Valvular heart disease remains a major problem worldwide and is responsible for over 20,000 deaths each year in the United States, with an estimated 99,000 inpatient valve procedures performed annually 1. The mitral and tricuspid valve complexes are comprised of the valve leaflets, valvular annulus, papillary muscles, and chordae tendineae, which anchor the leaflets to the papillary muscles. As papillary muscles contract, chordae tendineae transmit tension to the valves, directing the valve leaflets to their correct position during the cardiac cycle. Rupture of the chordae tendineae occurs as a consequence of infective endocarditis, myxomatous degeneration, rheumatic fever, or rarely, osteogenesis imperfecta or relapsing polychondritis 2. In addition, ischemia-induced dysfunction of papillary muscles can cause stretching and ultimately rupture of the chordae tendineae, as well as release of chordal attachment at sites of papillary muscle necrosis.
Chordae tendineae are similar to tendons connecting our skeletal muscles to bones, as both structures are avascular connective tissues. Chordae tendineae are approximately 80% collagen, while the remaining 20% is elastic fibers and layers of endothelial cells on a basal lamina 3. A wavy arrangement of collagen surrounded by elastic fibers is well adapted for the cyclic stresses to which the chordae are continuously subjected 3. Chordal disruption can be sudden and precipitate acute heart failure, and why these avascular structures suddenly rupture is often unclear. In this issue, Kimura et al. provide evidence that rupture of the chordae tendineae is associated with local angiogenesis accompanied by the absence of tenomodulin, a potent angiogenesis inhibitor 4. Their findings suggest a novel paradigm for rupture of the chordae tendineae in response to mechanical or hypoxic stress and suggest that vascularization, usually considered a friend in the myocardium, could be an enemy in the cardiac valves.
Neovascularization is a common feature of degenerative tendon diseases, as a marker but potentially also as a facilitator of inflammation 5. Kimura et al suggest a role of neovessel ingrowth in the pathogenesis of degenerative chordae 4. Under mechanical overload, the chordae tendineae undergo remodeling; however, if the mechanical overload continues, this remodeling could lead to a degenerative process. In this degeneration, loss of tenomodulin may play a role in promoting local angiogenesis and activation of matrix metalloproteinases (MMPs). This scenario is consistent with their previous study showing that dysregulation of an endogenous inhibitor of angiogenesis, chondromodulin-I, leads to local angiogenesis, thickening of the cardiac valves and valvular stenosis 6. Thus, neovascularization may be pathogenic in tendons, valves and chordae, and tonic inhibition of angiogenesis may be necessary to maintain normal functional properties of these avascular tissues.
An important question is how the invasion of neovessels in avascularized region results in weakening of the normal chordae structure. A variety of growth factors can regulate angiogenesis in connective tissues. Vascular endothelial growth factor (VEGF)-A has a key role in migration and proliferation of endothelial cells 7. VEGF-A is a potent inducer of matrix-degrading metalloproteinases 8, which play an essential role in VEGF-A-induced endothelial migration. To form new vessels, the endothelial cell layer must break down diverse extracellular matrix components including collagen, laminin, and fibronectin. Local production of proteolytic enzymes such as MMPs that degrade fibrillar collagen or proteoglycan core proteins permit endothelial cells to form a sprouting vessel by migration 9, 10. Since degradation of the extracellular matrix is required for the sprouting and invasion of new blood vessels into avascularized chordae, angiogenesis can weaken the chordal extracellular matrix 11. Thus, angiogenesis not only contributes to the repair and remodeling of tissues, but may also compromise mechanical stability by proteolysis of the extracellular matrix.
Under normal conditions in adults, angiogenesis is constitutively relatively quiescent, with a balance between angiogenic and angiostatic factors 12. Kimura et al. identify tenomodulin as a novel anti-angiogenic factor abundantly expressed in the outer layer of normal chordae tendineae 4. Tenomodulin is a type II transmembrane glycoprotein (317 amino acid residues) that contains a C-terminal domain with homology to the secreted form of chondromodulin-I, a cartilage-derived angiogenesis inhibitor 13. The secreted, truncated form of tenomodulin (the C-terminal domain) is abundant in chordae tendineae, indicating that the extracellular domain is cleaved and released from cells in a soluble form by ectodomain shedding. This finding is in agreement with a previous report showing the potential processing site (position 233–236) between the N-terminal glycosylation domain and the C-terminal action domain in tenomodulin protein 14. The C-terminal domain of tenomodulin has robust anti-angiogenic activity when expressed in a secreted form 13. In vitro angiogenesis assays confirmed that conditioned medium from chordae tendineae interstitial cells or the recombinant protein of the C-terminal domain of tenomodulin suppresses the angiogenic activity of endothelial cells 4, suggesting that anti-angiogenic actions by the C-terminal domain of tenomodulin involve an autocrine/paracrine loop. In contrast, mechanical stretch, hypoxia, and oxidative stress all suppress the endogenous expression of tenomodulin in chordae tendineae interstitial cells 4. Consistently, in the ruptured area of chordae tendineae, tenomodulin is locally downregulated, which promotes abnormal vessel formation with infiltration of inflammatory cells and induction of VEGF-A and MMPs 4.
It seems likely that tenomodulin coordinately maintains the avascular nature of chordae tendineae in an autocrine/paracrine manner, and under pathologic conditions, angiogenesis appears to be deregulated being dramatically enhanced by loss of tenomodulin through its association with VEGF-A (Figure 1). What then, is the relationship between tenomodulin shedding and the induction of VEGF-A? Do eight conserved Cys residues in the C-terminal domain of tenomodulin 14 play any regulatory role in the VEGF-A-stimulated DNA synthesis and tube morphogenesis in cells? If the shedding is blocked with an inhibitor of proteolysis or mutations of the potential cleaved site, is the regulation of VEGF-A by tenomodulin attenuated? It also remains to be determined whether regulation of VEGF-A is secondary to low oxygen, the mechanical overload or to other potential mechanisms in the chordae tendineae, as tenomodulin is probably not the only factor regulating angiogenesis in the chordae tendineae. For example, oxygen tension 15, the transcription factor, hypoxia inducible factor-1 (HIF-1) 16, a number of cytokines, hormones, and growth factors, such as epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and insulin-like growth factor (IGF)-1 all can regulate induction of VEGF-A.
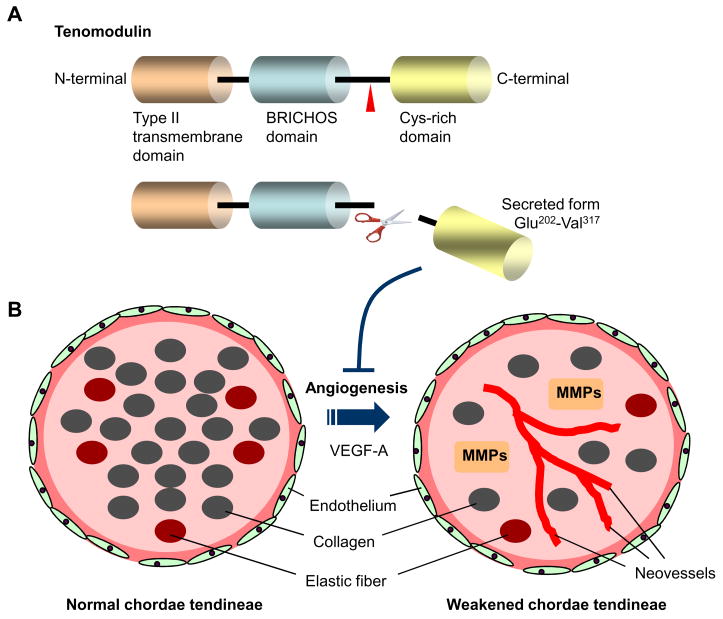
Figure 1.
(A) Schematic structure of human tenomodulin protein. The protein consists mainly of three domains; type II transmembrane domain at the N-terminus (Ile31-Lys51), a BRICHOS domain (Gly93-Ile186), and a cysteine-rich domain at the C-terminus (Phe255-Val317). There are two N-glycosylation sites within the BRICHOS domain, and the potential cleaved site (Arg-Xxx-Xxx-Arg) is located at position 233–236. The cysteine (Cys) rich domain contains eight cysteine residues. The C-terminal domain has a robust anti-angiogenic activity when expressed in a secreted form. (B) Schematic images of normal and pathogenic chordae tendineae. Cross-sectioned chordae show layers of endothelial cells on a basal lamina, the underlying layer consisting of collagen with occasional elastic fibers. Tenomodulin maintains a state of avascularity in healthy chordae tendineae. Loss of tenomodulin is associated with neoangiogenesis, probably mediated by vascular endothelial growth factor (VEGF)-A. Such changes lead to the degenerative process with matrix metalloproteases (MMPs). The reduced material properties can predispose the chordae for mechanical damage or in the long term for spontaneous rupture.
A ‘Frenemy’ is an enemy disguised as a friend; this term is a recent addition to popular lexicon, but the concept is as old as history. Angiogenesis is certainly a friend in ischemic tissues, but it can be an enemy in tumors, the retina, and connective tissues like tendon. Kimura et al. present the interesting possibility that neovascularization may be an important component of chordal disruption 4. Their finding that a proteolytically-cleaved glycoprotein, tenomodulin, may play a role in local angiogenesis of the chordae tendineae offers a new molecular insight into the sudden and sometimes dramatic event of chordal disruption.
Acknowledgments
Funding Sources
This work was supported by an American Heart Association grant 0835484N (to JY), and NIH grants HL073809 and HL048743 (to RTL).
Footnotes
Disclosures
None
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. In: Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 6. Braunwald E, Zipes DP, Libby P, editors. Philadelphia, Pa: Saunders Elsevier; 2001. p. 1654. [Google Scholar]
- 3.Millington-Sanders C, Meir A, Lawrence L, Stolinski C. Structure of chordae tendineae in the left ventricle of the human heart. J Anat. 1998;192:573–581. doi: 10.1046/j.1469-7580.1998.19240573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura N, Shukunami C, Hakuno D, Yoshioka M, Miura S, Docheva D, Kimura T, Okada Y, Matsumura G, Shin’oka T, Yozu R, Kobayashi J, Ishibashi-Ueda H, Hiraki Y, Fukuda K. Local tenomodulin absense, angiogenesis, and MMP activation are associated with the rupture of the chordae tendineae cordis. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.780031. [DOI] [PubMed] [Google Scholar]
- 5.Pufe T, Petersen WJ, Mentlein R, Tillmann BN. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005;15:211–222. doi: 10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka M, Yuasa S, Matsumura K, Kimura K, Shiomi T, Kimura N, Shukunami C, Okada Y, Mukai M, Shin H, Yozu R, Sata M, Ogawa S, Hiraki Y, Fukuda K. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med. 2006;12:1151–1159. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res. 1998;83:832–840. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Schonbeck U. Drilling for oxygen: angiogenesis involves proteolysis of the extracellular matrix. Circ Res. 2001;89:195–197. [PubMed] [Google Scholar]
- 10.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan L, Hoying JB, Nguyen H, Song H, Weiss JA. Interaction of angiogenic microvessels with the extracellular matrix. Am J Physiol Heart Circ Physiol. 2007;293:H3650–3658. doi: 10.1152/ajpheart.00772.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepper MS. Manipulating angiogenesis. From basic science to the bedside. Arterioscler Thromb Vasc Biol. 1997;17:605–619. doi: 10.1161/01.atv.17.4.605. [DOI] [PubMed] [Google Scholar]
- 13.Oshima Y, Sato K, Tashiro F, Miyazaki J, Nishida K, Hiraki Y, Tano Y, Shukunami C. Anti-angiogenic action of the C-terminal domain of tenomodulin that shares homology with chondromodulin-I. J Cell Sci. 2004;117:2731–2744. doi: 10.1242/jcs.01112. [DOI] [PubMed] [Google Scholar]
- 14.Shukunami C, Oshima Y, Hiraki Y. Chondromodulin-I and tenomodulin: a new class of tissue-specific angiogenesis inhibitors found in hypovascular connective tissues. Biochem Biophys Res Commun. 2005;333:299–307. doi: 10.1016/j.bbrc.2005.05.133. [DOI] [PubMed] [Google Scholar]
- 15.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 16.Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. Embo J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]