Abstract
The ability to regulate emotions is an important part of adaptive functioning in society. Advances in cognitive and affective neuroscience and biological psychiatry have facilitated examination of neural systems that may be important for emotion regulation. In this critical review we first develop a neural model of emotion regulation that includes neural systems implicated in different voluntary and automatic emotion regulatory subprocesses. We then use this model as a theoretical framework to examine functional neural abnormalities in these neural systems that may predispose to the development of a major psychiatric disorder characterized by severe emotion dysregulation, bipolar disorder.
Keywords: emotion regulation, voluntary emotion regulation, automatic emotion regulation, affective neuroscience, neurodevelopment, bipolar disorder
Introduction
Advances in the fields of cognitive and affective neuroscience and biological psychiatry have allowed us to identify the neural systems underlying emotion regulation and how abnormalities in these neural systems may be associated with the presence of symptoms of certain psychiatric disorders, particularly symptoms of mood disorders. We previously described a neural model of emotion perception and regulation based on a critical review of animal, human focal lesion and human neuroimaging studies.1 Here, the main focus was the development of a theoretical framework for the study of functional abnormalities in neural systems implicated in emotional dysregulation in various psychiatric disorders, including bipolar disorder (BD).
Since the publication of our neural model of emotion perception and regulation there have been several developments in the following research areas. First, there has been an increasing focus in the field of neuroimaging on the study of neural systems underlying emotion regulation in healthy, nonpsychiatric populations and the application of findings from these studies to the examination of psychiatric populations. Second, the recent research agenda for the Diagnostic and Statistical Manual of Mental Disorders, Version V (DSM-V) has emphasized the need to apply these basic and clinical neuroscience research findings to develop a framework for identifying biomarkers that reflect pathophysiological processes to facilitate earlier and more accurate diagnosis of psychiatric disorders.2–4 This is particularly relevant to BD, a disorder that is frequently misdiagnosed or diagnosed too late for early and successful treatment.5,6 For mood disorders in general, and BD in particular, these biomarkers could include functional and/or structural abnormalities in neural systems important for emotion regulation. In parallel with these clinical advances have been an increasing number of neuroimaging studies in BD that are beginning to show specific abnormalities in the structure and function of neural regions implicated in emotion regulation in individuals with the disorder. Finally, there is emerging work in the field of developmental affective neuroscience examining the development of neural systems of emotion and emotion regulation in youth with and without psychiatric disorders. Here, there is increasing interest in applying clinical neuroscience research findings to the study of populations who are genetically at risk of developing disorders such as BD, where the goal is to identify neural abnormalities that reflect high risk of future development of psychiatric disorder to target individuals for early, preventative interventions.
Such advances in the field of affective neuroscience and biological psychiatry have provided the motivation for the refinement of our original neural model, specifically to expand upon neural systems implicated in the different voluntary and automatic component processes and subprocesses inherent to emotion regulation and to develop a new neural model of emotion regulation. We then use this as a framework with which to examine findings from existing studies to increase understanding of the neural basis of emotion dysregulation in individuals with BD and in youth at risk for subsequent development of the disorder. There are two important longer-term implications of this approach. First, this can help facilitate identification of biomarkers reflecting pathophysiological mechanisms of BD to aid diagnosis. Second, this approach can help identify biomarkers reflecting abnormal neurodevelopmental processes that may characterize individuals most at risk of future development of BD.
The main goals of this review are therefore as follows:
Development of a new neural model of emotion perception to focus on examination of the neural systems implicated in the different voluntary and automatic component processes and subprocesses implicated in emotion regulation
Employment of this new neural model as a framework with which to examine the neural basis of emotion dysregulation in BD
Employment of this new neural model as a framework to examine abnormalities in the development of neural regions implicated in emotion regulation that may be already present in youth at risk for, and in youth with, BD, and that, in turn, may represent biomarkers associated with the development of the disorder.
Before introducing the components of our proposed neural model of emotion regulation, we first provide an overview of the anatomy of the prefrontal cortical regions upon which we focus in our model, and findings that have elucidated the functional connectivity between these prefrontal cortical regions and subcortical limbic regions implicated in emotion processing.
Anatomy of prefrontal cortical neural regions implicated in emotion regulation
The regions of the prefrontal cortex (PFC) that have most consistently been implicated in cognitive control processes, including decision-making and emotion regulation, include the orbitofrontal cortex (OFC), dorsomedial prefrontal cortex (MdPFC), anterior cingulate gyrus (ACG), dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC; for example see Krawczyk,7 and see following section). These regions in primates comprise a mixture of dysgranular, granular and agranular cortical areas, including an internal granular layer IV. Ventromedial areas of the PFC (that is, OFC, MdPFC and ACG) develop relatively early, and are involved especially in the control of emotional behaviors, whereas lateral prefrontal cortical regions (that is, DLPFC and VLPFC) develop late, and are principally involved in higher executive functions.8 The OFC, MdPFC and the ACG are large, heterogeneous cortical regions that cover the medial wall and ventral surface of the frontal cortex. The OFC, including Brodmann areas (BAs) 11–14 and medial BA 47, MdPFC (BA 10/32) and parts of the ACG, are regions of the PFC that are the most densely connected with the amygdala and other subcortical limbic and paralimbic regions.9 The lateral PFC can be divided into DLPFC, comprising BAs 9 and 46,10 and VLPFC, comprising BAs 45 and 47.11 BA 44, caudal to BA 46, subserves cognitive functions, in particular deductive reasoning and language perception,12 which make it functionally aligned with the DLPFC. The DLPFC is connected to the OFC, and to a variety of subcortical neural regions, including the thalamus, the dorsal striatum (dorsal caudate nucleus), the hippocampus and primary and secondary cortical association areas, including posterior temporal, parietal and occipital areas.13 There are also dense connections between OFC, MdPFC, and the ACG and DLFPC and VLPFC9 that provide indirect connections between subcortical regions implicated in processing emotional information and lateral prefrontal regions implicated in cognitive and higher-order executive processing.
The intrinsic cortico-cortical connections of the OFC and the MdPFC can further be described as reflecting two distinct networks.9 The ‘orbital prefrontal network’ involves multisensory inputs into the OFC and MdPFC regions and as such provides integration of sensory information, primarily information of an affective value.9 The ‘medial prefrontal network’ involves the integration of visceromotor information. Both of these networks have connections with subcortical limbic structures. In particular, the orbital network has connections with the ventral medial part of the basal nucleus of the amygdala whereas the medial network has connections with the ventral lateral part of the nucleus.9 There are also distinct connections between these neural networks and the striatum, thalamus, hypothalamus and brainstem.9 Although distinct, these networks have interconnections that allow convergence of sensorimotor integration and visceromotor control in the processing of emotionally salient information, and thereby facilitate the regulation of emotional behavior.
More recent work most relevant to the formulation of our following neural model of voluntary and automatic emotion regulation suggests that the pattern of projections to and from the OFC and the MdPFC and subcortical regions such as the amygdala allows for the identification of specific ‘feedback’ and focal ‘feedforward’ pathways. In particular, a recent study by Ghashghaei et al.14 examining output and input patterns of connections between prefrontal cortices and the amygdala in nonhuman primates confirmed that caudal OFC and ACG areas have strongest connections with the amygdala. Here, the authors provided novel evidence for connections from the amygdala to cortical layers I and II of these PFC regions, which they interpreted as being implicated in the focus of attention to motivationally relevant stimuli. Furthermore, they provided evidence for focal ‘feedforward’ projections from the amygdala to the middle layers of caudal OFC and ACG, which they interpreted as serving to provide information about the emotional salience or significance of external sensory stimuli, and potentially contributing to the role of the OFC in the rapid perception of reward contingencies.15 Input connections from cortical layer III (posterior OFC and areas of the ACG) to the amygdala were also identified and interpreted as involved in conveying information to the amygdala about internalized emotions such as jealousy, embarrassment and guilt. Finally, the authors demonstrated connections between OFC and lateral PFC, which may help mediate voluntary emotion regulation processes, given that lateral PFC regions have limited output to the amygdala (see section below).
Prefrontal cortical–subcortical limbic functional connectivity
Functional neuroimaging studies employing functional connectivity analyses afford the possibility of describing correlations between activities in different neural regions during task performance. Research in the field of affective neuroscience using functional connectivity analyses is still in its infancy. Nevertheless, the few studies that have been conducted to date have yielded findings that can inform understanding of the role of different prefrontal regions in emotion regulation. One recent study16 employing functional connectivity analyses to examine neural activity in response to fearful and angry faces in healthy adults yielded a path model that comprised a system linking the amygdala to subgenual ACG (BA25) to supragenual cingulate cortex (BA32) and back to the amygdala. In addition, the model indicated a significant information-processing path from OFC to DLPFC (BA46), and also between the amygdala and OFC. These findings support anatomical data demonstrating that the OFC is extensively and reciprocally connected with the lateral and dorsal regions of the PFC, and with the amygdala, and support the hypothesis that the OFC may mediate connections between higher-order dorsolateral prefrontal regions and subcortical limbic regions such as the amygdala during emotion regulation.
Other studies, using emotion-labeling paradigms, which are associated with voluntary control of responses to emotional stimuli—‘thinking rather than feeling about emotion’17—have also indicated that the right VLPFC may have an inhibitory role upon the amygdala, and that this may be mediated by the OFC.17–20 These findings are paralleled by reports of inverse patterns of activity in amygdala and OFC in response to ambiguous emotional stimuli, surprised facial expressions, depending upon whether these stimuli were interpreted positively or negatively21 More negative interpretations of surprised faces were associated with greater signal changes in the right ventral amygdala, although more positive interpretations, with greater signal changes in OFC. Another study provided further evidence for functional integration of OFC, MdPFC and VLPFC in the response to emotionally salient information. Here, functional connectivity analyses of neural activity to positive vs negative emotional sentence cues revealed a positive correlation between ventral striatal, OFC and MdPFC activity to positive sentences, and a positive correlation between ventral amygdala, MdPFC and VLPFC activities to negative sentences. Findings in this study also revealed a negative correlation between OFC, MdPFC and VLPFC activity to negative sentences, however, suggesting differential roles of OFC and VLPFC in the response to positive vs negative emotional stimuli.22
Functional connectivity findings have also indicated a dorsal-rostral division in ACC modulation of the thalamus—sensory cortex pathway in response to emotionally salient stimuli, with the dorsal ACC showing a positive modulation of this pathway, and the rostral ACC, an inverse relationship.23 Findings from this study also indicated an inverse interaction between the dorsal and rostral ACC with the direct thalamus–amygdala pathway, providing additional evidence for roles of dynamic functional relationships between thalamo–amygdala and cortical regions, and for a functional differentiation ventral/dorsal prefrontal neural systems.
These findings therefore implicate different regions of the PFC, including OFC, MdPFC, VLPFC and ACG, and further suggest distinguishable roles of these regions in the regulation of activity within subcortical limbic regions in the response to emotionally salient information. We next discuss specific neural models of emotion regulation that have been informed by these data.
Neural models of emotion regulation
Thompson24 proposed that ‘emotion regulation consists of extrinsic and intrinsic processes responsible for monitoring, evaluating, and modifying emotional reactions, especially their intensive and temporal features, to accomplish one’s goals’ (page 28). Gross and Thompson25 later added that these processes may be ‘automatic or controlled, conscious or unconscious, and may have their effects at one or more points in the emotion generative process’ (page 8). Although other definitions of emotion regulation have been proposed, this definition captures the complex nature of emotion regulation, including the existence of subprocesses that may be either voluntary or automatic. We highlight two previous models of emotion processing that focused on neural regions implicated in emotion regulation.
Phillips et al. model
In our previous model, we focused on neural regions implicated in processes involved in emotion perception, including the regulation of affective state and emotional behavior. Our review of the existing animal, human lesion and human functional neuroimaging literature led us to propose a neural model of emotion perception and regulation.1 We highlighted the role of a ventral neural system, including amygdala, insula, ventral striatum (that is, ventral caudate nucleus, putamen), ventral regions of the ACG and ventral regions of the PFC, in particular, ventromedial PFC/OFC, in the identification of emotionally salient stimuli, and mediation of autonomic responses to emotionally salient stimuli associated with the generation of an emotional state. In contrast, we highlighted the role of a dorsal neural system, including the hippocampus and dorsal regions of the ACG and PFC—brain regions that support cognitive processes such as selective attention, planning, performance monitoring and voluntary regulation of emotional states. We proposed that voluntary regulation of emotion is supported predominantly by the dorsal system. We also proposed, however, that reciprocal functional relationships may exist between the ventral and dorsal systems, which may be mediated by the ventral medial region of the PFC and which may support both voluntary and automatic regulation of emotion.
Ochsner and Gross model
More recently, Ochsner and Gross26 proposed a neural model of emotion regulation that focuses on the interaction between bottom-up emotion appraisal and top-down cognitive control processes centered on subcortical affective appraisal systems (amygdala, basal ganglia) and prefrontal cortical and cingulate systems, respectively.26–28 Their model includes two types of top-down appraisal systems. The first type involves dorsomedial and dorsolateral prefrontal cortical systems. These systems are implicated in top-down description-based appraisal, allowing the generation of mental representations of affective states, as well as reappraisal to regulate emotion. The second type involves ventral prefrontal cortical systems. These systems are implicated in top-down outcome-based appraisal, important for the learning of associations between outcomes and preceding choices or events to regulate emotion.
Commonalities and differences between these neural models of emotion regulation
Both of the above models implicate top-down processes in emotion regulation, and center top-down emotion regulatory processes in dorsal prefrontal cortical regions, and bottom-up emotion generation in subcortical, limbic neural regions. There are some differences, however, between the models.
One issue is the role of the ventral PFC, specifically the OFC, in emotion regulation. In our previous model, we argued for a role of OFC regions in the generation of emotional states based upon evidence from lesion analysis studies in humans, monkeys and rodents and neuroimaging studies in humans during normal and pathological emotional states.1,29,30 In contrast, Ochsner and Gross highlighted the role of this region in outcome-based appraisal as a process important for emotion regulation. This is paralleled by an increasing number of studies that point to the OFC, in tandem with ventral striatal regions implicated in expectancy or anticipation of emotionally salient future events, in outcome-based learning.31,32
Another major issue is the number of different subprocesses implicated in emotion regulation. In addition to the separation of emotion regulation into the two different types of appraisal described above, Ochsner and Gross28 proposed a hypothetical continuum to organize different subprocesses involved in the cognitive control of emotion. At one end of the continuum is the exclusive use of attentional control, including engagement or disengagement of attention to emotional stimuli, and at the other end is the exclusive use of cognitive change, including the above top-down appraisal and reappraisal processes. There is also a distinction between suppression and cognitive appraisal and re-appraisal as two different emotion regulatory strategies. Suppression, defined as a response-focused emotion regulatory strategy, involves the inhibition of ongoing emotion-expressive behavior. Although suppression alters the behavioral expression of emotion, it produces mixed physiological effects (for example, decreased heart rate and increased sympathetic activity).33–36 Reappraisal, defined as an antecedent-focused strategy, involves the attempt to alter the emotional meaning of originally salient stimuli but it does not lead to increased sympathetic arousal. It has therefore been argued that reappraisal is a more effective strategy for the regulation of negative affect.26,28,33,34,37 The extent to which these subprocesses are subserved by different neural systems remains unclear.
An important, but somewhat neglected issue is the extent to which emotion regulation can be subdivided into voluntary vs automatic component processes and subprocesses. It could be argued that these different subprocesses operate in parallel and possibly simultaneously with emotion appraisal and emotion generation processes and, as such, it may be deemed futile to attempt to study voluntary and automatic subprocesses as separate entities. If, however, we are to begin to understand the mechanisms of emotion regulation, the underlying neural systems, their development and subsequently how they may be altered in individuals with mood disorders, it will be necessary to identify the different subprocesses of emotion regulation and their corresponding neural systems.38 Understanding automatic emotion regulatory subprocesses may be particularly relevant to the study of emotion regulation development in childhood and adolescence and to the study of individuals with BD, in whom the inability to regulate emotion and resulting behavioral disturbances occur frequently without insight or subjective awareness.39 Although previous models,24,25 including our own,1 alluded to the fact that emotion regulation can occur either voluntarily or automatically, to our knowledge there has been no integration of these voluntary and automatic subprocesses into one neural model of emotion regulation.
The above models of emotion regulation can be integrated into a theoretical framework to include different types of voluntary and automatic behavioral and cognitive control subprocesses important for emotion regulation. These include voluntary behavioral control, attentional control and cognitive change, and automatic behavioral control, attentional control and cognitive change subprocesses (Table 1). We, therefore, next briefly review findings from animal studies and from human lesion and neuroimaging studies that can help to increase understanding of the neural systems implicated in these voluntary vs automatic emotion regulatory subprocesses. Our review of the recent literature is not exhaustive but focuses on examples of studies providing evidence for the presence of specific subprocesses of voluntary and automatic emotion regulation.
Table 1.
Voluntary and automatic subprocesses of emotion regulation—examples of research studies
| Components of neural model |
Examples of research studies |
Number of subjects |
Task | Key contrast | Method | Relevant brain regions |
|---|---|---|---|---|---|---|
| Voluntary subprocesses | ||||||
| Voluntary behavioral control | ||||||
| Suppression of emotion expression | Goldin et al.40 | 17 (healthy subjects) | Affect ratings after viewing film clips and still pictures (negative and neutral); conditions: watch, suppress, reappraise | Suppress > watch negative | fMRI | Amygdala R VLPFC |
| Beauregard et al.41 | 10 (healthy subjects) | Affect ratings after viewing film clips (sexually arousing and neutral); conditions: normal reaction, attempted inhibition | Sexual arousal minus neutral in the attempted inhibition condition | fMRI | R DLPFC R rostral ACG |
|
| Levesque et al.42 | 20 (healthy women) | Affect ratings after viewing film clips (sad and neutral); conditions: normal reaction, suppression | Sad minus neutral in the Suppression condition | fMRI | R OFC R DLPFC |
|
| Ohira et al.43 | 10 (healthy subjects) | Affect ratings after viewing pictures (negative, positive and neutral); conditions: attend, suppress | Suppress minus attend | PET | L MdPFC VLPFC Bi anteromedial and caudal OFC Rostral-ventral ACG |
|
| Gillath et al.44 | 20 (healthy women) | Thought suppression paradigm (negative, neutral); conditions: do not think, think, do not think relationship, think relationship | Do not think > think Do not think relationship > think relationship Do not think negative > think negative | fMRI | L dorsal ACG L MdPFC |
|
| Voluntary attentional control | ||||||
| Selective attention | Goldstein et al.45 | 14 (healthy subjects) | Emotional linguistic go/NoGo task | Contrasts containing Negative NoGo condition | fMRI | L amygdala Bi hippocampus and parahippocampus R dorsal ACG R DLPFC Bi OFC |
| Overcoming interference from emotional distractors | Erk et al.46 | 12 (healthy subjects) | Sternberg item recognition paradigm with emotional pictures as distractors | Valence by working memory load | fMRI | R amygdala R DLPFC L ventral striatum L DLPFC |
| Inhibition of emotional motor response | Perlstein et al.47 | 10 (healthy subjects) | Emotional working memory task with emotional pictures as cues and probes | Valence by working memory load | fMRI | R DLPFC Medial OFC |
| Luo et al.48 | 14 (healthy subjects) | Lexical decision-making task with supraliminal and subliminal emotional distractors | Valence by visibility | fMRI | L DLPFC R posterior parietal cortex R dorsal ACG |
|
| Voluntary cognitive change | ||||||
| Reappraisal expectancy of forthcoming events | Ochsner et al.49 | 15 (healthy subjects) | Cognitive reappraisal task (negative and neutral); conditions: attend, reappraise | Reappraise > attend | fMRI | R amygdale L OFC L DLPFC L VLPFC L MdPFC |
| Phan et al.50 | 14 (healthy subjects) | Cognitive reappraisal task (negative and neutral); conditions; maintain, suppress (reappraise) | Suppress (reappraise) > maintain | fMRI | L amygdale R DLPFC Bi dorsal ACG Bi OFC |
|
| Banks et al.51 | 14 (healthy subjects) | Cognitive reappraisal task (negative, positive and neutral); conditions: maintain, reappraise | Reappraise > maintain | fMRI | L amygdale Bi DLPFC R MdPFC R subgenual ACG Bi OFC |
|
| Urry et al.52 | 19 (healthy subjects) | Cognitive reappraisal task (negative and neutral); conditions; increase, decrease, attend | Decrease minus attend | fMRI | L amygdale Bi MdPFC |
|
| Corlett et al.53 | 14 (healthy subjects) | Contingency learning task | All retrospective revaluation events vs control items | fMRI | R lateral PFC |
|
| Kalish et al.84 | 15 (healthy subjects) | Anxiety Induction Task (for warning of possible electric painful stimuli); conditions: (1) anticipation vs no anticipation and (2) regulation vs no regulation | (Regulation > no Regulation)Anxiety > (Regulation > no regulation)No Anxiety | fMRI | R DLPFC | |
| Automatic subprocesses | ||||||
| Automatic behavioral control | ||||||
| Extinction | Rosenkranz et al.54 | Sample of rats | Pavlovian conditioning— in vivo | NA | Intracellular electrophysiological recordings | Lateral nucleus of the amygdala Infralimbic cortex (posterior subgenual ACG is the equivalent in humans) |
| Behavioral regulation | Vidal-Gonzalez et al.55 | 76 rats | Fear conditioning and extinction | NA | Microstimulation | Basal amygdale infralimbic cortex |
| Milad and Quirk56 | Sample of rats | Auditory fear conditioning | NA | Intracellular electrophysiological recordings and microstimulation | Infralimbic cortex | |
| Morgan et al.57 | Male Sprague- Dawley rats | Classic fear-conditioning paradigm with extinction and reacquisition | NA | Lesion study | Infralimbic cortex | |
| McGinty et al.58 | Male Sprague- Dawley rats | Pavlovian odor conditioning | NA | Intracellular electrophysiological recordings and micro-stimulation | Basolateral amygdala Infralimbic cortex Nucleus accumbens | |
| Phelps et al.59 | 18 (healthy subjects) | Simple discrimination, partial reinforcement paradigm | Conditioned stimulus (CS+) minus absence of conditioned stimulus (CS−) | fMRI | Bisubgenual ACG Bi MdPFC Dorsal ACG |
|
| Gottfried & Dolan60 | 18 (healthy subjects) | Olfactory aversive-conditioning and extinction paradigm | Conditioning minus extinction; extinction minus conditioning | fMRI | Amygdala OFC | |
| Milad et al.61 | 14 (healthy subjects) | Differential fear-conditioning paradigm | Cortical thickness; conditioned stimulus (CS+) minus absence of conditioned stimulus (CS−) | Structural MRI; fMRI | Dorsal ACG | |
| Automatic attentional control | ||||||
| Cognitive disengagement | Armony and Dolan62 | 10 (healthy subjects) | Fear-conditioning paradigm and attention-orienting task | Conditioned stimulus (CS+) minus absence of conditioned stimulus (CS−); incongruent minus congruent | fMRI | Left OFC |
| Repressive and avoidant personality styles | Pourtois et al.63 | 15 (healthy subjects) | Modified Dot-Probe task: designed to assess covert spatial selective attention modulated by threatening stimuli | Fear valid > fear invalid | fMRI | Lateral OFC Intraparietal sulcus |
| Whalen et al.64 | 10 (healthy subjects) | Masked emotional facial expressions block design paradigm; conditions: fearful, happy and fixation | Fearful face > happy face | fMRI | Amygdala | |
| Blair et al.65 | 22 (healthy subjects) | Affective Stroop with emotional pictures; conditions: negative, positive and neutral | Task by emotion | fMRI | L rostral ACG Bi DLPFC bilateral OFC R MdPFC |
|
| Etkin et al.66 | 19 (healthy subjects) | Emotion conflict resolution paradigm (emotional facial expressions with congruent and incongruent emotional words) | High conflict minus low conflict | fMRI; functional connectivity | Amygdala Rostral ACG |
|
| Vuilleumier et al.67 | 12 (healthy subjects) | Attention-emotion paradigm (pair of emotional facial expressions and houses) | (Attended fearful face—attended neutral face) > (unattended fearful face—unattended neutral face) | fMRI | Amygdala Fusiform cortex Dorsal ACG |
|
| Pessoa et αl.68 | 20 (healthy subjects) | Block design attention– emotion paradigm | Emotion by attentional load | fMRI | Amygdala Posterior cingulate MdPFC |
|
| Phillips et al.69 | 8 (healthy men) | Emotional facial expressions (fearful, disgust, neutral); conditions: overt, covert | Overt vs covert presentations of fear Overt vs covert presentations of disgust | fMRI | Amygdala—overt fearful facial expressions Insula—overt disgust facial expressions |
|
| Stein et al.16 | 83 (healthy subjects) | Block design face-matching paradigm | Emotional faces minus control task | fMRI; functional connectivity | Amygdala Subgenual ACG Lateral PFC OFC DLPFC |
|
| Williams et al.70 | 15 (healthy subjects) | Emotional facial expressions (fearful, neutral); conditions: conscious, nonconscious | Conscious: fearful minus neutral Nonconscious: fearful minus neutral | fMRI; functional connectivity | Bi Amygdala MdPFC Dorsal ACG Ventral ACG |
|
| Automatic cognitive change | ||||||
| Covert appraisal and reappraisal | Hunkin et al.71 | 8 (healthy subjects) | Associative encoding and single item encoding paradigms | Associative encoding of novel associations vs associative encoding of familiar associations | fMRI | Parahippocampus |
| Covert response (e.g., error) monitoring | van Veen and Carter72 | 12 (healthy subjects) | Eriksen flanker paradigm | Incorrect responses vs correct responses | ERP; source localization | Dorsal ACG |
| Covert learning that serves to automatically adjust behavior | Luks et al.73 | |||||
| Bechara et al.74 | 44 controls 7 subjects with damage to OPFC | Iowa gambling task | Advantageous decks (C + D) minus disadvantageous decks (A + B) | Neuropsychological data | OFC | |
| Ernst et al.75 | 20 (healthy subjects) | Risk-taking task: computerized gambling card game | Risk-taking task minus control task | PET | Bi OFC | |
| Fukui et al.76 | 19 (healthy subjects) | Iowa gambling task | Risky decisions minus safe decisions | fMRI | Bi MdPFC | |
| Lawrence et al., under review77 | 17 (healthy men) | Iowa gambling task (modified for rapid event-related fMRI) | Risky decisions minus safe decisions | fMRI | L MdPFC OFC |
Abbreviations: ACG, anterior cingulate gyrus; Bi, bilateral; DLPFC, dorsolateral prefrontal cortex; fMRI, functional magnetic resonance imaging; L, left; MdPFC, dorsomedial prefrontal cortex; NA, not applicable; OFC, orbital frontal cortex; R, right; VLPFC, ventrolateral prefrontal cortex.
Studies reviewed in this table serve as examples of empirical evidence in support of our neural model of emotion regulation and do not constitute an exhaustive review of the literature.
Voluntary subprocesses
Voluntary behavioral control (suppression)
A small number of human neuroimaging studies have examined the neural correlates of voluntary behavioral control in response to emotional stimuli using different methods to train volunteers to suppress emotional expression. One such method is to train volunteers to keep their face still while viewing film clips such that another person watching their face would not be able to detect the emotion being experienced.33,36,40 Here, suppression of emotion-expressive behavior, associated with reduced negative emotion experience and behavior, was associated with activity within bilateral dorsomedial PFC (MdPFC), right dorsolateral PFC (DLPFC) and left ventrolateral PFC (VLPFC). Greater, rather than reduced, bilateral subcortical limbic (that is, amygdala, insula) activity accompanied voluntary suppression, however, suggesting that although suppression may constitute an emotion regulation strategy, it may not be the most beneficial as a therapeutic strategy.28
Another behavioral control method is to train volunteers to view emotionally provoking stimuli as ‘detached observers’.41 Here, attempts to inhibit sexual arousal in response to erotic film excerpts were associated with greater right superior PFC/DLPFC and right rostral ACG activity. Using a similar approach, Levesque et al.42 showed that suppression of sadness was related to greater activity in right OFC and right DLPFC in healthy women. Positive correlations were also demonstrated between self-report ratings of sadness and activity in these regions.
In a recent study, Ohira et al.43 used positron emission tomography (PET) to examine regional cerebral blood flow changes in PFC that accompanied autonomic nervous system responses associated with emotion suppression (attempting to remain calm and diminish any emotional response to emotionally salient pictures). Suppression of emotional responses was associated with greater activity within left MdPFC, VLPFC, bilateral anteromedial and caudal OFC, as well as left rostral-ventral ACG. Suppression was also associated with increased amplitude of skin conductance responses, which, in turn, was positively correlated with right OFC activity. In a further study, suppression of neutral, negative and relationship-related thoughts was associated with activity within left dorsal ACG and MdPFC.44
Findings from the above studies indicate that neural regions consistently implicated in voluntary behavioral control of both positive41 and negative42 emotions include DLPFC and VLPFC, in particular, right DLPFC and left VLPFC. These regions may support processes involved in regulating inner states to achieve desired outcomes.78 Other regions implicated in voluntary behavioral control of emotional behaviors include left/bilateral MdPFC, left rostral ACG and right/bilateral OFC. Given that lateral regions of the PFC share only sparse direct connections with subcortical limbic regions such as the amygdala, it has been proposed that the modulation of subcortical limbic regions by lateral and dorsal regions of the PFC during voluntary behavioral control of emotion may occur indirectly via OFC,79 which has extensive reciprocal anatomical connections with both lateral prefrontal cortical and subcortical limbic structures,9,80 and has been implicated in regulating autonomic nervous system responses during emotion suppression.43
Voluntary attentional control
Included in voluntary attentional control are (1) selective attention, to direct or redirect attention toward goal-related stimuli, and (2) inhibition, to distract from goal-irrelevant stimuli. For example, using an affective Go/NoGo functional magnetic resonance imaging (fMRI) paradigm, Goldstein et al.45 examined the neural correlates of response inhibition to emotional words in healthy adult volunteers. The task involved responding to word stimuli written in normal font (Go trials) and inhibiting responses to words written in italics (NoGo trials). The valence of these words was positive, negative or neutral. Findings revealed a frontolimbic network associated with a valence by response inhibition interaction. Specifically, this network included prefrontal (right DLPFC, right dorsal ACG and bilateral OFC), left subcortical (amygdala), bilateral paralimbic (hippocampus/parahippocampus) and right parietal cortical regions, particularly during inhibition of responses to negative emotional stimuli.
In another study, Erk et al.46 used an affective working memory task. Here, healthy volunteers first viewed a display of six capital letters followed by a delay period, during which they viewed simultaneous presentations of a series of emotional pictures, each paired with a single lower case letter. During presentation of the latter, participants indicated whether the single letter was part of the initial letter set. Greater right DLPFC activity was associated with reduced right amygdala activity during presentation of negative emotional pictures, whereas greater left DLPFC activity was associated with reduced left ventral striatal activity during presentation of positive emotional pictures. The involvement of specific prefrontal regions in downregulating subcortical activity to negative vs positive emotion stimuli is consistent with previous findings using a different version of an affective working memory task.47 Moreover, recent findings indicate that activity in left DLPFC, right posterior parietal cortex and right dorsal ACG is greater as a function of the emotional salience and visibility of emotional information during a lexical decision-making task.48
Findings from the above studies, therefore, indicate that bilateral DLPFC, right dorsal ACG and right parietal cortex are important in supporting voluntary attentional control subprocesses. Again, it is possible that modulation of subcortical limbic regional activity by these lateral and dorsal regions of the PFC during voluntary control of attention away from emotional material may occur indirectly via the OFC. This latter region has been demonstrated to be part of the neural network recruited to support this voluntary emotion regulatory subprocess.45
Voluntary cognitive change: reappraisal
One of the first studies to use fMRI to examine the neural correlates of voluntary reappraisal processes involved volunteers viewing negative and neutral pictures while being instructed either to experience the emotion that each picture elicited, or to reappraise the emotional salience by reinterpretation of the negative pictures so that they no longer elicited a negative emotional response.49 Reappraisal was associated with significantly greater activity in left DLPFC, left VLPFC and left MdPFC. There was also reduced activity in the right amygdala and left OFC. Moreover, the magnitude of activity in left VLPFC was negatively correlated with magnitude of activity within left amygdala and left OFC.
Another study using a similar paradigm50 asked trained subjects to either transform a depicted scenario into a positive event or to rationalize the content of an emotional picture. Downregulation of negative emotions by the use of reappraisal strategies recruited several prefrontal regions, including right DLPFC, bilateral dorsal ACG and bilateral OFC. Activity in these regions was associated with decreased activity in subcortical limbic regions, including the left amygdala. In addition, activity in bilateral dorsal ACG, bilateral DLPFC, right anterior insula and bilateral VLPFC was inversely associated with the intensity of negative affect. A recent study also demonstrated that activity in left amygdala covaried with activity in bilateral DLPFC, right MdPFC, the right subgenual region of the ACG and bilateral OFC during a reappraisal-based strategy to downregulate negative affect.51 Moreover, the magnitude of amygdala—OFC/MdPFC coupling predicted successful emotion regulation. A further study52 demonstrated an inverse functional coupling between bilateral OFC and left amygdala during the employment of reappraisal strategies to downregulate negative affect.
Expectancy of forthcoming events and outcomes is an additional type of reappraisal of environmental stimuli, and may serve as an additional voluntary cognitive control strategy for emotion regulation. Animal studies81 have associated expectancy of subsequent outcomes with activity in lateral PFC, whereas human neuroimaging studies have shown that mismatch between expected and actual outcomes (prediction error) is associated with activity in right lateral PFC.53,82 It has been also recently demonstrated that prediction error-related activity in right lateral PFC is reduced in schizophrenia.83
Together, the above findings suggest that reappraisal of emotional stimuli to reduce negative affect is associated with activity in a network of prefrontal cortical regions, including bilateral DLPFC, bilateral MdPFC and bilateral dorsal ACG (see Ochsner and Gross26). A specific role of the right DLPFC in reappraisal has also been proposed.84 Activity in this lateral and medial prefrontal cortical network may, in turn, be mediated by bilateral OFC and the right subgenual ACG, as activity in these latter regions has also been observed during reappraisal of emotional information.51,52
Automatic subprocesses
Automatic behavioral control
Key subprocesses of automatic behavioral control include extinction of previously acquired behavior and inhibition of the stress response. Rodent studies have demonstrated that the infralimbic cortex, a region that shares extensive anatomical connections with the amygdala,85,86 has a role in inhibiting activity within the amygdala.54,55 The infralimbic cortex has also been implicated in the extinction of previously conditioned behaviors.56,57,87,88 A recent study in rodents has indicated that projections from the basolateral amygdala, which encodes affective information, and the MdPFC converge within the ventral striatum (nucleus accumbens; McGinty and Grace58), suggesting that the basolateral amygdala may recruit the MdPFC to drive specific sets of nucleus accumbens neurons and thereby influence behavioral responses to conditioned cues. Thus, both infralimbic cortex and MdPFC in rodents appear to be involved in regulation of amygdala-driven behaviors.
Functional neuroimaging studies in humans implicate bilateral regions of the subgenual region of the ACG, together with bilateral MdPFC and dorsal ACG in fear extinction.59 The posterior subgenual ACG has been identified as the human equivalent of the rat infralimbic cortex.86,89 Other findings highlight the role of bilateral OFC in extinction learning,60,61 and a role for the right OFC in extinction learning more than aversive conditioning.60
The infralimbic cortex has been also implicated, however, in the encoding of the predictive value of emotionally salient stimuli in rodents through close interconnections with the basolateral amygdala,9,90 whereas the OFC has been associated with the mediation of anxious temperament in nonhuman primates.91 In humans, the left OFC has been implicated in aversive conditioning and, in more lateral left OFC, the representation of the value of stimulus-outcome contingencies.60 The generation of sad mood92 and depression,93 monitoring of internal states in individuals with attachment-avoidant personality styles44 have all been associated with greater activity in left or bilateral subgenual ACG, whereas improvement in depression after pharmacotherapy94 and deep-brain stimulation93 have been associated with decreases in activity within right and left subgenual ACG, respectively. One study, for example, demonstrated in individuals with attachment-avoidant personality styles relative to individuals with nonavoidant personality styles reduced deactivation of bilateral subgenual ACG during attempted voluntary suppression of emotionally neutral, negative and/or relationship-related thoughts.44 The authors interpreted these findings as evidence of a failure of top-down control to decrease monitoring of internal states (mediated by bilateral subgenual ACG) in individuals with avoidant personality styles. These findings therefore suggest, in humans, roles of bilateral OFC and subgenual ACG in the automatic regulation of emotional behavior that may be intrinsically linked with the roles of these structures in the encoding of emotional salience.
Automatic attentional control
To examine neural systems implicated in automatic attentional control of emotion processing, studies have employed paradigms that implicitly direct attention toward or away from emotional material. One such example is the dot probe task.95 Here, a faster response to a probe that appears at the same location of a previously presented emotional stimulus relative to the response to a probe at the opposite location is interpreted as an automatic attentional bias toward the emotional stimulus. Recent findings implicate the left OFC in automatic disengagement of attention away from emotional stimuli (for example, fearful faces) during this type of paradigm.62,63
Another example is the emotional Stroop task. Here, individuals attend to a nonemotional stimulus feature (for example, color or number of stimuli) at the expense of the emotional content of the stimulus. Performance of this task has been associated with activity in left rostral ACG, specifically, greater activity in this region to negative emotional relative to neutral stimuli during early stages of task performance, although decreased activity in this region overall.64 The authors noted here that susceptibility artifacts may have resulted in signal dropout in more ventral regions of the PFC, such as the OFC. More recent findings confirm the role of the left rostral ACG, in addition to a wider network of bilateral DLPFC, bilateral OFC and right MdPFC, in performance of incongruent and congruent trials of an emotional counting Stroop paradigm.65 This study also demonstrated a role of the right MdPFC in performance of negative and positive incongruent trials. A modified emotional Stroop paradigm has also been developed.66 Here, the task was to identify the expression and ignore the emotion word label displayed by either congruent or incongruent facial expression—emotion label stimuli. Incongruent stimuli were further subdivided into implicitly high conflict resolution trials, where incongruent stimuli were preceded by incongruent stimuli, and implicitly low conflict resolution trials, where incongruent stimuli were preceded by congruent stimuli. Facial expression identification during the high conflict resolution, but not low conflict resolution, trials was associated with left rostral ACG, further highlighting a role for this region in the implicit resolution of emotional conflict.
A further type of paradigm involves redirection of attention away from facial expressions using coin-cidentally presented spatial cues and/or cognitive task performance. In one study,67 the task involved direction of attention toward fearful facial expression pairs, or away from these pairs and toward nonface pairs during a ‘same-different’ task within-pair labeling task. Here, the caudal region of the ACG was activated when fearful faces were task relevant and that the rostral region of the ACG was activated when the fearful faces were task irrelevant. These findings were interpreted as evidence for a role of the rostral ACG in the direction of attention away from emotional stimuli by inhibiting processing of emotional information.
Other studies have examined the extent to which the magnitude of amygdala activity to facial expressions is, in fact, dependent on available attentional resources by either systematically manipulating the attentional load of a coincidentally presented visuos-patial task68,96 or by presentation of facial expressions covertly, that is, below the level of subjective awareness.69,70,97,98 Findings from the former study68 indicated that increasing the attentional load required to perform a coincidentally presented visuospatial task was associated with reduced amygdala activity. Findings from the latter group of studies provide conflicting data of preserved97,98 but also absent69 amygdala activity to covert presentations of facial expressions. Functional connectivity analyses have furthermore suggested that covert processing of threat-related facial expressions may be associated with a positive feedforward subcortical pathway to the amygdala, whereas awareness of these facial expressions was associated with negative connectivity within both cortical and subcortical pathways to the amygdala.70 Reentrant feedback between the amygdala and cortical and subcortical regions may be necessary, therefore, for awareness of threat.70 Together, these studies suggest that processing emotionally salient stimuli per se may require a certain level of automatic or covertly directed attention, associated with amygdala activity, whereas automatic redirection of attention away from emotion stimuli is dependent upon rostral ACG.
Automatic cognitive change
Subprocesses included within automatic cognitive change comprise appraisal and reappraisal, monitoring of behavior and rule learning that all occur without subjective awareness. Implicit appraisal of novel contexts, covert error monitoring and covert risk learning paradigms, such as gambling tasks, are all examples of paradigms that are, therefore, appropriate paradigms for the examination of neural regions underlying automatic emotion regulation. Regarding the first of these subprocesses, implicit appraisal and reappraisal, Gray and McNaughton99 upon the hippocampus and septum, with the capacity to act as a general-purpose comparator to appraise potential conflict between different goal-directed behaviors, to facilitate exploratory rather than defensive patterns of behavior and to allow resolution of goal conflict. The parahippocampal gyrus, which has direct connections with the hippocampus and amygdala,100 has also been associated with implicit appraisal and reappraisal processes: for example, with novel face detection in humans (right parahip-pocampus101), and with the encoding of novel associations occurring without subjective awareness of the novelty of these associations (left parahippo-campus71). A recent functional connectivity study in humans has provided, furthermore, evidence of strong connections between the amygdala and the parahippocampus (including the hippocampus16).
Covert error monitoring and covert risk learning paradigms, such as gambling tasks are paradigms relevant for examination of neural regions underlying the second and third types of subprocesses described above that are involved in automatic emotion regulation: behavior monitoring and rule learning occurring without subjective awareness. Error monitoring has been linked with midline dorsal ACG72,102 More specifically, a role for dorsal ACG has been postulated that involves the monitoring of conflict as an index of task difficulty, and associated autonomic activity, during action or strategy selection.102 Informed preparation for conflict processing, furthermore, has been linked with decreased ACG activity73 The dorsal ACG has also been associated with the mediation of fear expression,61 in further support of the role of this structure in the mediation of autonomic activity.
Performance of gambling tasks has been most consistently associated with bilateral OFC in human lesion studies,74,103 and in neuroimaging studies,75 highlighting a role of the OFC in outcome-based appraisal. Recent neuroimaging findings also implicate bilateral (left more than right) MdPFC in the implicit learning of successful strategies during gambling task performance,76,77 however, that may relate to a potential role for this region in switching of attention between internally generated thoughts and external stimuli104 and the use of internal signals generated by the OFC to select goal-directed responses.77
Together, these findings implicate bilateral hippocampus and parahippocampus, midline dorsal ACG and bilateral OFC and MdPFC in automatic cognitive change subprocesses relevant to automatic emotion regulation. The connections between hippocampus and parahippocampus with OFC and ACG through the subiculum and entorhinal cortex9,90 make it plausible that these regions may function as a neural network during automatic emotion regulation.
Summary and integration: a new neural model of emotion regulation
Findings from human neuroimaging studies indicate roles of several prefrontal cortical regions in sub-processes associated with voluntary emotion regulation, including voluntary behavioral control, attention redirection and cognitive change. Dorsal prefrontal cortical regions, including bilateral DLPFC, bilateral MdPFC and bilateral dorsal ACG, have been most consistently linked with these subprocesses. The right DLPFC may be implicated in particular with voluntary cognitive change, that is, reappraisal. Activity in these regions may be mediated by bilateral ventromedial prefrontal cortical regions such as the OFC that have direct connections with subcortical neural regions underlying the identification and initial processing of emotional material. By contrast, lesion studies in humans and experimental animals and human neuroimaging studies highlight bilateral subgenual ACG, bilateral OFC, left rostral ACG and bilateral MdPFC, midline dorsal ACG, and contributing roles of the hippocampus and parahippocampus, in the different subprocesses associated with automatic emotion regulation.
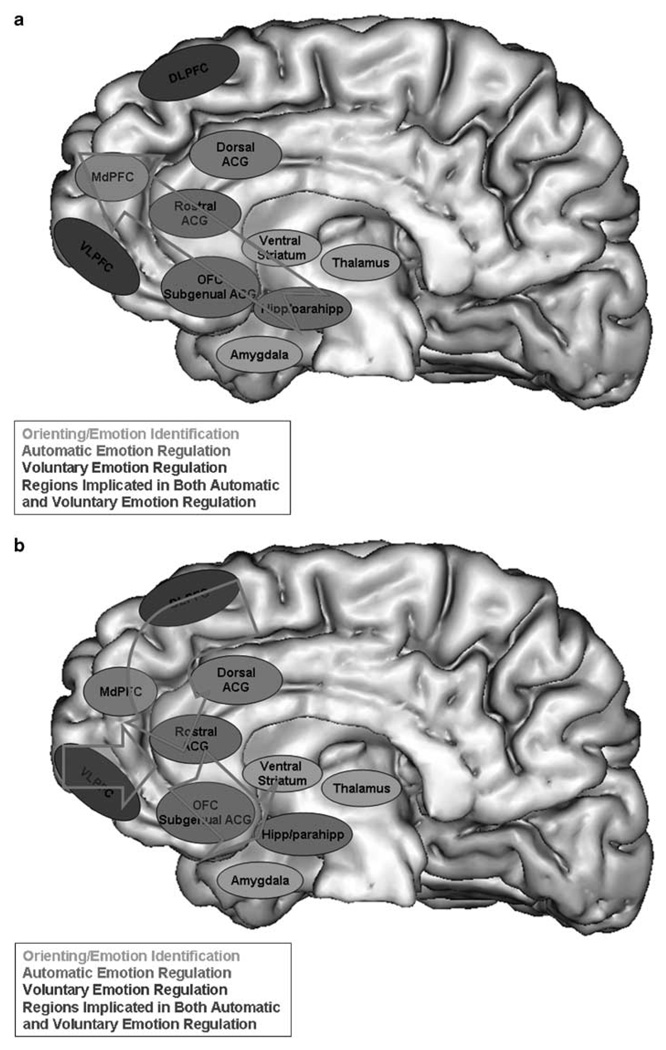
In our original neural model of emotion processing, we highlighted that two systems in PFC may be implicated in emotion regulation: a lateral prefrontal cortical system (including, for example, DLPFC and VLPFC) that is neocortical in origin and operates by a feedback mechanism, and a medial prefrontal cortical system (including, for example, OFC subgenual ACG, rostral ACG and MdPFC) that is paleocortical in origin and operates by a feedforward mechanism.105 Our overview of the more recent literature suggests that the former neural system may be involved in voluntary subprocesses, whereas the latter neural system may subserve automatic subprocesses. The MdPFC in particular may use feedforward inputs from the OFC, as signals of internal states, to select appropriate behaviors during automatic cognitive change paradigms. These two neural systems may be activated concurrently during regulation of emotional states and behaviors that are initially generated by orienting and emotion perceptual processes in amygdala, ventral striatum and thalamus (Figures 1a and b).
Figure 1.
Neural model of emotion regulation illustrating neural systems implicated in voluntary and automatic subprocesses of emotion regulation, (a) Feedforward pathway: medial prefrontal cortical system, including the OFC, subgenual ACG, rostral ACG, hippocampus and parahippocampus and MdPFC. (b) Feedback pathway: lateral prefrontal cortical system, including DLPFC and VLPFC. DLPFC, dorsolateral prefrontal cortex; MdPFC, dorsomedial prefrontal cortex; ACG, anterior cingulate gyrus; VLPFC, ventrolateral prefrontal cortex; OFC, orbital frontal cortex; hipp/parahip, hippocampus-parahippocampus region.
Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder
We previously emphasized the importance of our earlier neural model of emotion perception and regulation as a framework for understanding pathophysiological mechanisms underlying major psychiatric disorders, in particular, mood disorders such as BD.29 Using our new neural model of emotion regulation as the framework, we now examine the extent to which recent neuroimaging studies inform understanding of pathophysiological processes that lead to the development of BD. We first examine findings from structural and functional neuroimaging studies in adult BD. Informed by current understanding of the normal development of neural regions implicated in the different emotion regulatory sub-processes, we then review findings from existing neuroimaging studies of pediatric bipolar and bipolar at-risk populations that point to abnormal development of these neural regions in BD.
Adult bipolar disorder
Structural neuroimaging studies
There is a growing literature from volumetric structural neuroimaging studies indicating greater amygdala volumes in adult BD106,107 relative to age-matched healthy volunteers. More importantly, for the study of neural regions implicated in emotion regulation, studies have reported reduced volume and gray matter (GM) density in a variety of different prefrontal cortical regions in BD relative to healthy adults, but no consistent lateralization of these findings.
Findings include, for example, reduced GM volume and/or density in left or bilateral ACG and subgenual ACG108–112 and left, right or bilateral DLPFC, MdPFC and VLPFC,111,113–115 although there are some reports of greater bilateral VLPFC and dorsal ACG GM volume in adult BD116 or no significant abnormalities in PFC volumes.117 Recent findings also indicate GM volume reductions in left VLPFC in medicated adult BD compared with healthy individuals,118 although not in first-episode BD.119 Genetic risk for BD has been specifically associated with GM deficits in right ACG, including subgenual, rostral and more dorsal components, and ventral striatum,120 and a recent report provides evidence of an early neurodevelop-mental abnormality in bilateral ACG in adult BD.121
The pattern of volumetric abnormalities in BD resembles that of the changes in dendritic arborization during stress. Dendritic branching of pyramidal cells increases in some amygdala nuclei, but decreases in the medial PFC and hippocampus, in response to repeated stress in rodents.122–124 These findings raise the possibilities that impaired emotion regulation may contribute to the volumetric abnormalities found in these structures in BD, by permitting stress responses that are exaggerated in magnitude or duration, or by interfering with the modulation or resetting of stress-induced dendritic remodeling.123 Such changes could in turn further exacerbate the emotion dysregulation associated with BD, as in rodents the dendritic atrophy arising in the medial PFC during repeated stress was associated with impaired modulation (that is, extinction) of behavioral responses to fear-conditioned stimuli.125
In parallel, there are reports of reduced white matter density and volume in bilateral regions corresponding to subcortical white matter tracts connecting OFC, thalamus and striatal regions implicated in emotion processing in adult BD,112,126 and reduced120 white matter volume in left OFC. Other findings suggest more widespread127 or no white matter abnormalities114,128 in adult BD. These data thus appear compatible with evidence from postmortem studies indicating that BD subjects show abnormally decreased myelin staining in the deep white matter of the DLPFC129 and reduced expression of oligodendroglia-related genes in multiple PFC regions (reviewed in Sokolov130).
Diffusion tensor imaging studies that allow measurement of the structural integrity of specific white matter tracts have reported reduced integrity of white matter tracts in bilateral OFC in adult BD.131,132 Recent findings indicate abnormalities specifically in left OFC and bilateral dorsal PFC. Here, studies have reported a greater number of reconstructed white matter fibers between left subgenual ACG and amygdala133 and decreased integrity of bilateral white matter tracts in dorsal prefrontal cortical regions129,134 in adult BD.
Together, these findings point to structural abnormalities in BD in gray and white matter and in white matter tracts in left OFC, implicated in automatic emotion regulation, and in a variety of different lateral and dorsal prefrontal cortical regions implicated in voluntary emotion regulation.
Functional neuroimaging studies
Functional neuroimaging studies have consistently demonstrated greater subcortical limbic activity (including amygdala, ventral striatum and hippocampus) to emotional stimuli in adult BD relative to healthy individuals during mania,135,136 depression137 and even when euthymic.138–140 Findings in BD remission and depression also include reduced blood flow in bilateral MdPFC during sad mood induction relative to baseline,141 and reduced activity in bilateral regions of MdPFC, DLPFC and ACG during a modified word-based memory task designed to implicitly evoke affective change in euthymic adults with BD.142 While these studies suggest functional impairments in prefrontal cortical neural regions implicated in both voluntary and automatic emotion regulation, they did not focus on examination of neural activity during paradigms measuring emotion regulation per se.
Another approach for neuroimaging studies of BD, therefore, has been to examine neural activity during cognitive control paradigms with nonemotional stimuli. Some studies have employed voluntary attentional control paradigms, including executive function (for example, working memory; word generation), sustained attention (for example, continuous performance task) or response inhibition (for example, Go/NoGo) tasks. The majority of these studies show intact task performance but either reduced right DLPFC and right dorsal ACG,143,144 or greater bilateral medial and lateral145 prefrontal cortical activities in remitted BD vs healthy adults. Findings also show reduced DLPFC, and subgenual ACG metabolism in BD depression;146 and reduced activity in right OFC and right VLPFC,147,148 and also in right hippocampus and left dorsal ACG,147 in BD mania.
Other studies have employed automatic attentional control paradigms, such as the nonemotional Stroop color-word or counting task. Findings from these studies show reduced activity in remitted BD relative to healthy adults during these paradigms in left-sided regions, including OFC/VLPFC and MdPFC149,150 and right-sided regions, including MdPFC151 and dorsal ACG, but also increased activity in right DLPFC.152 Other studies show reduced left OFC activity,149 or no functional abnormalities in PFC,153 in BD depression; and reduced left OFC activity during BD mania149 relative to healthy volunteers. The majority of these studies show intact, although some show impaired,154 task performance in BD adults.
Together, these studies suggest functional abnormalities in adult BD, including both greater and reduced activity relative to healthy individuals, in lateral and dorsal PFC regions implicated in voluntary emotion regulation during voluntary attentional control paradigms, and, largely, reduced activity in OFC and MdPFC implicated in automatic, emotion regulation during automatic attentional control paradigms. These studies did not, however, specifically examine activity in these neural regions during performance of voluntary and automatic emotion regulation paradigms. We next examine findings from the few studies that have employed such paradigms to help determine the extent to which emotion dysregulation in BD may be associated with functional abnormalities in neural regions underlying voluntary, vs neural regions underlying automatic, emotion regulation.
Voluntary emotion regulation paradigms
One recent study employed an affective Go/NoGo paradigm to examine neural activity during voluntary attentional control of emotion in BD remission.140 Here, despite intact task performance, euthymic BD adults demonstrated abnormally increased activity in bilateral OFC and left dorsal ACG, in addition to increased activity in different subcortical limbic regions implicated in emotion processing, when inhibiting responses to emotional relative to neutral distractors. Another study employing a similar paradigm showed greater activity to sad relative to neutral distracters in right DLPFC and VLPFC, and greater activity to happy distracters in bilateral DLPFC, MdPFC and left subgenual ACG in manic BD vs healthy adults.155 One study156 used functional connectivity analysis to examine neural activity in manic BD adults using emotion labeling of facial expressions, a task previously associated with voluntary control of responses to emotional stimuli—‘thinking rather than feeling’ about emotion—and with greater VLPFC and reduced subcortical limbic activity than gender labeling of facial expression.17 Findings indicated that manic BD adults had significantly reduced right VLPFC regulation of the left amygdala response during the task that parallel previous reports of decreased VLPFC activity in BD adults relative to healthy adults during the above-described voluntary attentional control paradigms.147,149,150,155
Automatic emotion regulation paradigms
Two studies employing an emotional Stroop paradigm demonstrated decreased left OFC activity in euthymic BD adults,157,158 which was associated with slower task performance.158 Another study demonstrated decreased left MdPFC (BA10), and right VLPFC activity, although no performance deficit, in manic BD adults during risky decisionmaking—a gambling paradigm dependent upon automatic cognitive change as a strategy to maximize reward and minimize loss.159
Summary
There are inconsistent findings regarding function in lateral and dorsal prefrontal cortical regions implicated in voluntary emotion regulation in adult BD. For example, findings from studies employing voluntary attentional control paradigms per se demonstrate patterns of reduced but also increased activity in different bilateral lateral and dorsal prefrontal cortical regions in BD vs healthy adults. Findings from studies employing voluntary emotion regulation paradigms, however, indicate greater activity in BD than healthy adults bilaterally in these lateral and dorsal prefrontal cortical regions, together with greater activity in bilateral ventromedial prefrontal cortical regions implicated in automatic emotion regulation, which may mediate the voluntary emotion regulatory roles of the previous lateral and dorsal prefrontal cortical regions during voluntary emotion regulation. Structural findings indicate reductions in GM volume and density in lateral and dorsal prefrontal cortical regions implicated in voluntary emotion regulation, although there are inconsistent findings of increased GM volume or no abnormalities in these regions, in adult BD. The role of lateral prefrontal cortical regions in voluntary emotion regulation in adult BD therefore remains unclear. It is possible that increased activity in lateral prefrontal cortical regions during voluntary attentional control and voluntary emotion regulation paradigms may reflect inefficient utilization of these regions during cognitive control tasks in BD relative to healthy adults that has been previously proposed in adults with schizophrenia.160
There are more consistent findings regarding functional and structural changes in neural regions implicated in automatic emotion regulation in adult BD. Studies employing automatic attentional control paradigms show reduced activity predominantly in left-sided ventromedial PFC in BD relative to healthy adults. Studies employing automatic emotion regulation paradigms also show reduced activity predominantly within left-sided ventromedial prefrontal cortical regions implicated in automatic emotion regulation, both during remission and mania, in BD relative to healthy adults. These functional neuroimaging findings are paralleled by structural neuroimaging findings showing GM structural changes in left OFC, and abnormal integrity and number of white matter fibers connecting left OFC and subcortical limbic regions implicated in emotion processing, in adult BD. Interestingly, increased recruitment of DLPFC in BD vs healthy adults has been reported during an automatic attentional control paradigm. This suggests the employment of more effortful, voluntary, rather than automatic, regulatory control systems during performance of otherwise automatic control tasks in adult BD. Further studies are required to examine functional connectivity between ventromedial and medial prefrontal cortical systems implicated in automatic, vs lateral and dorsal prefrontal cortical systems implicated in voluntary, emotion regulation in adult BD.
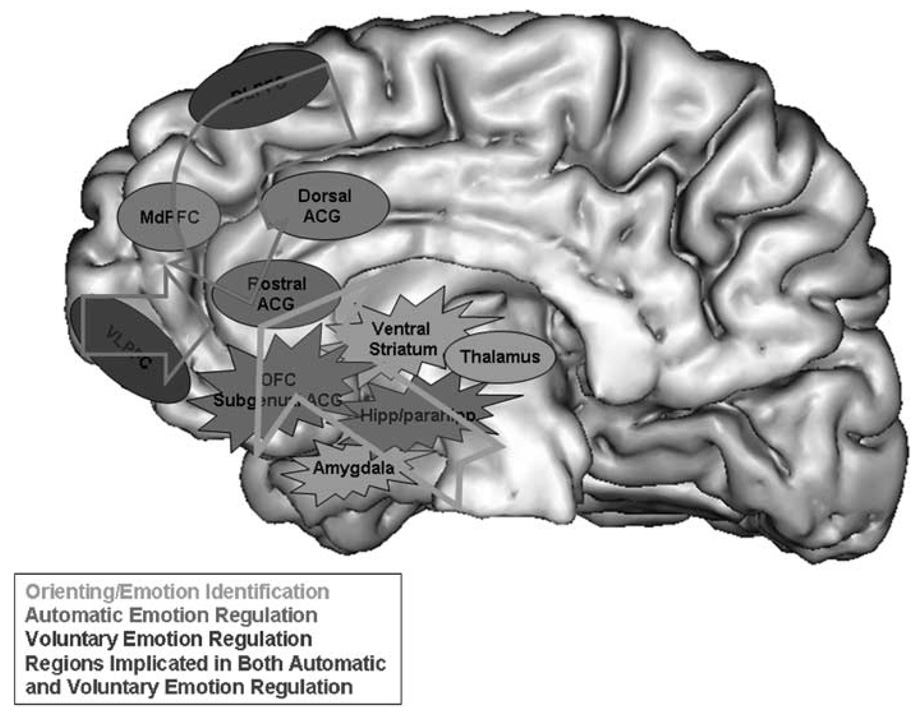
The combination of these functional and structural neural abnormalities, which appear to be decreases in activity and GM volume or density reductions predominantly within left-sided ventromedial prefrontal cortical regions implicated in automatic emotion regulation, may underlie the mood instability of adult BD (Figure 2). The significance of the laterality of these findings remains unclear, but may suggest a role for the left hemisphere, previously linked with perception of positive emotion163 in the pathophysiology of BD. These findings indicate that further insights into the neural basis of mood dysregulation in BD will come especially from studies that employ paradigms that measure functional integrity of neural regions during performance of automatic emotion regulation paradigms. A key focus of these future studies will be to identify persistent and mood-state-dependent functional and, potentially, structural abnormalities in these neural regions in adult BD to dissociate neural abnormalities reflecting pathophysiological mechanisms of BD vs neural abnormalities that may be secondary to depression or mania. Moreover, future studies should focus on examination of the relationship between functional and structural abnormalities in these neural regions in adult BD, and the extent to which specific symptom domains, including comorbid anxiety and substance abuse disorders, are associated with distinguishable patterns of abnormality in these neural regions.
Figure 2.
Neural model of emotion regulation illustrating possible functional and structural abnormalities in neural systems implicated in voluntary and automatic subprocesses of emotion regulation in adult bipolar disorder. These abnormalities, which appear to be predominantly within the left-sided ventromedial prefrontal cortical regions implicated in automatic emotion regulation, may underlie the mood instability of adult bipolar disorder (BD). For example, functional neuroimaging studies have demonstrated greater subcortical limbic activity (including amygdala, ventral striatum and hippocampus) to emotional stimuli in adult BD relative to healthy individuals during mania,136,147 depression137 and when euthy-mic.139,140,161,162 Studies employing automatic attentional control paradigms show reduced activity predominantly in left-sided ventromedial PFC in BD relative to healthy adults.149,150 Studies employing automatic emotion regulation paradigms also show reduced activity predominantly within left-sided ventromedial prefrontal cortical regions implicated in automatic emotion regulation, both during remission and mania in BD relative to healthy adults.157,159 Structural neuroimaging findings show gray matter structural changes in left OFC, and abnormal integrity and number of white matter fibers connecting left OFC and subcortical limbic regions implicated in emotion processing, in adult BD.112,120,126,131–133 There are more inconsistent findings regarding the roles of lateral and dorsal prefrontal cortical regions implicated in voluntary emotion regulation in adult BD. For example, findings from studies employing voluntary attentional control paradigms per se demonstrate patterns of reduced,143,144 although also increased,145,152 activity in different bilateral lateral and dorsal prefrontal cortical regions in BD vs healthy adults, whereas findings from studies employing voluntary emotion regulation paradigms indicate greater activity in BD than healthy adults bilaterally in these lateral and dorsal prefrontal cortical regions, together with greater activity in bilateral ventromedial prefrontal cortical regions implicated in automatic emotion regulation.140,155 The latter may mediate the voluntary emotion regulatory roles of the previous lateral and dorsal prefrontal cortical regions during voluntary emotion regulation.14 DLPFC, dorsolateral prefrontal cortex; MdPFC, dorsomedial prefrontal cortex; ACG, anterior cingulate gyrus; VLPFC, ventrolateral prefrontal cortex; OFC, orbital frontal cortex; hipp/parahip, hippocampus-parahippocampus region.
Normal development of neural systems of emotion regulation
To better understand how abnormalities in the neural systems of emotion regulation may contribute to the neurodevelopment of BD, we first briefly review findings from studies focusing on the normal development of neural regions implicated in emotion regulation.
During childhood and adolescence, there are important maturational changes that occur in brain structure. Cross-sectional164–166 and longitudinal167 studies have shown increases in white matter volumes, which are thought to reflect increasing myelination in childhood and adolescence. Maturational increases in white matter are assumed to be present globally, with specific increases shown in frontal, parietal and occipital cortices.166 The overall growth pattern of GM in the telencephalon is more heterogeneous.168 The general pattern reflects decreases in GM from early childhood to post-adolescence but this effect is nonlinear, likely reflecting selective pruning of neuronal connections.164,165,169 Regional analyses have shown that GM volumes peak at about 12 years of age in frontal and parietal cortical regions followed by a decline in post-adolescence thus yielding a net decrease.167
Furthermore, there is evidence of developmental changes in GM volume in subcortical structures implicated in processing emotional information. For example, in a study with 4- to 18-year olds, Giedd et al.170 showed that amygdala volume significantly increased with age, but only in men, whereas hippocampal volume significantly increased with age, but only in women.
Recent research has also revealed important ma-turational changes in brain function throughout childhood and adolescence, particularly in regions involved in the voluntary regulation of emotion. For example, several studies have reported greater activity with age in brain regions known to support cognitive control processes.171,172 Some studies, however, demonstrated changes that were specific to adolescence. One study examined neural activity during voluntary response suppression in 8- to 30-year olds using an anti-saccade task.173 In this study, adolescents had difficulty achieving adult levels of performance and compensated by engaging more frontostriatal circuitry compared with adults. These findings suggest that developmental changes in cognitive control processes in adolescence are associated with a more efficient and functionally integrated use of neural circuitry supporting these processes.174 These studies, however, involved none-motional stimuli.
Developmental changes in processing and regulating emotion may be associated with a refinement or increased efficiency in prefrontal—subcortical connections from childhood through adolescence.175 For instance, recent research suggests that explicit processing of emotional faces continues to develop from early childhood through adolescence.176–178 Neural systems implicated in voluntary emotion regulation may not be fully mature until adulthood. For instance, in a study examining neural activity during voluntary reappraisal in 8- and 10-year-old girls, Levesque et al.179 found that in contrast to adult women young girls recruited a greater number of prefrontal regions, possibly reflecting immaturity of prefrontal—limbic connections in childhood. During passive viewing of fearful faces, Monk et al.180 demonstrated that adolescents showed greater activity in right amygdala, bilateral OFC and ACG relative to adults, suggesting that when attention is unconstrained, adolescents may be more sensitive to emotionally salient stimuli.
Although research in the normal development of neural systems of emotion regulation (voluntary and automatic) remains in its infancy, evidence is emerging suggesting that adolescence may be a key period for the development of these neural systems.181–186 For example, there is evidence indicating important developmental changes during adolescence in neural systems involved in reward processing,187 response inhibition in the context of emotional stimuli,188 response conflict monitoring189–191 and risk taking.192,193 The impact of puberty upon the development of neural systems of emotion regulation remains to be fully examined.181,182,194 For example, recent studies indicate puberty-specific changes in brain structure195 and function,181,196 particularly in neural regions implicated in emotion processing and regulation (for example, amygdala, hippocampus).
Pediatric bipolar disorder and high-risk populations
We next examine findings from the few structural and functional neuroimaging studies that have examined key neural regions underlying voluntary and automatic emotion regulation in pediatric BD and individuals at high risk for developing BD.
Structural neuroimaging studies
These have revealed structural alterations in both cortical197 and subcortical161,198–200 regions. Studies in adolescent and first-episode BD198,199 as well as one longitudinal study of adolescents161 demonstrate smaller amygdalae and smaller200,201 or normal-sized hippocampi198,199 in adolescent BD, which differ from the above findings of enlarged amygdalae in adults.
Although earlier studies failed to detect any differences in PFC volumes in pediatric BD, a recent study using a more sensitive voxel-based morpho-metric automated technique showed abnormally decreased GM volume in left DLPFC. With less stringent statistical thresholds, results also revealed decreased left amygdala and left nucleus accumbens volumes in BD children and adolescents.197 Other recent findings indicate decreased bilateral OFC in BD adolescents.202 Given the discrepant findings between BD children and adolescents and BD adults in key areas such as the amygdala, further study must be done to determine possible differences in PFC volumes of those with early vs late onset and/or diagnosis, to characterize the effects of chronic mood stabilizer treatment on regional brain structure, and to track change over development within the context of possible compensatory mechanisms (including early treatment).
There are very few structural studies of individuals at risk for BD. A review of structural neuroimaging studies in individuals at risk for BD reported structural abnormalities in amygdala, striatum, hippocampus and subgenual ACG that were proposed as potential ‘candidate neuroanatomical’ risk markers for BD (see Hajek et al.203). A recent study, however, has shown, in children who became diagnosed with BD, increased GM over the left temporal cortex and decreased GM bilaterally in the ACG (including the subgenual ACG), which was observed most strikingly after the illness onset.204 This neurodevelopmental trajectory was observed also in children who had a diagnosis of multidimensional impairment (atypical psychosis), who did not convert to a diagnosis of BD. The authors suggested that this pattern of cortical development may reflect affective dysregulation (lability) in general. More recently, we found abnormally increased left hippocampal/parahippocampal GM volume in healthy offspring of parents diagnosed with BD.205 Moreover, this increase in GM volume in the BD offspring group was significantly positively correlated with pubertal stage (but not age). Given that these offspring of BD parents were healthy at the time of the study, it is possible that these differences in GM volume may reflect protective markers for BD. Longitudinal follow-up studies are required to relate these structural findings in BD at-risk individuals at high familial risk for BD to future clinical outcome.
Functional neuroimaging studies
BD children and adolescents demonstrate deficits in executive function206 and emotion processing,207 and impaired social cognition and response flexibility.208 As with adult BD, it has been proposed, therefore, that pediatric BD may be associated with functional abnormalities in neural systems supporting emotion regulation processes.209,210 There are several findings that support this. First, there is evidence indicating that children with BD exhibit deficits in response-reversal learning, suggesting altered functioning of neural systems implicated in automatic cognitive change subprocesses.206,211 Euthymic BD adolescents also show greater activity within subcortical limbic regions associated with emotion processing rather than prefrontal cortical regions such as OFC, rostral and dorsal ACG and MdPFC implicated in automatic attentional control during nonemotional Stroop task performance.212 In these BD adolescents, there was also no age-related increase in bilateral OFC activity demonstrated by age-matched healthy adolescents during the task.212 Abnormal increases in activity in bilateral DLPFC as well as subcortical limbic regions during voluntary attentional control (a working memory task) have also been shown in children and adolescents with BD.213
Regarding emotion-processing tasks, when attention is directed toward the emotional aspect of a stimulus, BD children and adolescents show abnormally increased left amygdala activity to neutral faces.207 Furthermore, functional connectivity analyses have recently revealed that compared with age-matched controls BD children and adolescents show less functional connectivity between left amygdala and a network of neural regions implicated in processing faces and emotional stimuli, including right posterior cingulate/precuneus and right fusiform gyrus/parahippocampal gyrus.214 These findings suggest that functional abnormalities in neural systems supporting emotion processing may underlie the pathophysiology of pediatric BD.207,209 Further studies are required, specifically studies employing voluntary and automatic emotion regulation paradigms, to elucidate the functional abnormalities in neural systems underlying emotion regulation in pediatric and adolescent BD.
There is a paucity of functional neuroimaging studies in individuals at high risk of BD. One recent neuroimaging study using PET contrasted neural activity during sad mood induction in adult BD and their healthy siblings.141 Here, healthy siblings relative to their affected probands showed greater activity in left MdPFC, a region activated in healthy individuals during paradigms involving automatic cognitive change. These differences in neural activity were interpreted as a potential resiliency marker for BD, given that the adult siblings were free of psychopathology. To our knowledge, there are no current published data examining the functioning of neural systems involved in emotion regulation in offspring of individuals with BD.
Summary
Findings from existing studies suggest structural abnormalities in dorsal and ventral prefrontal cortices and functional abnormalities within prefrontal cortical regions implicated in voluntary and automatic emotion regulatory subprocesses in pediatric BD. Clearly, there is a need for further studies to examine the extent to which both children and adolescents with, and those at risk for, BD have abnormal development of different prefrontal cortical regions implicated in automatic and voluntary emotion regulation using experimental paradigms that focus on these different emotion regulatory subprocesses.
Conclusion: understanding the pathophysiology and neurodevelopment of bipolar disorder
We have described a new neural model of emotion regulation that includes voluntary and automatic regulatory subprocesses, centered in different regions of PFC, hippocampus and parahippocampus. We have then used this model as a theoretical framework to examine functional neural abnormalities in these neural systems that may predispose to the development of BD, a major psychiatric disorder characterized by severe emotion dysregulation. The most consistent findings in adult BD are those of studies employing automatic attentional control and automatic emotion regulation paradigms. These findings indicate abnormally reduced activity in, predominantly, left-sided OFC and MdPFC during automatic attentional control and automatic emotion regulation paradigms in adult BD. Data further suggest structural abnormalities in dorsal and ventral prefrontal cortices, including left-sided abnormalities in the integrity of white matter tracts in OFC, in adult BD. Together, these findings point to left-sided abnormalities in prefrontal cortical regions implicated in automatic regulation in adult BD but further study is required to elucidate the nature of these abnormalities in emotion regulatory neural systems, and the extent to which persistent vs mood-state-dependent abnormalities in these neural systems can be dissociated in BD.
In pediatric BD, findings indicate functional abnormalities in bilateral prefrontal cortical regions and left amygdala during attentional control and emotion-processing tasks, respectively. These findings are paralleled by structural abnormalities in bilateral OFC and left amygdala. There is additional, emerging evidence of structural abnormalities in the left hippocampus/parahippocampus, implicated in automatic emotion regulation, in children and adolescents at risk of BD vs age-matched healthy volunteers. Further studies are needed in pediatric BD and at risk for BD population to establish the nature and laterality of the structural and functional abnormalities in PFC regions subserving voluntary and automatic emotion regulation subprocesses. This new neural model of emotion regulation is, therefore, a valuable framework with which to examine the neurodevelopment of BD, and to help identify biomarkers that represent risk for the disorder, through childhood, adolescence and adulthood.
Limitations and future directions
Although our review of the literature includes a large number of animal and human neuroimaging studies, it does not represent a complete and extensive review of the literature on the topic of neural systems of voluntary and automatic emotion regulation and related research in BD. Rather, our critical review focuses upon studies that have examined neural regions and systems underlying different subprocesses of voluntary and automatic emotion regulation and the extent to which these neural regions, particularly those implicated in automatic emotion regulation, are altered in BD and possibly contribute to the pathophysiology of the disorder. There is a need for more studies in adult and pediatric BD to elucidate the nature of abnormalities in these neural systems. There is, in parallel, a need for studies in individuals at risk for BD, particularly offspring of BD adults, to elucidate the neurodevelopmental trajectories that may contribute to the onset of BD in youth or young adults.
Acknowledgments
This study was supported by NIMH, R01(MH076971-01) to MLP; NARSAD Independent Investigator Award to MLP; NARSAD Young Investigator Award to CDL and NIMH DIRP support for WCD.
Glossary of terms
- (ACG)
Anterior cingulate gyrus
- Subgenual ACG
Brodmann areas (BAs) 25 posteriorly and 24 anteriorly
- Rostral ACG
Brodmann area 24
- Dorsal ACG
Brodmann areas 24, 32
- Orbital frontal cortex (OFC)
Brodmann areas 11—14 and medial 47
- Dorsomedial prefrontal cortex (MdPFC)
Brodmann areas 10/32
- Dorsolateral prefrontal cortex (DLPFC)
Brodmann areas 9, 44, 46
- Ventrolateral prefrontal cortex (VLPFC)
Brodmann areas 45, 47
References
- 1.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 2.Hasler G, Drevets WC, Gould T, Gottesman I, Manji H. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2005;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Phillips ML, Frank E. Redefining bipolar disorder: toward DSM-V. Am J Psychiatry. 2006;163:1135–1136. doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]
- 4.Charney DS, Barlow DH, Botteron K, Cohen JD, Goldman D, Gur RE, et al. Neuroscience research agenda to guided development of a pathophysiologically based classification system. In: Kupfer DJ, First MB, Regier DA, editors. A Research Agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002. pp. 31–83. [Google Scholar]
- 5.Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv. 2001;52:51–55. doi: 10.1176/appi.ps.52.1.51. [DOI] [PubMed] [Google Scholar]
- 6.Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84:117–125. doi: 10.1016/S0165-0327(03)00194-0. [DOI] [PubMed] [Google Scholar]
- 7.Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci Biohehav Rev. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- 8.Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:375–383. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- 9.Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 10.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 11.Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 12.Reverberi C, Cherubini P, Rapisarda A, Rigamonti F, Caltagirone C, Frackowiak RS, et al. Neural basis of generation of conclusions in elementary deduction. Neuroimage. 2007;38:752–762. doi: 10.1016/j.neuroimage.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 13.Procyk E, Goldman-Rakic PS. Modulation of dorsolateral prefrontal delay activity in self-organized behavior. J Neurosci. 2006;26:11313–11323. doi: 10.1523/JNEUROSCI.2157-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolls ET. The orbitofrontal cortex. Philos Trans R Soc Land B Biol Sci. 1996;351:1143–1433. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- 16.Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SCR, et al. Task instructions modulate neural responses to fearful facial expressions. Biol Psychiatry. 2003;53:226–232. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- 18.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman MD, Hariri A, Jarcho JJ, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat Neurosci. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, et al. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- 23.Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, et al. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26:141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 24.Thompson RA. Emotion regulation: a theme in search of definition. In: Fox NA, editor. The Development of Emotion Regulation: Biological and Behavioral Considerations. Chicago, IL: University of Chicago Press; 1994. pp. 25–52. [PubMed] [Google Scholar]
- 25.Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- 26.Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 87–109. [Google Scholar]
- 27.Ochsner KN, Gross JJ. Thinking makes it so: a social cognitive neuroscience approach to emotion regulation. In: Baumeister RF, Vohs KD, editors. Handbook of Self-Regulation: Research, Theory, and Applications. New York: Guildford Press; 2004. pp. 229–255. [Google Scholar]
- 28.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 30.Drevets WC. Orbitofrontal cortex function and structure in depression. Ann NY Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- 31.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 32.Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. J Abnorm Psychol. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- 34.Gross JJ, Sutton SK, Ketelaar TV. Relations between affect and personality: support for the affect-level and affective-reactivity views. Pers Soc Psychol Bull. 1998;24:279–288. [Google Scholar]
- 35.Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 36.Hagemann T, Levenson RW, Gross JJ. Expressive suppression during an acoustic start. Psychophysiology. 2006;43:104–112. doi: 10.1111/j.1469-8986.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 37.Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. J Pers Soc Psychol. 1993;64:970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- 38.Goldsmith HH, Davidson RJ. Disambiguating the components of emotion regulation. Child Dev. 2004;75:361–365. doi: 10.1111/j.1467-8624.2004.00678.x. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin FK, Jamison KR. Manic-Depressive Illness. Oxford: Oxford University Press; 1990. [Google Scholar]
- 40.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2002;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- 43.Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, et al. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 44.Gillath O, Bunge SA, Shaver PR, Wendelken C, Mikulincer M. Attachment-style differences in the ability to suppress negative thoughts: exploring the neural correlates. Neuroimage. 2005;28:835–847. doi: 10.1016/j.neuroimage.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein M, Brendel G, Tuescher O, Pan H, Epstein J, Beutel M, et al. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage. 2007;36:1026–1040. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 46.Erk S, Kleczar A, Walter H. Valence-specific regulation effects in a working memory task with emotional context. Neuroimage. 2007;37:623–632. doi: 10.1016/j.neuroimage.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Perlstein WM, Elbert T, Stenger VA. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proc Natl Acad Sci USA. 2002;99:1736–1741. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo Q, Mitchell D, Jones M, Mondillo K, Vythilingam M, James R, et al. Common regions of dorsal anterial cingulate and prefrontal-parietal cortices provide attentional control of distracters varying in emotionality and visibility. Neuroimage. 2007;38:631–639. doi: 10.1016/j.neuroimage.2007.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 50.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 51.Banks SJ, Eddy KT, Angstadt M, Pradeep NJ, Phan LK. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urry H, van Reekum C, Johnstone T, Kalin N, Thurow M, Schaefer H, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of Cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corlett P, Aitken M, Dickinson A, Shanks D, Honey G, Honey R, et al. Prediction error during retrospective revaluation of causal associations in humans: fMRI evidence in favor of an associative model of learning. Neuron. 2004;44:877–888. doi: 10.1016/j.neuron.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vidal-Gonzalez IB, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 57.Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 58.McGinty VB, Grace AA. Selective activation of medial prefrontal-to-accumbens projection neurons by amygdala stimulation and Pavlovian conditioned stimuli. Cereb Cortex. 2007;223:1–12. doi: 10.1093/cercor/bhm223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and VMPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 60.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1144–1152. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 61.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 62.Armony JL, Dolan RJ. Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsycho-logia. 2002;40:817–826. doi: 10.1016/s0028-3932(01)00178-6. [DOI] [PubMed] [Google Scholar]
- 63.Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. Neural systems for orienting attention to the location of threat signals: an event-related fMRI study. Neuroimage. 2006;31:920–933. doi: 10.1016/j.neuroimage.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 64.Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 65.Blair KS, Smith BW, Mitchell DG. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 67.Vuilleumier P, Armony JL. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 68.Pessoa L, Padmala S, Morland T. Fate of the unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28:249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, et al. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21:1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 70.Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26:9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunkin NM, Mayes AR, Gregory LJ, Nicholas AK, Nunn JA, Brammer MJ, et al. Novelty-related activation within the medial temporal lobes. Neuropsychologia. 2002;40:1456–1464. doi: 10.1016/s0028-3932(01)00200-7. [DOI] [PubMed] [Google Scholar]
- 72.van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- 73.Luks TL, Simpson GV, Dale CL, Hough MG. Preparatory allocation of attention and adjustments in conflict processing. Neuroimage. 2007;35:949–958. doi: 10.1016/j.neuroimage.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 75.Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, et al. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- 76.Fukui H, Murai T, Fukuyama E, Hayashi T, Hanakawa T. Functional activity related to risk anticipation during performance of the Iowa Gambling Task. Neuroimage. 2005;24:253–259. doi: 10.1016/j.neuroimage.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 77.Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions during the Iowa gambling task. Cereb Cortex. doi: 10.1093/cercor/bhn154. in press. [DOI] [PubMed] [Google Scholar]
- 78.Beauregard M, Levesque P, Paquette V. Neural basis of conscious and voluntary self-regulation of emotion. In: Beauregard M, editor. Consciousness, Emotional Self-Regulation and the Brain. Amsterdam: John Benjamins; 2004. pp. 163–194. [Google Scholar]
- 79.Beauregard M. Mind does really matter: evidence from neuroimaging studies of emotional self-regulation, psychotherapy, and placebo effect. Prog Neurobiol. 2007;81:218–236. doi: 10.1016/j.pneurobio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 80.Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suare F. The anatomical connections of the macaque monkey orbitofrontal cortex. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- 82.Fletcher PC, Anderson JM, Shanks DR, Honey R, Carpenter TA, Donovan T, et al. Responses of human frontal cortex to surprising events are predicted by formal associative learning theory. Nat Neurosci. 2001;4:1043–1048. doi: 10.1038/nn733. [DOI] [PubMed] [Google Scholar]
- 83.Corlett PR, Murray GK, Honey GD, Aitken MRF, Shanks DR, Robbins TW, et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130:2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalisch R, Wiech K, Herrmann K, Dolan RJ. Neural correlates of self-distraction from anxiety and a process model of cognitive emotional regulation. J Cogn Neurosci. 2006;18:1266–1276. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 86.Öngür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 87.Morgan M, Romanski L, LeDoux J. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 88.Rhodes SEV, Killcross AS. Lesions of rat infralimbic cortex result in disrupted retardation but normal summation test performance following training on a Pavlovian conditioned inhibition procedure. Eur J Neurosci. 2007;26:2654–2660. doi: 10.1111/j.1460-9568.2007.05855.x. [DOI] [PubMed] [Google Scholar]
- 89.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann NY Acad Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- 91.Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mayberg HS. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 93.Mayberg HS. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 94.Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, Mclntyre RS, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007;164:778–788. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- 95.Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: the role of awareness. Br J Clin Psychol. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 96.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morris JS, Friston KJPCJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role of the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 98.Whalen PJ, Rauch SL, Etcoff NL, Mclnerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. 2nd edn. Oxford: Oxford University Press; 2000. [Google Scholar]
- 100.Stefanacci L, Suzuki WA, Amaral DG. Organization of connections between amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol. 1996;375:552–582. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 101.Schroeder U, Hennenlotter A, Erhard R, Haslinger B, Stahl R, Lange KW, et al. Functional neuroanatomy of perceiving surprised faces. Hum Brain Mapp. 2004;23:181–187. doi: 10.1002/hbm.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Bechara A, Van Der L. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- 104.Burgess PW, Gilbert SJ, Dumontheil I. Functional and localization within rostral prefrontal cortex (BA10) Philos Trans R Soc Lond B Biol Sci. 2007;362:887–889. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanides F. Functional Architecture of Motor and Sensory Cortices in Primates in the Light of a New Concept of Neocortex Evolution. New York: Appleton-Century-Crofts; 1970. [Google Scholar]
- 106.Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- 107.Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, E F, et al. MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 108.Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 109.Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by Optimized voxel-based morphometry. Biol Psychiatry. 2004;55:1154–1162. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 110.Sassi R, Brambilla P, Hatch J, Nicoletti M, Mallinger A, Frank E, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 111.Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 112.Bruno SD, Barker GJ, Cercignani M, Symms M, Ron MA. A study of bipolar disorder using magnetization transfer imaging and voxel-based morphometry. Brain. 2004;127:2433–2440. doi: 10.1093/brain/awh274. [DOI] [PubMed] [Google Scholar]
- 113.Frangou S. The Maudsley bipolar disorder project. Epilepsia. 2005;46:19–25. doi: 10.1111/j.1528-1167.2005.463005.x. [DOI] [PubMed] [Google Scholar]
- 114.Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 115.Sharma V, Menon R, Carr TJ, Densmore M, Mazmania D, Williamson PC. An MRI study of subgenual prefrontal cortex in patients with familial and non-familial bipolar I disorder. J Affect Disord. 2003;77:167–171. doi: 10.1016/s0165-0327(02)00109-x. [DOI] [PubMed] [Google Scholar]
- 116.Adler CM, Levine AD, Delbello MP, Strakowski SM. Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry. 2005;58:151–157. doi: 10.1016/j.biopsych.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 117.Brambilla P, Nicoletti M, Harenski K, Sassi RB, Mallinger AG, Frank E, et al. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology. 2002;27:792–799. doi: 10.1016/S0893-133X(02)00352-4. [DOI] [PubMed] [Google Scholar]
- 118.Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 119.Farrow T, Whitford T, Williams LL, Gomes L, Harris A. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58:713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 120.McDonald C, Bullmore ET, Sham P, Chitnis X, Wickham H, Bramon E, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 121.Fornito A, Malhi GS, Lagopoulos J, Ivanovski B, Wood SJ, Saling MM, et al. Anatomical abnormalities of the anterior cingulate and paracingulate cortex in patients with bipolar I disorder. Psychiatry Res. 2008;162:123–132. doi: 10.1016/j.pscychresns.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 122.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarjo S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 124.Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendric retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mcintosh AM, Job DE, Moorhead TW, Harrison LK, Lawrie SM, Johnstone EC. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biol Psychiatry. 2005;58:254–257. doi: 10.1016/j.biopsych.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 127.McDonald E, Bullmore P, Sham X, Chinis J, Suckling J, MacCabe M, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: a computational morphometry study. Br J Psychol. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- 128.Lim KO, Rosenbloom MJ, Faustman WO, Sullivan EV, Pfefferbaum A. Cortical gray matter deficit in patients with bipolar disorder. Schizophr Res. 1999;40:219–227. doi: 10.1016/s0920-9964(99)00063-8. [DOI] [PubMed] [Google Scholar]
- 129.Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley CK. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder and unipolar major depression. Psychiatry Res. 2007;151:179–188. doi: 10.1016/j.psychres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 130.Sokolov BP. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies. Int J Neuropsychopharmacol. 2007;10:547–555. doi: 10.1017/S1461145706007322. [DOI] [PubMed] [Google Scholar]
- 131.Beyer JL, Taylor WD, MacFall JR, Kuchibhatla M, Payne ME, Provenzale JM, et al. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30:2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 132.Haznedar MM, Roversi F, Pallanti S, Baldini-Rossi N, Schnur DB, Licalzi EM, et al. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol Psychiatry. 2005;57:733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 133.Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, et al. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdal-hippocampal complex. Mol Psychiatry. 2007;11:1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 134.Adler CM, Holland SK, Schmithorst V, Wilke M, Weiss KL, Pan H, et al. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;6:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 135.Altshuler LL, Bookheimer SY, Townsend J. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 136.Chen CH, Lennox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 137.Malhi GS, Lagopoulos J, Ward PB, Kumari V, Mitchell PB, Parker GB, et al. Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci. 2004;19:741–754. doi: 10.1111/j.0953-816x.2003.03159.x. [DOI] [PubMed] [Google Scholar]
- 138.Hassel S, Almeida JRC, Kerr N, Nau S, Ladouceur CD, Fissel K, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008 doi: 10.1111/j.1399-5618.2008.00641.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, Ketter T. Is a lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disord. 2007;9:345–357. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 140.Wessa M, Houenou J, Paillere-Martinot ML, Berthoz S, Artiges E, Leboyer M, et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- 141.Krüger S, Aida M, Young LT, Goldapple K, Parikh S, Mayberg HS. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings. Am J Psychiatry. 2006;163:257–264. doi: 10.1176/appi.ajp.163.2.257. [DOI] [PubMed] [Google Scholar]
- 142.Malhi GS, Lagopoulos J, Owen AM, Ivanovski B, Shnier R, Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. J Affect Disord. 2007;97:109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 143.Lagopoulos J, Ivanovski B, Mahli GS. An event-related functional MRI study of working memory in euthymic bipolar disorder. J Psychiatry Neurosci. 2007;32:174–184. [PMC free article] [PubMed] [Google Scholar]
- 144.Monks PJ, Thompson JM, Bullmore ET, Suckling J, Brammer MJ, Williams SCR, et al. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for tasks-specific dysfunction. Bipolar Disord. 2004;6:550–564. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 145.Strakowski SM, Adler CM, Holland SK, Mills NP, Delbello MP. A preliminary fMRI study of sustained attention in euthymic bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 146.Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- 147.Altshuler L, Bookheimer S, Proenza MA. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 148.Blumberg HP, Stern E, Ricketts S, Martinez D, White T, Epstein J, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156:1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 149.Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, et al. A functional magnetic resonance imaging study of bipolar disorder: state-and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 150.Kronhaus DM, Lawrence NS, Williams AM, Frangou S, Brammer MJ, Williams SC, et al. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 151.Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal fMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. A J Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 152.Gruber SA, Rogowska J, Yurgelun-Todd DA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord. 2004;82:191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 153.Marchand WR, Lee JN, Thatcher GW, Jensen C, Stewart D, Dilda V, et al. A functional MRI study of a paced motor activation task to evaluate frontal-subcortical circuit function in bipolar depression. Psychiatry Res. 2007;155:221–230. doi: 10.1016/j.pscychresns.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 154.Strakowski S, DelBello M, Adler C. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 155.Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 156.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lagopoulos J, Malhi GS. A functional magnetic resonance imaging study of emotional Stroop in euthymic bipolar disorder. Neuroreport. 2007;18:1583–1587. doi: 10.1097/WNR.0b013e3282efa07a. [DOI] [PubMed] [Google Scholar]
- 158.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005;7:58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 159.Rubinsztein JS, Fletcher PC, Rogers RD, Ho LW, Aigbirhio FI, Paykel ES, et al. Decision-making in mania: a PET study. Brain. 2001;124:2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- 160.Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 161.Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, Durkin K, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 162.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 163.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 164.Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal brain development and aging: quantitative analysis at in vivo MRI imaging in healthy volunteers. MD Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 165.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 166.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 167.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 168.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 169.Huttenlocher PR. Synaptic density in human frontal cortex: developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 170.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 171.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 172.Marsh R, Zhu RT, Schultz RT, Quakenbush G, Royal J, Skudlarski R, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr Bull. 2001;27:443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]
- 174.Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Ann NY Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 175.Herba C, Phillips ML. Development of facial expression recognition from childhood to adolescence: behavioural and neurological perspectives. J Child Psychol Psychiatry. 2004;45:1185–1198. doi: 10.1111/j.1469-7610.2004.00316.x. [DOI] [PubMed] [Google Scholar]
- 176.Camras LA, Allison K. Children’s understanding of emotional facial expressions and verbal labels. J Nonverbal Behav. 1985;9:84–94. [Google Scholar]
- 177.Chung MS, Thomson DM. Development of face recognition. Br J Psychol. 1995;86:55–87. doi: 10.1111/j.2044-8295.1995.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 178.De Sonneville LMJ, Verschoor CA, Njiokiktjien C, Op het Veld V, Toorenaar N, Vranken M. Facial identity and facial emotions: speed, accuracy, and processing strategies in children and adults. J Clin Exp Neuropsychol. 2002;24:200–213. doi: 10.1076/jcen.24.2.200.989. [DOI] [PubMed] [Google Scholar]
- 179.Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux J-M, Bourgouin RM, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 180.Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 181.Blakemore SJ, Choudhury S. Brain development during puberty: state of the science. Dev Sci. 2006;9:11–14. doi: 10.1111/j.1467-7687.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 182.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. NY Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 183.Nelson EE, Leibenluft E, McClure E, Pine DS. The social-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- 184.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 185.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yurgelun-Todd DA. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 187.Galvan A, Hare T, Parra C, Penn J, Voss H, Glover G, et al. Earlier development of accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 189.Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7–25-year-olds. Dev Neuropsychol. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- 190.Ladouceur CD, Dahl RE, Carter CS. ERP correlates of action monitoring in adolescence. Ann NY Acad Sci. 2004;1021:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- 191.Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev Sci. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- 192.Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 193.Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neurop-sychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 195.Giedd JN, Ciasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 196.Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, et al. Reversal of neurosteroid effects at alpha4beta2deltaGABA(A) receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney D, Pine DS, et al. Frontotemporal alternations in pediatric bipolar disorder. Arch Gen Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 198.Chang K, Barnea-Goraly N, Karchemskiy A, Simeonava D, Barnes P, Ketter T, et al. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biol Psychiatry. 2005;58:197–203. doi: 10.1016/j.biopsych.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 199.Chen H, Nicoletti M, Hatch J, Sassi R, Axelson DA, Brambilla R, et al. Abnormal left superior temporal gyrus volumes in children and adolescents with bipolar disorder: a magnetic resonance imaging study. Neurosci Lett. 2004;363:65–68. doi: 10.1016/j.neulet.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 200.Blumberg P, Kaufman J, Martin A, Whiteman R, Zhang J, Gore J, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 201.Frazier J, Chiu S, Breeze J, Makris N, Lange N, Kennedy D, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 202.Blumberg HP, Frdericks C, Wang F, Kalmar J, Spencer L, Papademetris X, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents with bipolar disorder. Bipolar Disord. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hajek T, Carrey N, Aida M. Neuroanatomical abnormalities as risk factors for bipolar disorder. Bipolar Disord. 2005;7:393–403. doi: 10.1111/j.1399-5618.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- 204.Gotgay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 205.Ladouceur CD, Almeida JRC, Birmaher B, Axelson DA, Nau S, Kalas C, et al. Subcortical gray matter volume abnormalities in healthy bipolar offspring: potential neuroanatomical risk marker for bipolar disorder? J Am Acad Child Adolesc Psychiatry. 2008;47:532–539. doi: 10.1097/CHI.0b013e318167656e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, et al. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- 207.Rich B, Vinton D, Roberson-Nay R, Hommer R, Berghorst L, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.McClure EB, Treland J, Snow J, Schmajuk M, Dickstein DP, Towbin K, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 209.Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biol Psychiatry. 2003;53:1009–1020. doi: 10.1016/s0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 210.Dickstein DP, Leibenluft E. Emotion regulation in children and adolescents: boundaries between normalcy and bipolar disorder. Dev Psychopathol. 2006;18:1105–1131. doi: 10.1017/S0954579406060536. [DOI] [PubMed] [Google Scholar]
- 211.Gorrindo T, Blair RJR, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- 212.Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. [Comparative Study. Journal Article. Research Support, Non-U.S. Gov’t. Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.] Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 213.Chang K, Adleman NE, Dienes K, Simeonava D, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 214.Rich B, Fromm S, Berghorst L, Dickstein DP, Brotman MA, Pine DS, et al. Neural connectivity in children with bipolar disorder: impairment in the face of emotion processing circuit. J Child Psychol Psychiatry. 2008;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]