparation using a PI-based flow cytometric method adapted from a previously described technique.29 As a positive control for visualization of rWBCs, whole blood was stained and PI+ events corresponding to WBCs were enumerated (Figure 1A). rWBCs were easily detectable in whole blood (Figure 1A middle panel), and this was not the result of background signal, as no events were observed in unstained samples (Figure 1A left panel). Filter leukoreduction resulted in a considerable decrease in rWBCs (Figure 1A right panel). Enumeration of rWBCs demonstrated that, on average, there were fewer than 1000 rWBCs per 100 μL unit transfused. At the human scale, this would represent 2.5 × 106 rWBCs per 250 mL unit of packed RBCs. Although volumes of RBCs per human unit vary, this constitutes less than 5 × 106 residual leukocytes per unit, which is the standard for blood transfusion in the United States.23
Figure 1.
Flow cytometric enumeration of residual WBCs in leukoreduced blood and measurement of T-cell chimerism in recipient mice. Each leukoreduced unit of blood was analyzed for residual WBCs. (A) A representative analysis is shown. Leukoreduction resulted in fewer than 1000 rWBCs on average. Peripheral blood from recipient and control mice was stained with antibodies against Thy1.1 and Thy1.2 and analyzed by flow cytometry after gating on CD3+ events. (B) Representative plots of a mouse with engraftment and a mouse displaying rejection of BMT are shown.
B6.Thy1.1 mice were given weekly intravenous transfusions of 100 μL packed, leukoreduced RBCs from BALB.B donors. This amount of blood approximates the volume of a human RBC unit in the context of mouse whole blood volume. Negative control mice were transfused with 100 μL packed leukoreduced RBCs from B6 donors. Both groups received 4 weekly transfusions. One week after the last blood transfusion, mice were conditioned and given transplants of bone marrow from BALB.B donors. Engraftment of the BMT was evaluated 6 weeks after transplantation by staining peripheral WBCs of recipient mice with antibodies against the Thy1.1 (recipient) and Thy1.2 (donor) congenic markers. Representative plots of mice with BMT engraftment and rejection are shown (Figure 1B).
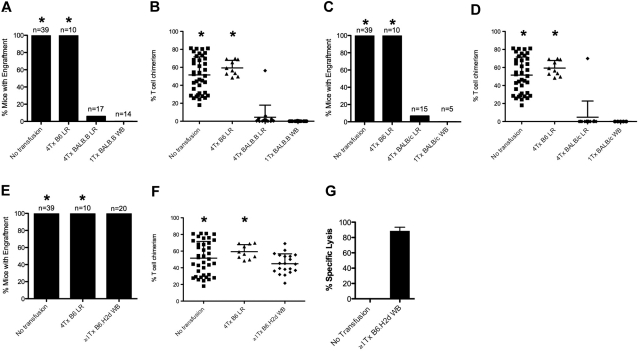
Whereas 100% of naive mice displayed BMT engraftment, transfusion of leukoreduced BALB.B blood into B6.Thy1.1 recipients induced BMT rejection (Figure 2A; Table 1). Combined analysis of 3 separate experiments with 17 total recipient mice demonstrated a rejection rate of 94% (P < .001) after transfusion with leukoreduced BALB.B blood. This effect was not the result of nonspecific factors in the transfused RBCs, as no rejection was observed in recipients receiving B6 RBCs (Figure 2A). In this model system, mice displaying engraftment had a range of T-cell chimerism from 18% to 81% and 48% to 70% for untransfused and B6 transfused mice, respectively (Figure 2B). In mice that rejected BMT, essentially no donor Thy1.2-positive cells were detectable (Figure 2B; see Figure 1B for representative flow cytometric data). Early experiments showed that BALB.B whole blood was more potent than leukoreduced blood in inducing BMT rejection; a single transfusion of whole blood induced 100% rejection, whereas rejection was variable with fewer than 3 transfusions of leukoreduced RBCs (data not shown). Together, these data demonstrate that transfusion of mHA-mismatched RBCs induces BMT rejection and that, although leukoreduction decreases the immunogenicity of RBC transfusion compared with whole blood, it is insufficient to prevent transfusion-induced BMT rejection.
Figure 2.
Transfusion of mHA-mismatched leukoreduced blood induces rejection of subsequent BMT. Transfusion with mHA-mismatched leukoreduced blood induces rejection of BMT from BALB.B donors. (A) The number of mice with engraftment was significantly reduced after 4 transfusions with mHA-mismatched blood (group BALB.B LR) compared with mice transfused with syngeneic blood (group B6 LR; P < .001, 2-tailed Fisher exact test). (B,D,F) Chimerism in engrafted animals was somewhat variable from experiment to experiment but resulted in a clear determination of engraftment versus rejection. (C) Use of BALB/c RBC donors induces rejection of BALB.B bone marrow, suggesting that the indirect pathway is active for donor RBCs (P < .001, 2-tailed Fisher exact test). (E) Transfusion-induced mHA-mismatched BMT rejection is not the result of alloimmunization against H-2d MHC molecules, as no rejection is seen in mice transfused with whole blood from B6.H2d mice (group B6.H2d WB). (G) Specific lysis against target cells from B6.H2d donor mice measured in an in vivo killing assay, indicating that anti-H2d alloimmunization occurred. Data shown are the combined results of at least 3 separate experiments in each figure. *The data corresponding to the no transfusion and syngeneic transfusion groups were combined for all the experiments and are presented in multiple panels.
Table 1.
Summary of strains used in the research
| Figure no. | RBC donor | Recipient | BMT donor | Main conclusion |
|---|---|---|---|---|
| 2A-B | ||||
| Strain | BALB.B | B6 | BALB.B | Exposure to mHAs in leukoreduced RBC units induce BMT rejection. |
| MHC | H-2b | H-2b | H-2b | |
| mHA | BALB* | B6 | BALB* | |
| 2C-D | ||||
| Strain | BALB/c | B6 | BALB.B | The indirect presentation pathway is sufficient to induce BMT rejection. |
| MHC | H-2d* | H-2b | H-2b | |
| mHA | BALB* | B6 | BALB* | |
| 2E-G | ||||
| Strain | B6.H2d* | B6 | BALB.B | Immunization against H-2d is not responsible for mHA-mismatched BMT rejection. |
| MHC | H-2d* | H-2b | H-2b | |
| mHA | B6 | B6 | BALB* | |
| 3 | ||||
| Strain | Male B10.BR | Female B6 | Male B6 | Removal of leukocyte-associated mHAs by filtration leukoreduction is sufficient to prevent BMT rejection. |
| MHC | H-2k* | H-2b | H-2b | |
| mHA | HY* | — | HY* |
— indicates not applicable.
MHC and mHA mismatches to the recipient.
Direct and indirect pathways in mHA-based transfusion-induced BMT rejection
To study the contribution of the indirect pathway of antigen presentation in transfusion-induced BMT rejection, we modified our model using BALB/c mice instead of BALB.B mice as RBC donors while keeping BALB.B mice as BMT donors (Table 1). As both B6 recipients and BALB.B BMT donors encode the H-2b MHC haplotype, any rejection of BALB.B marrow across mHA barriers will occur in response to peptides presented on MHC encoded by H-2b. BALB.B and BALB/c mice are congenic and differ only at the MHC locus (BALB/c mice express H-2d MHC molecules). Thus, BALB/c APCs are incapable of directly presenting mHAs peptides in MHC molecules encoded by H-2b. Accordingly, BALB/c cells cannot induce an immune response to BALB mHAs on H-2b MHC through the direct pathway. However, the indirect presentation pathway remains unaltered, and mHAs on donor cells may be processed and presented on H-2b MHC by the B6.Thy1.1 recipient's APCs.
B6.Thy1.1 mice were transfused weekly with 100 μL packed leukoreduced RBCs from BALB/c donors. Four weekly transfusions were given; 1 week after the last blood transfusion, all mice were conditioned and transplanted with BALB.B bone marrow. Whereas control mice receiving no transfusion or autologous B6 RBCs had 100% engraftment, 93% of mice receiving leukoreduced BALB/c RBCs rejected the BMT (Figure 2C). These data suggested that the indirect pathway was sufficient for transfusion-induced BMT rejection; however, it remained possible that the major alloresponse against H-2d MHC on BALB/c blood crossreacted with mHAs on BALB.B to the extent that it induced BMT rejection. To assess this possibility, we used B6 mice congenic for the H-2d MHC as RBC donors (B6.H2d), thus isolating the H-2d component of the immune response (Table 1). To enhance the alloresponse, recipient mice were transfused up to 3 times with packed whole blood (not leukoreduced). One week after the last transfusion, mice were conditioned and BALB.B marrow was transplanted. No rejection was observed in any of the transfused animals (Figure 2E). There was a trend to lower levels of engraftment in mice receiving H-2d blood (Figure 2F), but this trend did not reach statistical significance; no difference was seen between H-2d–transfused and untransfused groups. Lack of BMT rejection was not the result of lack of alloimmunization, as in vivo killing assays demonstrated elimination of the H-2d targets (Figure 2G). These data demonstrate that a robust anti–H-2d response does not cause rejection of BALB.B marrow, and rules out that the anti–H-2d response caused BMT rejection after transfusion of BALB/c RBCs. It is worth noting that, despite their differences in MHC molecule sequence, a fraction of mHA peptides may be presented by MHC from both H-2d and H-2b haplotypes. This may particularly be the case for MHC class II, as the MHC II molecules I-Ab and I-Ad have relatively few differences and are known to present some of the same peptides.30 Thus, we cannot unequivocally rule out that a low level of direct presentation is possible to the extent that a T cell specific for an H-2d/mHA complex may crossreact with an H-2b/mHA complex. However, these findings suggest that the direct pathway is not required and suggest that the indirect presentation of mHAs on transfused leukoreduced blood is sufficient to induce an immune response that causes BMT rejection.
Leukoreduction prevents BMT rejection induced by HY mHA-mismatched blood
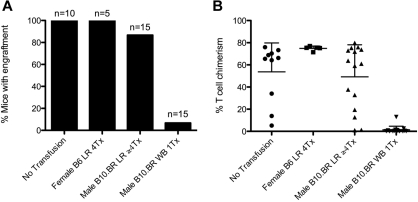
The data from the two preceding sections demonstrate that transfusion of RBC units induces BMT rejection. Although the units are stringently leukoreduced by the same methodologies used on human blood, leukocytes are nevertheless not completely eliminated. Thus, 2 hypotheses are equally consistent with the observed data: (1) mHAs on RBCs induce BMT rejection, and (2) mHAs on the low number of rWBCs are sufficient to induce rejection. To assess the contribution of rWBCs, we narrowed the transplantation barrier to a known minor antigen system, the male antigen (HY).20 We chose this system specifically because the HY antigens, Smcy and Uty in H-2b animals,31 are predicted nuclear proteins (http://www.uniprot.org/uniprot/Q62240, http://www.uniprot.org/uniprot/P79457). As RBCs are anucleate cells, one would not predict Smcy or Uty to be present in RBCs; indeed, the male antigens have not been detected in mature RBCs.32,33 We used this system to assess whether the extent of leukoreduction was sufficient to prevent BMT rejection to an mHA present on leukocytes but not RBCs.
Female B6.Thy1.1 mice were transfused with RBCs from male B10.BR mice, followed by bone marrow transplantation with male B6 donor marrow. B10.BR mice, which are H-2k, were used to minimize the effects of direct presentation analogous to the BALB/c–BALB.B combination, as any rWBCs would not present Smcy or Uty in the context of H-2b MHC (Table 1). Whereas a single transfusion of whole blood induced BMT rejection in 100% of mice, 4 weekly transfusions of leukoreduced male RBCs induced BMT rejection in only 13% of mice (Figure 3). These data demonstrate that the extent of filter leukoreduction is generally sufficient to eliminate transfusion-induced BMT rejection in response to an antigen on leukocytes, which is not on RBCs. This also suggests that residual leukocytes are not sufficient to cause BMT rejection in the previous experiments transfusing BALB.B or BALB/c blood into B6.Thy1.1 recipients. This supports the hypothesis that mHAs on RBCs are contributory.
Figure 3.
Leukoreduction prevents transfusion-induced rejection of HY+ BMT. Transfusion of whole blood but not leukoreduced blood from HY+ (male) donors into female B6.Thy1.1 causes rejection of HY+ BMT. Injection with male whole blood induced rejection in 93% of the recipients compared with untransfused mice or mice transfused with syngeneic blood (P = .001, 2-tailed Fisher exact test). (A) Transfusions with male LR blood did not significantly affect the number of mice displaying BMT engraftment. (B) Chimerism in engrafted animals was variable with the observation of microchimerism in some animals. Data shown are the compounded results of 2 separate experiments.
Generation of a novel transgenic donor mouse with RBC-specific expression of defined mHAs
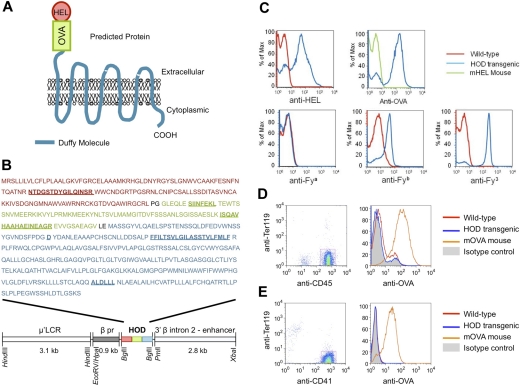
Although the data in previous sections suggest a role for mHAs on RBCs in transfusion-induced BMT rejection, an RBC-specific mHA was required to focus our analysis on the role of RBCs to the exclusion of WBC antigens. In addition, one would need sufficient analytic tools to study the immune response to the RBC-specific mHA. To achieve these criteria, we chose to generate a novel transgenic mouse with RBC-specific expression of existing model antigens for which a wide array of analytic tools already existed. We engineered a fusion protein of a model humoral antigen (HEL) and a model cellular antigen (OVA). To allow linkage to the RBC by natural means, the HEL-OVA protein was further fused in frame to the human Duffy blood group antigen; the Duffy b (Fyb) variant was used. The ORF codes for the triple-fusion protein HOD (Figure 4A). To provide RBC-specific expression, the HOD ORF was ligated into an expression vector containing the human β-globin gene promoter, β-globin locus control region (μ′LCR), and the β-globin gene intron 2 and 3′ enhancer (Figure 4B). This construct has previously been reported to confer RBC-specific expression in a transgenic mouse.34
Figure 4.
RBC-specific expression of a model fusion antigen by the HOD mouse. (A) Predicted structure of the HOD antigen. (B) Structure of the HOD construct and amino acid sequence of the HOD antigen. The HOD antigen is under control of the RBC-specific μ′LCR promoter and is a fusion of the full sequence of HEL, a fragment of OVA containing both the OT-I T cell–specific epitope (SIINFEKL) and the OT-II–specific epitope (ISQAVHAAHAEINEAGR), and the human Duffy blood group antigen. Additional features are indicated, including the “Duffy b” polymorphism (D), the first transmembrane domain of the molecule (FFILTSVLGILASSTVLFMLF), and the epitope for the Duffy 3 specific MIMA 29 monoclonal antibody (ALDLLL). The features described in this figure legend are underlined in panel B; amino acids were added at each fusion junction because of introduction of restriction sites Sma I and XhoI (PG and LE, in black). Staining of HOD blood demonstrates the predicted epitopes on RBCs, with no detectable HOD on CD45+TER-119− leukocytes or CD41+TER-119− platelets (C-E). Whereas only the OVA antigen was used in the course of the presented research, the HEL and Duffy antigens were included for alternate research purposes outside the scope of the current report.
A founder mouse that had germline integration of the transgene was identified and bred to generate a working colony. The transgene was transmitted with Mendelian segregation and litter sizes were normal, suggesting no deleterious effects of transgene integration (data not shown). Using RBCs from wild-type mice as a negative control, HOD RBCs stained positive with anti-HEL, anti-OVA, anti-Fyb, and anti-Fy3 (Figure 4C). The staining with anti-OVA was not an artifact of HEL contamination of the OVA used to generate anti-OVA antiserum, as anti-OVA did not stain cells from a mouse with ubiquitous expression of surface HEL (mHEL mouse,25 Figure 4C). HOD RBCs stained negative with control mouse serum (data not shown). HOD RBCs also stained negative with anti-Fya (as predicted because the Fyb Duffy variant was used in the HOD construct).
Expression on nonerythroid cells was assessed by gating on CD45+ leukocytes or CD41+ platelets and assessing anti-OVA staining. No HOD expression was detected on CD45+TER-119− leukocytes (Figure 4D). This was not an artifact of the stain not working, as leukocytes from the mOVA mouse were strongly positive. Likewise, no expression of HOD was detected on CD41+TER-119− platelets (Figure 4E). Together, these data indicate that the HOD antigen is expressed in a conformation that preserves the different epitopes from the fusion partners and in an RBC-specific fashion.
mHAs on transfused RBCs are cross-presented on MHC I of recipient APCs
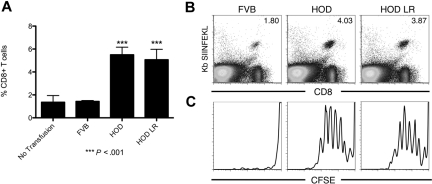
On the basis of the ability of leukoreduced RBCs to induce BMT rejection, we hypothesized that mHAs on transfused RBCs were capable of inducing anti-mHA immunity after processing and presentation by recipient APCs. In the field of transfusion medicine, it is well established that RBC antigens can induce activation of recipient CD4+ T cells and antibody-secreting B cells. Here, we further hypothesized that mHAs on transfused RBCs could be crosspresented into recipient MHC class I and stimulate CD8+ T cells. To test this hypothesis, we used OT-I transgenic mice, which express a T-cell receptor that recognizes a peptide from OVA (SIINFEKL) presented by H-2Kb MHC-I. OT-I splenocytes were adoptively transferred to wild-type B6.Thy1.1 recipient mice. Transferred OT-I T cells were visualized by an H-2Kb/SIINFEKL tetramer reagent. To allow an assessment of proliferation, OT-I T cells were labeled with CFSE before adoptive transfer. Transfusion of HOD RBCs induced a substantial expansion of OT-I T cells compared with negative control mice that received wild-type FVB blood (Figure 5A-B). Expansion of OT-I T cells was not significantly decreased by leukoreduction of HOD RBCs. The expansion was a function of CD8+ T-cell proliferation, as CFSE was diluted out in mice that received HOD whole blood or leukoreduced HOD RBCs, but not wild-type FVB RBCs (Figure 5C). These data indicate that mHAs expressed on transfused RBCs can be processed by recipient APCs and crosspresented on MHC class I in a fashion that leads to expansion of mHA-specific CD8+ T cells.
Figure 5.
mHAs on transfused leukoreduced HOD blood are crosspresented into MHC class I by recipient APCs. (A) Expansion of OT-I T cells in the spleen of recipient mice after transfusion with HOD whole blood or HOD LR blood, control mice were transfused with blood from FVB donors. (B) Representative flow cytometry plots. (C) CFSE dilution of OT-I T cells in response to transfusion of HOD blood. Data shown are representative of 3 separate experiments with similar results.
Discussion
Currently, the primary immunologic focus of transfusion medicine is to monitor the generation of antibody responses against blood group antigens on transfused RBCs. Indeed, providing crossmatch-compatible RBCs to patients is essential to avoid hemolytic transfusion reactions, which may lead to substantial morbidity and, in some cases, mortality. However, several clinical observations have demonstrated a correlation between the number of transfused units and the likelihood that a patient will reject BMT.13,14 This observation is equally consistent with 2 distinct hypotheses. First, repeat transfusion is causal for subsequent BMT rejection. Second, the severity of the underlying illness both necessitates more frequent transfusion and increases the likelihood of BMT rejection (ie, transfusion is correlative to rejection but not causal). Although severity of illness may certainly play a role in the likelihood of BMT rejection, the data presented herein demonstrate a causal link between transfused RBC units and BMT rejection across mHA barriers in a murine model. This defines a novel immunologic sequela of RBC transfusion, one that is currently neither monitored nor addressed by clinical transfusion immunology.
It is our hypothesis that exposure to mHAs on RBCs is alone sufficient to mediate transfusion-induced BMT rejection, as rejection occurred despite stringent leukoreduction. Of course, rWBCs cannot be entirely eliminated, thus making it impossible to unequivocally rule out that trace levels of rWBCs contribute to the effect. From the standpoint of establishing the transfusion-induced BMT rejection phenomenon, this issue is not practically relevant as, like our murine model, units of human leukoreduced RBCs likewise have rWBCs present. However, identifying the immunizing component of the RBC unit is central to devising a strategy to address the problem. If rWBCs are indeed responsible, then efforts should focus on developing better leukoreduction methods. However, if mHAs on RBCs are sufficient, then improving leukoreduction will not remedy the problem. The observation that leukoreduction is sufficient to prevent transfusion-induced BMT rejection in response to a leukocyte-specific antigen (HY antigen; Figure 3) indicates that the number of rWBCs after leukoreduction is insufficient to cause BMT rejection, suggesting that mHAs on a non-WBC component contribute to inducing rejection in leukoreduced transfusion-induced BMT rejection in the BALB.B-B6 strain combination. However, it is important to consider that the BALB.B-B6 strain combination has numerous antigenic differences and represents a greater immunologic barrier than the HY system. Thus, it is also possible that rWBCs are more potent in the BALB.B-B6 system.
We further tested the hypothesis that exposure to RBC mHAs is sufficient to induce immunity by creating the HOD mouse and observing that exposure to an RBC-specific mHA can induce a cellular response in the CD8+ compartment. These results build on our previous observations that CD8+ T cells responded to OVA chemically crosslinked to leukoreduced RBCs.35 However, in these previous experiments, as OVA was crosslinked to rWBCs, the crosslinking procedure may have altered the biology of RBCs. Using an RBC-specific genetic approach in the HOD mouse, the current data circumvent these caveats and provide substantial additional support to the notion that processing and presentation of mHAs on RBCs stimulates CD8+ T cells in the absence of contaminating donor WBCs. It is worth acknowledging that we cannot rule out that trace levels of HOD expression on WBCs, which are below our limits of detection, are responsible for activating CD8+ T cells. However, given the lack of detectable HOD on WBCs by flow cytometry and the similar expansion of OT-I T cells with leukoreduced RBCs and whole blood, we consider this possibility doubtful.
By minimizing the direct antigen presentation pathway in the BALB/c-C57BL/6 strain combination, our data suggest that the indirect pathway of antigen presentation is sufficient for transfusion-induced BMT rejection. The direct pathway was likewise minimized in the induction of OT-I activation by HOD RBCs, as the HOD mouse is on an FVB background and does not express H-2b. Together, these data indicate that processing of the blood by the recipient's APCs and presentation to the recipient's T cells are sufficient to activate an immune response against an mHA present on RBCs. However, the OT-I data only represent activation of CD8+ T cells, not established immunity, and thus represent a potential afferent mechanism of induction of immune responses by allogeneic RBCs. The effector mechanism of transfusion-induced BMT rejection has yet to be determined. However, the known activation of CD4+ T cells and B cells by transfused RBCs and the current HOD data indicating activation of CD8+ T cells provide several potential pathways of rejection.
In aggregate, the data presented herein strongly suggest that exposure to mHAs present on RBCs is capable of inducing cellular immunity and BMT rejection. Of course, the applicability of these findings to the human setting depends on the parallels between mouse and human biology. Nevertheless, these findings may explain the increased rates of BMT rejection in multiply transfused patients. Moreover, to the extent that mHAs are shared on RBCs and solid organs, these data predict increased rejection of solid organs in multiply transfused patients, as it has been suggested in recent years.36 Finally, as transfusion may induce memory cells specific for donor antigens, multiple transfusions may decrease efficacy of immunomodulatory approaches such as costimulatory blockade, which are less effective at inhibiting memory versus naive T cells. Extension of these studies into the human setting may consist of analyzing chronically transfused patients for T-cell immunity against known human mHAs through tetramer analysis of freshly isolated or restimulated peripheral T cells.
In the context of the data presented herein indicating that exposure to mHAs on RBCs can induce immunity, it is our prediction that no extent of leukoreduction will prevent transfusion-induced BMT rejection. Exposure clearly cannot be eliminated, as patients need the therapeutic effect of the transfused RBCs. At first consideration, it seems doubtful that matching donor and recipient mHAs during transfusion of patients likely to receive a transplant will be feasible, as the involved mHAs may be undefined and/or too varied to allow matching. However, it is possible that the specific mHAs are limited to antigen present on both mature RBCs and expressed by hematopoietic stem cells. In such a case, the number of relevant antigens may be limited, and matching may ultimately become feasible. In the absence of matching, development of immunomodulation strategies and/or improved conditioning regimens that can overcome existing immunity while limiting toxicity will probably be required.
Acknowledgments
The authors thank Dr John T. Horan for insightful discussion and help with the manuscript.
This work was supported by National Institutes of Health grants R21HL086312 and R01HL092977 (J.C.Z.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.D. and J.C.Z. designed the research, performed experiments, analyzed the data, and wrote the article; C.M.C. performed experiments and analyzed the data; and R.N. and K.R.P. contributed to construction and functional testing of the HOD construct.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James C. Zimring, Center for Transfusion and Cellular Therapies, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Woodruff Memorial Bldg, Suite 7107, 101 Woodruff Cir, Atlanta, GA 30322; e-mail: jzimrin@emory.edu.
References
- 1.Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117:850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia M, Walters MC. Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 2008;41:109–117. doi: 10.1038/sj.bmt.1705943. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman E, Wagner JE. Hematopoietic stem cell transplantation in childhood inherited bone marrow failure syndrome. Bone Marrow Transplant. 2008;41:127–132. doi: 10.1038/sj.bmt.1705960. [DOI] [PubMed] [Google Scholar]
- 4.Vlachos A, Ball S, Dahl N, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008;142:859–876. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georges GE, Storb R. Stem cell transplantation for aplastic anemia. Int J Hematol. 2002;75:141–146. doi: 10.1007/BF02982018. [DOI] [PubMed] [Google Scholar]
- 6.Fleitz J, Rumelhart S, Goldman F, et al. Successful allogeneic hematopoietic stem cell transplantation (HSCT) for Shwachman-Diamond syndrome. Bone Marrow Transplant. 2002;29:75–79. doi: 10.1038/sj.bmt.1703321. [DOI] [PubMed] [Google Scholar]
- 7.Roy V, Perez WS, Eapen M, et al. Bone marrow transplantation for diamond-blackfan anemia. Biol Blood Marrow Transplant. 2005;11:600–608. doi: 10.1016/j.bbmt.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Schrier SL, Angelucci E. New strategies in the treatment of the thalassemias. Annu Rev Med. 2005;56:157–171. doi: 10.1146/annurev.med.56.082103.104718. [DOI] [PubMed] [Google Scholar]
- 9.Iannone R, Casella JF, Fuchs EJ, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant. 2003;9:519–528. doi: 10.1016/s1083-8791(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsohn DA, Duerst R, Tse W, Kletzel M. Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. Lancet. 2004;364:156–162. doi: 10.1016/S0140-6736(04)16628-2. [DOI] [PubMed] [Google Scholar]
- 11.Krishnamurti L. Hematopoietic cell transplantation: a curative option for sickle cell disease. Pediatr Hematol Oncol. 2007;24:569–575. doi: 10.1080/08880010701640531. [DOI] [PubMed] [Google Scholar]
- 12.Viollier R, Socié G, Tichelli A, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008;41:45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]
- 13.Deeg HJ, Self S, Storb R, et al. Decreased incidence of marrow graft rejection in patients with severe aplastic anemia: changing impact of risk factors. Blood. 1986;68:1363–1368. [PubMed] [Google Scholar]
- 14.Gluckman E, Horowitz MM, Champlin RE, et al. Bone marrow transplantation for severe aplastic anemia: influence of conditioning and graft-versus-host disease prophylaxis regimens on outcome. Blood. 1992;79:269–275. [PubMed] [Google Scholar]
- 15.Champlin RE, Horowitz MM, van Bekkum DW, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. 1989;73:606–613. [PubMed] [Google Scholar]
- 16.Storb R, Epstein RB, Rudolph RH, Thomas ED. The effect of prior transfusion on marrow grafts between histocompatible canine siblings. J Immunol. 1970;105:627–633. [PubMed] [Google Scholar]
- 17.Storb R, Rudolph RH, Graham TC, Thomas ED. The influence of transfusions from unrelated donors upon marrow grafts between histocompatible canine siblings. J Immunol. 1971;107:409–413. [PubMed] [Google Scholar]
- 18.Storb R, Deeg HJ. Failure of allogeneic canine marrow grafts after total-body irradiation: allogeneic “resistance” versus transfusion-induced sensitization. Transplantation. 1986;42:571–580. doi: 10.1097/00007890-198612000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman ZF, Levy RB. MiHA reactive CD4 and CD8 T-cells effect resistance to hematopoietic engraftment following reduced intensity conditioning. Am J Transplant. 2006;6:2089–2098. doi: 10.1111/j.1600-6143.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 20.Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: a history of transplantation, immune response genes, sex determination and expression cloning. Annu Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- 21.Champlin RE, Perez WS, Passweg JR, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern M, Passweg JR, Locasciulli A, et al. Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia. Transplantation. 2006;82:218–226. doi: 10.1097/01.tp.0000226156.99206.d1. [DOI] [PubMed] [Google Scholar]
- 23.American Association of Blood Banks. Standards for Blood Banks and Transfusion Services. 24th Ed. Bethesda, MD: American Association of Blood Banks; 2006. [DOI] [PubMed] [Google Scholar]
- 24.Council of Europe. Guide to the Preparation, Use and Quality Assurance of Blood Components. 13th Ed. Strasbourg, France: Council of Europe; 2007. [Google Scholar]
- 25.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 26.Hendrickson JE, Desmarets M, Deshpande SS, et al. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–1536. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 27.van Os R, Sheridan TM, Robinson S, Drukteinis D, Ferrara JL, Mauch PM. Immunogenicity of Ly5 (CD45)-antigens hampers long-term engraftment following minimal conditioning in a murine bone marrow transplantation model. Stem Cells. 2001;19:80–87. doi: 10.1634/stemcells.19-1-80. [DOI] [PubMed] [Google Scholar]
- 28.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 29.Mandy F, Brando B. Enumeration of absolute cell counts using immunophenotypic techniques. Curr Protoc Cytom. 2001 doi: 10.1002/0471142956.cy0608s13. Chapter 6:Unit 6.8. [DOI] [PubMed] [Google Scholar]
- 30.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323-339 epitope. J Immunol. 2000;164:4706–4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 31.Roopenian D, Choi EY, Brown A. The immunogenomics of minor histocompatibility antigens. Immunol Rev. 2002;190:86–94. doi: 10.1034/j.1600-065x.2002.19007.x. [DOI] [PubMed] [Google Scholar]
- 32.Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood. 2006;108:791–801. doi: 10.1182/blood-2005-11-007799. [DOI] [PubMed] [Google Scholar]
- 33.Voogt PJ, Goulmy E, Fibbe WE, Veenhof WF, Brand A, Falkenburg JH. Minor histocompatibility antigen H-Y is expressed on human hematopoietic progenitor cells. J Clin Invest. 1988;82:906–912. doi: 10.1172/JCI113697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson KR, Fedosyuk H, Zelenchuk L, et al. Transgenic Cre expression mice for generation of erythroid-specific gene alterations. Genesis. 2004;39:1–9. doi: 10.1002/gene.20020. [DOI] [PubMed] [Google Scholar]
- 35.Zimring JC, Hair GA, Deshpande SS, Horan JT. Immunization to minor histocompatibility antigens on transfused RBCs through crosspriming into recipient MHC class I pathways. Blood. 2006;107:187–189. doi: 10.1182/blood-2005-07-3059. [DOI] [PubMed] [Google Scholar]
- 36.Lerut E, Van Damme B, Noizat-Pirenne F, et al. Duffy and Kidd blood group antigens: minor histocompatibility antigens involved in renal allograft rejection? Transfusion. 2007;47:28–40. doi: 10.1111/j.1537-2995.2007.01060.x. [DOI] [PubMed] [Google Scholar]